ABSTRACT
Purpose
Whether the association between objectively assessed physical activity and mortality differs between adults with versus those without frailty is unclear. We investigated this association in community-dwelling older adults.
Methods
This prospective study used the data of 4165 older adults 65 yr or older from the Kyoto–Kameoka study in Japan who wore a triaxial accelerometer (EW-NK52). The number of steps was classified by quartiles using the average daily value of data obtained from the accelerometer across four or more days. Frailty was evaluated using the validated Kihon Checklist. We evaluated the association between mortality and daily steps using a multivariable Cox proportional hazards analysis and restricted spline model.
Results
The average daily steps for the first, second, third, and fourth quartiles were 1786, 3030, 4452, and 7502, respectively. In total, 113 deaths were recorded during a median follow-up of 3.38 yr (14,061 person-years). After adjusting for confounders, the top quartile was associated with a lower hazard ratio (HR) for mortality than the bottom quartile (HR = 0.39, 95% confidence interval = 0.18–0.85). In a stratified model by frailty status, the daily step count dose–response curve at which the HR for mortality plateaued among nonfrail individuals was approximately 5000–7000 steps per day. By contrast, the daily step count showed an inverse relationship with mortality at approximately 5000 steps or more per day in frail individuals.
Conclusions
The relationship between daily steps and mortality is different between those with and those without frailty, and people with frailty may require more daily steps than those with nonfrailty to achieve the inverse relationship with mortality. These findings may be useful for informing future physical activity guidelines.
Key Words: DAILY STEP COUNT, PHYSICAL ACTIVITY, FRAILTY, ALL-CAUSE MORTALITY, SPLINE MODEL, KIHON CHECKLIST
Frailty is a condition in which the integrity of multiple physiological systems is impaired due to a loss of healthy equilibrium caused by stress responses associated with multidimensional risk factors such as physical, cognitive, and psychosocial abilities (1,2). A meta-analysis comprising adults older than 50 yr in 62 countries and territories reported a frailty rate of 12%–24% (3), demonstrating a strong association between frailty and adult mortality (4,5). Therefore, it is important to provide evidence of proper care for older adults with frailty at high risk of mortality.
Inadequate physical activity is a major correctable cause of adverse health effects (6,7), and its prevalence has been increasing over time in high-income countries (8). Deaths associated with lower physical activity contribute to an economic burden of approximately $13 billion due to productivity loss worldwide (7). Because the daily step count is a simple objective measure of physical activity that anyone can easily understand, it is an effective tool for setting physical activity goals and motivating individuals to increase physical activity (9,10). Daily step counts have been shown to vary greatly across 111 countries and territories (11). Furthermore, step count distribution differs depending on age, sex (11,12), and frailty status (12). Therefore, to extend life span, it is necessary to set daily step count goals that frail and nonfrail individuals can achieve every day.
Several prospective cohort studies have reported an inverse association between step count and mortality (13–20). A previous study reported that engaging in sports/recreation 1–2 times per week is inversely associated with mortality in sedentary men who have no risk factors such as smoking, overweight, history of hypertension, and hypercholesterolemia, whereas men with these high-risk factors required more physical activity (21). However, it is unclear whether the association between objectively assessed physical activity and mortality in older adults differs between those with versus those without frailty. An investigation in this regard may provide essential findings for setting targets and developing public policies on step counts for sedentary older adults, particularly frail individuals (12). This study aimed to evaluate the dose-dependent relationship between step count and all-cause mortality in a community-based longitudinal cohort study of older adults with and without frailty. Given that frailty in older adults is closely associated with mortality (4,5), we hypothesized that those with frailty require a higher daily step count to achieve an inverse association with mortality than those without frailty with reference to a previous study (21).
METHODS
Study population and assessment of baseline characteristics
The Kyoto–Kameoka study is a population-based prospective cohort study of older adults 65 yr or older living in Kameoka, Kyoto, Japan. Details of the study are explained elsewhere (5,12,22–26). Briefly, after the first baseline survey, the Health and Nutrition Status Survey (pretrial [second] survey) was conducted by postal mail in the district on February 14, 2012, obtaining responses from 8370 residents (response rate = 69.8%; Fig. 1). Of these, residents who were assigned to the comprehensive geriatric intervention program as part of the Kyoto–Kameoka cluster randomized controlled trial (n = 524), residents who could not be identified (n = 30), and those who moved out of the city or died (n = 282) were excluded. Accelerometers were distributed to the remaining 7534 people from April to November 2013 (third survey). A step count measurement was performed on 4368 people (response rate = 57.9%) for at least 1 d. This research protocol was approved by the Institutional Review Boards of the following institutions: the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN-76-2); Kyoto University of Advanced Science (No. 20-1); and Kyoto Prefectural University of Medicine (RBMR-E-363). All participants provided informed consent when responding to the mail survey. For reporting this study, we followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (27).
FIGURE 1.
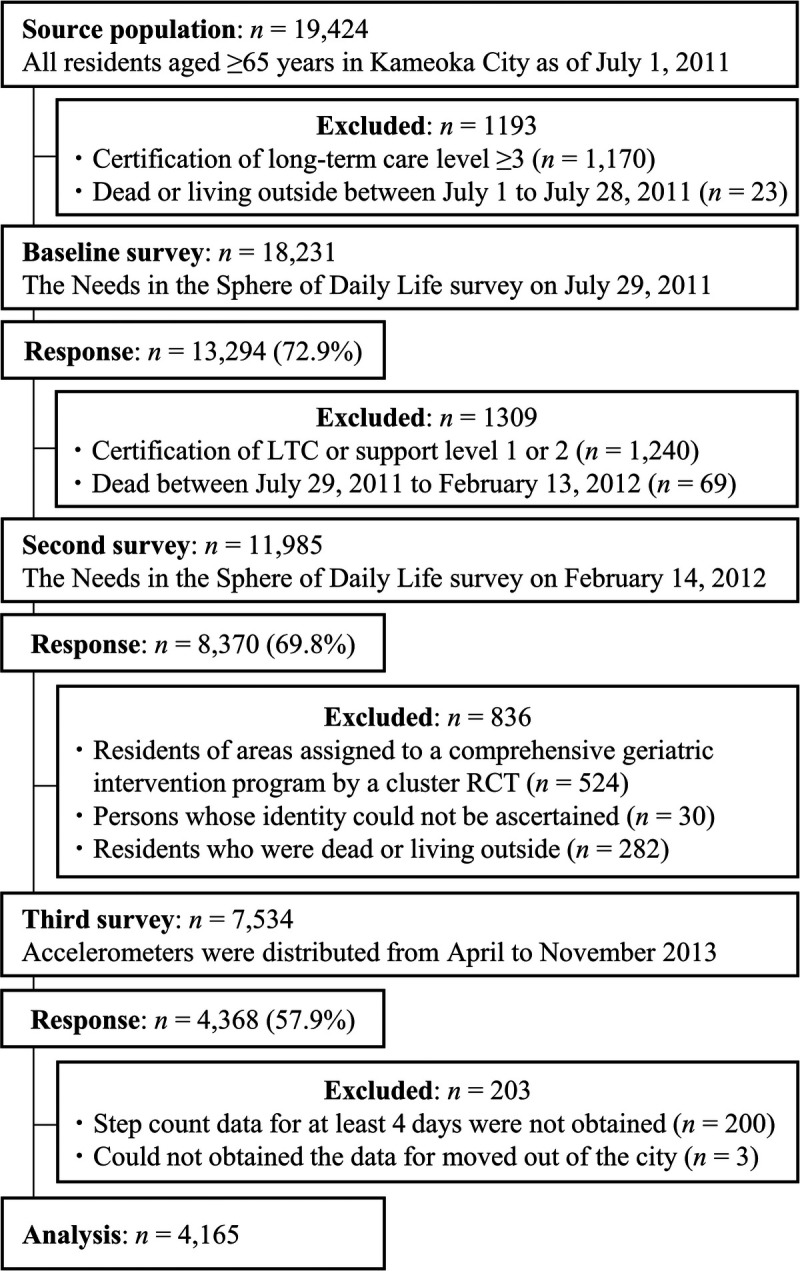
Participant flow diagram for the analysis of daily step counts and mortality in the Kyoto–Kameoka study. RCT, randomized controlled trial, LTC, long-term care.
From the participants included at baseline (n = 4368), we excluded individuals with missing data on the step count, as measured by not having appropriate accelerometer use (n = 200) (12), and those with an unknown date of moving away from the community (n = 3). Ultimately, 4165 participants were included in this study.
Assessment of daily steps counts
We measured the number of steps as an objective physical activity index using a triaxial accelerometer (EW-NK52; Panasonic Co., Ltd, Osaka, Japan), the details of which are described in a previous study (12). From April 1 to November 15, 2013, we mailed residents an accelerometer and printed material describing how to use it and asked them to wear it for 10 d or more. Participants were instructed to wear the accelerometer on their waists from when they woke up to when they went to bed, but not when sleeping, bathing, and swimming; except for this, they were asked to continue with their routine tasks as usual. The daily step count was determined using the manufacturer’s step algorithm. Because we could not obtain the wear time of the accelerometer, we excluded from analysis any data points lower than the 1st percentile (499 steps for males and 653 for females) of step counts in older adults 60–69 yr old as reported in the National Health and Nutrition Surveys Japan (NHNS-J) (28) to eliminate individuals with lower adherence to wear time of the accelerometer (12). To calculate the daily step count, we summed the steps surveyed during four or more days (including one holiday day) and divided this by the number of adhered days to obtain the average daily step count. Individual male and female measurements estimated based on a 4-d step survey had a correlation coefficient (r) of 0.90 with respect to the “true” average step count, suggesting that a 4-d accelerometer study was sufficient to reflect the individuals’ habitual step counts (12).
Definition of frailty
Frailty was assessed using a validated, self-administered Kihon Checklist (KCL), which consists of 25 question items (5,24). The KCL evaluates frailty from multiple aspects, including physical, social, and cognitive factors (comprehensive frailty). Frailty was defined as having at least seven positives in the 25-item list (5,24). A prospective cohort study has shown that KCL scores are associated with the risk of death in older adults during the 5-yr study period (5).
Outcomes
The life status of the participants during the follow-up period, current to November 30, 2016, was evaluated using the information in the Basic Resident Register managed by the Kameoka City Hall. Residents whose records were administratively removed or who moved out of the municipality were censored (20 individuals [36 person-years] of 4165 individuals [14,061 person-years]).
Statistical analysis
We needed 3658 participants to estimate the “true” mean step count of the group within a 95% confidence interval (CI) and error margin of 2.5% (12). Using “power cox” in STATA, we estimated that a sample size of 138 would have 80% power (1 − beta error) with a level of significance of 5% to detect a 38% difference in the hazard ratios (HR) of mortality when comparing the second and the first quartiles of daily step counts according to previous studies in older adults 60 yr or older (13). Therefore, the sample available for this study was sufficiently large.
Daily step counts were classified into four groups by quartiles. Descriptive statistics for continuous and categorical variables are shown as means and standard deviations and numbers and percentages, respectively. Where information pertaining to body mass index (n = 6; 0.1%), family structure (n = 269; 6.5%), socioeconomic status (n = 170; 4.1%), education attainment (n = 405; 9.7%), smoking status (n = 150; 3.6%), alcohol use (n = 124; 3.0%), denture use (n = 107; 2.6%), medications (n = 285; 6.8%), or frailty status (n = 493; 11.8%) was missing, these covariates were complemented from five data sets created by the multiple imputation method using the Multivariate Imputation by Chained Equation package of R (29) to prevent a systematic error appearing due to selection bias. All missing values were assumed missing at random.
The rate of all-cause mortality for each daily step count quartile is shown as the number of events per 1000 person-years. We used a multivariable Cox proportional hazards model that included baseline covariates to adjust for confounding factors associated with step count and all-cause mortality. The assumption for the Cox proportional hazards models was confirmed using the Schoenfeld residuals test (P = 0.138). Multivariable analysis was verified using two models. Model 1 adjusted for age (continuous), sex (female or male), population density (≥1000 or <1000 people per km2), and the step count assessment season (spring, summer, or autumn). Model 2 adjusted for all variables in model 1 and body mass index (continuous), smoking status (never smoked, past smoker, or current smoker), alcohol drinking (yes or no), living alone (yes or no), educational attainment (<9, 10–12, or ≥13 yr), socioeconomic status (high or low), denture use (yes or no), medication use (continuous), number of chronic diseases (continuous), and frailty (yes or no). These adjustment factors were determined after referencing previous studies (13–20). Health-related information, including medical history, socioeconomic status, smoking, and alcohol consumption, was extracted from the first and second (pretrial) surveys (Fig. 1). The results of these analyses are presented as HR and 95% CI. HR values were calculated using the first quartile as a reference. The P value of the linear trend was calculated by treating the step count exposure variable as a continuous variable. These analyses were also performed after stratifying by the frailty status. The HR and the 95% CI values for mortality per 1000 steps per day categories were calculated using those with less than 1000 steps per day as a reference (14). HR and corresponding 95% CI were estimated for a 1000 steps per day increase in daily step count stratified by approximately 5000 steps (<5000 or ≥5000) in total and in nonfrail individuals and approximately 2500 steps (<2500 or ≥2500) in frail individuals (12) because the curvilinear relationship between daily step count or total physical activity and mortality is also well-documented within the literature (13). For the sensitivity analysis, we used the following two methods: 1) to avoid the possibility of a reversed causal relationship, death events in the first 1 yr of the follow-up study (12 men) were excluded from the analysis, and 2) a similar analysis was performed using a case data set that did not contain missing values (30).
We estimated the propensity scores for assignment into the daily step count quartiles using a multivariable logistic regression model that included the variables in model 2 and created multivariable-adjusted Kaplan–Meier survival curves using inverse probability weighting methods. We also created Nelson–Aalen cumulative hazard curves for mortality according to quartiles of daily step count using age as the time scale (22). Further, we used a restricted cubic spline model with three knots (5th, 50th, and 95th percentiles) based on the distribution of steps to evaluate the curvilinearity of the relationship between the step counts and the all-cause mortality (5,12,14,22,23). These results are presented as HR and 95% CI, with the HR calculated using the average value of the first step count quartile as the reference value. Because the data were sparse, we truncated the analysis at 12,000 steps per day (99% of the distribution) (14,22). Statistical significance of nonlinearity was assessed using a Wald test, comparing the likelihood ratio of the spline model with the linear model, and P values of <0.05 were regarded as indicating a statistically significant nonlinear relationship between the exposure and the outcome.
A two-tailed probability of less than 5% was considered significant for all statistical analyses. Statistical analysis was performed using STATA MP, Version 15.0 (StataCorp LP, College Station, TX, USA) and R software 3.4.3 (R Core Team, Vienna, Austria).
RESULTS
Participant characteristics
Table 1 shows the participant characteristics by the daily step count quartiles in the analyzed cohort. The mean daily step count (standard deviation) for the entire population was 4192 (2395). The higher the number of steps, the larger the number of people not taking medication, drinking alcohol, and males. These participants were younger, had fewer diseases, and fewer were frail. Furthermore, this study included fewer participants with frailty and fewer mortality events than in the baseline (pretrial) survey (Supplemental Table 1, Supplemental Digital Content, Characteristics of participants with baseline and additional surveys and accelerometer study in the Kyoto–Kameoka Study, http://links.lww.com/MSS/C795).
TABLE 1.
Baseline characteristics of the study participants by quartile of daily step count.
| Total (n = 4165) | Quartile of the Daily Step Count | ||||
|---|---|---|---|---|---|
| Q1 (n = 1042) | Q2 (n = 1040) | Q3 (n = 1042) | Q4 (n = 1041) | ||
| Age (yr)a | 72.3 ± 5.4 | 74.7 ± 6.1 | 72.6 ± 5.3 | 71.4 ± 4.7 | 70.4 ± 4.3 |
| Women, n (%)b | 2028 (48.7) | 544 (52.2) | 564 (54.2) | 526 (50.5) | 394 (37.8) |
| PD ≥1000 people per km2, n (%)b | 2037 (48.9) | 517 (49.6) | 535 (51.4) | 513 (49.2) | 472 (45.3) |
| Body mass index (kg·m−2)a | 22.7 ± 3.2 | 22.8 ± 3.6 | 22.8 ± 3.5 | 22.6 ± 2.9 | 22.4 ± 2.6 |
| Current smoke, n (%)b | 422 (10.1) | 127 (12.2) | 94 (9.0) | 104 (10.0) | 97 (9.3) |
| Alcohol drinker, n (%)b | 2892 (69.4) | 680 (65.3) | 679 (65.3) | 734 (70.4) | 799 (76.8) |
| Living alone, n (%)b | 481 (11.5) | 121 (11.6) | 134 (12.9) | 131 (12.6) | 95 (9.1) |
| Education ≥13 yr, n (%)b | 989 (23.7) | 215 (20.6) | 234 (22.5) | 248 (23.8) | 292 (28.0) |
| HSES, n (%)b | 1469 (35.3) | 342 (32.8) | 388 (37.3) | 367 (35.2) | 372 (35.7) |
| Denture use, n (%)b | 2432 (58.4) | 650 (62.4) | 610 (58.7) | 614 (58.9) | 558 (53.6) |
| No medication, n (%)b | 1020 (24.5) | 209 (20.1) | 223 (21.4) | 265 (25.4) | 323 (31.0) |
| Hypertension, n (%)b | 1506 (36.2) | 430 (41.3) | 367 (35.3) | 358 (34.4) | 351 (33.7) |
| Stroke, n (%)b | 124 (3.0) | 32 (3.1) | 37 (3.6) | 22 (2.1) | 33 (3.2) |
| Heart disease, n (%)b | 464 (11.1) | 151 (14.5) | 128 (12.3) | 85 (8.2) | 100 (9.6) |
| Diabetes, n (%)b | 393 (9.4) | 110 (10.6) | 90 (8.7) | 90 (8.6) | 103 (9.9) |
| Hyperlipidemia, n (%)b | 450 (10.8) | 107 (10.3) | 133 (12.8) | 122 (11.7) | 88 (8.5) |
| Digestive disease, n (%)b | 353 (8.5) | 106 (10.2) | 91 (8.8) | 88 (8.4) | 68 (6.5) |
| Respiratory disease, n (%)b | 172 (4.1) | 59 (5.7) | 45 (4.3) | 37 (3.6) | 31 (3.0) |
| Urological diseases, n (%)b | 274 (6.6) | 72 (6.9) | 62 (6.0) | 57 (5.5) | 83 (8.0) |
| Cancer, n (%)b | 120 (2.9) | 40 (3.8) | 35 (3.4) | 29 (2.8) | 16 (1.5) |
| No. of chronic diseasesa,c | 0.93 ± 0.95 | 1.06 ± 1.03 | 0.95 ± 0.95 | 0.85 ± 0.91 | 0.84 ± 0.91 |
| Frailty, n (%)b | 1029 (24.7) | 371 (35.6) | 266 (25.6) | 225 (21.6) | 167 (16.0) |
| Daily step count (steps per day)a | 4192 ± 2395 | 1786 ± 418 | 3030 ± 352 | 4452 ± 492 | 7502 ± 2061 |
Missing values were supplemented using the multivariate imputation method: body mass index (n = 6; 0.1%), family structure (n = 269; 6.5%), socioeconomic status (n = 170; 4.1%), education attainment (n = 405; 9.7%), smoking status (n = 150; 3.6%), alcohol drinker (n = 124; 3.0%), denture use (n = 107; 2.6%), medications (n = 285; 6.8%), and frailty status (n = 493; 11.8%). Body mass index was calculated as body weight (kg) divided by height squared (m2). Q1 to Q4 consist of daily step counts of <2433, 2433–3639, 3641–5350, and ≥5357 steps, respectively.
aContinuous values are shown as mean ± SD.
bCategorical values are shown as number (percentage).
cFrom the data obtained on disease status (including the presence of hypertension, stroke, heart disease, diabetes, hyperlipidemia, digestive disease, respiratory disease, urological diseases, and cancer), the comorbidity scores were summed to obtain a total score ranging from 0 (no comorbidity) to 9 (poor status).
Q, quartiles; PD, population density; HSES, high socioeconomic status.
Step counts and mortality
Table 2 and Figure 2A show the relationship between the number of daily steps and the all-cause mortality. The median follow-up period for all participants was 3.38 yr (interquartile range = 3.28 to 3.53). In total, 113 people (2.7%) died during the follow-up period (14,061 person-years). After adjusting for confounding factors, an inverse association was observed between the number of daily steps and the all-cause mortality (Q1: reference; Q2: HR = 0.84 [95% CI = 0.53–1.32]; Q3: HR = 0.57 [95% CI = 0.31–1.03]; Q4: HR = 0.39 [95% CI = 0.18–0.85]). The Nelson–Aalen cumulative hazard curves using age as the time scale were similar to these results and showed an association between higher mortality and the first step count quartile (Fig. 2B). The HR values (95% CI) for all-cause mortality when the number of daily steps increased by 1000 were 0.77 (95% CI = 0.56–0.98) and 1.04 (95% CI = 0.68–1.40) in participants with fewer than 5000 steps and those with 5000 steps or more, respectively. Similar results were obtained in the sensitivity analysis (Supplemental Tables 2 and 3, Supplemental Digital Content, Results of sensitivity analysis for the relationship between all-cause mortality and daily step count using complete case data; and Results of sensitivity analysis for the relationship between all-cause mortality and daily step count after excluding participants with an event in the first one year of follow-up, http://links.lww.com/MSS/C795) and age- and sex-stratified analysis (Supplemental Table 4, Supplemental Digital Content, Hazard ratios for daily step counts and all-cause mortality calculated using age- and sex-stratified multivariable Cox proportional hazards analysis, http://links.lww.com/MSS/C795). Figure 3A shows the dose–response relationship between number of daily steps and mortality using the restricted cubic spline model. The first daily step count quartile (1786 steps per day) was used as the standard. The daily step count at which the HR for mortality plateaued among the total participants was approximately 5000–7000 steps per day (P for nonlinearity = 0.019). This spline analysis model fitted the data well compared with the linear regression analysis (Akaike Information Criterion; 1661 vs 1666).
TABLE 2.
Multivariable cox proportional hazard model of the mortality risk according to daily step counts.
| Step Counts | n | Event | PY | Event/1000 PY | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate (95% CI) | HR (95% CI) | HR (95% CI) | |||||||
| Quartile (mean) | |||||||||
| Q1 (1786 steps) | 1042 | 60 | 3447 | 17.4 (13.5–22.4) | 1.00 (Ref) | 1.00 (Ref) | |||
| Q2 (3030 steps) | 1040 | 30 | 3502 | 8.6 (6.0–12.3) | 0.83 (0.53–1.30) | 0.84 (0.53–1.32) | |||
| Q3 (4452 steps) | 1042 | 15 | 3544 | 4.2 (2.6–7.0) | 0.55 (0.31–0.99) | 0.57 (0.31–1.03) | |||
| Q4 (7502 steps) | 1041 | 8 | 3568 | 2.2 (1.1–4.5) | 0.35 (0.16–0.76) | 0.39 (0.18–0.85) | |||
| 1000 step units | |||||||||
| <1000 | 40 | 6 | 123 | 48.8 (21.9–108.6) | 1.00 (Ref) | 1.00 (Ref) | |||
| 1000–1999 | 618 | 42 | 2022 | 20.8 (15.3–28.1) | 0.75 (0.32–1.78) | 0.80 (0.33–1.90) | |||
| 2000–2999 | 895 | 32 | 2985 | 10.7 (7.6–15.2) | 0.61 (0.25–1.47) | 0.63 (0.26–1.54) | |||
| 3000–3999 | 787 | 16 | 2629 | 6.1 (3.7–9.9) | 0.47 (0.18–1.24) | 0.51 (0.19–1.35) | |||
| 4000–4999 | 625 | 7 | 2112 | 3.3 (1.6–7.0) | 0.30 (0.10–0.91) | 0.33 (0.11–1.01) | |||
| 5000–5999 | 443 | 5 | 1518 | 3.3 (1.4–7.9) | 0.29 (0.09–0.98) | 0.31 (0.09–1.06) | |||
| ≥6000 | 297 | 5 | 2672 | 1.9 (0.8–4.5) | 0.20 (0.06–0.69) | 0.23 (0.07–0.81) | |||
| P for trendc | 0.001 | 0.003 | |||||||
| 1000 steps increment | |||||||||
| Total | 4165 | 0.79 (0.66–0.92) | 0.81 (0.68–0.94) | ||||||
| <5000 steps | 2943 | 0.74 (0.54–0.95) | 0.77 (0.56–0.98) | ||||||
| ≥5000 steps | 1222 | 1.05 (0.72–1.38) | 1.04 (0.68–1.40) | ||||||
Q1 through Q4 include daily step counts of <2437, 2437–3653, 3654–5378, and ≥5379 steps, respectively, in total participants. The step counts are expressed as quartiles (mean values).
aModel 1: adjusted for age, sex, population density, and season of wear.
bModel 2: adjusted for model 1 and body mass index, smoking status, alcohol consumption status, family structure, educational attainment, economic status, denture use, medication use, number of chronic diseases, and frailty status.
cLinear trend P values were calculated using the likelihood ratio test and a continuous variable of daily step counts.
PY, person-years; Q, quartile; Ref, reference.
FIGURE 2.
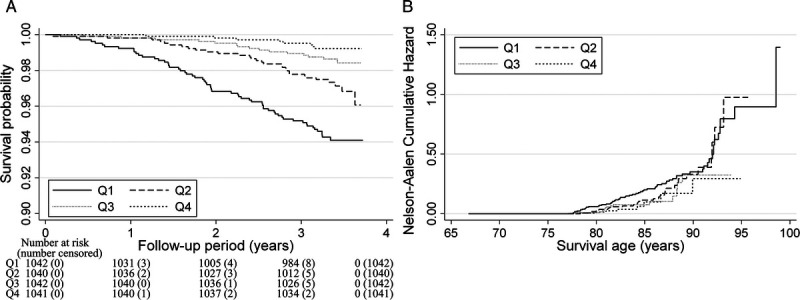
Survival analysis for all-cause mortality according to daily step counts among older adults. A, Multivariable-adjusted Kaplan–Meier survival curves using inverse probability weighting according to quartiles (Qs) of daily step counts. B, Nelson–Aalen cumulative hazard curves using age as the time scale. The adjustment factors were age, sex, population density, season of wear, body mass index, smoking status, alcohol consumption status, family structure, educational attainment, economic status, denture use, medication use, number of chronic diseases, and frailty status.
FIGURE 3.
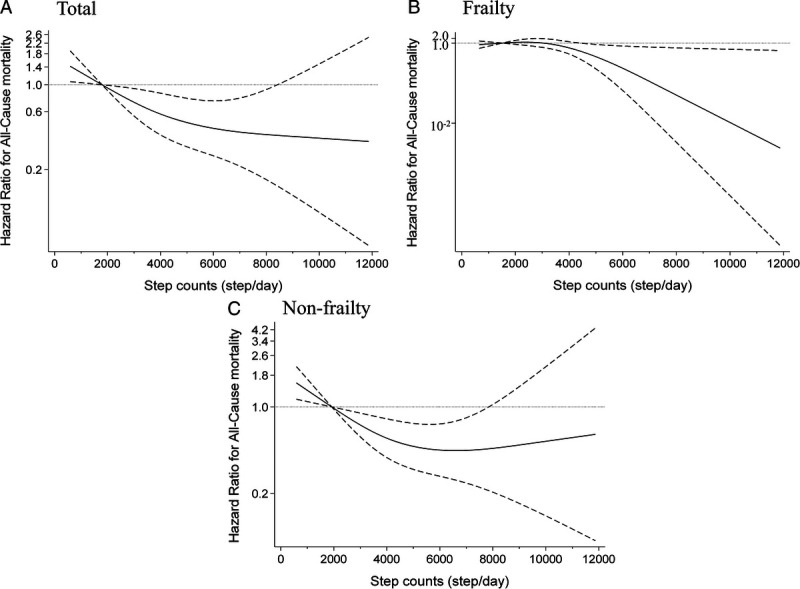
Restricted cubic spline regression model between daily step count and risk of mortality. Solid lines represent HR, dashed lines represent 95% CI, and the HR based on 1786 steps per day in the total participants (n = 4124, P for nonlinearity = 0.019) (A), 1514 steps per day in frail individuals (n = 1022, P for nonlinearity = 0.021) (B), and 1922 steps per day in nonfrail individuals (n = 3102, P for nonlinearity = 0.012) (C) as the reference (mean step counts for the first quartile value) were calculated. We estimated that P < 0.05 when the 95% CI of the HR did not exceed 1.00, and P ≥ 0.05 when the 95% CI of the HR exceeded 1.00. The adjustment factors were age, sex, population density, season of wear, body mass index, smoking status, alcohol consumption status, family structure, educational attainment, economic status, denture use, medication use, number of chronic diseases, and frailty status.
Stratified model by the state of frailty
Table 3 shows the relationship between step counts and mortality in individuals with and without frailty. The prevalence of frailty in this population was 24.7% (95% CI = 23.4 to 26.0). The multivariable-adjusted HR values (95% CI) of mortality for a 1000 steps per day increment were 0.28 (95% CI = 0.07–0.97) and 0.68 (95% CI = 0.40–0.96) in frail individuals with 2500 steps or more and nonfrail individuals with fewer than 5000 steps, respectively. These results showed significant differences for frailty status (P = 0.048), which means more pronounced results in frail individuals with a higher step count and nonfrail individuals with a lower step count. Similar results were obtained in the spline models; the daily step count at which the HR for mortality plateaued among nonfrail individuals was approximately 5000–7000 steps per day, whereas the daily step count showed an inverse relationship with mortality at approximately 5000 steps or more per day in frail individuals (Figs. 3B and 3C).
TABLE 3.
HR for daily step counts and all-cause mortality calculated using the multivariable Cox proportional hazards model in frail and nonfrail individuals.
| Step Counts | n | Event | PY | Event/1000 PY | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate (95% CI) | HR (95% CI) | HR (95% CI) | |||||||
| Frailty | |||||||||
| Q1 (1514 steps) | 257 | 21 | 847 | 24.8 (16.2–38.0) | 1.00 (Ref) | 1.00 (Ref) | |||
| Q2 (2484 steps) | 257 | 14 | 859 | 16.3 (9.7–27.5) | 0.94 (0.47–1.87) | 0.86 (0.42–1.74) | |||
| Q3 (3704 steps) | 257 | 8 | 870 | 9.2 (4.6–18.4) | 0.65 (0.28–1.52) | 0.64 (0.27–1.52) | |||
| Q4 (6434 steps) | 258 | 1 | 885 | 1.1 (0.2–8.0) | 0.10 (0.01–0.78) | 0.10 (0.01–0.73) | |||
| P for trendc | 0.011 | 0.010 | |||||||
| 1000 steps increment | |||||||||
| Total | 1029 | 0.66 (0.41–0.92) | 0.65 (0.39–0.92) | ||||||
| <2500 steps | 387 | 1.02 (0.17–1.87) | 1.05 (0.19–1.92) | ||||||
| ≥2500 steps | 642 | 0.26 (0.06–0.93) | 0.28 (0.07–0.97) | ||||||
| Nonfrailty | |||||||||
| Q1 (1922 steps) | 784 | 37 | 2598 | 14.2 (10.3–19.7) | 1.00 (Ref) | 1.00 (Ref) | |||
| Q2 (3232 steps) | 784 | 15 | 2650 | 5.7 (3.4–9.4) | 0.71 (0.38–1.32) | 0.75 (0.40–1.40) | |||
| Q3 (4692 steps) | 784 | 10 | 2667 | 3.7 (2.0–7.0) | 0.55 (0.27–1.13) | 0.58 (0.28–1.21) | |||
| Q4 (7785 steps) | 784 | 7 | 2684 | 2.6 (1.2–5.5) | 0.51 (0.22–1.21) | 0.59 (0.25–1.41) | |||
| P for trendc | 0.027 | 0.057 | |||||||
| 1000 steps increment | |||||||||
| Total | 3136 | 0.83 (0.68–0.98) | 0.86 (0.71–1.00) | ||||||
| <5000 steps | 2115 | 0.66 (0.38–0.94) | 0.68 (0.40–0.96) | ||||||
| ≥5000 steps | 1021 | 1.07 (0.73–1.40) | 1.04 (0.68–1.40) | ||||||
| P for between groups | 0.045 | 0.048 | |||||||
Q1 through Q4 include daily step counts of <2027, 2028–3019, 3021–4473, and ≥4474 steps, respectively, in frail individuals and <2620, 2621–3892, 3893–5640, and ≥5642 steps, respectively, in nonfrail individuals. The step counts are expressed as quartiles (mean values).
aModel 1: adjusted for age, sex, population density, and season of wear.
bModel 2: adjusted for model 1 and body mass index, smoking status, alcohol consumption status, family structure, educational attainment, economic status, denture use, medication use, and number of chronic diseases.
cLinear trend P values were calculated using the likelihood ratio test and a continuous variable of daily step counts.
PY, person-years; Q, quartile; Ref, reference.
DISCUSSION
Main findings
The daily step count dose–response curve at which the HR for mortality plateaued among nonfrail individuals was approximately 5000–7000 steps per day. By contrast, the daily step count showed an inverse relationship with mortality at approximately 5000 steps or more per day in frail individuals. To the best of our knowledge, this was the first study demonstrating that the association between objectively assessed physical activity and mortality in older adults differs between those with versus those without frailty.
Comparison with previous studies
Several studies have shown an inverse association between step counts and mortality (13–20). A pooled analysis of 47,471 adults from 15 international cohorts indicated that daily step count showed a strong inverse dose-dependent association with mortality up to approximately 8000–10,000 steps per day in individuals younger than 60 yr, but no significant differences were observed thereafter (L-shaped relationship) (13). However, the daily step counts showed a strong inverse relationship with mortality up to about 6000–8000 steps in those older than 60 yr, and then a weak, dose-dependent, inverse relationship up to about 16,000 steps (13). Our results are consistent with the results of these previous studies. Data from our dose–response curves and previous studies showed that daily step count and mortality were strongly inversely associated, especially in individuals with low step counts. These findings suggest that increasing the number of steps might particularly benefit older adults with numerous sedentary behaviors including low daily step counts.
A previous study observed that those most physically active based on self-reports (≥10,000 MET-min·wk−1) had a high mortality rate (31). However, like in previous studies (13,14,19,20), this tendency was not observed in the relationship between the objectively evaluated daily step count and mortality in this study. Because step count can differ by 20% or more between devices (for example, a difference of about 1000 steps per day for a value of 5000 steps per day), care must be taken when interpreting absolute step count values (32). However, the step counts measured by these devices have a high correlation coefficient between devices (r ≥ 0.80) (32). Similar results were reported by a study that compared the steps measured by 13 selected consumer-based and research-grade wearable devices in young and middle-age Japanese individuals (33). Therefore, measurement error should be considered when extrapolating our and previous studies’ results.
Differences between those with and without frailty
Our findings showed that people with frailty may require more daily steps to achieve an inverse association with mortality than those without frailty. Similar results have been observed in a previous study that compared American men with and without risk factors such as smoking, overweight, and history of disease (21). A previous study also reported that physical activity (inactive or active) assessed using a single self-reported question was inversely associated with mortality in frail, prefrail, and robust people, which is consistent with our results (34). However, this previous study could not evaluate the dose–response relationship between physical activity and mortality in individuals with and without frailty because physical activity was evaluated from a single question and divided into two groups (34). Self-reported physical activity assessment can lead to increases in systematic reporting biases because the participants modify their responses in the desired direction independently of actual behavior change (35). Therefore, further well-designed prospective cohort studies are needed to evaluate the differences in the association between objectively assessed physical activity and mortality in older adults with versus without frailty. Quantifying the dose–response relationship between daily steps and mortality stratified by those with and without frailty may be useful for informing future physical activity guidelines.
Mechanism
The detailed mechanism by which physical activity is inversely associated with mortality remains unclear, but several previous studies suggested two possible reasons. First, step count was inversely associated with the risk of diabetes (36), cardiovascular disease (36–38), and fractures (38). In fact, the World Health Organization 2020 guidelines for physical activity and sitting behavior (39) showed that physical activity in older adults improved health outcomes and helped prevent functional decline. Interrupting sedentary behavior with low-intensity walking has been demonstrated to improve the adverse effects of sitting on superficial femoral artery endothelial function (40). Our data supported these previous reports by showing that those with higher step counts had a lower mortality risk. Second, the number of steps is inversely associated with the maintenance of skeletal muscle mass and the proportion of frailty. We reported that the number of steps was inversely associated with the proportion of individuals with frailty (12). Higher step counts might be associated with improvements in the frailty status, which was associated with higher mortality risk. A mail-based walking intervention study showed that the walking intervention group had higher levels of serum dehydroepiandrosterone and insulin-like growth factor as anabolic hormones and skeletal muscle mass than the control group (41), and these studies may support our findings. Because our study could not prove why frail individuals need more physical activity to achieve an inverse association with mortality than nonfrail individuals, a detailed evaluation of these relationships through intervention studies and basic research is necessary.
Perspective
It is important to set achievable goals to improve the physical activity of older adults (9,10). A typical target daily step count in software programs for wearable devices and smartphones is 10,000 steps. Although this common goal can be traced back to Japanese walking clubs and a business slogan about 60 yr ago, it is not evidence based (14,42). The distribution of our step count data suggests that this target is difficult for older adults to achieve. An effort called “+10” in Japan encourages individuals to add 10 min·d−1 of moderate–vigorous physical activity, as this exceeds 3 METs or more and can be performed in their daily lives (43). This is also recommended by the Japanese official physical activity guidelines for health promotion (44). A meta-analysis of 26 cohort studies showed that “+10” was associated with a 3.2% reduction in the relative risk of composite outcomes, including death, lifestyle-related diseases, cancer, and dementia (45). According to the NHNS-J 2010, 66.8% of those 60–69 yr old and 50.8% of those 70 yr or older answered that they could increase their physical activity by 10 min·d−1 (46). Therefore, our results suggest that a “+10” plan that aims to increase the number of daily steps slightly to improve health status including life span (about 1000 steps per day) could be an achievable goal for many older adults.
Strengths and limitations
The strength of this study is that it examined the relationship between the number of daily steps measured by an accelerometer and the mortality in a large cohort of community-dwelling older adults. A previous study has reported that objective physical activity, assessed by a device, was more strongly associated with health outcomes than self-reported measurements (47). Additionally, the sample size and the number of assessment days for step counts in this study were sufficient (12), providing strong support for these relationships. However, this study has some methodological limitations. First, it was impossible to ensure the obtained step count data from the participants were unbiased. The characteristics and mortality risk of those included in this study and those answering the baseline (pretrial) survey were different (Supplemental Table 1, Table, Supplemental Digital Content, http://links.lww.com/MSS/C795). In addition, participants may have been more health conscious than the general population of older adults when wearing the device. The daily step counts may have been underestimated by including days of low accelerometer adherence because we could not obtain the wear time of the accelerometer. Previous studies used criteria based on the distribution of step counts to eliminate individuals with lower adherence to wear time for pedometers (cannot obtain objective wear time) (28) and wearable devices (36). Despite performing data cleaning, this study may have introduced a measurement error. Nevertheless, the average daily step counts estimated from our study (mean age = 72.3 yr) did not differ significantly compared with the median step counts in older adults 70–79 yr old as reported in the NHNS-J in 2016 (28). Second, our study had a relatively short observation period, explaining the wider 95% CI in the spline analysis and the small number of mortality events in those with a high step count (13,18,20). Previous studies have shown a stronger association between step counts and mortality in shorter than in longer follow-up studies (13,48). This is because the HR estimated from the data analysis might change over time, and the results based on a shorter observation period might overestimate by confounding as a result of preexisting conditions or poor health on the relationship between exposure factors and outcomes and invert causal relationships (48). Furthermore, because data on the cause of death could not be obtained, it was impossible to examine its association with the number of daily steps. Third, the step count measurements were taken only at baseline, and the participants’ step count may have changed during the follow-up period. However, a study of Japanese older adults showed that the individual step count ranking at baseline was well maintained for 8 yr for both males and females (rank correlation coefficient ≥0.6) (49). This study’s participants may also have had a stable individual step count ranking during the follow-up period. Finally, our study adjusted for several confounding factors, but residual confounders in the association between step count and all-cause mortality may remain. Therefore, further prospective studies with randomly sampled participants and longer-term follow-up are needed to assess the association of step count trajectories with specific-cause death.
CONCLUSIONS
Our results showed that the relationship between daily step count and mortality is different between those with and without frailty, and people with frailty may require more daily steps to achieve an inverse association with mortality than those without frailty. Given the recent increase in the degree of frailty in most adult age-groups in the United States (1), our findings are encouraging news to older adults, including many with frailty or a sedentary lifestyle. These findings may be useful for informing future physical activity guidelines.
Supplementary Material
Acknowledgments
The authors express their appreciation to all the participants of this study and to all individuals involved in the data collection. They acknowledge several administrative staff of Kameoka city and Kyoto prefecture. They thank the Kyoto–Kameoka Study Group who contributed their resources to the development of this study. They also thank Editage (www.editage.jp) for English-language editing.
The Kyoto–Kameoka study was conducted with JSPS KAKENHI and was supported by a research grant provided to Misaka Kimura (24240091), Yosuke Yamada (15H05363), and Daiki Watanabe (21K17699); a grant and administrative support by the Kyoto Prefecture Community-based Integrated older adults Care Systems Promotion Organization since 2011; Kameoka City under the program of the Long-term Care Insurance and Planning Division of the Health and Welfare Bureau for the older adults, Ministry of Health, Labour, and Welfare; and the World Health Organization (WHO) Collaborating Centre on Community Safety Promotion.
D. W., T. Y., and Y. W. conceived the concept of the study. M. K. and Y. Y. managed the project. T. Y., Y. W., M. K., and Y. Y. performed data acquisition or entry. D. W. and M. M. performed the literature review. D. W. performed data analysis and graphics. D. W. and M. M. wrote the first draft of the manuscript. All authors contributed to the writing of the manuscript. All authors have read and approved the final manuscript.
The authors declare that they have no competing interests. The authors declare that the results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Availability of data and materials: All data sharing and collaboration requests should be directed to the corresponding author (d2watanabe@nibiohn.go.jp), T. Y. (t-yoshida@nibiohn.go.jp), and Y. Y. (yamaday@nibiohn.go.jp).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
TSUKASA YOSHIDA, Email: t-yoshida@nibiohn.go.jp.
YUYA WATANABE, Email: yu15-watanabe@my-zaidan.or.jp.
YOSUKE YAMADA, Email: yamaday@nibiohn.go.jp.
MOTOHIKO MIYACHI, Email: cardiovascular0327@mac.com.
MISAKA KIMURA, Email: kimura.misaka@kuas.ac.jp.
REFERENCES
- 1.Blodgett JM, Rockwood K, Theou O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev. 2021;2(2):e96–104. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Caoimh R Sezgin D O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104. [DOI] [PubMed] [Google Scholar]
- 4.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe D Yoshida T Yamada Y, et al. Combined use of two frailty tools in predicting mortality in older adults. Sci Rep. 2022;12(1):15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding D Lawson KD Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311–24. [DOI] [PubMed] [Google Scholar]
- 8.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):e1077–86. [DOI] [PubMed] [Google Scholar]
- 9.Patel MS Bachireddy C Small DS, et al. Effect of goal-setting approaches within a gamification intervention to increase physical activity among economically disadvantaged adults at elevated risk for major adverse cardiovascular events: the ENGAGE randomized clinical trial. JAMA Cardiol. 2021;6(12):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett DR, Jr, Toth LP, LaMunion SR, Crouter SE. Step counting: a review of measurement considerations and health-related applications. Sports Med. 2017;47(7):1303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Althoff T, Sosic R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017;547(7663):336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe D Yoshida T Watanabe Y Yamada Y Kimura M, Group KS . Objectively measured daily step counts and prevalence of frailty in 3,616 older adults. J Am Geriatr Soc. 2020;68(10):2310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paluch AE Bajpai S Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7(3):e219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med. 2019;179(8):1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jefferis BJ Parsons TJ Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53(16):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dwyer T Pezic A Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10(11):e0141274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto N Miyazaki H Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18(1):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paluch AE Gabriel KP Fulton JE, et al. Steps per day and all-cause mortality in middle-aged adults in the coronary artery risk development in young adults study. JAMA Netw Open. 2021;4(9):e2124516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saint-Maurice PF Troiano RP Bassett DR Jr, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323(12):1151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen BH Dalene KE Ekelund U, et al. Step by step: association of device-measured daily steps with all-cause mortality-a prospective cohort study. Scand J Med Sci Sports. 2020;30(9):1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee IM, Sesso HD, Oguma Y, Paffenbarger RS, Jr. The “weekend warrior” and risk of mortality. Am J Epidemiol. 2004;160(7):636–41. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe D Yamada Y Yoshida T, et al. Association of the interaction between physical activity and sitting time with mortality in older Japanese adults. Scand J Med Sci Sports. 2022;32(12):1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe D Yoshida T Nanri H, et al. Association between the prevalence of frailty and doubly labeled water-calibrated energy intake among community-dwelling older adults. J Gerontol a Biol Sci Med Sci. 2021;76(5):876–84. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Miyachi M, Kimura M. Validation of the Kihon Checklist and the frailty screening index for frailty defined by the phenotype model in older Japanese adults. BMC Geriatr. 2022;22(1):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada Y Nanri H Watanabe Y, et al. Prevalence of frailty assessed by Fried and Kihon Checklist indexes in a prospective cohort study: design and demographics of the Kyoto–Kameoka Longitudinal Study. J Am Med Dir Assoc. 2017;18(8):733.e7–e15. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe D Yoshida T Watanabe Y Kimura M Yamada Y, Kyoto–Kameoka Study Group . Doubly labelled water-calibrated energy intake associations with mortality risk among older adults. J Cachexia Sarcopenia Muscle. 2022;14(1):214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E Altman DG Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamiya T, Inoue S. Trends in step-determined physical activity among Japanese adults from 1995 to 2016. Med Sci Sports Exerc. 2019;51(9):1852–9. [DOI] [PubMed] [Google Scholar]
- 29.Buuren SV, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 30.Sterne JA White IR Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFina LF Radford NB Barlow CE, et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4(2):174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth LP, Park S, Springer CM, Feyerabend MD, Steeves JA, Bassett DR. Video-recorded validation of wearable step counters under free-living conditions. Med Sci Sports Exerc. 2018;50(6):1315–22. [DOI] [PubMed] [Google Scholar]
- 33.Nakagata T Murakami H Kawakami R, et al. Step-count outcomes of 13 different activity trackers: results from laboratory and free-living experiments. Gait Posture. 2022;98:24–33. [DOI] [PubMed] [Google Scholar]
- 34.Higueras-Fresnillo S Cabanas-Sanchez V Lopez-Garcia E, et al. Physical activity and association between frailty and all-cause and cardiovascular mortality in older adults: population-based prospective cohort study. J Am Geriatr Soc. 2018;66(11):2097–103. [DOI] [PubMed] [Google Scholar]
- 35.Taber DR Stevens J Murray DM, et al. The effect of a physical activity intervention on bias in self-reported activity. Ann Epidemiol. 2009;19(5):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Master H Annis J Huang S, et al. Association of step counts over time with the risk of chronic disease in the All of Us Research Program. Nat Med. 2022;28(11):2301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates T Haffner SM Schulte PJ, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383(9922):1059–66. [DOI] [PubMed] [Google Scholar]
- 38.Harris T Limb ES Hosking F, et al. Effect of pedometer-based walking interventions on long-term health outcomes: prospective 4-year follow-up of two randomised controlled trials using routine primary care data. PLoS Med. 2019;16(6):e1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bull FC Al-Ansari SS Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc. 2015;47(4):843–9. [DOI] [PubMed] [Google Scholar]
- 41.Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail-based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community-dwelling Japanese older adults: the INE (Intervention by Nutrition and Exercise) study. J Am Med Dir Assoc. 2015;16(8):654–60. [DOI] [PubMed] [Google Scholar]
- 42.Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. [DOI] [PubMed] [Google Scholar]
- 43.Miyachi M, Tripette J, Kawakami R, Murakami H. “+10 min of physical activity per day”: Japan is looking for efficient but feasible recommendations for its population. J Nutr Sci Vitaminol (Tokyo). 2015;61Suppl:S7–9. [DOI] [PubMed] [Google Scholar]
- 44.Ministry of Health, Labour and Welfare, National Institute of Health and Nutrition . Japanese Official Physical Activity Guidelines for Health Promotion-ActiveGuide. 2013. [accessed on 12 August 2022]; Available online: https://www.nibiohn.go.jp/eiken/programs/pdf/active2013-e.pdf.
- 45.Murakami H, Tripette J, Kawakami R, Miyachi M. “Add 10 min for your health”: the new Japanese recommendation for physical activity based on dose–response analysis. J Am Coll Cardiol. 2015;65(11):1153–4. [DOI] [PubMed] [Google Scholar]
- 46.Ministry of Health, Labour and Welfare . [The National Health and Nutrition Survey, Japan 2010]. Report in Japanese. See p. 129. [accessed on 12 August 2022]; Available online: http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h22-houkoku-01.pdf. [Google Scholar]
- 47.Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews CE Troiano RP Salerno EA, et al. Exploration of confounding due to poor health in an accelerometer-mortality study. Med Sci Sports Exerc. 2020;52(12):2546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto N Shimada M Nakagawa N, et al. Tracking of pedometer-determined physical activity in healthy elderly Japanese people. J Phys Act Health. 2015;12(10):1421–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


