Abstract
In 2014, we proposed that orexin signaling transformed motivationally relevant states into adaptive behavior directed toward exploiting an opportunity or managing a threat, a process we referred to as motivational activation. Advancements in animal models since then have permitted higher-resolution measurements of motivational states; in particular, the behavioral economics approach for studying drug demand characterizes conditions that lead to the enhanced motivation that underlies addiction. This motivational plasticity is paralleled by persistently increased orexin expression in a topographically specific manner—a finding confirmed across species, including in humans. Normalization of orexin levels also reduces drug motivation in addiction models. These new advancements lead us to update our proposed framework for the orexin function. We now propose that the capacity of orexin neurons to exhibit dynamic shifts in peptide production contributes to their role in adaptive motivational regulation and that this is achieved via a pool of reserve orexin neurons. This reserve is normally bidirectionally recruited to permit motivational plasticity that promotes flexible, adaptive behavior. In pathological states such as addiction, however, we propose that the orexin system loses capacity to adaptively adjust peptide production, resulting in focused hypermotivation for drug, driven by aberrantly and persistently high expression in the orexin reserve pool. This mechanistic framework has implications for the understanding and treatment of several psychiatric disorders beyond addiction, particularly those characterized by motivational dysfunction.
THE OREXIN SYSTEM AS A MOTIVATIONAL ACTIVATION SYSTEM
The brain orexin/hypocretin (referred to here simply as orexin) system originates solely from neurons in the posterior hypothalamus but includes projections throughout the central nervous system (1). Studies over the past 20 years since the orexin system was discovered (2,3) have implicated these hypothalamic neurons in several behaviors. Originally, this system was linked to feeding (3), narcolepsy, and arousal (4,5), and subsequent studies from our laboratory showed that the system also played an important role in reward and reward dysfunctions including addiction (6). Additional groups showed roles for orexin neurons in cognition, motor activity, and stress, as well as cardiovascular, respiratory, and other homeostatic functions (7). The widespread orexin innervation and multiple functional roles imply that this system plays a general role in the central nervous system function that contains and integrates many functions. We proposed that the general role of the orexin system was the ability to activate and coordinate adaptive motivational responses to imperative environmental as well as interoceptive signals, a role we termed motivational activation (7). In this view, the ability of orexin neurons to drive waking and coordinated responses to rewarding stimuli, as well as to regulate homeostatic activities such as waking, reflects engagement of this system to modulate multiple disparate networks in the service of activating adaptive motivational responses to an opportunity and/or threat at hand.
Inappropriate activity in such a key behavioral system would, of course, be expected to participate in multiple behavioral disorders. Indeed, evidence indicates that orexins play roles in disorders of stress, affect/emotion, and cognitive processes. Here, we focus on a role of this system in responses to highly salient rewards, including drugs of abuse. Early studies found not only that these neurons are activated by, for instance, acute withdrawal from chronic opioids (8,9), but also that these cells become hyperactivated in response to stimuli that were previously associated with highly salient rewards such as drugs of abuse. Our laboratory was the first to show that such orexin hyperactivity plays an important role in driving a strong motivational response for previously experienced drugs and is a key element in multiple behaviors that characterize addiction to many drugs including opioids and stimulants as well as salient food (6). This finding sparked a great deal of additional work that consistently has shown a major role for orexin signaling in reward and reward dysfunctions.
The main topic of this review focuses on a new vista that has appeared in the past few years and substantially extends our understanding of how the orexin system functions in reward and addiction. Recent studies by us and others have found significant plasticity in the expression of orexin, such that the number of orexin-expressing neurons increases substantially after chronic exposure to highly salient rewards, such as drugs of abuse including cocaine (10,11), opioids (12,13), and alcohol (14-16). This increased expression provides a novel source of enhanced signaling in association with such motivational disorders. Below, we review evidence for this changed expression, discuss its functional implications, and posit a novel population of reserve orexin neurons that can be brought to bear for adaptive and maladaptive periods of high motivation.
MOTIVATIONAL PLASTICITY
Motivation is a critical element in adaptive behavior. An adaptive behavioral response must take into account many factors and become energized (motivated) in one direction and compete with others. Although the importance of this construct has been long recognized, defining motivation has been challenging.
Classical fixed ratio (FR) self-administration or conditioned place preference tasks do not readily translate into measures of motivation. One method of measuring motivation is through increasing FR schedules, where higher ratios require more effort to obtain a reward (17). One widely used version of this is the progressive ratio schedule, where the FR required to obtain a reward increases (usually geometrically) after each reward within a session. The primary result of progressive ratio studies is the breakpoint, which is the final ratio performed and results in a reward within a set criterion time period. Although such schedules capture many elements of motivation for a reward, they have serious drawbacks as well: 1) Reward does not occur for each behavioral response, and the time between rewards increases during the session and can reach many minutes; this can introduce a delay-discounting confound. 2) Long periods of time between rewards exert a demand on working memory in the task, and manipulations that influence working memory could have confounding effects. 3) Each session typically produces a single result—the breakpoint. Therefore, these tasks have relatively coarse and infrequent data points.
We define motivation operationally using a behavioral economics (BE) approach. This has the advantage of providing a quantitative measure of motivation, separate from other reward-related behaviors such as free consumption or hedonic set point [see (18-20) for review of BE methods and capabilities]. For many of our studies, we used a within-session dose-reduction BE procedure in rats (21-23), in which the price (effort) required per milligram of drug increases in 10-minute intervals within a session because the dose administered per lever press decreases. We then used an exponential demand equation to fit demand curves (which express consumption as a function of price) to data and allow estimation of the key parameters, α (demand elasticity, inverse of motivation) and Q0 (amount consumed at nil effort); this procedure is outlined in (18,24). For many drugs, α and Q0 are unrelated and vary independently of each other. This is important, as this means that the measure of motivation in this procedure (α) is not confounded by changes in consumption (reflected in Q0). This also is consistent with the fact that α is self-normalizing, as it is the slope of the demand curve, which varies independently of the y intercept of the curve (which is Q0). Another advantage of BE over other behavioral procedures for measuring properties such as motivation and hedonic value is that BE does not require working memory and is not confounded by delay-discounting because subjects receive a reward for every lever press. Note that there are other BE methods that use changes in FR response to vary the effort required per milligram of drug or fixed unit of other rewards (e.g., food)—these are important for long time-course drugs such as methamphetamine (25), or in the case of food, to avoid satiety (26). Results within a drug for dose-reduction versus FR methods are comparable (24).
PLASTICITY IN OREXIN PEPTIDE PRODUCTION
Although the role of the orexin system in motivation has been known for some time, this has conventionally been thought to result from changes in the activity of orexin cells. Indeed, orexin neurons exhibit diurnal fluctuations in activity across the day/night cycle, with the highest activity during the active period when opportunities to engage in motivated behavior (e.g., feeding, sex, social play) are highest (27,28). Moreover, homeostatic challenges that demand adaptive responses, including stress exposure and food deprivation, robustly enhance orexin neuron firing, as does the presentation of stimuli that predict the availability of salient rewards (29-34). Not surprisingly therefore, a major goal in addiction neuroscience (and related fields) has been to determine mechanisms driving changes in orexin neuronal excitability (31). However, emerging literature now points to an additional, previously underappreciated dimension of orexin system plasticity that contributes to altered motivational states: dynamic and persistent changes in the number of orexin-expressing neurons, driven by chronic exposure to highly salient rewarding stimuli.
The number of cells that are immunoreactive for orexin fluctuates in relation to several factors, including the time of day [discussed below; (35)]. This plasticity in expression likely permits dynamic changes in orexin peptide availability and, in combination with stimulus-driven changes in cell activity, permits adaptive behavioral responses to threats and opportunities (motivational plasticity). Two studies published by independent groups highlighted the possibility that this plasticity in orexin peptide production may be decreased following chronic drug exposure, such that the number of orexin-producing neurons is persistently elevated in addiction. Siegel and colleagues (13) reported a 54% increase in the number of orexin-immunoreactive neurons in postmortem brains from a small number (n = 5) of patients with opioid (heroin) use disorder, relative to matched control subjects. This effect was recapitulated in a dose-dependent manner by daily morphine injections (experimenter-administered) in mice and persisted for up to 4 weeks into withdrawal. Morphine-induced increase in orexin cell numbers was not the result of neurogenesis, as treatment groups did not differ in the number of cells labeled for BrdU or doublecortin, markers of new neurons. In the other study, our group showed an approximately 25% increase in the number of orexin-immunoreactive neurons in rats after 2 weeks of intermittent cocaine self-administration, a schedule of intake that promotes several addiction-relevant behaviors, including higher drug motivation (10). Notably, these cell number changes were persistent (differences remained at 5 months after cocaine self-administration). Moreover, this increased orexin cell number was causally linked to motivational plasticity for drug, as normalization of the number of orexin neurons using a morpholino-antisense approach (~25% knockdown) reduced cocaine demand to levels similar to those observed prior to intermittent cocaine self-administration [although see (36)]. [These changes in orexin levels mirror previous demonstrations of drug-induced changes in the orexin messenger RNA (mRNA) levels (37), discussed below.] In the time since the publication of these 2 studies, this phenomenon has been confirmed by several groups, across multiple drugs of abuse, using various models of addiction. One group reported that extended access to cocaine (6 hours/day, continuous access), which promotes escalation of intake, is associated with a persistent increase in orexin cell numbers in rats (11). We reported similar increases following intermittent fentanyl self-administration, also in rats (12). Moreover, in a series of studies in both rats and zebrafish, Leibowitz and colleagues (14-16,38) reported increased orexin neuron numbers following prenatal ethanol exposure. Notable also is that drugs of abuse do not appear to result in a general upregulation of hypothalamic neuropeptide production, as several of the abovementioned studies reported no change in the number of melanin-concentrating hormone–immunoreactive neurons, which are interdigitated but not overlapping with orexin neurons. Taken together, several drugs of abuse reliably promote an increase in the number of orexin-immunoreactive neurons, and this is reproducible across several species including humans. Moreover, within the hypothalamus, these effects appear to be specific to orexin-producing neurons, pointing to potentially unique mechanisms underlying their dynamic expression (discussed in more detail below). Notably, similar persistent increases in orexin neuron numbers and mRNA have been observed in animals exposed to high-fat diets (39-42), indicating that this phenomenon may reflect a general upregulation of the orexin system function in response to a highly salient reward.
Topography of Orexin Plasticity
There is evidence that the upregulation of orexin levels is not uniform across the hypothalamus but rather occurs in a topographically specific manner. Orexin-containing neurons range mediolaterally from the dorsomedial hypothalamus (DMH) through the perifornical area (PF) to the lateral hypothalamus (LH, lateral to the fornix in rat) (2,3). In the first paper to directly link the orexin system with reward, our laboratory reported that the activity of orexin neurons in rat LH (lateral subpopulation) but not DMH/PF (medial subpopulation) was directly correlated with drug- (or food-) seeking behavior (6). In a subsequent study, we reported that medial but not lateral orexin neurons were recruited by stress, leading us to hypothesize a functional dichotomy of the orexin system function, whereby (at least in rats) medial orexin neurons convey stress/arousal, whereas the lateral subpopulation is important for reward (30). This idea is consistent with the observation that wake-associated Fos activity in orexin neurons is found in the medial but not in the lateral subpopulation (27). In further support, previous studies had reported that lateral orexin neurons are exclusively activated by antipsychotics that induce weight gain, whereas the activity of medial neurons was preferentially associated with wakefulness and exposure to various stressors (27,43). Consistent with this proposed subregional specificity, we showed that the persistent increases in orexin cell numbers following intermittent cocaine self-administration in rats occurred only in the LH (10). This was true also for increased orexin mRNA levels following chronic ethanol consumption in rats [discussed in more detail below; (37)]. We also reported that the number of LH but not DMH/PF orexin neurons predicts individual variability in cocaine-demand elasticity (44). Moreover, the increased orexin cell numbers in the hypothalamus of heroin addicts and morphine-exposed mice were proportionally and statistically greatest in the LH (13).
One exception to these observations is a recent study in which we found that increased orexin cell numbers after fentanyl self-administration in rats occurred at a similar magnitude in medial and lateral subregions—a finding we speculate may result from a stronger and more persistent stress phenotype for opioid withdrawal (compared with cocaine) (12). Intriguingly, data from zebrafish reveal another potential source of topographical specificity; both prefertilization and embryonic exposure to ethanol result in increased orexin cell numbers exclusively in the left hemisphere of the brain (15,16). It is unclear whether these effects are species specific or occur only during early development, as studies examining the effect of drug exposure in rodents and humans, including our own, reported total cell counts across both hemispheres in adults.
Plasticity in Orexin Function
Intriguingly, we found that increased orexin cell numbers in addiction models were often associated with an increased potency of orexin receptor antagonism. For example, following intermittent access to cocaine or fentanyl, which promoted increased orexin cell expression, suvorexant (dual orexin 1/2 receptor antagonist) or SB-334867 (orexin 1 receptor antagonist) decreased drug demand and cued relapse at doses approximately one third of what is needed to have similar effects in short access or FR1 rats (10,12,45,46). Similarly, a selective orexin 2 receptor antagonist reduced drug intake following daily extended (6-hour), but not 1-hour, access to heroin (47), a procedure that promotes an addictedlike state and promotes higher orexin expression (11). Moreover, when examining individual differences in drug motivation following limited drug access to cocaine or alcohol, we and others routinely have found that animals with high baseline motivation (who likely have higher endogenous orexin cell numbers—discussed below) are most susceptible to the ability of SB-334867 to decrease addiction behaviors (45,48,49). Similar findings have been reported for food, whereby an orexin receptor antagonist reduces motivated response for highly palatable foods at doses that generally do not affect regular food intake (50-55) [although see (56)]. Together, these data indicate that addiction behaviors become more “orexin dependent” in association with increased orexin expression.
Therapeutic Implications
These findings have important potential therapeutic implications—they indicate that orexin receptor antagonists may be able to reduce addiction behaviors at low doses that have limited off-target effects (57,58). Indeed, as noted above, the orexin system is critically involved in the regulation of several physiological processes, including feeding and sleep/wake processes, and it would be clinically important that treatments with orexin antagonists do not interfere with these functions. Studies that have tested this possibility to date are encouraging; in animals that exhibit strong addiction behaviors, SB-334867 inhibits drug-seeking at doses that do not interfere with homeostatic feeding (51,59) or cognition (50,60). Moreover, the dual orexin receptor antagonist suvorexant, a compound that is Food and Drug Administration–approved for the treatment of insomnia and could readily be repurposed for addiction, can be used in low, nonsedating doses to reduce drug craving in rats (46,60). Indeed, we note that several clinical trials are currently underway to further characterize the potential utility of such compounds for the management of substance use disorders (57,58,61-65).
Data linking the number of orexin neurons with addiction behaviors following drug exposure (state differences) also raise the interesting possibility that individual baseline differences in orexin levels (trait differences) may underlie propensity to abuse drugs. Indeed, we recently reported that the number of LH orexin neurons in rats correlates closely with baseline motivation for cocaine, measured after limited cocaine self-administration training and before the induction of an addiction-like state (44). We also observed a similar relationship with the ultrashort-acting opioid remifentanil (A. Mohammadkkani, Ph.D., et al., unpublished data, January 2018), again indicating that these findings might be generalized across drugs of abuse. These findings align well with the clinical observation that human patients with narcolepsy, who, postmortem studies revealed, have 85%–95% fewer orexin neurons than normal, rarely develop stimulant abuse, despite long-term treatment with stimulant medications, including amphetamines (66,67). Unfortunately, there are currently no positron emission tomography ligand or other techniques for live cranial imaging that allow for estimation of orexin cell numbers in people; the development of such approaches may have significant value in identifying individuals at risk of developing addictions.
OREXIN CELL RESERVE: A POSSIBLE WAY TO MODULATE MOTIVATIONAL GAIN AND DIRECTION
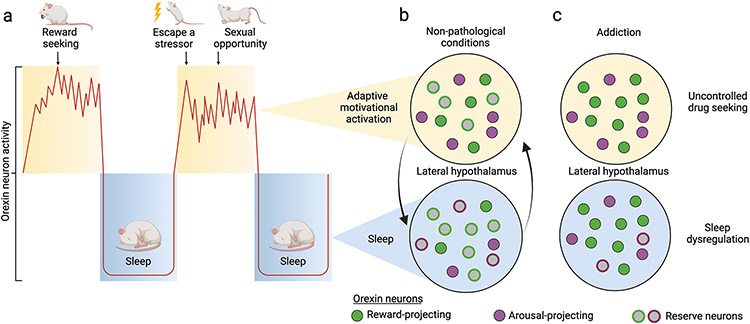
A detailed understanding of the mechanisms underlying plasticity in orexin expression remains unknown. We submit here a conceptual framework to foster hypotheses that can be addressed by future studies (Figure 1). We propose that the orexin cell field in the hypothalamus contains a reserve population of potential orexin neurons that can be transiently recruited under circumstances of high motivation to promote adaptive behavior; by “recruited,” we mean increased orexin peptide production in these reserve cells (discussed below). Recruitment of this reserve population would, for example, promote vigorous food foraging behavior under circumstances of brief caloric deprivation. In the case of drug addiction, however, we postulate that chronic exposure to drugs of abuse persistently upregulates orexin peptide production in a select group of neurons, such that the manifestation of addiction is coincident with long-term recruitment of reserve orexin neurons. The result is persistently hypermotivated behavior that is predominantly drug-directed, including—when necessary—the expression of flexible behaviors to procure drug (68). We predict that this has potential detrimental consequences for other orexin-dependent physiological processes, including reduced homeostatic behaviors such as eating and sleeping.
Figure 1.
(A) Orexin neuron activity (red line) follows a circadian pattern, with peak activity during the active period. Overlaid on this diurnal activity pattern are phasic bursts during waking; these reflect involvement of the orexin neurons in translating motivationally relevant states into adaptive behavior directed toward managing or exploiting a threat or opportunity, a process we referred to as motivational activation. (B) Motivational activation is also serviced by state-dependent changes in orexin peptide production. Under nonpathological conditions, a reserve population of orexin neurons with projections to key arousal sites is preferentially recruited during the active period to support wake/arousal. Some reserve neurons with projections to reward centers are also recruited to facilitate adaptive motivated behaviors (e.g., feeding). Recruitment of these reserve cells is primarily governed by circadian factors. Note that nonreserve neurons may also increase their peptide production as a function of behavioral state (not shown here). (C) Addiction to drugs is characterized by a persistent and preferential recruitment of orexin reserve neurons that project to reward regions, resulting in sustained, hypermotivated, and uncontrolled drug seeking behavior. A loss of plasticity in peptide production by the reserve population also contributes to inappropriate arousal during the inactive period, resulting in sleep dysregulation in addiction. [Figure adapted from (7)].
Plasticity in orexin gene expression is well established. Lawrence and colleagues (37) reported approximately 15 years ago that chronic ethanol consumption in rats was associated with an increase in LH prepro-orexin mRNA expression. Similarly, repeated nicotine exposure (experimenter-administered) increased prepro-orexin mRNA in the hypothalamus (69), as did naloxone-precipitated withdrawal in morphine-dependent mice (8). Importantly, nondrug stimuli have also been shown to promote changes in orexin gene expression, including acute food restriction (3) and high-fat diet exposure (40,42), as well as exposure to stressors (70,71). Together, these data indicate that orexin gene expression is normally dynamically altered by exposure to environmental and pharmacological stimuli—particularly those with motivational relevance.
Despite these well-documented changes in orexin gene expression, little consideration has been given to whether these are linked to changes in orexin peptide production. This question was brought to the fore in 2015 upon the discovery that a number of orexin-immunoreactive neurons in mice exhibit diurnal fluctuations, such that higher numbers are observed in the active (dark) period compared with the inactive (light) period (35). These findings indicate that during the light phase, there is a population of neurons that contains the molecular machinery necessary for producing orexin peptide but is undetectable to an orexin antibody owing to extremely low mRNA and/or peptide production. Consistent with this interpretation, this same study reported that treatment with colchicine, which promotes the accumulation of neuropeptide in the cell body, resulted in an increase in the overall number of detectable orexin-expressing neurons (35). Combined with evidence that drug-induced increases in cell numbers may not result from neurogenesis (13), these data point to a reserve population of orexin-producing neurons that, under normal circumstances, is transiently recruited to facilitate arousal. It is also notable that the number of detectable melanin-concentrating hormone neurons did not change following colchicine treatment (35), perhaps pointing to a unique and highly specialized role for orexin neuronal reserves.
We posit that a similar reserve of orexin neurons supports motivational plasticity. Under nonpathological conditions, this reserve is transiently recruited to provide additional orexin peptide in the service of motivational activation, allowing animals to optimally exploit environmental circumstances (Figure 1B). During the early phases of addiction, these neurons might be recruited by stimuli that predict drug availability; however, this recruitment may be transient and readily over-ridden by other neural systems (e.g., executive control networks) that control drug use. As drug use becomes more chronic, we posit that reserve orexin neurons are repeatedly recruited, but in a way that results in a gradual loss of plasticity in peptide production and a persistent upregulation of their orexin expression (Figure 1C). As a result, motivational plasticity is lost, leading to maladaptive and focused hypermotivation for drug.
An additional implication of increased peptide production could be the amplification of the effects of firing activity in orexin neurons: more peptide expression may mean more peptide released per action potential. This increased “gain” in orexin signaling may interact with downstream targets to augment particular elements in behavioral repertoires. We also posit that increased orexin signaling (perhaps via altered receptor number or altered intracellular signaling mechanisms) accompanies the increased orexin terminals found in specific target areas (11,47); this may result in enhanced behavioral impact of orexin input after chronic drug exposure. This, in turn, may contribute to the abovementioned enhanced potency of orexin receptor antagonists in addicted rats. We note, however, that potential changes in orexin receptor number and signaling need to be explored further in addiction models.
Dynorphin Coexpression
Neurons that produce the orexin peptide also coexpress other neurotransmitters including dynorphin (72), which is coreleased with orexin at terminal sites (73) and generally has opposing effects to orexin on postsynaptic activity (particularly in the ventral tegmental area) and motivated behavior (74-76). Cocaine and other drugs increase expression of dynorphin in several reward regions (77); however, it is unclear whether these changes occur in orexin reserve neurons. It is enticing to hypothesize that exposure to drugs of abuse increases orexin peptide expression while leaving dynorphin levels unaffected, resulting in a net increase in excitatory actions. However, short hairpin RNA-mediated alterations in orexin levels are paralleled by compensatory (similar) changes in dynorphin expression in the hypothalamus (78), and unpublished studies from our laboratory indicate that dynorphin increases along with orexin in response to drug exposure. Thus, any imbalance of orexin versus dynorphin signaling in addiction might be dependent on alterations in their actions at target regions, possibly owing to differential release probability, changes in postsynaptic receptor expression, or other signaling properties.
IMPLICATIONS OF THE OREXIN RESERVE HYPOTHESIS: FUTURE CONSIDERATIONS
There are several potential consequences of the proposed orexin reserve described above. The reserve orexin neurons appear to have topographical specificity within the hypothalamus (discussed previously). We propose that they may also have select targets, so that in response to chronic addictive drugs, orexin expression is increased especially in neurons that project to reward regions where orexin regulates motivated behavior, such as the ventral tegmental area (42,79-85) and nucleus accumbens (86,87). This would be consistent with our recent results that orexin innervation of the ventral tegmental area preferentially originates from the LH (79). This arrangement could provide a target-defined specificity/direction to the increased motivation induced by the orexin reserve cells engaged. It is also interesting to consider whether reserve orexin neurons and their related circuits encode motivation that is specific to the drug whose chronic exposure engaged them, or whether their enhanced output also alters motivation for other rewards (e.g., food) or drugs of abuse. Indeed, recruitment of the orexin reserve may serve as a common mechanism underlying high rates of polysubstance abuse (88) and increased risk of overeating disorders (89) in addiction.
Alternatively, it is possible that there exist multiple “pools” of reserve orexin neurons that drive behavioral responsivity to distinct motivationally relevant stimuli. For example, stress upregulates orexin mRNA, peptide, and receptor levels in a way that is functionally linked with stress reactivity (90-93); this might be achieved via recruitment of unique reserve neurons with preferential input to arousal and emotional centers. The extent to which drugs, stress, and other stimuli recruit overlapping versus distinct reserve orexin neurons should be a focus of studies going forward, especially in view of the large comorbidities among stress, sleep dysregulation, and substance use disorders (54,62,65,94).
Mechanisms Regulating Orexin Expression
Other questions relate to the molecular machinery underlying dynamic shifts in orexin gene and protein production, the time scale on which these occur, and how these are perturbed by drugs of abuse. We propose that at baseline, reserve orexin neurons express very little prepro-orexin mRNA and, therefore, very little orexin peptide, but can increase both in the context of high motivational relevance. Some reserve neurons may express little or no mRNA or peptide until stimulated by threat or opportunity. Indeed, it is unclear whether there is a stable population of orexin neurons that is supplemented by an additional population of reserve neurons when needed, or whether there are many populations that wax and wane in orexin expression depending on the environment and the target of that neuron. Moreover, it is interesting to consider whether reserve neurons coexpress dynorphin and other neurotransmitters (e.g., glutamate) when orexin peptide production is dormant.
Several transcription factors have been identified as regulating prepro-orexin gene expression, both in vitro and in vivo (95-98), with one (FOXA2) shown to bind the orexin promoter specifically under fasting conditions (96); it is plausible that a similar mechanism mediates orexin gene (and peptide) upregulation in orexin reserve cells when motivational plasticity is required and that these processes are dysregulated by chronic drug exposure. Orexin mRNA expression might also be mediated by other mechanisms, including epigenetic modifications (e.g., DNA methylation, histone modifications, chromatin remodeling) and changes in the expression of noncoding RNAs such as microRNAs (99-101); these possibilities are yet to be fully explored. Recruitment of the “reserve” orexin neurons may also be activity dependent, resulting from changes in the balance between excitatory versus inhibitory inputs arising from specific circuits and cell types (31,102,103). In light of the evidence reviewed above indicating that the orexin cell reserve might be more numerous in the rat LH, the mechanisms governing orexin expression may differ for LH versus DMH/PF populations.
Sex Differences
Finally, work on sex differences in orexin and addiction is limited. Greater increases in orexin mRNA are observed in female versus male offspring prenatally exposed to ethanol (38) or chronic stress (90). Moreover, orexin mRNA concentrations in the hypothalamus vary across the estrus cycle, with higher levels observed in proestrus compared with other phases (104). These data indicate that a dynamic, drug-sensitive orexin reserve may exist in females; however, more work is required to fully characterize sex differences and their underlying mechanisms.
CONCLUSIONS
Additional orexin neurons are observed in response to multiple drugs and across several species, including humans, pointing to enhanced orexin expression as a common neural signature of addiction. We propose that this phenomenon reflects a disruption in the normal plasticity of the orexin cell reserve, which, in a nonpathological state, is transiently recruited in the service of facilitating adaptive motivational behaviors. In this view, dynamism of this orexin reserve is lost in addiction, such that orexin peptide production is persistently upregulated, and the behavior becomes hypermotivated, focused, and compulsive. The framework outlined here may be a starting point for future efforts to more comprehensively characterize orexin system plasticity in substance use and related disorders.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by grants from the National Institute of Health (Grant No. R00 DA045675 [to MHJ] and Grant No. R01 DA006214 [to GA-J]).
Figures were created in BioRender.com.
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Morgan H. James, Brain Health Institute, Rutgers University and Rutgers Biomedical and Health Sciences, Piscataway, New Jersey; Department of Psychiatry, Robert Wood Johnson Medical School, Rutgers University and Rutgers Biomedical and Health Sciences, Piscataway, New Jersey
Gary Aston-Jones, Brain Health Institute, Rutgers University and Rutgers Biomedical and Health Sciences, Piscataway, New Jersey; Department of Psychiatry, Robert Wood Johnson Medical School, Rutgers University and Rutgers Biomedical and Health Sciences, Piscataway, New Jersey.
REFERENCES
- 1.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. (2000): A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6:991–997. [DOI] [PubMed] [Google Scholar]
- 2.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. (1998): The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. (1998): Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- 4.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. (1999): Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98:437–451. [DOI] [PubMed] [Google Scholar]
- 5.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. (1999): The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98:365–376. [DOI] [PubMed] [Google Scholar]
- 6.Harris GC, Wimmer M, Aston-Jones G (2005): A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. [DOI] [PubMed] [Google Scholar]
- 7.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014): Motivational activation: A unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. (2003): Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23:3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharf R, Sarhan M, Dileone RJ (2008): Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry 64:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G (2019): Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry 85:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matzeu A, Martin-Fardon R (2021): Cocaine-seeking behavior induced by orexin A administration in the posterior paraventricular nucleus of the thalamus is not long-lasting: Neuroadaptation of the orexin system during cocaine abstinence. Front Behav Neurosci 15:620868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fragale JE, James MH, Aston-Jones G (2021): Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addict Biol 26:e12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thannickal TC, John J, Shan L, Swaab DF, Wu MF, Ramanathan L, et al. (2018): Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med 10:eaao4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier AD, Halkina V, Min SS, Roberts MY, Campbell SD, Camidge K, Leibowitz SF (2019): Embryonic ethanol exposure affects the early development, migration, and location of hypocretin/orexin neurons in zebrafish. Alcohol Clin Exp Res 43:1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collier AD, Min SS, Campbell SD, Roberts MY, Camidge K, Leibowitz SF (2020): Maternal ethanol consumption before paternal fertilization: Stimulation of hypocretin neurogenesis and ethanol intake in zebrafish offspring. Prog Neuropsychopharmacol Biol Psychiatry 96:109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier AD, Yasmin N, Khalizova N, Campbell S, Onoichenco A, Fam M, et al. (2021): Sexually dimorphic and asymmetric effects of embryonic ethanol exposure on hypocretin/orexin neurons as related to behavioral changes in zebrafish. Sci Rep 11:16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts DC, Loh EA, Vickers G (1989): Self-administration of cocaine on a progressive ratio schedule in rats: Dose–response relationship and effect of haloperidol pretreatment. Psychopharmacol (Berl) 97:535–538. [DOI] [PubMed] [Google Scholar]
- 18.Bentzley BS, Fender KM, Aston-Jones G (2013): The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacol (Berl) 226:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hursh SR, Silberberg A (2008): Economic demand and essential value. Psychol Rev 115:186–198. [DOI] [PubMed] [Google Scholar]
- 20.Oleson EB, Roberts DC (2009): Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology 34:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmer BA, Oleson EB, Roberts DCS (2012): The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siciliano CA, Saha K, Calipari ES, Fordahl SC, Chen R, Khoshbouei H, Jones SR (2018): Amphetamine reverses escalated cocaine intake via restoration of dopamine transporter conformation. J Neurosci 38:484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calipari ES, Siciliano CA, Zimmer BA, Jones SR (2015): Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology 40:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentzley BS, Jhou TC, Aston-Jones G (2014): Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111:11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G (2017): Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol Psychiatry 81:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman LR, Bentzley BS, James MH, Aston-Jones G (2021): Sex differences in demand for highly palatable foods: Role of the orexin system. Int J Neuropsychopharmacol 24:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. (2001): Fos expression in orexin neurons varies with behavioral state. J Neurosci 21:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mileykovskiy BY, Kiyashchenko LI, Siegel JM (2005): Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furlong TM, Vianna DM, Liu L, Carrive P (2009): Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30:1603–1614. [DOI] [PubMed] [Google Scholar]
- 30.Harris GC, Aston-Jones G (2006): Arousal and reward: A dichotomy in orexin function. Trends Neurosci 29:571–577. [DOI] [PubMed] [Google Scholar]
- 31.Yeoh JW, James MH, Adams CD, Bains JS, Sakurai T, Aston-Jones G, et al. (2019): Activation of lateral hypothalamic group III metabotropic glutamate receptors suppresses cocaine-seeking following abstinence and normalizes drug-associated increases in excitatory drive to orexin/hypocretin cells. Neuropharmacology 154:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurose T, Ueta Y, Yamamoto Y, Serino R, Ozaki Y, Saito J, et al. (2002): Effects of restricted feeding on the activity of hypothalamic orexin (OX)-A containing neurons and OX2 receptor mRNA level in the paraventricular nucleus of rats. Regul Pept 104:145–151. [DOI] [PubMed] [Google Scholar]
- 33.Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, et al. (2012): Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav 107:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorman DE, James MH, Kilroy EA, Aston-Jones G (2016): Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci 43:710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGregor R, Shan L, Wu MF, Siegel JM (2017): Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLOS ONE 12:e0178573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad AA, McNally GP (2014): Effects of vivo morpholino knockdown of lateral hypothalamus orexin/hypocretin on renewal of alcohol seeking. PLoS One 9:e110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006): The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang GQ, Collier AD, Karatayev O, Gulati G, Boorgu DSSK, Leibowitz SF (2020): Moderate prenatal ethanol exposure stimulates CXCL12/CXCR4 chemokine system in radial glia progenitor cells in hypothalamic neuroepithelium and peptide neurons in lateral hypothalamus of the embryo and postnatal offspring. Alcohol Clin Exp Res 44:866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemus MB, Bayliss JA, Lockie SH, Santos VV, Reichenbach A, Stark R, Andrews ZB (2015): A stereological analysis of NPY, POMC, orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology 156:1701–1713. [DOI] [PubMed] [Google Scholar]
- 40.Wortley KE, Chang GQ, Davydova Z, Leibowitz SF (2003): Peptides that regulate food intake: Orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol 284:R1454–R1465. [DOI] [PubMed] [Google Scholar]
- 41.Morganstern I, Chang GQ, Karatayev O, Leibowitz SF (2010): Increased orexin and melanin-concentrating hormone expression in the perifornical lateral hypothalamus of rats prone to overconsuming a fat-rich diet. Pharmacol Biochem Behav 96:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. (2009): Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29:11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fadel J, Bubser M, Deutch AY (2002): Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci 22:6742–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantazis CB, James MH, Bentzley BS, Aston-Jones G (2020): The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addict Biol 25:e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James MH, Bowrey HE, Stopper CM, Aston-Jones G (2019): Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci 50:2602–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connor SL, Fragale JE, James MH, Aston-Jones G (2020): The dual orexin/hypocretin receptor antagonist suvorexant reduces addiction-like behaviors for the opioid fentanyl. bioRxiv. 10.1101/2020.04.25.061887. [DOI] [Google Scholar]
- 47.Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, et al. (2015): Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology 40:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moorman DE, James MH, Kilroy EA, Aston-Jones G (2017): Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res 1654:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammadkhani A, Fragale JE, Pantazis CB, Bowrey HE, James MH, Aston-Jones G (2019): Orexin-1 receptor signaling in ventral pallidum regulates motivation for the opioid remifentanil. J Neurosci 39:9831–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiskerke J, James MH, Aston-Jones G (2020): The orexin-1 receptor antagonist SB-334867 reduces motivation, but not inhibitory control, in a rat stop signal task. Brain Res 1731:146222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vickers SP, Hackett D, Murray F, Hutson PH, Heal DJ (2015): Effects of lisdexamfetamine in a rat model of binge-eating. J Psychopharmacol 29:1290–1307. [DOI] [PubMed] [Google Scholar]
- 52.Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I (2014): Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: Pharmacological and molecular evidence of orexin involvement. Behav Brain Res 272:93–99. [DOI] [PubMed] [Google Scholar]
- 53.Rorabaugh JM, Stratford JM, Zahniser NR (2014): A relationship between reduced nucleus accumbens shell and enhanced lateral hypothalamic orexin neuronal activation in long-term fructose bingeing behavior. PLoS One 9:e95019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehr JB, Mitchison D, Bowrey HE, James MH (2021): Sleep dysregulation in binge eating disorder and “food addiction”: The orexin (hypocretin) system as a potential neurobiological link. Neuropsychopharmacology 46:2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jupp B, Krivdic B, Krstew E, Lawrence AJ (2011): The orexin1 receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res 1391:54–59. [DOI] [PubMed] [Google Scholar]
- 56.Khoo SY, Clemens KJ, McNally GP (2018): Palatable food self-administration and reinstatement are not affected by dual orexin receptor antagonism. Prog Neuropsychopharmacol Biol Psychiatry 87:147–157. [DOI] [PubMed] [Google Scholar]
- 57.James MH, Aston-Jones G (2020): Introduction to the Special Issue: “Making orexin-based therapies for addiction a reality: What are the steps from here?”. Brain Res 1731:146665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, Aston-Jones G (2020): Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: Why sleep on this any longer? Neuropsychopharmacology 45:717–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole S, Mayer HS, Petrovich GD (2015): Orexin/Hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep 5:16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, Muschamp JW (2018): Effects of suvorexant, a dual orexin/hypocretin receptor antagonist, on impulsive behavior associated with cocaine. Neuropsychopharmacology 43:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasmussen K, White DA, Acri JB (2019): NIDA’s medication development priorities in response to the Opioid Crisis: Ten most wanted. Neuropsychopharmacology 44:657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell EJ, Marchant NJ, Lawrence AJ (2020): A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res 1731:145902. [DOI] [PubMed] [Google Scholar]
- 63.Campbell EJ, Norman A, Bonomo Y, Lawrence AJ (2020): Suvorexant to treat alcohol use disorder and comorbid insomnia: Plan for a phase II trial. Brain Res 1728:146597. [DOI] [PubMed] [Google Scholar]
- 64.Matzeu A, Martin-Fardon R (2020): Targeting the orexin system for prescription opioid use disorder. Brain Sci 10:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fragale JE, James MH, Avila JA, Spaeth AM, Aurora RN, Langleben D, Aston-Jones G (2021): The insomnia-addiction positive feedback loop: Role of the orexin system. Front Neurol Neurosci 45:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akimoto H, Honda Y, Takahashi Y (1960): Pharmacotherapy in narcolepsy. Dis Nerv Syst 21:704–706. [PubMed] [Google Scholar]
- 67.Nishino S, Mignot E (1997): Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol 52:27–78. [DOI] [PubMed] [Google Scholar]
- 68.Singer BF, Fadanelli M, Kawa AB, Robinson TE (2018): Are cocaine-seeking “habits” necessary for the development of addiction-like behavior in rats? J Neurosci 38:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD (2000): Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology 141:3623–3629. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Li S, Kirouac GJ (2014): Blocking of corticotrophin releasing factor receptor-1 during footshock attenuates context fear but not the upregulation of prepro-orexin mRNA in rats. Pharmacol Biochem Behav 120:1–6. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, et al. (2014): Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct 219:2103–2118. [DOI] [PubMed] [Google Scholar]
- 72.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. (2001): Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21:RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, van den Pol AN (2006): Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci 26:13037–13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. (2014): Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A 111:E1648–E1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baimel C, Lau BK, Qiao M, Borgland SL (2017): Projection-target-defined effects of orexin and dynorphin on VTA dopamine neurons. Cell Rep 18:1346–1355. [DOI] [PubMed] [Google Scholar]
- 76.Matzeu A, Martin-Fardon R (2018): Drug seeking and relapse: New evidence of a role for orexin and dynorphin co-transmission in the paraventricular nucleus of the thalamus. Front Neurol 9:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spangler R, Unterwald EM, Kreek MJ (1993): ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res 19:323–327. [DOI] [PubMed] [Google Scholar]
- 78.Schmeichel BE, Matzeu A, Koebel P, Vendruscolo LF, Sidhu H, Shahryari R, et al. (2018): Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats. Neuropsychopharmacology 43:2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pantazis CB, James MH, O’Connor S, Shin N, Aston-Jones G (2022): Orexin-1 receptor signaling in ventral tegmental area mediates cue-driven demand for cocaine. Neuropsychopharmacology 47:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang B, You ZB, Wise RA (2009): Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: Independence from the local corticotropin-releasing factor network. Biol Psychiatry 65:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mahler SV, Smith RJ, Aston-Jones G (2013): Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacol (Berl) 226:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV (2011): Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol 14:684–690. [DOI] [PubMed] [Google Scholar]
- 83.Borgland SL, Storm E, Bonci A (2008): Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci 28:1545–1556. [DOI] [PubMed] [Google Scholar]
- 84.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006): Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601. [DOI] [PubMed] [Google Scholar]
- 85.Mehr JB, Bilotti MM, James MH (2021): Orexin (hypocretin) and addiction. Trends Neurosci 44:852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei K, Kwok C, Darevsky D, Wegner SA, Yu J, Nakayama L, et al. (2019): Nucleus accumbens shell Orexin-1 receptors are critical mediators of binge intake in excessive-drinking individuals. Front Neurosci 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei K, Wegner SA, Yu JH, Mototake A, Hu B, Hopf FW (2016): Nucleus accumbens shell and mPFC but not insula Orexin-1 receptors promote excessive alcohol drinking. Front Neurosci 10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.James MH, Fragale JE, O’Connor SL, Zimmer BA, Aston-Jones G (2021): The orexin (hypocretin) neuropeptide system is a target for novel therapeutics to treat cocaine use disorder with alcohol coabuse. Neuropharmacology 183:108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Escrivá-Martínez T, Herrero R, Molinari G, Rodríguez-Arias M, Verdejo-García A, Baños RM (2020): Binge eating and binge drinking: A two-way road? An integrative review. Curr Pharm Des 26:2402–2415. [DOI] [PubMed] [Google Scholar]
- 90.Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S (2017): Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol Psychiatry 81:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, et al. (2015): Anti-depressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis 79:59–69. [DOI] [PubMed] [Google Scholar]
- 92.Yaeger JDW, Krupp KT, Jacobs BM, Onserio BO, Meyerink BL, Cain JT, et al. (2022): Orexin 1 receptor antagonism in the basolateral amygdala shifts the balance from pro- to antistress signaling and behavior. Biol Psychiatry 91:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gyawali U, James MH (2022): Orexin (hypocretin) signaling in the basolateral amygdala contributes to individual differences in stress sensitivity. Biol Psychiatry 91:775–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gyawali U, James MH (2022): Sleep disturbance in substance use disorders: The orexin (hypocretin) system as an emerging pharmacological target [published online ahead of print]. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanaka S (2012): Chapter Five: Transcriptional regulation of the hypocretin/orexin gene. In: Litwack G, editor. Vitamins & Hormones. Cambridge, United Kingdom: Academic Press, 75–90. [Google Scholar]
- 96.Silva JP, von Meyenn F, Howell J, Thorens B, Wolfrum C, Stoffel M (2009): Regulation of adaptive behaviour during fasting by hypothalamic Foxa2. Nature 462:646–650. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka S, Kodama T, Nonaka T, Toyoda H, Arai M, Fukazawa M, et al. (2010): Transcriptional regulation of the hypocretin/orexin gene by NR6A1. Biochem Biophys Res Commun 403:178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honda M, Eriksson KS, Zhang S, Tanaka S, Lin L, Salehi A, et al. (2009): IGFBP3 colocalizes with and regulates hypocretin (orexin). PLoS One 4:e4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nestler EJ, Lüscher C (2019): The molecular basis of drug addiction: Linking epigenetic to synaptic and circuit mechanisms. Neuron 102:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bali P, Kenny PJ (2019): Transcriptional mechanisms of drug addiction. Dialogues Clin Neurosci 21:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kenny PJ (2014): Epigenetics, microRNA, and addiction. Dialogues Clin Neurosci 16:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rao Y, Mineur YS, Gan G, Wang AH, Liu ZW, Wu X, et al. (2013): Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin-producing neurons in the lateral hypothalamus in mice. J Physiol 591:1951–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV (2012): Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol 590:3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D (2004): Orexin A and B levels in the hypothalamus of female rats: The effects of the estrous cycle and age. Eur J Endocrinol 150:737–742. [DOI] [PubMed] [Google Scholar]