Abstract
Purpose
The German Asthma Net (GAN) operates a Severe Asthma Registry that provides an overview of the clinical presentation and management of patients with severe asthma. Based upon data from the GAN registry, the MepoGAN study aimed to describe clinical profiles and treatment outcomes of patients who were treated with the anti-IL-5 monoclonal antibody mepolizumab (NucalaTM) in routine practice in Germany.
Patients and Methods
The MepoGAN study is a descriptive retrospective non-interventional cohort study. Mepolizumab patients enrolled in the GAN registry were evaluated with results being described in two different data sets: Cohort 1 (n=131) started on mepolizumab when the patients entered the registry. Results were reported after 4 months of therapy. Patients in Cohort 2 (n=220) were on treatment with mepolizumab at the time of enrollment and follow-up data were collected after a further year of treatment. Outcome measures included asthma control, lung function, disease symptoms, OCS use, and exacerbations.
Results
Patients enrolled in the registry who started on mepolizumab in Cohort 1 had a mean age of 55 years, were former smokers in 51% of the cases, had a mean blood eosinophil count of 500 cells/μL, and frequently had maintenance OCS use (55%). In this real-world setting, mepolizumab therapy was associated with a clinically relevant reduction in blood eosinophils (−445.7 cells/μL), OCS use (−30%), and improvement in asthma control. Fifty-five percent (vs 10% at baseline) of the patients reported controlled or partially controlled asthma 4 months after starting therapy. In patients who were already treated with mepolizumab at registry enrollment (Cohort 2), asthma control and lung function remained stable after a further year of observation.
Conclusion
The GAN registry data confirm the effectiveness of mepolizumab in a real-world setting. Treatment benefits are maintained over time. While the asthma of patients treated in routine practice was more severe, the results observed with mepolizumab are broadly consistent with RCTs.
Keywords: severe asthma, registry, real-world, mepolizumab, patient profile, asthma control
Introduction
Patients with severe persistent asthma represent the highest unmet medical need among the asthmatic population.1,2 Severe asthma, as defined by GINA, is asthma that is uncontrolled despite adherence with optimized high-dose ICS-LABA treatment and management of contributory factors, or that worsens when high-dose treatment is decreased.3 A common phenotype, eosinophilic asthma, is associated with persistent eosinophilic inflammation, late onset of disease, impaired lung function, poor asthma control, recurrent exacerbations, and, in some patients, dependence on high-dose oral corticosteroids (OCS).4
The development of monoclonal antibodies has transformed the management of severe asthma, allowing a more personalized treatment approach.5–7 During the past decade, several antibodies have been approved: anti-IgE (omalizumab) against IgE-mediated asthma, anti-interleukin-5 (anti-IL5, mepolizumab, reslizumab) and anti-interleukin-5 receptor α (anti-IL5R, benralizumab) for eosinophilic asthma, and recently anti-interleukin-4 receptor α (anti-IL4R, dupilumab) for asthmatics with elevated eosinophils and/or exhaled nitric oxide (FeNO) levels.8 Tezepelumab, an anti-TSLP antibody, is the latest biologic for severe asthma.
Mepolizumab was the first-in-class monoclonal antibody available to target IL-5, which is responsible for development, maturation, and survival of eosinophils.9 The fully humanized immunoglobulin (IgG) antibody is indicated as an add-on treatment for severe uncontrolled eosinophilic asthma in adults, adolescents and children aged 6 years and older, and has recently gained EU approval for use in three additional eosinophil-driven diseases (eosinophilic granulomatosis with polyangiitis [EGPA], hypereosinophilic syndrome [HES], and chronic rhinosinusitis with nasal polyps).9 For severe eosinophilic asthma, a comprehensive clinical trial program consisting of four randomized, double-blind, parallel-group clinical studies of between 24 and 52 weeks duration evaluated the efficacy and safety of mepolizumab.10 The dose-ranging efficacy study DREAM11 and the exacerbation reduction study MENSA12 were accompanied by SIRIUS,13 an oral corticosteroid-sparing study, and MUSCA,14 a quality-of-life (QoL) study. COLUMBA, a 4.5-year-long extension study to DREAM, investigated the long-term safety and efficacy of mepolizumab.15 All these studies demonstrated that treatment with mepolizumab was well tolerated and resulted in a meaningful decrease in blood eosinophils and a significant reduction of intake/dosage of OCS, reduction of exacerbations, and, in one study, improvement of QoL.10 Despite this convincing evidence, controlled studies are derived in a narrowly framed selected population with limited information for treatment implementation in a real-world environment. It is unknown whether these patients respond differently to therapy when compared with patients from controlled trials.16 More recently, data generation on the mepolizumab real-world impact in routine clinical practice has started to emerge,4,7,16–24 though there are still limitations in knowledge, particularly at the national level with different healthcare systems.
National and International Asthma Registries Provide Real-World Data
Disease registries are well-established tools to advance the quality of care and gather information on disease epidemiology, patient management and outcomes. As such, asthma registries provide data on the effectiveness and safety of treatment regimens in a real-world setting.1,2,5,17,25–32 They describe common challenges (eg, management of elderly or comorbid patients, adherence to treatment, different schedules of dosing or administration) and allow deeper insight of what can be achieved with a treatment in routine clinical practice.26 Thus, asthma disease registries provide a suitable source of real-world information, which also can be turned into actionable insights for decision-making for biological therapies.2
Severe Asthma Treatment in Routine Practice in Germany: The GAN Registry
In December 2011, the Severe Asthma Registry in Germany was set up, which is hosted by the German Asthma Net (GAN, www.german-asthma-net.de). The objectives of the registry are to improve the understanding of underlying mechanisms and disease progression and to characterize the parameters related to severe disease in a prospective assessment. Patients are enrolled in a real-life setting in dedicated centers and followed over time using a database management system. Recruitment of patients with severe asthma into the registry is ongoing. All patients with a physician-diagnosed severe asthma are eligible for inclusion. Enrolled patients should be treatment compliant and educated in correct inhaler use. They undergo detailed clinical and functional evaluations, including patients’ medical history and comorbidities, allergy and lung function tests, concomitant medication, asthma control and quality of life. Patients are followed up at least once a year.1 Currently, more than 75 asthma centers in Germany are contributing patients to the GAN registry.8
The MepoGAN study aimed to describe clinical profiles and treatment outcomes of patients for whom mepolizumab is prescribed in routine practice in Germany based upon data from the GAN registry.
Methods
The MepoGAN study is a retrospective analysis of patients with severe asthma who were treated with mepolizumab and were included in the Severe Asthma Registry of the German Asthma Net (GAN). We included two cohorts: 1) Cohort 1: a “4-month cohort with baseline history”; patients who were initiated on mepolizumab when they entered the registry and for whom effectiveness and safety results were reported after 4 months of therapy, and 2) Cohort 2: a “≥12-month cohort”; patients, most of whom were already receiving mepolizumab at the time of enrollment and who had a follow-up of at least 12 months in the registry.
For patients documented in the GAN registry, the participating centers completed a detailed documentation sheet at baseline. After 1 year of follow-up, documentation was collected again (Cohort 2). Following the launch of the registry, the GAN introduced an additional visit (4-month visit) in 2018 exclusively for patients in whom a biological agent was initiated at the time of registry enrollment. In these patients, the baseline history and subsequent documentation of effectiveness and safety following 4 months of mepolizumab therapy were evaluated (Cohort 1).
The GAN registry was approved by the ethics committee of the University of Mainz, as well as by local IRBs of participating centers. All patients gave written informed consent to participation in the registry. No additional vote was required for this retrospective analysis of the anonymized registry data.
Objectives
The primary objective was to describe the clinical characteristics of patients with severe eosinophilic asthma treated with mepolizumab. Variables evaluated included demographics, disease characteristics, medication, disease control, lung function, allergic sensitization and comorbidities. The secondary objective was to describe the treatment outcomes, including 1) in Cohort 1: GINA control status, lung function, use of maintenance OCS, differential blood count, and adverse events, and 2) in Cohort 2: GINA control status, disease status according to asthma control and quality of life questionnaires (ACQ-5, ACT, mini AQLQ), lung function, disease symptoms during day and night, use of reliever medication, user of maintenance OCS, and frequency of exacerbations. The deviating outcome measurements in the two cohorts result from the fact that the data collection in the registry for Cohort 1 and Cohort 2 included partly different parameters.
Study Population and Data Collection
The GAN registry includes a broad population of patients with a medical diagnosis of severe asthma. In the present study, all patients aged 18 and over who were admitted to the registry by July 10, 2020, and treated with mepolizumab were included for analysis. Data collection was performed by health-care professionals in the participating centers.
Information on asthma control and quality of life of the patients was documented using the ACQ-5 (Asthma Control Questionnaire),33 the ACT (Asthma Control Test),34,35 and the mini AQLQ (Asthma Quality of Life Questionnaire).36
Statistical Analysis
Continuous variables were summarized using the number of observations, mean, and standard deviation (SD). The categorical variables were based on the number of observations and relative frequencies (percentages). Statistical tests for dependent data, Chi-square test, and paired sample t-test were used to compare the change from baseline to follow-up.
The statistical analyses of the changes from baseline to 4-month follow-up for Cohort 1, or 1-year follow-up for Cohort 2, followed a complete case strategy. The analyzed population only included patients who had a baseline and the respective follow-up value, no missing values were imputed. To validate the assumption that the follow-up data was missing at random, the baseline data of patients with and without follow-up data were compared by appropriate exploratory statistical tests for difference depending on the nature of the data, eg, Chi-square test, Fisher’s exact test for categorical/nominal data, t-test for continuous normal distributed data and Mann–Whitney U-test for continuous non-normal distributed data. Changes from baseline were quantified by pairwise p-values (paired t-test in case of normal distribution or Wilcoxon signed rank test in case of non-normal distribution). Within single items, only parameters for which at least 70% of patient data were available were analyzed further in the case of longitudinal comparison.
Results
Cohort 1: “4-Month Cohort with Baseline History”
For the analysis in Cohort 1, data from 131 patients from 36 centers were available. The cohort comprised patients who had started on mepolizumab therapy at the time of enrollment in the registry and were then followed up for 4 months. More than half of the patients entered the registry as of 2019.
Patient Profiles at Entry into the Registry (Baseline Characteristics)
The mean age (±SD) of the patients was 55.0 (±14.5) years (range: 10–83). Seventy-seven patients (58.8%) were female and the mean (±SD) body mass index was 27.0 (±5.6) kg/m2. Slightly more than half of the patients (n=66/130; 50.8%) were former smokers with an average of 14.7 ± 17.7 pack-years. Two patients (1.5%) stated that they were current smokers (mean pack years: 9.0 ± 5.7). The mean (±SD) asthma disease duration was 17.7 (±14.2) years (range: 0–63). In almost 90% of the patients, the asthma was classified as uncontrolled at baseline, ie, before commencement of mepolizumab. Table 1 displays clinical characteristics and treatment in the past at registry entry.
Table 1.
Cohort 1 (N=131): Clinical Characteristics and Maintenance Medication in the Past at Entry into the GAN Registry, ie, Before Starting Mepolizumab Therapy
| Baseline characteristics (N=131*) | ||
|---|---|---|
| Exacerbations during the past 12 months | n | % |
|
4 | 3.1 |
|
10 | 7.6 |
|
83 | 63.4 |
|
28 | 21.4 |
|
6 | 4.6 |
| GINA control status | ||
|
3 | 2.3 |
|
11 | 8.4 |
|
117 | 89.3 |
| Systemic steroids/OCS as maintenance medication | 72 | 55.0 |
| Lung function, pre-bronchodilator | Mean (±SD) | |
| FEV1, liter (N=104) | 2.70 (±7.44) | |
| FEV1, % (N=105) | 65.1 (±24.9) | |
| Eosinophils, cells/μL (N=107) | 500 (±438) | |
| Leukocytes, cells/nL (N=108) | 8.6 (±2.5) | |
Note: *Unless otherwise stated.
Abbreviations: GINA, Global Initiative for Asthma; OCS, oral corticosteroids.
4-Month Follow-Up Data After Initiating Mepolizumab
In Cohort 1, data from 87 patients were eligible for the analysis of follow-up data after 4 months of mepolizumab treatment. Table 2 provides an overview of the changes in asthma control, lung function, eosinophil and white blood cell counts, and maintenance therapy with OCS. At the end of the 4-month period, more than half of the patients (55.1%) reported controlled or partially controlled asthma. In this cohort, 40/87 patients remained unchanged, 4/87 had deteriorated and 43/87 improved (p<0.0001). This was accompanied by an improvement in lung function, measured by means of pre-bronchodilator FEV1 (FEV1, %: p=0.003; FEV1, liter: p=0.0088). Mepolizumab also resulted in a marked reduction in peripheral blood eosinophil count (p<0.0001) and white blood cell count as well (p=0.001). The proportion of patients taking OCS as maintenance medication decreased by almost 30.0% after 4 months of mepolizumab therapy. And 1/87 patients remained unchanged, 1/87 had deteriorated (no OCS at baseline, OCS after 4 months) and 15/87 improved (OCS at baseline, no OCS after 4 months) (p=0.0005).
Table 2.
Cohort 1: Clinical Characteristics in the Subset of Patients (N=87) with Available 4-Month Follow-Up Data After Initiating Mepolizumab Therapy
| Baseline | After 4-Month FU | |||
| GINA control status (N=87)* | n (%) | |||
|
3 (3.4) | 21 (24.1) | ||
|
6 (6.9) | 27 (31.0) | ||
|
78 (89.7) | 39 (44.8) | ||
| Systemic steroids/OCS as maintenance medication (N=87)* | 47 (54.0) | 33 (37.9) | ||
| Lung Function (N=66), Pre-Bronchodilator* | Mean (±SD) | Difference to Baseline | p-value | |
| FEV1, liter | 3.21 (±9.27) | 3.42 (±9.49) | 0.21 | 0.0088 |
| FEV1, % | 68.4 (±23.6) | 74.8 (±24.4) | 6.4 | 0.0030 |
| Eosinophils (N=52), cells/μL* | 534.8 (±420.1) | 89.1 (±83.8) | −445.7 | <0.0001 |
| Leukocytes, cells/nL | 8.7 (±2.5) | 7.5 (±2.6) | −1.3 | 0.0010 |
Note: *Patients with available data.
Abbreviations: FU, follow-up; GINA, Global Initiative for Asthma; OCS, oral corticosteroids.
A total of 11 adverse events were documented in 7/87 patients (8.0%) during mepolizumab therapy. These included headache (2), muscle/joint pain (3), stomach pain (1), sore throat (1), itching/rash/tingle (3), and unable to walk (1). Treatment with mepolizumab was continued in 76 patients (87.4%) beyond the 4-month observation period.
Cohort 2: “≥12-Month Cohort”
The full baseline data set of the GAN registry included 2033 patients with severe asthma, 220 of whom received mepolizumab (Cohort 2) in 33 participating dedicated asthma centers. The majority of patients were enrolled between 2017 and 2019, and most patients were on treatment with mepolizumab at the time of enrollment.
Patient Profiles at Entry into the Registry (Baseline Characteristics)
The mean age of the mepolizumab-treated severe asthma population in Cohort 2 (N=220) was 56 (range: 22–87) and mean (±SD) disease duration 16.7 (±13.3; range: 0–63, data from 167 patients available) years. Cohort 2 consisted of 58.2% women. The mean body mass index (±SD) was 27.1 (±5.6) kg/m2. Approximately half of the patients (n=112; 50.9%) were reported as never-smokers. Three patients (1.4%) were current smokers and 105 patients (47.7%) former smokers, with a mean (±SD) of 13.7 (±13.0) pack-years. Eighty-six patients (39.1%) stated being exposed to passive smoking. In total, 121 patients (55.0%) reported at least one allergic comorbidity; 134/219 patients (61.2%) suffered from chronic sinusitis, 67/153 patients (43.8%) had nasal polyps, and 133/219 patients reported frequent (ie, > 2× per year) lower respiratory tract infections (Table 3).
Table 3.
Cohort 2 (N=220): Asthma Symptoms, Other Allergic Conditions/Comorbidities, and Rescue Medication at Entry into the GAN Registry
| Asthma symptoms | n | %* |
|---|---|---|
| Total number of patients with symptoms | 192 | 87.3** |
| Dyspnea | 159 | 82.8 |
|
157 | 81.8 |
|
23 | 12.0 |
| Chest tightness/chest pain | 43 | 22.4 |
| Coughing | 125 | 65.1 |
| Rhonchus or wheezing | 79 | 41.1 |
| Prolonged expiration | 18 | 9.4 |
| Reduced exercise capacity | 138 | 71.9 |
| Bronchial hyperresponsiveness (BHR) | 129 | 67.2 |
| Symptoms during the day | n | %* |
|
5 | 2.6 |
|
48 | 25.0 |
|
46 | 24.0 |
|
80 | 41.7 |
|
13 | 6.8 |
| Symptoms during the night | n | %* |
|
52 | 27.1 |
|
37 | 19.3 |
|
24 | 12.5 |
|
29 | 15.1 |
|
44 | 22.9 |
|
6 | 3.1 |
| Allergic condition/Comorbidity (patients with available data) | n | % |
| Allergic rhinitis/rhinoconjunctivitis | 101 (220) | 45.9 |
| Food allergy | 39 (220) | 17.7 |
| Atopic eczema | 12 (220) | 5.5 |
| Other allergic conditions | 21 (220) | 9.5 |
| Chronic sinusitis | 134 (219) | 61.2 |
| Nasal polyps | 67 (153) | 43.8 |
| Frequent LRTI (>2× per year) | 133 (219) | 60.7 |
| GERD | 73 (219) | 33.3 |
| Asthma rescue medication | n | %** |
|
62 | 28.2 |
|
53 | 24.1 |
|
42 | 19.1 |
|
54 | 24.5 |
|
9 | 4.1 |
Notes: *Of total number of patients with symptoms (n=192). **Of total cohort population (N=220).
Abbreviations: GERD, gastroesophageal reflux disease; LRTI, lower respiratory tract infection.
At the time of entry into the registry (“baseline”), the duration of mepolizumab therapy amounted to (mean ± SD) 11.7 (±12.1) months (range: 0–49; n=212). The duration of previous mepolizumab therapy was 0–3 months in 25.5%, 4–6 months in 12.2%, 7–12 months in 20.7%, and 12–24 months in 14.0% of the patients. More than a quarter (27.4%) had received mepolizumab for more than 24 months. The mean (±SD) eosinophil count before starting mepolizumab therapy was 549.1 (±637.2) cells/µL (N=220). 79/220 patients (35.9%) received maintenance therapy with an oral corticosteroid (OCS), with a mean (±SD) prednisolone dose of 9.5 (±9.2) mg per day.
In total, 192 patients (87.3%) reported persistent symptoms at baseline, the most common of which were dyspnea (82.8%), reduced exercise capacity (71.9%), and cough (65.1%) (Table 3). At least 41.7% and 22.9% of the patients experienced diurnal asthma symptoms and frequent nocturnal symptoms, respectively. Around a quarter (24.5%) reported rescue medication intake at least once a day. The analysis of Cohort 2 also included data from validated symptom questionnaires. At enrollment into the registry, the overall mean (±SD) score from the ACT, mini AQLQ and ACQ-5 was 17.1 (±5.5), 4.5 (±1.4), and 2.1 (±1.4) points, respectively. The analysis of lung function showed a mean (± SD) pre-bronchodilator absolute FEV1 value of 2.06 (0.84) liters (range: 0.53–4.97; data from 187 patients available). The mean (± SD) FEV1 (% predicted) was 69.0 (±21.9) (range: 24.0–125.0), the mean (± SD) FEV1/FVC ratio 67.7 (±15.0) (range: 29.0–106.0), and the mean (± SD) peak expiratory flow (PEF) 6.98 (±19.75) liters per second (range: 0.82–269.42).
Baseline information on exacerbations was available for 198 patients. Of these, 139 (70.2%) had at least one disease exacerbation in the previous 12 months. Moreover, 59/137 (43.1%) and 45/138 (32.6%) patients reported emergency medical treatment or asthma-related hospitalization during the past year. In about half of the patients, the GINA control status of asthma was classified as controlled or partly controlled (controlled: n=52, 23.6%; partly controlled: n=58, 26.4%; uncontrolled: n=110, 50.0%).
1-Year Follow-Up Data from the GAN Registry
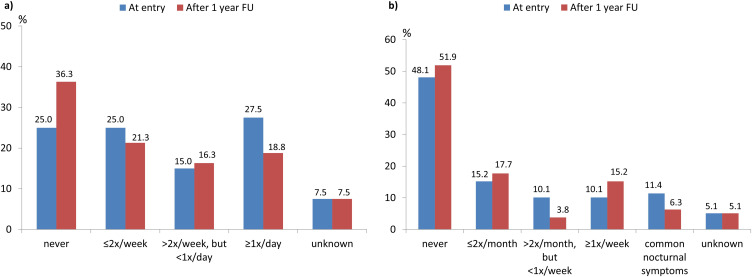
A total of 89 patients on mepolizumab in Cohort 2 were eligible for analysis of the 1-year follow-up data; 80.2% reported ongoing symptoms both upon entry into the registry and after 1 year of follow-up. However, the proportion of patients who experienced diurnal symptoms decreased. Overall, 47.1% of the patients were unchanged, 34.3% had improved, and 18.6% had worsened (p=0.0705 for “improved” vs “worsened”). In particular, there were fewer patients with asthma symptoms several times per day (Figure 1a). The nocturnal symptom burden remained unchanged (p=n.s. for “improved” vs “worsened”; Figure 1b). The proportion of patients reporting the use of rescue medication decreased from 67.5% at baseline to 60.0% during the 12-month observation period (p=n.s.). After the end of the 1-year follow-up, 12.5% of the patients still required daily rescue medication compared to 17.5% at entry into the registry (p=n.s.).
Figure 1.
Cohort 2: Percentage of patients with symptoms (a) during the day or (b) during the night in the subset of patients with available 1-year follow-up data after entry into the GAN registry (N=80 and N=79 with available data).
Abbreviation: FU, follow-up.
The number of patients with at least one exacerbation during the past 12 months dropped from n=48 (66.7%) at baseline to n=39 (45.8%) after 1 year of follow-up (data from 72 patients available). In particular, the proportion of patients with frequent exacerbations (≥2 per year) decreased (45.8% at registry enrollment and 26.4% after 1 year of follow-up). Overall, 33/70 (47.1%) patients remained unchanged with regard to exacerbations, 27/70 (38.6%) had improved and 10/70 (14.3%) had worsened (p=0.0052 for “improved” vs “worsened”). The lung function (FEV1, FEV1/FVC, PEF) remained unchanged during the 1-year observation period. This also applied to symptom control, which was measured by means of ACT, mini AQLQ and ACQ-5 questionnaires (Table 4). After 1 year of follow-up, 23.5% of the patients required maintenance therapy with OCS compared to 28.4% at baseline (p=0.1573 for “improved” vs “worsened”). The required mean prednisolone dose decreased from 8.6 (±5.4) at registry entry to 7.1 (±2.6) after 1 year of follow-up (p=n.s.).
Table 4.
Cohort 2: Lung Function and Symptom Control in the Subset of Patients (N=89) with Available 1-Year Follow-Up Data After Entry into the GAN Registry
| At Entry Into Registry | After 1-Year FU | Difference to Entry | p-value | |
|---|---|---|---|---|
| Lung function pre-bronchodilator, mean (±SD) | ||||
| FEV1, liter N=57* | 2.09 (±0.89) | 2.13 (±0.91) | 0.05 | 0.5020 |
| FEV1, % N=56* | 71.36 (±24.77) | 73.68 (±26.84) | 2.32 | 0.3106 |
| FEV1/FVC N=56* | 67.93 (±16.64) | 68.74 (±15.54) | 0.81 | 0.5165 |
| PEF, liter per second | 5.51 (±2.22) | 5.67 (±2.54) | 1.66 | 0.4766 |
| Symptom control mean (±SD) points | ||||
| ACQ-5 N=66* | 1.78 (±1.41) | 1.75 (±1.54) | −0.03 | 0.8818 |
| ACT N=71* | 18.5 (±5.2) | 18.6 (±5.6) | 0.0 | 0.9823 |
| Mini AQLQ N=63* | 4.75 (±1.43) | 5.04 (±1.38) | 0.29 | 0.0876 |
Note: *Patients with available data.
Abbreviations: ACQ-5, Asthma Control Questionnaire, 5-item version; ACT, Asthma Control Test; AQLQ, Asthma Quality of Life Questionnaire; FEV1, forced expiratory volume in 1 second; FU, follow-up; FVC, forced vital capacity; PEF, peak expiratory flow; SD, standard deviation.
Discussion
In controlled clinical studies, the IL-5 monoclonal antibody mepolizumab has been shown to reduce exacerbation rates and the use of oral corticosteroids as well as to improve asthma control and health-related quality of life compared with placebo in patients with severe eosinophilic asthma.19 Although data from RCTs can provide significant insights into the clinical efficacy and safety of a therapy, these studies often include a highly selected homogenous population, which is not representative of the general asthma population, due to narrow eligibility criteria. It is therefore important to obtain data on the effects of a treatment outside the constraints of a formal clinical trial.23 Registries may offer an important source of real-life data for scientific research to understand and improve disease burden, treatment patterns and patient outcomes.2
The size of the GAN registry cohort offers the opportunity to understand outcomes of severe asthma patients in routine clinical practice, including real-world experience with biologicals like mepolizumab. In contrast to RCTs with selected, homogeneous populations, the GAN registry includes a real-world heterogeneous population, with a broad spectrum of comorbidities and a relatively large proportion of patients with a positive smoking history. Compared to the RCTs MENSA and MUSCA,12,14 MepoGAN patients were older, more often ex-smokers, had higher OCS dosages (Cohort 1 and 2), and a higher baseline ACQ-5 score (Cohort 2) as well as higher baseline blood eosinophil levels (Cohort 1). More than half of the patients were using OCS when they entered the GAN registry. This is in line with results from the International Severe Asthma Registry (ISAR), which collected data from a large cohort of patients with severe asthma treated in the United States, Europe, and the Asia-Pacific region. Within ISAR, a total of 51.1% of the patients were receiving intermittent oral corticosteroids on a regular basis.28 Smoking patients or ex-smokers with a history of ⩾10 pack-years are almost never included in asthma trials due to the risk of confounding effects of smoking. Our results are consistent with data from 11 national registries for severe asthma, which could show that in practice; however, a significant proportion of patients with severe asthma are smokers or ex-smokers.32
While the asthma and comorbidities of patients treated in routine practice are more severe, clinically relevant reductions in blood eosinophils, OCS use, and improvement in asthma control were achieved with mepolizumab, similar to the RCTs. Richards et al extracted study criteria and patient characteristics of the RCTs SIRIUS, DREAM and MENSA and compared them with real-world data from the Dutch severe asthma registry. The authors found that a large proportion of the real-life, mepolizumab-treated population with severe asthma would be excluded from trial participation, and significant differences in population characteristics exist. Regardless, a large fraction of ineligible patients in clinical care could reduce maintenance OCS dosage under mepolizumab therapy.16 A single-center, observational cohort study from Germany found that antibody treatments (including mepolizumab), when added to standard asthma therapies, were as efficacious in ex-smokers suffering from severe asthma as they are in nonsmokers, by improving the asthma control, exacerbation rate and lung function of these patients.37
Evaluations from a number of other real-world databases also underscore the importance of everyday clinical experience with mepolizumab. The findings provide efficacy results that are similar to the GAN data and the results from controlled clinical trials.4,18,19 In our cohort of mepolizumab-naïve severe asthma patients, the anti-IL-5 treatment improved asthma control over a short observation period of only 4 months. In patients with predominantly ongoing mepolizumab treatment, outcomes were stable or even showed ongoing improvement over a one-year observation period.
The MepoGAN study revealed a high frequency of relevant comorbidities in severe asthma patients (nasal polyposis, chronic sinusitis, allergic rhinitis). The Severe Asthma Network in Italy (SANI), which analyzed data from 437 patients, identified rhinitis and nasal polyposis as most common comorbidities as well.26 Indeed, asthma and some chronic rhinosinusitis subtypes are mediated by similar pathophysiologic mechanisms.38 Crimi et al performed a single-center retrospective study in a real-world setting in patients with severe asthma and presence of comorbidities (eg, nasal polyps, allergic rhinitis, GERD, non-allergic rhinitis with hypereosinophilic syndrome). Nearly 84% of the patients were classified as responsive to mepolizumab treatment. Neither the comorbidities nor other characteristics (sex, BMI, age, smoking) influenced treatment response.39
Some limitations should be considered when interpreting the results from the GAN registry. Retrospective studies are designed to analyse pre-existing data and are subject to numerous potential biases. The investigator cannot assess the selection bias because it is unknown which patients have not been included in the registry. Another limitation of real-world studies includes the capturing of data from standard clinical care recording; therefore, there may be missing information. Additionally, patient behaviour may be harder to control in real-world studies. Beyond this, our study included two cohorts. Cohort 1 had a follow-up of only 4 months. Cohort 2 was a mixture of new and experienced mepolizumab users, but with a majority of patients treated for more than 4 months. In Cohort 2, we were losing more than half of the patients from baseline to the 1-year follow-up (inclusion criteria). However, the pre-specified statistical analyses showed that follow-up data was missing at random. Finally, MepoGAN is not a placebo-controlled study. Therefore, outcomes may represent a combination of treatment effect and other behavioural changes. The generalizability of the findings outside of the GAN registry may be limited.
In conclusion, the data from the GAN registry provide evidence that the efficacy of the anti-IL-5 monoclonal antibody mepolizumab is replicated in a real-world setting, outside of the controlled environment of a clinical trial.
Acknowledgment
Editorial support (in the form of writing assistance under the direction and guidance of the authors, assembling tables and figures, grammatical editing and referencing) was provided by Dr Anja Luetke (medinform, Ratekau, Germany). This study was funded by GSK (GSK ID: 213579). Statistical support was provided by Annette Holtdirk (Kottmann GmbH & Co KG, Hamm, Germany) and was funded by GlaxoSmithKline GmbH & Co. KG, Munich, Germany.
Funding Statement
This study was funded by GSK (GSK ID: 213579). Editorial support for the manuscript (in the form of writing assistance under the direction and guidance of the authors) was provided by Dr. Anja Luetke (medinform, Ratekau, Germany), and was funded by GlaxoSmithKline GmbH & Co. KG, Munich, Germany. Statistical support was provided by Annette Holtdirk (Kottmann GmbH & Co KG, Hamm, Germany), and was funded by GlaxoSmithKline GmbH & Co. KG, Munich, Germany.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Stephanie Korn received consulting fees from Astra Zeneca, Boehringer Ingelheim, GSK, Novartis, Sanofi and Teva.
Kathrin Milger received consulting fees from AstraZeneca, GSK, Novartis and Sanofi, honoraria for lectures from AstraZeneca, GSK, Novartis and Sanofi and travelling grants from AstraZeneca.
Dirk Skowasch reports personal fees and/or grants from AstraZeneca, Bayer, Boehringer-Ingelheim, Chiesi, GSK, Janssen-Cilag, Sanofi, Novartis and Pfizer; grants from DFG and BMBF, outside the submitted work.
Roland Buhl received grants to Mainz University from Boehringer Ingelheim, GSK, Novartis and Roche, honoraria for lectures from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, Cipla, GSK, Novartis, Roche, Sanofi and Teva and consulting fees for advisory boards from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, Novartis, Roche and Sanofi. He is a member of the GINA science committee, and co-author of the German asthma guidelines.
Christian Schulz reports personal fees from AstraZeneca, personal fees from Novartis, personal fees from Boehringer Ingelheim, outside the submitted work.
Cordula Mohrlang, Michael Hennig and Thomas Paulsson are employees and shareholders of GSK.
Martin Wernitz received consulting fees from GSK for designing, writing and managing the study. Martin Wernitz is an independent consultant for GlaxoSmithKline GmbH & Co. KG, Munich, Germany. The authors report no other conflicts of interest in this work.
References
- 1.Korn S, Hübner M, Hamelmann E, Buhl R. The German severe asthma registry. Pneumologie. 2012;66(6):341–344. [DOI] [PubMed] [Google Scholar]
- 2.FitzGerald JM, Tran TN, Alacqua M, et al. International severe asthma registry (ISAR): protocol for a global registry. BMC Med Res Methodol. 2020;20(1):212. doi: 10.1186/s12874-020-01065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma. Global strategy for asthma management and prevention; 2022. Available from: http://www.ginasthma.org. Accessed July 19, 2022.
- 4.Harrison T, Canonica GW, Chupp G, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J. 2020;56(4):2000151. doi: 10.1183/13993003.00151-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson DJ, Busby J, Pfeffer PE, et al. Characterisation of patients with severe asthma in the UK severe asthma registry in the biologic era. Thorax. 2021;76(3):220–227. doi: 10.1136/thoraxjnl-2020-215168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers FC, Licskai C, Chanez P, et al. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806. doi: 10.1016/j.rmed.2019.105806 [DOI] [PubMed] [Google Scholar]
- 7.Bagnasco D, Massolo A, Bonavia M, et al. The importance of being not significant: blood eosinophils and clinical responses do not correlate in severe asthma patients treated with mepolizumab in real life. Allergy. 2020;75(6):1460–1463. doi: 10.1111/all.14135 [DOI] [PubMed] [Google Scholar]
- 8.Milger K, Korn S, Buhl R, et al. Age- and sex-dependent differences in patients with severe asthma included in the German asthma net cohort. Respir Med. 2020;162:105858. doi: 10.1016/j.rmed.2019.105858 [DOI] [PubMed] [Google Scholar]
- 9.European Medicine Agency (EMA). Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/nucala. Accessed July 19, 2022.
- 10.Miyokawa R, Kivler C, Louie S, et al. Self-administered mepolizumab in the management of severe asthma: usability and patient acceptance. Patient Prefer Adherence. 2020;14:1669–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 12.Ortega HG, Liu MC, Pavord ID, et al; MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 13.Bel EH, Wenzel SE, Thompson PJ, et al; SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 14.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 15.Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143:1742–1751.e1747. [DOI] [PubMed] [Google Scholar]
- 16.Richards LB, van Bragt JJMH, Aarab R, et al. Treatment eligibility of real-life mepolizumab-treated severe asthma patients. J Allergy Clin Immunol Pract. 2020;8(9):2999–3008.e1. doi: 10.1016/j.jaip.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 17.Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5):1902420. doi: 10.1183/13993003.02420-2019 [DOI] [PubMed] [Google Scholar]
- 18.Llanos JP, Ortega H, Bogart M, et al. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. J Asthma Allergy. 2020;29(13):77–87. doi: 10.2147/JAA.S236609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortega H, Hahn B, Bogart M, et al. Impact of mepolizumab on exacerbations in severe asthma: results from a U.S. insurance claims data base. Allergy Asthma Proc. 2020;41(5):341–347. doi: 10.2500/aap.2020.41.200043 [DOI] [PubMed] [Google Scholar]
- 20.Pelaia C, Crimi C, Pelaia G, et al. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020;50(7):780–788. doi: 10.1111/cea.13613 [DOI] [PubMed] [Google Scholar]
- 21.Renner A, Marth K, Patocka K, et al. Effectiveness of mepolizumab therapy in patients with severe eosinophilic asthma: Austrian real-life data. Pulm Pharmacol Ther. 2020;64:101946. doi: 10.1016/j.pupt.2020.101946 [DOI] [PubMed] [Google Scholar]
- 22.Enríquez Rodríguez AI, Valverde TH, Romero AP, et al. Results in clinical practice in the treatment of severe eosinophilic asthma with mepolizumab: a real-life study. J Asthma. 2022;59(5):1005–1011. doi: 10.1080/02770903.2021.1897835 [DOI] [PubMed] [Google Scholar]
- 23.Taillé C, Chanez P, Devouassoux G, et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J. 2020;55(6):1902345. doi: 10.1183/13993003.02345-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas D, Harvey ES, McDonald VM, et al. Mepolizumab and oral corticosteroid stewardship: data from the Australian mepolizumab registry. J Allergy Clin Immunol Pract. 2021;9(7):2715–2724.e5. doi: 10.1016/j.jaip.2021.01.028 [DOI] [PubMed] [Google Scholar]
- 25.Bulathsinhala L, Eleangovan N, Heaney LG, et al. Development of the International Severe Asthma Registry (ISAR): a modified delphi study. J Allergy Clin Immunol Pract. 2019;7(2):578–588.e2. doi: 10.1016/j.jaip.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 26.Heffler E, Blasi F, Latorre M, et al. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract. 2019;7(5):1462–1468. doi: 10.1016/j.jaip.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 27.Graff S, Vanwynsberghe S, Brusselle G, et al. Chronic oral corticosteroids use and persistent eosinophilia in severe asthmatics from the Belgian severe asthma registry. Respir Res. 2020;21(1):214. doi: 10.1186/s12931-020-01460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi: 10.1016/j.chest.2019.10.053 [DOI] [PubMed] [Google Scholar]
- 29.Canonica GW, Malvezzi L, Blasi F, et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. 2020;166:105947. doi: 10.1016/j.rmed.2020.105947 [DOI] [PubMed] [Google Scholar]
- 30.Senna G, Latorre M, Bugiani M, et al. Sex differences in severe asthma: results from severe asthma network in Italy-SANI. Allergy Asthma Immunol Res. 2021;13(2):219–228. doi: 10.4168/aair.2021.13.2.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang F, Busby J, Heaney LG, et al. Factors associated with frequent exacerbations in the UK severe asthma registry. J Allergy Clin Immunol Pract. 2021;9(7). doi: 10.1016/j.jaip.2020.12.062 [DOI] [PubMed] [Google Scholar]
- 32.van Bragt JJMH, Adcock IM, Bel EHD, et al. Characteristics and treatment regimens across ERS SHARP severe asthma registries. Eur Respir J. 2020;55(1):1901163. doi: 10.1183/13993003.01163-2019 [DOI] [PubMed] [Google Scholar]
- 33.Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 34.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 35.Schatz M, Sorkness CA, Li JT, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. Allergy Clin Immunol. 2006;117(3):549–556. doi: 10.1016/j.jaci.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 36.Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14(1):32–38. doi: 10.1034/j.1399-3003.1999.14a08.x [DOI] [PubMed] [Google Scholar]
- 37.Morobeid H, Pizarro C, Biener L, et al. Impact of prior smoking exposure and COPD comorbidity on treatment response to monoclonal antibodies in patients with severe asthma. ERJ Open Res. 2021;7(7):00190–2021. doi: 10.1183/23120541.00190-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajpai S, Marino MJ, Rank MA, et al. Benefits of biologic therapy administered for asthma on co-existent chronic rhinosinusitis: a real-world study. Int Forum Allergy Rhinol. 2021;11(8):1152–1166. doi: 10.1002/alr.22774 [DOI] [PubMed] [Google Scholar]
- 39.Crimi C, Campisi R, Cacopardo G, et al. Real-life effectiveness of mepolizumab in patients with severe refractory eosinophilic asthma and multiple comorbidities. World Allergy Organ J. 2020;13(9):100462. doi: 10.1016/j.waojou.2020.100462 [DOI] [PMC free article] [PubMed] [Google Scholar]