ABSTRACT
Purpose:
Several risk classifications based on various preoperative factors have been proposed to prognosticate the immediate survival of children operated for esophageal atresia. A major drawback of these classifications is that they only focus on immediate survival while ignoring the long-term morbidity and mortality in these children. Our study aims to bridge this gap in knowledge by studying the impact of one such classification (Okamoto's classification) on mortality and morbidity during a period of 1 year after discharge from the hospital in operated cases of esophageal atresia.
Materials and Methods:
After institutes ethical clearance, 106 children operated for esophageal atresia-tracheoesophageal fistula between 2012 and 2015 were studied prospectively for a period of 1 year after their discharge. The children were graded as per Okamoto classification. The primary objective was to determine the efficacy of this classification in predicting the survival rates in infancy and the secondary objective was to compare the complication rates in these children based on the classification.
Results:
Sixty-nine children met the inclusion criteria. There were 40, 15, 10, and 4 children in Okamoto Classes I, II, III, and IV, respectively. Twenty-one patients (30%) died during the follow-up period with the maximum number of deaths occurring in Okamoto Class IV (75%) and the minimum in Okamoto Class I (17.5%) (P = 0.003). There was a significant correlation between the Okamoto classes with the incidence of poor weight gain (P = 0.001), lower respiratory tract infection (P = 0.007), and failure to thrive (P = 0.01) higher in Okamoto IV and III as compared to I and II.
Conclusion:
Okamoto prognostic classification during the initial hospitalization is relevant even at 1 year follow-up with increased mortality and morbidity in Okamoto Class IV as compared to Class I.
KEYWORDS: Esophageal atresia, intermediate outcomes, morbidity, mortality, prognostic classification
INTRODUCTION
Management of esophageal atresia has always been a challenge for Pediatric surgeons. Apart from the anomaly per se, many other factors determine the outcome in these neonates. Several risk classifications based on the various preoperative factors have been proposed to prognosticate immediate survival and these in addition also help in the comparison of results between various institutions. The best-known classification is named after Waterston (1962);[1] however, classifications given by Spitz (1994)[2] and later by Okamoto (2009)[3] have also gained popularity. A major drawback of these classifications is that they only focus on immediate survival while ignoring the long-term morbidity and mortality in these children. In addition, the impact of these classifications on mortality and morbidity after discharge from the hospital has not been studied so far. Our study aims to bridge this gap in knowledge by studying the impact of one such classification [Okamoto's classification - Table 1] on mortality and morbidity during a period of 1 year after discharge from the hospital in all operated cases of esophageal atresia.
Table 1.
Okamoto risk classification
| Risk category | Birth weight (g) | Cardiac anomaly |
|---|---|---|
| Class I (low risk) | >2000 | Absent |
| Class II (moderate risk) | <2000 | Absent |
| Class III (relatively high risk) | >2000 | Present |
| Class IV (high risk) | <2000 | Present |
Aims and objectives
The aim of this study was to evaluate the morbidity and mortality outcomes in patients operated for esophageal atresia with/without tracheoesophageal fistula (EA ± TEF) using the Okamoto classification during the 1st year after discharge from hospital. The primary objective was to determine the efficacy of this classification in predicting the survival rates in infancy and the secondary objective was to compare the complication rates in these children based on the classification.
MATERIAL AND METHODS
This prospective observational study was conducted after obtaining appropriate institutional ethical clearance over a period of 3 years (2012–2015) in a tertiary care pediatric surgical center in India. All patients who were operated for esophageal atresia and who were successfully followed-up for a period of 1 year following discharge and those who died during this follow-up period were included. Data collection included birth weight, cardiac anomaly, type of esophageal atresia, surgical management, complications, and mortality during the study period. Major cardiac defects were defined as any congenital cyanotic cardiac anomalies or noncyanotic congenital heart disease requiring any form of medical/surgical management. The follow-up patient data were collected either during routine hospital visits or telephonically and their well-being enquired with regards to weight gain, lower respiratory tract infections (LRTIs), esophageal stricture, repeat hospitalizations, or mortality if any. We defined failure to thrive if the weight for age recorded at two or more occasions was less than the 10th percentile as per the WHO growth charts; LRTI was diagnosed only if seen clinically and radiologically. Esophageal strictures were diagnosed if patients were symptomatic and further confirmed by upper gastrointestinal contrast studies.
Statistical analysis
This was done using SSPS version 23.0 (IBM Corp, Armonk, NY, USA) software. The risk of developing complications after discharge was calculated for the entire cohort, the risk estimates were compared between various Okamoto classes, and the P value was calculated. Univariate analysis was done for comparison between the groups and any P ≤ 0.05 was considered significant.
RESULTS
One hundred and six patients of EA ± TEF were admitted to the hospital during the study period, of which 101 patients underwent surgery. Twenty five patients died in the postoperative period (24.7%) and the remaining 76 patients who were discharged home (75.3%) became our study population. Six patients were lost to follow-up (8%) and adequate data was lacking in one; thus 69 (91%) patients formed the final study group out of which 43 (62%) were males and 26 females (38%) [Figure 1]. The mean gestational age was 37.3 ± 3 weeks and the mean birth weight was 2316 ± 850 gm. Associated anomalies were seen in 51 (74%) with the majority being cardiac anomalies (72%) [Table 2]. The distribution of these 69 patients as per the Okamoto classification based on the birth weight and major cardiac anomaly was Okamoto I-40 (57.9%), Okamoto II-15 (21.7%), Okamoto III-10 (14.4%), and Okamoto IV-4 (5.8%) [Figure 2].
Figure 1.
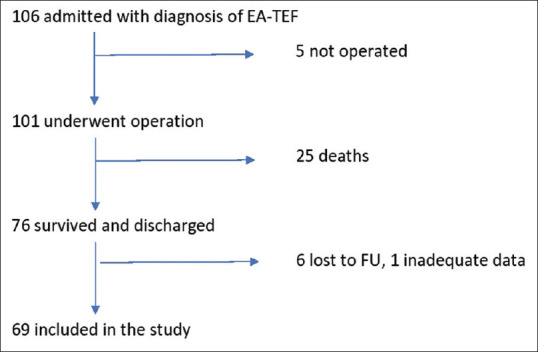
Flowchart of cohort selection for the study
Table 2.
Gestational age, birth weight, cardiac anomaly, and type of esophageal atresia distribution among various Okamoto groups
| Okamoto class (n) | Mean gestational age (weeks) | Mean birth weight (g) | Associated noncardiac anomalies, n (%) | Percentage of major cardiac anomaly | Gross type |
|---|---|---|---|---|---|
| I (40) | 38.2 | 2510 | 5 (12.5) | 0 | C-37, A-2, D-1 |
| II (15) | 34.6 | 1670 | 3 (20) | 0 | C-12, A-3 |
| III (10) | 38.5 | 2678 | 4 (40) | 100 | C-10 |
| IV (4) | 36 | 1905 | 1 (25) | 100 | C-4 |
Figure 2.
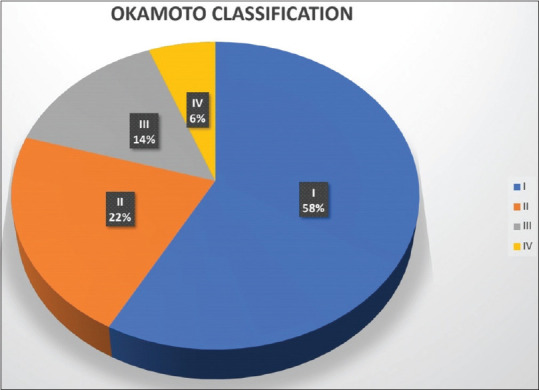
Pie chart distribution of patients into various Okamoto class
Forty seven patients (68%) had their esophageal continuity restored successfully in the initial surgery. Fourteen patients (20%) underwent primary diversion in view of having esophageal atresia without TEF, while 8 patients (12%) underwent esophageal diversion subsequent to a major anastomotic leak not amenable to conservative management. Twenty-one of these 69 patients (30%) died during the 12-month follow-up period with the maximum deaths occurring in Okamoto Class IV (75%) and minimum in Okamoto Class I (17.5%) (P = 0.003) [Table 3].
Table 3.
Mortality in relation to various Okamoto groups and types of surgical management
| Okamoto class (n) | Restored esophageal continuity, n (%) | Primary diversion, n (%) | Diversion after leak, n (%) | Mortality, n (%) |
|---|---|---|---|---|
| Class 1 (40) | 30 (75) | 7 (17.5) | 3 (7.5) | 7 (17.5) |
| Class II (15) | 9 (60) | 5 (33.3) | 1 (6.6) | 7 (46.6) |
| Class III (10) | 8 (80) | 0 | 2 (20) | 4 (40) |
| Class IV (4) | 0 | 2 (50) | 2 (50) | 3 (75) |
On follow-up, out of the 47 patients who had their esophageal continuity restored, 15 patients (32%) developed esophageal strictures of which there were 9 patients in Class I, 3 each in Class II, and Class III. The stricture rates were comparable among all four groups (P = 0.94). Overall, 27 patients (39%) showed signs of poor weight gain and the percentages increased from 22.5% in Class I to 75% in Class IV (P = 0.001). Nineteen patients (28%) out of the study population had failure to thrive and the percentages increased from 12.5% in Okamoto Class I to 75% in Class IV (P = 0.01).
Twenty-eight patients (41%) were hospitalized and treated for LRTI during the study period. More number of children in Okamoto Class III and IV (80% and 75%, respectively) developed LRTI as compared to Okamoto Class I and II (30% and 33.3%, respectively) (P = 0.007).
There was a significant correlation between the Okamoto classes when the incidence of poor weight gain (P = 0.001) and LRTI (P = 0.007) was compared between the classes. The stricture rate did not show any significant correlation (P = 0.94) [Table 4].
Table 4.
Distribution of patients in various Okamoto classes and postoperative outcomes in these groups
| Okamoto class | Total (n) | Anastomotic stricture, n (%) | Poor weight gain, n (%) | LRTI, n (%) |
|---|---|---|---|---|
| Class I | 40 | 9 (30) | 9 (23) | 12 (30) |
| Class II | 15 | 3 (33) | 8 (53) | 5 (33) |
| Class III | 10 | 3 (37) | 7 (70) | 8 (80) |
| Class IV | 4 | NA (all diverted) | 3 (75) | 3 (75) |
| P | 0.94 | 0.001 | 0.007 |
LRTI: Lower respiratory tract infections, NA: Not available
One-year overall survival was 82%, 53.3%, 60%, and 25% in Okamoto I, II, III, and IV, respectively [Figure 3]. Okamoto Class I demonstrated significantly better survival than class II (P = 0.02) and class IV (P = 0.003). However, the difference between Class I and III was not significant (P = 0.17).
Figure 3.
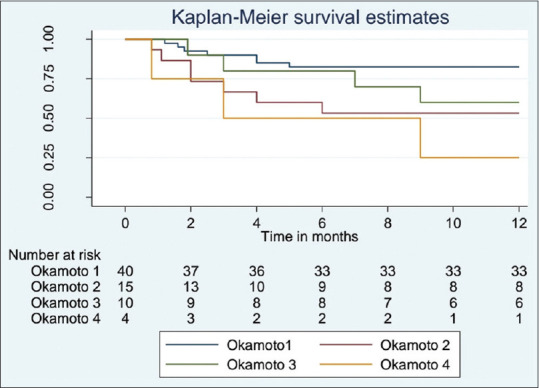
Kaplan-Meier survival estimate according to Okamoto class (n = 69)
DISCUSSION
Esophageal atresia, with an incidence of 1 in 4099 live births[4] is one of the most common congenital anomaly requiring surgical correction. The survival outcomes have improved dramatically over the last eight decades from 46% in 1950 to 91% in 2018.[5] With the advent of time and better technical analysis, various prognostic classifications like Waterson, Spitz, and Okamoto have been proposed to determine the survival outcomes. None of these classifications, though, have been studied to determine the short-long term outcomes of these children. We employed the Okamoto classification to follow-up 69 children of EA ± TEF over a period of 1 year looking into their survival outcomes and the complications related to EA ± TEF.
Forty-one percent of our patients required hospitalizations for respiratory infections with the incidence increasing with the increasing Okamoto classification. Respiratory symptoms including recurrent pneumonia and wheezing are seen in about 60% of children operated for EA ± TEF with the common etiologies being localized atelectasis, residual tracheal diverticulum, bronchiectasis, tracheal vascular compression, tracheomalacia, and esophageal diverticulum.[6] Early associated complications like duration of postoperative ventilation and recurrent pneumonia due to aspiration in patients with stricture and gastroesophageal reflux have a significant impact on long-term pulmonary outcomes. Almost 63% of patients are reported to have abnormal lung functions especially restrictive ventilatory defects.[7]
The esophageal stricture rates vary widely from 17% to 52%[8] mainly due to a lack of standard definition of postoperative anastomotic stricture in EA ± TEF patients, whether it is based on symptoms, contrast studies, or the need for dilatations. However, it is clear that long gap EA ± TEF and postoperative gastroesophageal reflux disease are definite risk factors for developing a stricture.[9] The criteria for diagnosing a stricture in this study was the presence of symptoms like dysphagia along with radiological evidence of stricture on contrast esophagogram and the incidence of stricture was 32% within one year of follow-up Routine postoperative proton pump inhibitor is advised to all patients on discharge along with advice to nurse in a propped up position for at least a year to decrease the risk of gastroesophageal reflux.
Another documented sequelae of EA ± TEF is impaired nutritional status and poor growth in childhood due to higher rate of hospitalizations, feeding difficulties, repeated respiratory infections, strictures, and gastroesophageal reflux.[10] The incidence of malnutrition, including both muscle wasting and stunting, depending on the age at assessment ranges from 15% to 29%.[11] The overall percentage of poor weight gain and failure to thrive in the present study was 39% with incidence increasing across the Okamoto classification. The presence of another noncardiac anomaly in some patients could also have contributed as cause for poor weight gain.
The survival rates as quoted in the literature are 100% for Okamoto class I, 82% for class II, 72% for Class III, and 27% for Class IV.[3] These survival rates refer to those patients who are discharged and not for any specific follow-up period. In the present study, the survival during 1 year after discharge for the various Okamoto risk groups had been documented. Approximately 60% of deaths occurred during the 1st 3 months after discharge and 85%of the death occurs by the end of 6 months. This underlines the need for stricter and more frequent follow-up of these patients in the first 6 months. The follow-up schedule should be suitably tailored to address the high-risk groups. This is especially true for developing country like India where medical help close to home may not be easily available. Parental education and economic status may also have an impact on the outcome of these patients though there is insufficient literature on this issue.
Similar studies on esophageal atresia related mortality in developing countries have shown an overall mortality of 21% during the admission for management of EA ± TEF, and most of these deaths occur in children with low birth weight and severe cardiac anomalies.[12] Another study involving 693 neonates managed over a 25 year period showed a mortality rate of 36% in children managed for esophageal atresia. This study also showed a positive correlation between mortality and perinatal factors like prematurity, low birth weight, congenital anomalies, and major cardiac defects.[13] Li et al. found that apart from low birth weight, other factors contributing to mortality are anastomotic leak, respiratory failure, and postoperative sepsis. Notably, any efforts to reduce these risk factors may reduce the mortality rate in EA.[14]
Amongst the classifications-: Montreal, Spitz and Waterston classifications are equally accurate in predicting mortality.[15] Yamoto et al proposed a classification based on weight with cut-off values at 1000 g and 2000 g, and the presence of complex cardiac anomaly to predict survival in esophageal atresia which they claim to predict mortality better than Spitz classification.[16] The drawbacks of all the above studies are that the mortality has been calculated after the primary surgery (in hospital mortality) and not during any specific follow-up period.
The limitations of the current study are that the follow-up period is limited to 1 year and patients contacted telephonically may have erred in reporting events like LRTI and failure to thrive. In addition, the strict criteria for diagnosing LRTI and esophageal strictures may have resulted in underdiagnosing patients, thereby acting as a confounding factor. Another independent factor that could have affected nutritional status directly and mortality indirectly is esophageal diversion; children with gastrostomy do face feeding difficulties in spite of the best of care and have poor weight gain. The study's strength rests in showing that the Okamoto classification holds relevance even after discharge and children at risk require closer monitoring and early aggressive interventions to improve overall outcomes. This is the first study where an existing prognostic classification has been used to predict mortality during follow-up period.
CONCLUSION
Children operated for EA ± TEF are at high risk for mortality and morbidity in the months following discharge. Okamoto prognostic classification during the initial hospitalization is relevant even at 1 year follow-up with significantly increased mortality and morbidity in higher risk Okamoto groups. Further prospective multicenter studies involving other classifications and prolonged follow-up beyond 1 year would help throw more light on this subject.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Waterston DJ, Carter RE, Aberdeen E. Oesophageal atresia: Tracheo-oesophageal fistula. A study of survival in 218 infants. Lancet. 1962;1:819–22. doi: 10.1016/s0140-6736(62)91837-8. [DOI] [PubMed] [Google Scholar]
- 2.Spitz L, Kiely EM, Morecroft JA, Drake DP. Oesophageal atresia: At-risk groups for the 1990s. J Pediatr Surg. 1994;29:723–5. doi: 10.1016/0022-3468(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto T, Takamizawa S, Arai H, Bitoh Y, Nakao M, Yokoi A, et al. Esophageal atresia: Prognostic classification revisited. Surgery. 2009;145:675–81. doi: 10.1016/j.surg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Nassar N, Leoncini E, Amar E, Arteaga-Vázquez J, Bakker MK, Bower C, et al. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94:893–9. doi: 10.1002/bdra.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmer J, Eaton S, Murchison LE, De Coppi P, Ure BM, Dingemann C. State of play: Eight decades of surgery for esophageal atresia. Eur J Pediatr Surg. 2019;29:39–48. doi: 10.1055/s-0038-1668150. [DOI] [PubMed] [Google Scholar]
- 6.Porcaro F, Valfré L, Aufiero LR, Dall’Oglio L, De Angelis P, Villani A, et al. Respiratory problems in children with esophageal atresia and tracheoesophageal fistula. Ital J Pediatr. 2017;43:77. doi: 10.1186/s13052-017-0396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dittrich R, Stock P, Rothe K, Degenhardt P. Pulmonary outcome of esophageal atresia patients and its potential causes in early childhood. J Pediatr Surg. 2017;52:1255–9. doi: 10.1016/j.jpedsurg.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Koivusalo AI, Pakarinen MP, Rintala RJ. Modern outcomes of oesophageal atresia: Single Centre experience over the last twenty years. J Pediatr Surg. 2013;48:297–303. doi: 10.1016/j.jpedsurg.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Lu YH, Yen TA, Chen CY, Tsao PN, Lin WH, Hsu WM, et al. Risk factors for digestive morbidities after esophageal atresia repair. Eur J Pediatr. 2021;180:187–94. doi: 10.1007/s00431-020-03733-1. [DOI] [PubMed] [Google Scholar]
- 10.Menzies J, Hughes J, Leach S, Belessis Y, Krishnan U. Prevalence of malnutrition and feeding difficulties in children with esophageal atresia. J Pediatr Gastroenterol Nutr. 2017;64:e100–5. doi: 10.1097/MPG.0000000000001436. [DOI] [PubMed] [Google Scholar]
- 11.Pelizzo G, Destro F, Selvaggio GG, Maestri L, Roveri M, Bosetti A, et al. Esophageal atresia: Nutritional status and energy metabolism to maximize growth outcome. Children (Basel) 2020;7:228. doi: 10.3390/children7110228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Salem AH, Kothari M, Oquaish M. Morbidity and mortality in esophageal atresia and tracheoesophageal fistula: A 20-year review. Ann Pediatr Surg. 2013;9:93–8. [Google Scholar]
- 13.Rattan KN, Singh J, Dalal P. Clinical profile and short-term outcome of neonates with esophageal atresia and tracheoesophageal fistula at tertiary care Center in a developing country: A 25 year experience. J Clin Neonatol. 2017;6:225–30. [Google Scholar]
- 14.Li XW, Jiang YJ, Wang XQ, Yu JL, Li LQ. A scoring system to predict mortality in infants with esophageal atresia: A case-control study. Medicine (Baltimore) 2017;96:e7755. doi: 10.1097/MD.0000000000007755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters RT, Ragab H, Columb MO, Bruce J, MacKinnon RJ, Craigie RJ. Mortality and morbidity in oesophageal atresia. Pediatr Surg Int. 2017;33:989–94. doi: 10.1007/s00383-017-4124-1. [DOI] [PubMed] [Google Scholar]
- 16.Yamoto M, Nomura A, Fukumoto K, Takahashi T, Nakaya K, Sekioka A, et al. New prognostic classification and managements in infants with esophageal atresia. Pediatr Surg Int. 2018;34:1019–26. doi: 10.1007/s00383-018-4322-5. [DOI] [PubMed] [Google Scholar]


