Abstract
Background
Information on variant-specific vaccine protection and the effect of previous infection variant is scarce in children. We aimed to ascertain the level of protection conferred by BNT162b2 COVID-19 vaccination against omicron variant infection (BA.4 or BA.5, and XBB) in a previously infected national paediatric cohort. We also explored the association between sequence of previous infection (variant) and vaccination on protection.
Methods
We did a retrospective, population-based cohort study using the national databases of all confirmed SARS-CoV-2 infections, vaccines administered, and demographic records maintained by the Ministry of Health, Singapore. The study cohort consisted of children aged 5–11 years and adolescents aged 12–17 years who had a previous SARS-CoV-2 infection from Jan 1, 2020, to Dec 15, 2022. People who were infected during the pre-delta period or were immunocompromised (received three vaccination doses [children 5–11 years old] and four vaccinations doses [adolescents 12–17 years old]) were excluded. Those who had multiple episodes of infection before the study start date, were not vaccinated before infection but completed three doses, received bivalent mRNA vaccine, or received non-mRNA vaccine doses were also excluded. All SARS-CoV-2 infections confirmed by reverse transcriptase polymerase chain reaction or rapid antigen testing were grouped into delta, BA.1, BA.2, BA.4 or BA.5, or XBB variants using a combination of whole-genome sequencing, S-gene target failure results, and imputation. For BA.4 or BA.5, the study outcome period was June 1–Sept 30, 2022, and for XBB variants the outcome period was Oct 18–Dec 15, 2022. Incidence rate ratios between vaccinated and unvaccinated were derived using adjusted Poisson regressions and vaccine effectiveness was estimated as (1–risk ratio) × 100%.
Findings
135 197 people aged 5–17 years (79 332 children and 55 865 adolescents) were included in the cohort for the vaccine effectiveness analysis against omicron BA.4 or BA.5, and 164 704 people aged 5–17 years (97 235 children and 67 469 adolescents) were included for the analysis against omicron XBB. Approximately 47% of participants were female and 53% were male. Among those previously infected, vaccine effectiveness against BA.4 or BA.5 infection in fully vaccinated children (two doses) was 74·0% (95% CI 67·7–79·1) and in adolescents (three doses) was 85·7% (80·2–89·6). Against XBB, protection conferred with full vaccination was lower at 62·8% (95% CI 42·3–76·0) in children and 47·9% (20·2–66·1) in adolescents. In children, receipt of two-dose vaccination before first SARS-CoV-2 infection provided them with the highest protection against subsequent BA.4 or BA.5 infection at 85·3% (95% CI 80·2–89·1); however, this was not shown to be the case for adolescents. First infection variant had an effect on vaccine effectiveness against omicron BA.4 or BA.5 reinfection in the following descending order: BA.2 conferred the highest protection (92·3% [95% CI 88·9–94·7] in children and 96·4% [93·5–98·0] in adolescents) followed by BA.1 (81·9% [75·9–86·4] in children and 95·0% [91·6–97·0] in adolescents), and delta which conferred the lowest protection (51·9% [5·3–75·6] in children and 77·5% [63·9–86·0] in adolescents).
Interpretation
In previously infected children and adolescents, BNT162b2 vaccination provided additional protection against omicron BA.4 or BA.5 and XBB variants compared with those who remained unvaccinated. Hybrid immunity against XBB was lower than against BA.4 or BA.5, especially in adolescents. Early vaccination of previously uninfected children before their first SARS-CoV-2 exposure could potentially strengthen population immunity resilience against future variants.
Funding
None.
Introduction
The evolution of the COVID-19 pandemic will be determined by identification of strategies to improve population protection and resilience against current and future variants of the SARS-CoV-2 virus. Several vaccines have been approved and used in multiple countries, but their effectiveness is continually being challenged with the emergence of new variants such as omicron (B.1.1.529).1, 2 As additional boosters are being recommended to counteract the effect of variants, there is also increasing unwillingness to get further vaccine doses or an increased feeling of so-called vaccine fatigue.3 Globally, only 34·7% of the population have completed their booster shots, whereas in the USA, bivalent booster coverage is currently about 17% among those aged 5–65 years.4, 5 People who had been infected with SARS-CoV-2 with or without previous vaccination increasingly question the benefit of getting vaccinated after their recovery.
Research in context.
Evidence before this study
We searched PubMed on March 20, 2023, for papers published in English, using the terms (“SARS-CoV-2” or “COVID-19) AND (“effectiveness” or “protection” or “reinfection”) AND (“Omicron” or “BA.4/BA.5/XBB”) AND (“BNT162b2” or “Pfizer” or “vaccination”). Most studies focused on adults and show that hybrid immunity (ie, a combination of vaccination and SARS-CoV-2 infection) can result in better immune responses than infection alone, with improved protection against omicron variants. mRNA vaccination was reported to be 65·7% effective in protecting previously infected children aged 5–11 years against omicron BA.1 or BA.2 infections. In adolescents aged 12–17 years, vaccine boosters protected against BA.1 or BA.2 infections at 56% effectiveness. However, up-to-date vaccine effectiveness data in the paediatric population against omicron BA.4 or BA.5 and XBB are scarce. Furthermore, the optimum vaccination schedule that infected children and adolescents should receive after they have recovered, or whether the sequence of previous SARS-CoV-2 infection variant and vaccination would affect overall protection, remains unknown.
Added value of this study
Using the national SARS-CoV-2 testing and BNT162b2 COVID-19 vaccination reports in Singapore, we showed that BNT162b2 vaccination in previously infected children and adolescents provided additional protection against omicron BA.4 or BA.5 and XBB infection compared with those who remained unvaccinated. Hybrid immunity against XBB was lower than against BA.4 or BA.5, especially in adolescents. Early vaccination of previously uninfected children before their first SARS-CoV-2 exposure could potentially strengthen population immunity resilience against future variants. Protection derived from previous or first infection variant against omicron BA.4 or BA.5 reinfection was in the following order: BA.2 which conferred the most protection; followed by BA.1; and finally delta, which conferred the least protection.
Implications of all the available evidence
Our analysis showed the additional protection against omicron BA.4 or BA.5 and XBB variant reinfections that BNT162b vaccination provided for children and adolescents with previous SARS-CoV-2 infection. The reduced hybrid immunity against the XBB variant compared with the BA.4 or BA.5 variants, especially in adolescents, could warrant the use of bivalent boosters in this age group. Vaccination before a first SARS-CoV-2 infection provided the greatest protection against subsequent omicron BA.4 or BA.5 reinfections in children. Moreover, for unvaccinated children and adolescents who recovered from a past SARS-CoV-2 infection, there were clear additional benefits of getting at least one dose of vaccination in terms of protection against omicron BA.4 or BA.5 infections. These findings have important implications for public health discussions on paediatric vaccination programmes to help improve population immunity resilience against future variants. There is a need for continued paediatric-specific monitoring of vaccine effectiveness as well as studies to understand the scientific basis of vaccination priming to enhance protection.
Studies have shown that hybrid immunity (ie, a combination of vaccination and SARS-CoV-2 infection) can result in better immune responses with improved protection against variants compared with infection alone.6, 7, 8 However, there is no clear scientific evidence to inform the optimum doses infected individuals should receive after they have recovered or whether the sequence of infection and vaccination would have an effect on protection from emerging SARS-CoV-2 variants. Furthermore, real-world vaccine effectiveness data against omicron BA.4, BA.5, and XBB variants in children are scarce.9 These questions are especially important in the paediatric population since the development, availability, and rollout of COVID-19 vaccines in children lag behind the adult population. By contrast to the adult population, many children acquired COVID-19 immunity via natural infection rather than vaccination.10, 11, 12 Understanding how much additional protection COVID-19 vaccination offers to current strains in previously infected people, and the potential option to strengthen population immunity resilience against emergent variants, will be crucial for public health.
Singapore experienced a large wave of SARS-CoV-2 infections from September to December, 2021, driven by the delta variant (B.1.617.2).13 This was followed by an even larger wave from January to April, 2022, driven by the omicron (BA.1 and BA.2) variant.12, 13 Further omicron waves swept through the country from June to September, 2022 (dominated by the BA.4 and BA.5 variants), and October to December, 2022 (dominated by XBB).13 Early introduction of the BNT162b2 vaccine (tozinameran; Pfizer–BioNTech) to individuals aged 12–17 years in May, 2021, and strict compliance with various public health interventions, including masking in educational settings, ensured that paediatric case numbers in Singapore remained fairly low before the delta wave (<0·1% of reported paediatric cases).13 The vaccination programme for children aged 5–11 years with two 10 μg doses of BNT162b2 started at the end of December, 2021. In January, 2022, a third BNT162b2 vaccine dose as booster was recommended for children aged 12–17 years who had completed their second dose more than 5 months previously.
In this study, we aimed to analyse protection conferred by monovalent BNT162b2 vaccine in previously infected children and adolescents (age 5–17 years) against omicron BA.4 or BA.5, and XBB infection. We further explored whether the sequence of SARS-CoV-2 infection (variant) and vaccination dose had an effect on the protection conferred against omicron BA.4 and BA.5.
Methods
Study design and participants
This was a retrospective, population-based cohort study of children aged 5–11 years and adolescents aged 12–17 years in Singapore who had a previous SARS-CoV-2 infection confirmed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) or antigen rapid testing from Jan 1, 2020, to Dec 15, 2022. All SARS-CoV-2 positive cases were required by law to be notified to the Ministry of Health, Singapore under the Infectious Diseases Act. People who were infected during the pre-delta period (<0·1% of cases), and who were immunocompromised (ie, those who received three vaccination doses [children 5–11 years old] and four vaccinations doses [adolescents 12–17 years old]) were excluded. Those who had multiple episodes of infection before the study start date, were not vaccinated before infection but completed three doses subsequently, received bivalent mRNA vaccine, or received non-mRNA vaccine doses were also excluded. The study was done to support policy decision making and evaluation of the public health response to COVID-19 under the Infectious Diseases Act, Singapore, and therefore ethics review by an Institutional Review Board or written informed consent was not required.
Procedures
Data were extracted by DP and KBT from official databases maintained by the Ministry of Health, Singapore, including national records of all confirmed SARS-CoV-2 infections, vaccines administered, age, sex, ethnicity, and housing type (proxy measure of socioeconomic status). Data regarding sex were extracted from the National Birth Registry (options included male or female). Information on dates of first infection, receipt of vaccination doses, and subsequent reinfection during the study periods were used for analysis of sequence on vaccine effectiveness. Variant and sub-lineage classifications were determined according to whole-genome sequencing and S-gene target failure (SGTF) results if available. SGTF positive status was used as a proxy criterion for omicron BA.4 or BA.5 variant infection after April, 2022, corresponding to the timing of omicron variant waves. Infections with SGTF failure were attributed to be omicron XBB variant from October, 2022, with it being the dominant variant. In the absence of whole-genome sequencing or SGTF, imputation of variant and sublineage was performed.14 Details of genomic surveillance in Singapore as well as imputation methods and results are available in the appendix (p 4).
Outcomes
The primary outcome measure was the incidence of all reported SARS-CoV-2 infections confirmed by RT-PCR or antigen rapid testing between June 1, 2022, and Sept 30, 2022 (omicron BA.4 or BA.5 wave) and between Oct 18, 2022, and Dec 15, 2022 (omicron XBB wave) in the cohort. Cases of COVID-19 among children and adolescents in Singapore were identified through testing of symptomatic children and adolescents who presented with acute respiratory illness at any health-care facility including primary care clinics, community testing centres, or hospitals. Additionally, asymptomatic cases were also detected mainly as part of recommendations for testing of close contacts of people with COVID-19. Individuals were considered to have an infection if they had tested positive at least 90 days after their previous infection.
Statistical analysis
For individuals who did not have an infection during our study period, person-days at risk were calculated based on the length of our study period (ie, June 1 2022, to Sept 30, 2022, for omicron BA.4 or BA.5 and Oct 18, 2022, to Dec 15, 2022, for omicron XBB. For individuals who had an omicron BA.4 or BA.5, or XBB infection during our study period, person-days at risk were calculated from the corresponding June 1, 2022, or Oct 18, 2022, respectively to the date of their infection. Generalised linear Poisson regression models, which can overcome overdispersion,15 were used to estimate incidence rate ratios of SARS-CoV-2 infection with omicron BA.4 or BA.5, with individuals who were unvaccinated serving as the reference group. Vaccine effectiveness was calculated by: 1 – (incidence rate ratio of vaccinated to unvaccinated) × 100.16, 17 Regressions were adjusted for age, sex, ethnicity, housing type (as a proxy for socioeconomic status), calendar week (to account for varying force of infection across time), vaccination status, time from last vaccine dose, variant of previous (first) infection, and time from previous infection. In counting person-days, we accounted for the time-varying nature of vaccination status and infection status: the same individual could contribute person-time to unvaccinated, one-dose vaccinated, two-dose vaccinated, and three-dose vaccinated groups as well as uninfected and previously infected groups depending on the period. Children aged 5–11 years were analysed separately from adolescents aged 12–17 years as three-dose vaccination had been recommended only for the adolescent group. To explore the effect of sequence and variant, various permutations or strains were stratified for comparison of vaccine effectiveness. Analysis were done with Stata Statistical Software Release 17.
Role of the funding source
There was no funding source for this study.
Results
After exclusion of those who did not meet the inclusion criteria, the study cohorts consisted of 135 197 people aged 5–17 years (79 332 children and 55 865 adolescents) for the vaccine effectiveness analysis against omicron BA.4 or BA.5, and 164 704 people aged 5–17 years (97 235 children and 67 469 adolescents) for the analysis against omicron XBB (appendix p 1). The demographic distribution of the study cohorts including sex, ethnicity, and housing type as a proxy measure of socioeconomic status are summarised in table 1 . Approximately 47% of participants were female and 53% were male. In the vaccine effectiveness against omicron BA.4 or BA.5 cohort, the 5–11-year-old age group (children) contributed 8·0 million person-days and the 12–17-year-old age group (adolescents) contributed 5·8 million person-days of observation to the study. Against omicron XBB, there were 4·8 million person-days for children and 3·5 million person-days for adolescent groups.
Table 1.
Characteristics of the study cohort
|
BA.4 or BA.5 |
XBB |
|||||
|---|---|---|---|---|---|---|
| Children aged 5–11 years, (number of person-days in millions) | Adolescents aged 12–17 years (number of person-days in millions) | Children aged 5–11 years, (number of person-days in millions) | Adolescents aged 12–17 years (number of person-days in millions) | |||
| Total | 8·0 | 5·8 | 4·8 | 3·5 | ||
| Sex | ||||||
| Female | 3·8 (46·9%) | 2·7 (46·9%) | 2·3 (47·4%) | 1·7 (47·2%) | ||
| Male | 4·3 (53·1%) | 3·1 (53·1%) | 2·5 (52·6%) | 1·8 (52·8%) | ||
| Ethnic group | ||||||
| Chinese | 5·2 (64·6%) | 3·5 (61·4%) | 3·2 (67·2%) | 2·3 (65·0%) | ||
| Malay | 0·9 (10·6%) | 0·7 (12·0%) | 1·0 (19·9%) | 0·7 (19·9%) | ||
| Indian | 1·7 (21·4%) | 1·3 (23·0%) | 0·5 (9·7%) | 0·4 (11·9%) | ||
| Other | 0·3 (3·4%) | 0·2 (3·7%) | 0·2 (3·3%) | 0·1 (3·3%) | ||
| Housing | ||||||
| Public housing | 6·9 (86·3%) | 4·9 (84·6%) | 4·1 (84·8%) | 2·9 (83·3%) | ||
| One or two rooms | 0·4 (4·8%) | 0·3 (5·0%) | 0·2 (4·4%) | 0·1 (4·1%) | ||
| Three rooms | 1·4 (17·4%) | 1·0 (16·8%) | 0·8 (15·8%) | 0·5 (15·7%) | ||
| Four rooms | 3·0 (37·7%) | 1·9 (33·5%) | 1·8 (38·2%) | 1·2 (33·7%) | ||
| Five rooms | 2·1 (26·4%) | 1·7 (29·3%) | 1·3 (26·4%) | 1·0 (29·8%) | ||
| Private housing | 1·1 (13·7%) | 0·9 (15·4%) | 0·7 (15·2%) | 0·6 (16·7%) | ||
| Vaccination status | ||||||
| Unvaccinated | 1·8 (23·0%) | 0·1 (2·1%) | 1·5 (31·0%) | 0·1 (3·4%) | ||
| One dose | 2·1 (26·6%) | 0·1 (1·3%) | 0·9 (18·5%) | 0·1 (3·2%) | ||
| Two dose | 4·1 (50·4%) | 1·9 (32·2%) | 2·4 (50·5%) | 0·9 (26·7%) | ||
| Three dose | NA | 3·7 (64·3%) | NA | 2·3 (66·7%) | ||
| Previous SARS-CoV-2 infection variant | ||||||
| Delta | 1·0 (12·5%) | 0·6 (9·6%) | 0·4 (8·2%) | 0·2 (6·3%) | ||
| BA.1 | 3·4 (42·2%) | 2·6 (45·3%) | 1·3 (27·7%) | 1·0 (29·0%) | ||
| BA.2 | 3·6 (45·3%) | 2·6 (45·1%) | 2·1 (44·1%) | 1·4 (40·9%) | ||
| BA.4 or BA.5 | NA | NA | 1·0 (19·9%) | 0·8 (23·8%) | ||
Data are number of person-days in millions (%). Percentages might not add up correctly due to rounding techniques used. NA=not applicable.
Vaccination rates as per recommended schedule at the time of the study periods (two doses in children and three doses in adolescents) in our previously infected cohort were about 50% among children and 65% among adolescents (table 1). The median time interval between the first and second dose was 29 days (IQR 22–42) for children and 30 days (27–42) for adolescents. The median time interval between the second and third dose was 189 days (IQR 167–234) for adolescents.
Overall, there were 1495 (1·9%) of 79 332 children and 599 (1·1%) of 55 865 adolescents who tested positive for SARS-CoV-2 infection attributed to omicron BA.4 or BA.5 variants. For XBB variant, 783 (0·8%) of 97 235 children and 767 (1·1%) of 67 469 adolescents tested positive.
In previously infected children, the overall vaccine effectiveness of two-dose vaccination against omicron BA.4 or BA.5 infections was 74·0% (95% CI 67·7–79·1; table 2 ). In previously infected adolescents, two-dose vaccination conferred additional protection against omicron BA.4 or BA.5 infections at a vaccine effectiveness of 84·9% (77·0–90·1), while a third dose did not substantially improve the level of protection (85·7% [80·2–89·6]). Additional analysis of vaccine effectiveness stratified by the number of days after a second dose in children and the number of days after a third dose in adolescents found no clear decline in vaccine effectiveness over time (appendix p 2). Therefore, these findings were not due to bias from waning of vaccine effectiveness.
Table 2.
Adjusted vaccine effectiveness against omicron BA.4 or BA.5 and XBB infection in previously infected children and adolescents
|
Children aged 5–11 years |
Adolescents aged 12–17 years |
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of person-days (in millions) | Number of infections | Crude incidence (number of infections per 100 000 person-days) | Adjusted vaccine effectiveness (95% CI)* | Number of person-days (in millions) | Number of infections | Crude incidence (number of infections per 100 000 person-days) | Adjusted vaccine effectiveness (95% CI)* | |
| BA.4 or BA.5 | ||||||||
| Unvaccinated | 1·8 | 485 | 26·2 | 1 (ref) | 0·1 | 57 | 47·4 | 1 (ref) |
| One dose | 2·1 | 746 | 34·9 | 44·0% (32·2–53·7) | 0·1 | 44 | 57·5 | 70·4% (53·3–81·2) |
| Two doses | 4·1 | 264 | 6·5 | 74·0% (67·7–79·1) | 1·9 | 176 | 9·5 | 84·9% (77·0–90·1) |
| Three doses | NA | NA | NA | NA | 3·7 | 322 | 8·7 | 85·7% (80·2–89·6) |
| XBB | ||||||||
| Unvaccinated | 1·5 | 221 | 14·9 | 1 (ref) | 0·1 | 36 | 29·9 | 1 (ref) |
| One dose | 0·9 | 231 | 25·9 | 59·1% (35·7–73·9) | 0·1 | 47 | 41·6 | 54·5% (22·1–73·4) |
| Two doses | 2·4 | 331 | 13·6 | 62·8% (42·3–76·0) | 0·9 | 183 | 19·6 | 57·9% (33·6–73·3) |
| Three doses | NA | NA | NA | NA | 2·3 | 501 | 21·5 | 47·9% (20·2–66·1) |
NA=not applicable.
Vaccine effectiveness was calculated by 1 – (incidence rate ratio of vaccinated to unvaccinated) × 100. Poisson regression was used to estimate incidence rate ratios of SARS-CoV-2 infection with omicron BA.4 or BA.5 adjusted for age, sex, ethnicity, housing type (as a proxy for socioeconomic status), calendar week (to account for varying force of infection across time), vaccination status and time from last vaccine dose, variant of previous infection, and time from previous infection.
Protection against omicron XBB reinfection from hybrid immunity was generally lower than protection against omicron BA.4 or BA.5. In previously infected children, protection with two-dose vaccination was 62·8% (95% CI 42·3–76·0). For previously infected adolescents, one-dose vaccine effectiveness was 54·5% (22·1–73·4), two-dose effectiveness was 57·9% (33·6–73·3), and three-dose effectiveness was 47·9% (20·2–66·1) against omicron XBB (table 2).
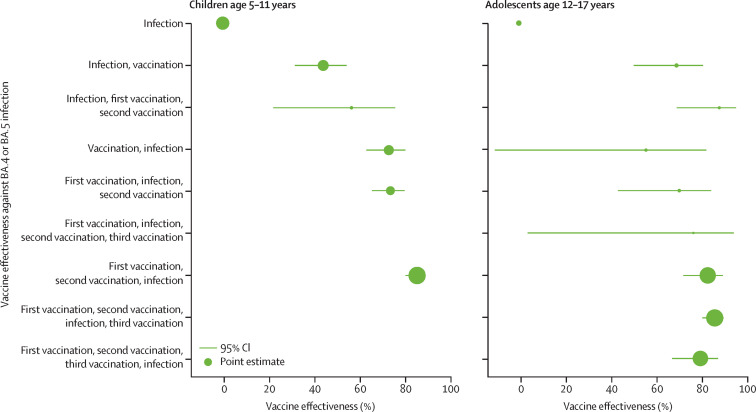
The adjusted vaccine effectiveness against omicron BA.4 or BA.5 infection by sequence of vaccination dose (vaccination) and SARS-CoV-2 infection status (infection) are summarised in table 3 and figure 1 . A single-dose vaccination in children after their first SARS-CoV-2 infection provided vaccine effectiveness against omicron BA.4 or BA.5 infection of 44·1% (95% CI 31·7–54·3). Protection improved to 56·5% (22·2–75·7) with a subsequent second dose. Similarly, in unvaccinated adolescents, a single dose vaccination after recovery from first SARS-CoV-2 infection provided vaccine effectiveness against omicron BA.4 or BA.5 infection of 69·2% (50·4–80·8), which increased to 88·0% (69·3–95·3) following a second dose.
Table 3.
Adjusted vaccine effectiveness against omicron BA.4 or BA.5 infection stratified by sequence of vaccination dose and SARS-CoV-2 infection
|
Children aged 5–11 years |
Adolescents aged 12–17 years |
|||
|---|---|---|---|---|
| Crude incidence (number of BA.4 or BA.5 infections per 100 000 person-days) | Adjusted vaccine effectiveness against BA.4 or BA.5 reinfection (95% CI)* | Crude incidence (number of BA.4 or BA.5 infections per 100 000 person-days) | Adjusted vaccine effectiveness against BA.4 or BA.5 reinfection (95% CI)* | |
| Unvaccinated before infection | ||||
| Infection | 26·2 | 1 (ref) | 47·4 | 1 (ref) |
| Infection, vaccination | 52·2 | 44·1% (31·7 to 54·3) | 69·2 | 69·2% (50·4 to 80·8) |
| Infection, first vaccination, second vaccination | 30·4 | 56·5% (22·2 to 75·7) | 24·6 | 88·0% (69·3 to 95·3) |
| Vaccinated with one dose before infection | ||||
| Vaccination, infection | 13·5 | 72·9% (63·1 to 80·1) | 27·8 | 55·8% (−10·5 to 82·3) |
| First vaccination, infection, second vaccination | 11·1 | 73·6% (65·5 to 79·8) | 45·7 | 70·4% (43·5 to 84·4) |
| First vaccination, infection, second vaccination, third vaccination | NA | NA | 48·6 | 76·6% (3·9 to 94·3) |
| Vaccinated with two doses before infection | ||||
| First vaccination, second vaccination, infection | 5·2 | 85·3% (80·2 to 89·1) | 8·8 | 82·9% (72·2 to 89·5) |
| First vaccination, second vaccination, infection, third vaccination | NA | NA | 10·8 | 86·0% (80·6 to 89·9) |
| Vaccinated with three doses before infection | ||||
| First vaccination, second vaccination, third vaccination, infection | NA | NA | 5·9 | 79·7% (67·3 to 87·4) |
NA=not applicable.
Vaccine effectiveness was calculated by 1 – (incidence rate ratio of vaccinated to unvaccinated) × 100. Poisson regression was used to estimate incidence rate ratios of SARS-CoV-2 infection with omicron BA.4 or BA.5 adjusted for age, sex, ethnicity, housing type (as a proxy for socioeconomic status), calendar week (to account for varying force of infection across time), vaccination status and time from last vaccine dose, variant of previous infection, and time from previous infection.
Figure 1.
Adjusted vaccine effectiveness against omicron BA.4 or BA.5 infection stratified by sequence of vaccination dose and SARS-CoV-2 infection
The size of the dots is proportional to the number of person-days within each age group, hence, the size of the dots is not comparable across age groups.
Individuals who received two doses of vaccination before their first infection had the highest protection against omicron BA.4 or BA.5 infection with vaccine effectiveness of 85·3% (95% CI 80·2–89·1) in children and 82·9% (72·2–89·5) in adolescents (table 3). There was a trend of greater protection against omicron BA.4 or BA.5 infection in children who were vaccinated before their first SARS-CoV-2 infection (figure 1). The vaccine effectiveness estimates for those who were vaccinated before infection were generally higher than those who were infected and then vaccinated (44·1% and 56·5% vs 72·9%, 73·6%, and 85·3%). Among adolescents, receipt of an additional third-dose vaccination after recovering from their breakthrough SARS-CoV-2 infection did not result in substantial improvement in protection against omicron BA.4 or BA.5 infection (vaccine effectiveness 86·0% [80·6–89·9]).
In children who had received only one dose of vaccination before a first infection, vaccine effectiveness against omicron BA.4 or BA.5 infection was 72·9% (95% CI 63·1 to 80·1; table 3). Receipt of a second-dose vaccination did not provide additional protection with a vaccine effectiveness of 73·6% (65·5 to 79·8). However, in adolescents who received only one dose of vaccination before their first infection, vaccine effectiveness against omicron BA.4 or BA.5 infection was found to increase from 55·8% (–10·5 to 82·3) to 70·4% (43·5 to 84·4) with a second-dose vaccination.
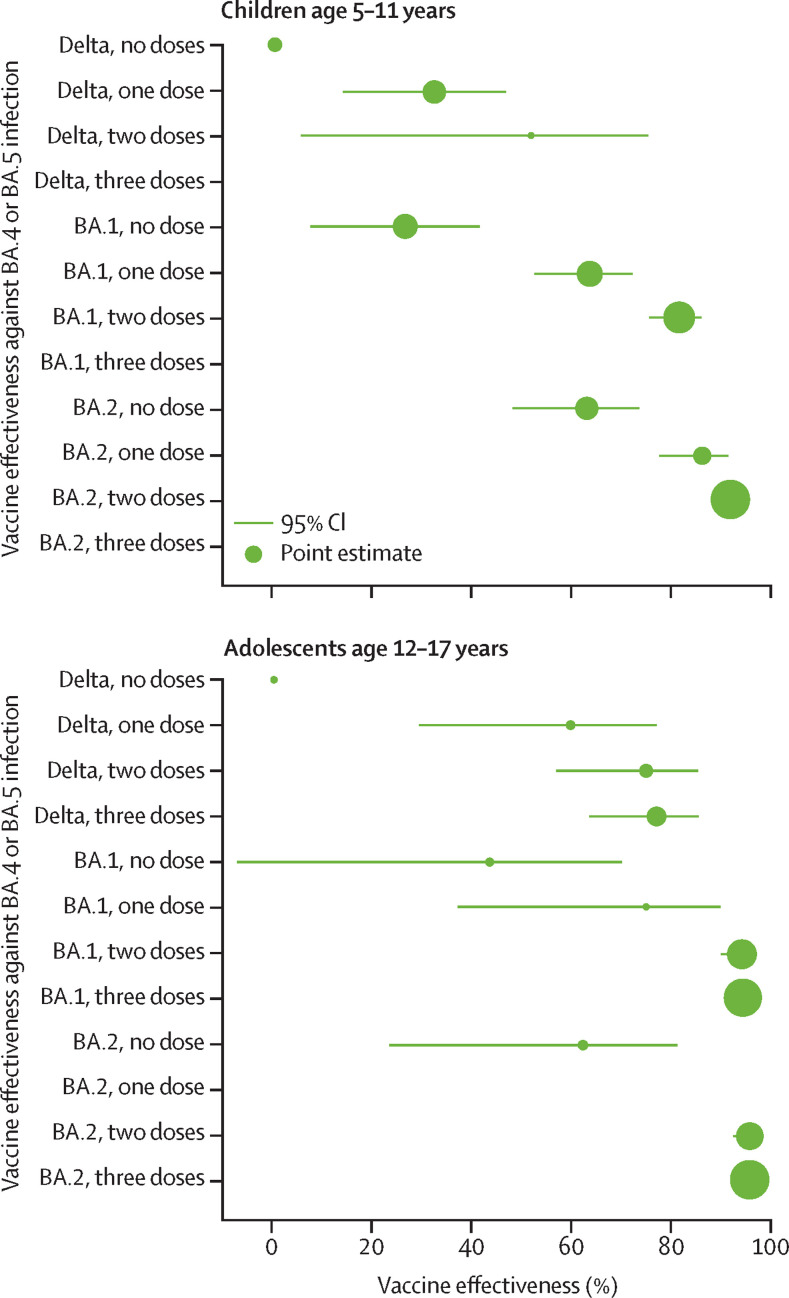
Among children previously infected with delta, omicron BA.1, or omicron BA.2 variants, vaccine effectiveness of two-dose vaccination against omicron BA.4 or BA.5 reinfections was 51·9% (95% CI 5·3–75·6) for delta, 81·9% (75·9–86·4) for BA.1, and 92·3% (88·9–94·7) for BA.2 variants (appendix p 7). A similar trend was observed in previously infected adolescents with vaccine effectiveness of two-dose vaccination at 75·4% (57·2–85·9) for delta, 94·8% (90·5–97·1) for BA.1, and 96·4% (93·0–98·2) for BA.2 variants. These findings show that against omicron BA.4 or BA.5 reinfection, hybrid immunity from a first infection with BA.2 conferred the highest protection whereas those whose first infection was with delta had the lowest protection (figure 2 ).
Figure 2.
Adjusted vaccine effectiveness against omicron BA.4 or BA.5 infection stratified by previous SARs-CoV-2 infection variant
The size of the dots is proportional to the number of person-days within each age group, hence, the size of the dots is not comparable across age groups.
Discussion
In children and adolescents with a previous episode of SARS-CoV-2 infection, receipt of two-dose and three-dose BNT162b2 vaccination provided protection against omicron BA.4 or BA.5 infections at 74·0% (two doses) and 85·7% (three doses), respectively. Against XBB reinfection, hybrid immunity was reduced in children with two-dose vaccination to 62·8% and in adolescents with three-dose vaccination to 47·9%. In children and adolescents who were unvaccinated before their first infection, subsequent receipt of two-dose vaccination generated better protection against BA.4 or BA.5 reinfection than did one dose, especially in adolescents. We found that receipt of two doses of BNT162b2 vaccination before first SARS-CoV-2 exposure provided the greatest protection against subsequent BA.4 or BA.5 infection, especially in children (vaccine effectiveness 85·3%). This trend was less clear in adolescents. Low numbers of adolescents were infected before vaccination resulting in vaccine effectiveness with wide confidence intervals. Finally, after adjustment for vaccination status, our results indicated an upward trend in protection against BA.4 or BA.5 reinfection if the first SARS-CoV-2 infection strain was delta, followed by BA.1 and then BA.2.
We confirmed that vaccination continues to provide protection against omicron BA.4 or BA.5 and XBB variants in previously infected children and adolescents. A recent preprint study of children age 6–14 years found that vaccination resulted in the highest level of neutralising antibodies, even when compared with those infected by two omicron variants.18 Published studies also report vaccination and infection eliciting increased cross-variant antibodies.19, 20 Vaccine effectiveness estimates against XBB variants were lower than against BA.4 or BA.5 variants, especially for adolescents in our study. Vaccine effectiveness against XBB available from a recently published study in adults aged 18 years or older from Singapore reported a protective hybrid immunity ranging between 30% and 51%, which was comparable with our findings.21 By contrast to our results, hybrid immunity against XBB in adults was only detected in vaccination-boosted individuals. These differences highlight the importance of monitoring real-world vaccine effectiveness in the paediatric population, whose young and evolving immune system differs substantially from the adult or older population. On the basis of our findings, bivalent boosters might be warranted for adolescents to improve protection against XBB, but less so in children aged 5–11 years. More research and data from other settings and countries are required to confirm this.
Our exploratory analysis of vaccine effectiveness against omicron BA.4 or BA.5 by sequence of vaccination dose and SARS-CoV-2 infection yielded important insights to guide public health vaccination policy and development of optimum vaccination schedules for the paediatric population. First, our study showed that vaccination before a SARS-CoV-2 infection provided the greatest protection against subsequent omicron BA.4 or BA.5 infection in children. This finding has implications for public health discussions of paediatric vaccination programmes in young children who have not yet been exposed to SARS-CoV-2, to help improve population immunity resilience against future variants. Second, for unvaccinated children and adolescents who recovered from a past SARS-CoV-2 infection, there were clear additional benefits of getting at least one dose of a vaccination in terms of protection against omicron BA.4 or BA.5 infection. The better protection from a second-dose vaccination observed in adolescents compared with children could be attributed to the larger antigen dose in the vaccine used in adolescents (30 μg) compared with in children (10 μg). Third, our study found that children who received one dose before getting their first infection might not need to complete their second dose vaccination upon recovery as there was no incremental protection against subsequent omicron BA.4 or BA.5 infection (vaccine effectiveness 72·9% vs 73·6%). By contrast, a substantial improvement in protection was observed in adolescents with receipt of two doses (vaccine effectiveness 55·8% vs 70·4%). Additional doses of vaccination did not result in significant boosting of immune responses to translate to clinical protection in previously infected individuals. A larger antigen dose, such as the ones used in vaccination of adolescents or bivalent vaccines, might be needed to overcome such immune effects to improve protection against new variants.
Among previously infected children and adolescents, vaccine effectiveness against omicron BA.4 or BA.5 variant infection was found to vary depending on the first SARS-CoV-2 infection variant. Those infected with the more recent omicron BA.1 or BA.2 variant had better protection than did those infected with a previous delta variant. Our results concur with a test-negative study from Qatar in which protection from infection with previous non-omicron variants was lower than protection from infection with previous BA.1 or BA.2 variants.22 Antigenic cartography studies have shown that omicron sublineages are separated from earlier SARS-CoV-2 variants with omicron BA.4 or BA.5 being the most distant.11
Although the study was based on a comprehensive national data set of paediatric infections and vaccination history, there are a number of inherent limitations as an observational study. We adjusted for potential confounders such as demographics, socioeconomic status, and calendar time. Furthermore, our primary analysis of vaccine effectiveness against BA.4 or BA.5 and XBB also adjusted for the variant of the first SARS-CoV-2 infection, which has been shown to have an effect on hybrid immunity. However, we were not able to exclude residual confounders such as all comorbidities, underlying parents’ decision to vaccinate their children, exposure risk, and health-seeking behaviour. Differences in health-seeking behaviour might result in bias in case ascertainment between vaccinated and unvaccinated individuals. We did not specifically evaluate duration of protection but additional analysis by days since vaccination showed no waning of vaccine effectivenes. Genomic sequencing was not routinely done on all confirmed cases of SARS-CoV-2 infection and hence, attributed causative variant utilised imputation methods, as described. We did not account for multiplicity resulting from analysis of more than one group of participants.
In previously infected children and adolescents, vaccination provided additional protection against omicron BA.4 or BA.5 and XBB infections compared with those who remained unvaccinated. Vaccine effectiveness was lower against the XBB variant than the BA.4 or BA.5 variant, especially in adolescents. Among children, those who were vaccinated before their first SARS-CoV-2 infection had the highest protection against subsequent omicron BA.4 or BA.5 infections. This finding could suggest that early vaccination of children who have not yet been exposed to SARS-CoV-2 could potentially strengthen population resilience against future SARS-CoV-2 variants. Our study provides important vaccine effectiveness insights regarding the possible importance of priming with vaccination before the first SARS-CoV-2 exposure to guide public health vaccination policies and optimisation of schedules for the paediatric population.
Data sharing
Requests for data sharing should be addressed to the corresponding author and will be handled in line with the data access and sharing policy of Ministry of Health, Singapore.
Declaration of interests
CFY declares funding to attend conferences and honorarium from Sanofi, Pfizer, and Takeda, outside of the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
There was no funding source for this study. We would like to thank members of the Ministry of Health's COVID Data Management and Analytics Team and Vaccination Operations Data Fusion Centre for their assistance in maintaining the datasets used for the analysis.
Contributors
CFY as lead author is the guarantor and accepts full responsibility for the work. CFY, DP, and KBT conceived the study. CFY, DP, KQK, CYC, and KBT designed the study. DP and KBT acquired the data. CFY, DP, KBT, BO, and DCL analysed and interpreted the data. DP and KBT accessed and verified the data. CFY and DP wrote the initial draft. All authors critically revised the manuscript and approved the final version. CFY affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors confirm they had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Tan SHX, Cook AR, Heng D, et al. Effectiveness of BNT162b2 Vaccine against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:525–532. doi: 10.1056/NEJMoa2203209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the omicron (B.1·1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Z, Cheshmehzangi A, McDonnell D, da Veiga CP, Xiang YT. Mind the “vaccine fatigue”. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.839433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Our World in Data COVID-19 vaccine boosters administered. https://ourworldindata.org/covid-vaccinations
- 5.CDC COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- 6.Bates TA, McBride SK, Leier HC, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abn8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammerman A, Sergienko R, Friger M, et al. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med. 2022;386:1221–1229. doi: 10.1056/NEJMoa2119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemaitelly H, Almukdad S, Ayoub HH, et al. COVID-19 vaccine protection among children and adolescents in Qatar. N Eng J Med. 2022;387:1865–1876. doi: 10.1056/NEJMoa2210058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yung CF, Saffari SE, Mah SYY, et al. Analysis of neutralizing antibody levels in children and adolescents up to 16 months after SARS-CoV-2 infection. JAMA Pediatr. 2022;176:1142–1143. doi: 10.1001/jamapediatrics.2022.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena S, Skirrow H, Wighton K. Vaccinating children aged under 5 years against covid-19. BMJ. 2022;378 doi: 10.1136/bmj.o1863. [DOI] [PubMed] [Google Scholar]
- 12.Kam KQ, Maiwald M, Chong CY, et al. SARS-CoV-2 antigen rapid tests and universal screening for COVID-19 Omicron variant among hospitalized children. Am J Infect Control. 2023;51:255–260. doi: 10.1016/j.ajic.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health Singapore COVID-19 statistics. https://www.moh.gov.sg/covid-19/statistics
- 14.Tan CY, Chiew CJ, Pang D, et al. Vaccine effectiveness against delta, omicron BA.1, and BA.2 in a highly vaccinated Asian setting: a test-negative design study. Clin Microbiol Infect. 2023;29:101–106. doi: 10.1016/j.cmi.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melliana A, Setyorini Y, Eko H, Rosi S, Purhadi The comparison of generalized Poisson regression and negative binomial reression methods in overcoming overdispersion. IJSTR. 2013;2:255–258. [Google Scholar]
- 16.Collie S, Saggers RT, Bandini R, et al. Association between regular physical activity and the protective effect of vaccination against SARS-CoV-2 in a South African case–control study. Br J Sport Med. 2023;57:205–211. doi: 10.1136/bjsports-2022-105734. [DOI] [PubMed] [Google Scholar]
- 17.Lefèvre B, Tondeur L, Madec Y, et al. Beta SARS-CoV-2 variant and BNT162b2 vaccine effectiveness in long-term care facilities in France. Lancet Healthy Longev. 2021;2:e685–e687. doi: 10.1016/S2666-7568(21)00230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowell AC, Lancaster T, Bruton R, et al. Immunological imprinting of humoral immunity to SARS-CoV-2 in children. bioRxiv. 2023 doi: 10.1101/2022.07.26.501570. published online March 9. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan CW, Lim BL, Young BE, et al. Comparative neutralisation profile of SARS-CoV-2 omicron subvariants BA.2·75 and BA.5. Lancet Microbe. 2022;3:e898. doi: 10.1016/S2666-5247(22)00220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonsenso D, Cusenza F, Passadore L, Bonanno F, De Guido C, Esposito S. Duration of immunity to SARS-CoV-2 in children after natural infection or vaccination in the omicron and pre-omicron era: a systematic review of clinical and immunological studies. Front Immunol. 2023;13 doi: 10.3389/fimmu.2022.1024924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan CY, Chiew CJ, Pang D, et al. Protective immunity of SARS-CoV-2 infection and vaccines against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfections in Singapore: a national cohort study. Lancet Infect Dis. 2023 doi: 10.1016/S1473-3099(23)00060-9. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data sharing should be addressed to the corresponding author and will be handled in line with the data access and sharing policy of Ministry of Health, Singapore.