Abstract
Novel targeted cancer therapies have revolutionized oncology therapies, but these treatments can have cardiovascular complications, which include heterogeneous cardiac, metabolic, and vascular sequelae. Vascular side effects have emerged as important considerations in both cancer patients undergoing active treatment and cancer survivors. Here, we provide an overview of vascular effects of cancer therapies, focusing on small molecule kinase inhibitors and specifically inhibitors of Bruton tyrosine kinase (BTK), which have revolutionized treatment and prognosis for B-cell malignancies. Cardiovascular side effects of BTK inhibitors include atrial fibrillation, increased risk of bleeding, and hypertension, with the former two especially providing a treatment challenge for the clinician. Cardiovascular complications of small molecule kinase inhibitors can occur through either “on-target” (targeting intended target kinase) or “off-target” kinase inhibition. We will review these concepts and focus on the case of BTK inhibitors, highlight the emerging data suggesting an off-target effect which may provide insights into development of arrhythmias, specifically atrial fibrillation. We believe that cardiac and vascular sequelae of novel targeted cancer therapies can provide insights into human cardiovascular biology.
Keywords: Cardio-oncology, vascular biology, cell biology/structural biology, basic science research, complications
Introduction
Cardio-oncology represents the intersection of cancer and its therapies with cardiology and has emerged as a new discipline in cardiology. While originated as a result of cardiomyopathies associated with traditional cancer therapies such as anthracyclines, cardio-oncology has expanded due to the explosion of novel oncology therapies. These therapies have a wide range of cardiac, metabolic and vascular toxicities1. Beyond the impact of new treatments, the recognition that common risk factors predispose patients to cancer and cardiovascular diseases have further linked the two disciplines and represents a new dimension to cardio-oncology2. These risk factors include genetic ones such as clonal hematopoiesis of intermediate potential (CHIP), somatic mutations in the peripheral blood cells, which predispose to both cancer and vascular disease3, 4. Further, there is growing recognition of “reverse cardio-oncology”, whereby cardiac disease can potentiate cancer5-7.
The study of cardiovascular sequelae from targeted oncologic therapies has provided novel insights into cardiovascular biology and disease pathogenesis through studying the impact of targeting specific cellular signaling pathways8. This concept was perhaps first recognized with the observation that cardiomyopathy unexpectedly occurred after treatment with trastuzumab, a monoclonal antibody that recognizes HER2 (also called erbB2), a receptor tyrosine kinase amplified in breast cancer9. This recognition uncovered new questions about the role of HER2 in the myocardium, as well as the potential for modulating the pathway for the treatment of heart failure10. Similar paradigms exist with vascular toxicities. For example, vascular endothelial growth factor (VEGF) inhibitors are associated with hypertension and proteinuria in a manner reminiscent of pre-eclampsia11. Soluble VEGF receptor 1 (also referred to as soluble fms-like tyrosine kinase 1, sFLT1), a soluble protein that exerts antiangiogenic effects by binding to and inhibiting the biological activity of proangiogenic proteins VEGF and which is secreted by the placenta, plays a pathophysiological role in preeclampsia12.
Many of the novel cancer treatments are kinase inhibitors. While kinases have critical functions in cardiac, vascular and metabolic hemostasis, aberrant kinase signaling also plays an important role in tumorigenesis, and thus treatment-related kinase inhibition can lead to pathologic changes in cardiovascular physiology13. Kinase inhibitors (KIs) – especially small molecules - may interact with more than one kinase because of the high degree of homology of ATP binding site across the kinome. In this regard, KIs may have both on-target and off-target effects14. Utilizing basic and translational approaches to study the mechanisms by which KIs result in cardiovascular side effects can elucidate novel pathways in cardiovascular pathophysiology and can help guide future oncologic drug design.
In this review, we will focus on the emerging medical and scientific data with the Bruton tyrosine kinase (BTK) inhibitor ibrutinib, which has revolutionized treatment for several B-cell malignancies15-18. Early experience with ibrutinib showed an unexpected association with atrial fibrillation and less frequently more serious arrhythmias, such as ventricular tachyarrhythmias19-22. At the same time, vascular complications, especially hypertension were also reported with ibrutinib23. Further complicating the clinical picture was an increased risk of bleeding in the setting of ibrutinib, a finding that had implications for anti-coagulation therapy for the associated atrial fibrillation24. A wide range of adverse effects was demonstrated in clinical trials where ibrutinib, used in a front-line setting, had an increased risk of sudden death during treatment compared with conventional chemotherapy despite considerable oncologic efficacy of the drug25. Cardiovascular toxicities were suspected as the underlying culprit and confirmed in a real-world population with the reporting of sudden cardiovascular death (including both arrhythmias and bleeding)26. The success of ibrutinib has led to the introduction of multiple BTK inhibitors to the market; however, whether the cardiovascular sequelae are a class effect remains to be determined.
Elucidating the pathway by which ibrutinib results in cardiovascular toxicities has resulted in recognition of novel mechanisms for the development of cardiovascular pathology27. Basic and translational approaches to studying the mechanisms by which BTK inhibition results in cardiovascular disease has successfully identified both on-target effects of inhibition (such as bleeding) and off-targets effects (such as atrial fibrillation). The aim of this review is to describe the mechanisms by which inhibitors of BTK result in cardiovascular pathology and the toxicities that clinicians may encounter. In addition, we will discuss clinical management strategies clinicians must consider when treating patients with concurrent cardiovascular disease and BTK-inhibitor therapy.
Overview of Novel Targeted Cancer Therapies
In the last two decades, targeted cancer therapies have revolutionized oncology treatment. Recognition of distinct pathways that have been hijacked by cancer cells to promote tumor growth has facilitated development of novel therapies targeting these pathways. As an example, the recognition that angiogenesis (mediated by VEGF) plays a causal role in renal cell carcinoma and other cancer types has led to pharmacological targeting of this pathway with the use of VEGF inhibitors28-30. Targeted cancer drugs come in 2 varieties: biologics (that are given intravenously and inhibit the ligand or the receptor extracellularly) and small molecule inhibitors (given orally and act intracellularly)1 (Figure 1). Cancer drugs target other fundamental cellular pathways, including both cancer cell intrinsic pathways as well as the cancer microenvironment. For example, protein degradation, a highly selective and regulated process, is often mediated by “tagging” proteins with ubiquitin, which leads to proteosome-mediated degradation31. The specificity is conferred by individual E3 ubiquitin ligases, which function as adaptor molecules that recognize the substrates through protein-protein interactions, rendering this a highly specific process. There are more than 600 E3 ubiquitin ligases--more than identified protein kinases—so targeting ubiquitin machinery becomes an important strategy for drug development32. Proteolysis-targeting chimera (PROTAC) and related molecules that promote or inhibit the E3 ubiquitin ligases have already proven effective drug molecules33. Finally, harnessing the immune system has been especially effective for treatment of cancers that were considered hopeless34. These therapies include immune checkpoint inhibitors (ICI) which are effective and are used for up to 50% of cancer therapies35. Cardiovascular disease become increasingly important following such transformation in prognosis for cancer patients36, 37.
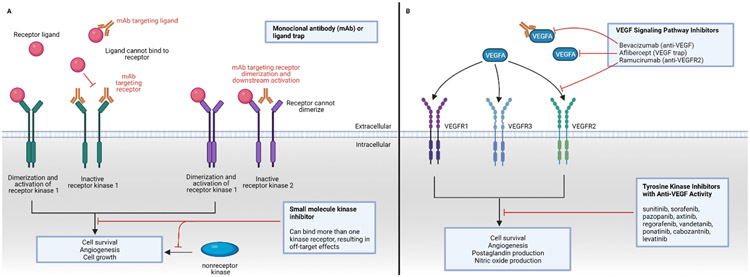
Figure 1:
Targeting kinases and VEGFR in the treatment of cancer. Panel A: Kinase inhibition can be achieved through targeting the kinase receptor ligand or kinase receptor, usually through binding of a monoclonal antibody (mAb) that is given as an intravenous infusion. Small-molecule kinase inhibitors, taken orally, work intracellularly and may bind more than one kinase. Panel B: VEGF-targeted therapies include a monoclonal antibody and ligand trap against circulating VEGFA, a monoclonal antibody against VEGF receptor 2 (VEGFR2), and multitargeted tyrosine kinase inhibitors with anti-VEGF activity.
Vascular Cardio-Oncology: Emerging Discipline
Cancers are inherently associated with vascular disease, especially venous thromboembolism (VTE)38. VTE, including superficial thrombophlebitis, deep vein thrombosis, in-dwelling catheter–associated thrombosis, and pulmonary embolism, likely represents the most common cardiovascular complication of malignancy. The presence of malignancy increases the risk of venous thromboembolic events 7 to 8-fold. The risk can be as high as 15-fold in the year following cancer diagnosis and reach as high as 28-fold with hematological malignancies39, 40. In addition, growing evidence suggests that there are risk factors that increase the risk of both cancer and cardiovascular disease2. These may include both environmental risk factors (e.g., tobacco abuse) or biological risk factors (e.g., inflammation). For example, in support of the latter, the recent results of CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) showed that pharmacological inhibition of IL-1β reduced both cardiac events and lung cancer incidence and mortality41, 42. Genetic factors may also contribute to this risk. Clonal hematopoiesis of indeterminate potential (CHIP) is the presence of an expanded somatic blood cell clone in individuals without other hematologic abnormalities. It is usually due to a driver somatic mutation of specific genes (DNMT3A, ASXL1 and TET2)4. CHIP both increases the risk for hematologic cancer and may well serve as an important risk factor for myocardial infarction and stroke3, 43.
Vascular disease and metabolic disorders have emerged as important sequelae of novel classes of oncology therapies and can affect both cancer patients undergoing active therapy, as well as cancer survivors following completion of treatment38, 44, 45. Immunomodulators (IMiDs, such as thalidomide and lenalidomide) bind cereblon, a component of a E3 ubiquitin ligase, causing selective ubiquitination and proteasome mediated degradation of key lymphoid transcription factors and have proven effective for multiple myeloma and other plasma disorders46, 47. However, IMiDs are associated with thromboembolism, which can occur in both arterial and venous circulation, the latter necessitating thromboprophylaxis48. Carfilzomib, a proteasome inhibitor used in the treatment of multiple myeloma, also potentiates vascular disease including thrombosis, hypertension and cardiac ischemia49, 50. Immunotherapies, including ICI, can result in vascular disease though the precise nature and mechanisms are just beginning to be studied. ICIs are associated with vasculitis, especially polymyalgia rheumatica and temporal arteritis, which can result in morbidity (up to 28% of temporal arteritis patients can have blindness or impaired vision) and mortality (up to 6%)51. Recent data also suggest increased atherosclerotic cardiovascular events, including myocardial infarction, coronary revascularization and ischemic stroke after exposure to ICI. One possible mechanism may be an increased rate of atherosclerotic plague progression37. On the other hand, CAR T and other cellular immunotherapies can cause myocardial injury, arrhythmia and refractory hypotension, usually in the context of cytokine release syndrome (CRS) and excessive release of proinflammatory cytokines52, 53. By far, the largest class of novel cancer therapies, however, have been kinase inhibitors, which will be the main focus of this review manuscript.
Properties of small molecule kinase inhibitors
Kinases are fundamental to regulation of cell signaling and act by transferring a phosphate group from adenosine triphosphate (ATP) to a specific protein or lipid substrate. The human genome project predicts at least 518 protein kinases in the human genome; at least 70% of all human proteins are phosphorylated by these kinases54, 55. Kinases are further divided structurally into 2 groups as either serine/threonine kinase or tyrosine/tyrosine-like kinases, depending on the amino acid substrate on the target protein56. Kinase protein structures include both an ATP-binding pocket (where the ATP binds) and an allosteric pocket (where the phosphorylated protein binds). Kinase inhibitors (KI) may act on either the ATP pocket or the allosteric pocket for effective inhibition. While the majority of KI binding is reversible, KI may also bind covalent and thus irreversibly57.
Small molecule KI are categorized based on the protein domain in the kinase by which the KI interacts and the mechanism by which this interaction occurs58. X-ray crystal structures of small molecule kinase inhibitors bound to their targets are utilized to make this classification59. Types I and II bind to the ATP binding pocket; type I inhibitors bind to the active protein kinase conformation whereas type II inhibitors bind to the inactive conformation. Types III and IV bind allosterically, with Type III binding next to the ATP-pocket and Type IV inhibitors binding neither to the ATP nor peptide substrate binding sites. Type V inhibitors bind bivalently to two different regions of the protein kinase domain.
Types I-V are reversible, whereas Type VI kinase inhibitors bind covalently to their protein kinase target59. Type VI kinases form a covalent, irreversible bond with cysteine or other nucleophilic residues within the ATP-active site of the kinase protein. This irreversible binding allows for sustainable inhibitory effects which are desired in treatment of cancer; however, irreversible binding can be deleterious if off-target binding occurs. Type VI kinase inhibitors have a number of potential therapeutic advantages including prolonged pharmacodynamics, suitability for rational design, and high potency and have been successfully developed for a number of cancers60. While the development of synthetic irreversible KIs was initiated with the goal of targeting epidermal growth factor receptor (EGFR), kinase sequence alignments indicated that other kinases possess similar conserved cysteine residues and may serve as drug targets. This included Tec family of kinases, including Bruton’s tyrosine kinase (BTK)61. A number of type VI KIs, such as afatinib, osimertinib, dacomitinib, and neratinib have been FDA approved to treat a variety of cancers62. Inhibitors of BTK will be discussed in detail below.
Cardiovascular Sequelae of Kinase Inhibitors
Vascular sequelae – with both beneficial and detrimental implications – have emerged as important clinical considerations in increasing number of cancer patients treated with KI63. These effects can be “on-target” (due to inhibition of intended cancer target) or “off-target” (resulting from inhibition of other kinase or non-kinase targets). Type I-II KI are theoretically the biggest culprits for off-target effects due to the high degree of homology of ATP binding sites across the kinome14. Off-target effects of KI may also include non-kinase targets64. Given that kinases also play a crucial role in vascular homeostasis, alteration of kinase activity as a result of KI treatment can lead to pathological effects on the vasculature. Scientifically, careful dissection of the vascular complications from both on-target and off-target effects of KI can inform new mechanisms of cardiovascular signaling8.
Small molecular inhibitors targeting VEGF receptors, were one of the early examples where vascular sequelae were observed and represented on-target effects. Nearly all the patients treated with VEGF inhibitors have an increase in blood pressure, often in a dose-dependent and transient manner, within 1 week after treatment65. The incidence of hypertension ranges from 20 to 25% with bevacizumab and sunitinib (the initially approved drugs in this class) to more than 50% with newer approved agents; the association of hypertension with every tested or approved drug in the class suggests an ‘on target’ effect63. Multiple mechanisms of VEGF inhibitors associated hypertension exist including an imbalance between vasodilators and vasoconstrictors, loss of capillary circulation, and alteration in glomerular function1. The concomitant presence of proteinuria in many patients with VEGF-inhibitor and the biological role for sFLT1 (i.e., sVEGF1) being a pathologic driver of similar clinical syndrome of preeclampsia provides support for an on-target, VEGF-dependent effect.
Vascular complications may also be a result of “off-target” effects of KI. This is best exemplified with KI used to treat chronic myelogenous leukemia (CML), where inappropriate activation of ABL1 kinase contributes to tumorigenesis66. Multiple small molecule inhibitor KI have been approved that inhibit the ABL1 kinase and have transformed the treatment for and nature course of CML, making it effectively a chronic disease67. Imatinib was the first KI approved in this class with later generation KI, initially developed to overcome imatinib resistance, but which were soon tested and approved in front-line (treatment naïve) patients. From a cardiovascular standpoint, whereas imatinib has proven safe, a heterogeneous spectrum of vascular toxic effects are observed with other KI68, 69. Dasatinib was associated with cardiopulmonary complications including pulmonary hypertension; both nilotinib and ponatinib were associated with vascular disease70, 71. The vascular sequelae with ponatinib were most problematic because of the drug’s considerable efficacy and its unique role in treating drug-resistant mutant CML; however, in a trial testing the efficacy of ponatinib in patients with CML in whom treatment with other tyrosine kinase inhibitors had failed, the cumulative rates of vascular events at a median follow-up of 15 months were 7.1% for cardiac events, 3.6% for cerebrovascular events, and 4.9% for peripheral-artery vascular events72. While the specific mechanisms of toxicity of cardiovascular complications of CML KIs are yet to be determined, the heterogeneous nature of toxicity suggest an off-target effect68, 73.
BTK and BTK inhibition for the Treatment of Cancer
BTK inhibitors are promising novel small molecules that have revolutionized treatments for B-cell malignancies and autoimmune diseases; however, clinical trials with BTK inhibitors showed increased cardiac and vascular side effects74, 75. BTK activity plays a crucial role in B cell differentiation, proliferation, and survival, and inhibition of BTK signaling has transformed treatment of B cell malignancies76. BTK acts downstream in the signal transduction pathway of the B cell receptor (BCR). Signaling via the BCR is essential for B cell survival and development, as well as antibody production in both physiologic and pathologic conditions77. Abnormal BCR signaling is associated with the development and progression of B cell malignancies and immunological diseases, including autoimmune disorders.
BTK is a member of the TEC family of non-receptor tyrosine kinase and contains five different protein interaction domains. These domains include an amino plecktein homology (PH) domain, a proline-rich TEC homology (TH) domain, two Src homology domains (SH2 and SH3), and a kinase domain with enzymatic activity. BTK is a cytoplasmic protein, although BTK activation occurs upon recruitment to the cell membrane through interaction with the PH domain76.
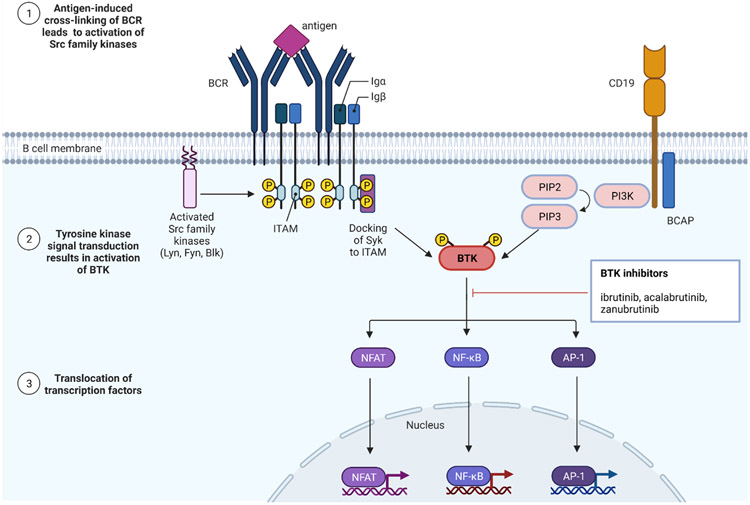
BTK is a crucial and proximal component of BCR signaling pathways. In the absence of BTK, BCR-induced proliferation and survival are impaired78. The immunoglobulin M (IgM) BCR has a very short cytoplasmic domain which cannot signal directly and thus associates with disulphide-linked Ig-α/Ig-β heterodimers. These transmembrane proteins contain immunoreceptor tyrosine-based activation motifs (ITAMs) in their cytoplasmic domain which participate in intracellular signal transduction. Following antigen-induced cross-linking of the B-cell receptor, Src-family protein tyrosine kinases (such as Lyn, Fyn, and Blk) phosphorylate ITAM and create docking sites for tyrosine protein kinase Syk (Syk). Src-family kinases also activate the B-cell co-receptor CD19 and the B-cell PI3K adaptor (BCAP) protein which facilitates recruitment and activation of PI3 kinase (PI3K)79. PI3K phosphorylates phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol-3,4,5-triphoshate (PIP3). The BTK PH domain binds PIP3 and localized BTK to the plasma membrane80. BTK is then phosphorylated and activated by Syk resulting in further downstream signaling cascades, including the transcription factors NFAT, NF-κB, and AP-1. These transcription factors are transported to the nucleus where they regulate genes responsible for both the innate and adaptive immune response, leading to increased B-cell differentiation, migration, and proliferation76, 81.
Loss-of-function mutations in BTK result in X-linked agammaglobulinemia (XLA), a primary inherited immunodeficiency82, 83. As one of the first recognized inborn errors of immunity, originally described in 1952 by Col. Ogden Bruton, MD, XLA is a primary immunodeficiency characterized by recurrent bacterial infections. The gene affected in XLA encodes BTK and is located on the X-chromosome84. This loss-of-function mutation results in universal B cell deficiency (<2%) and absent precursor B cell differentiation. Lymphocytes from patients with a pathogenic mutation fail to generate plasma cells and have severely decreased production of all classes of immunoglobulins with a markedly defective antibody response.
BTK plays a crucial role in proliferation and survival of leukemic cells in many B cell malignancies, including chronic lymphocytic leukemia (CLL), Waldenstrom’s macroglobulinemia, mantle cell lymphoma, marginal zone lymphoma, diffuse large B cell lymphoma, as well as chronic graft versus host disease15, 16, 18, 85-87. BTK inhibitors have revolutionized therapy for these B cell malignancies based on higher efficacy in patients with high-risk disease features as better tolerability in elderly and frail patients when compared with conventional chemotherapy88. For example, in a phase 3 study of first-line treatment with a BTK inhibitor (ibrutinib) versus conventional chemotherapy for CLL or small lymphocytic lymphoma (SLL), BTK inhibitor treatment improved progression-free survival (PFS) improved to 70% vs 12% (HR [95% CI]: 0.146 [0.098–0.218] and overall survival (OS) benefit to 83% vs 68% HR [95% CI]: 0.450 [0.266–0.761]) when compared with conventional chemotherapy, at a median follow-up of 60 months89.
Three orally available small molecule inhibitors of BTK - ibrutinib, acalabrutinib and zanubrutinib - have received FDA approval for different therapeutic indications90. First-in-class of the BTK inhibitors, ibrutinib was first approved in March 2016 for CLL, with later approval for mantle cell lymphoma, marginal zone lymphoma, and graft vs. host disease90. More recently, newer generation BTK inhibitors have been FDA approved including acalabrutinib and zanubrutinib. Acalabrutinib first received FDA approval in October 2017 for relapsed or refractory mantle cell lymphoma, and acalabrutinib has since gained FDA approval for CLL or small lymphocytic lymphoma (SLL)91, 92. Zanubrutinib was approved in November 2019 for patients with mantle cell lymphoma who have received at least one prior therapy93. A fourth orally available small molecule inhibitor of BTK, tirabrutinib, was approved in Japan for the treatment of recurrent or refractory primary central nervous system lymphoma, and clinical development in the USA and Europe is ongoing94.
Cardiac and Vascular side effects of BTK inhibitors
Following the first report of treatment with the BTK inhibitor ibrutinib for chronic/relapsed CLL in 2012, the toxicity profile of ibrutinib has been well characterized, and includes increased risk of bleeding, diarrhea, infection, arthralgia, as well as cardiovascular toxicities95-97. Among the most severe cardiovascular side effects are supraventricular and ventricular arrythmias, hypertension, conduction system disorders, increased risk of bleeding and central nervous system (CNS) hemorrhage89, 98-100. Second generation BTK inhibitors have also shown side effects, including increased risk of bleeding and hypertension101, 102. While the side effect profile of the first generation BTK inhibitor ibrutinib has been well described, the overall cardiovascular risks of the second generation BTK inhibitors acalabrutinib and zanubrutinib are less clear, given the more recent approval. A direct comparison of side effects between ibrutinib and other BTK inhibitors is also difficult given the limitations of cross-trial comparisons103.
Ibrutinib was associated with increased risk of development of supraventricular arrythmias, including atrial fibrillation when compared to similar patients treated with other therapies19. In a pooled analysis of four randomized clinical trials involving over 1500 patients with CLL or mantle cell lymphoma, with a mean follow-up of 16.6 months, the incidence of AF among patients receiving ibrutinib was 6.5% compared with 1.6% for comparator. Ibrutinib treatment, prior history of AF, and age greater than 65 years were independent risk factors for AF19. The majority (85.7%) of patients with AF did not discontinue ibrutinib and more than half received common anticoagulation/antiplatelet medications during the study. Second generation BTK inhibitors resulted in lower rates of atrial fibrillation, with AF rates with acalabrutinib of 3% and zanubrutinib of 3% compared with 6% for ibrutinib however, there are inherent challenges in such cross-trial comparisons with limited data103, 104.
Other forms of arrhythmia are also reported with ibrutinib. Ventricular arrythmias, sudden cardiac death, and conduction system disorders have been reported with treatment with the BTK inhibitor ibrutinib20, 21.Analysis of data from a large U.S.-based Comprehensive Cancer Center registry showed a cumulative incidence of ventricular arrhythmias (VA) at 0.03% at 6 years from onset of treatment with ibrutinib compared to less than 0.01% vs an ibrutinib-free population control21. In a randomized trial with 2 ibrutinib-containing arms in the front-line setting, reported death rates of 7% in each ibrutinib arm, compared with 1% in each control arm, with many of these deaths labeled “unexplained/unwitnessed death” or cardiac in nature25
Real world data from ibrutinib shows a more concerning cardiovascular picture. Utilizing the World Health Organization’s global database of individual case safety reports, Vigibase, where a disproportionality analysis was performed to define cardiovascular toxicities associated with ibrutinib treatment, 7 broad cardiovascular entities were identified to be significantly increased with ibrutinib compared with other drugs in the database 26. The reporting odds ratio (ROR), a measure of relative risk of a specific complication associated with ibrutinib, was significantly increased with ventricular arrhythmias (ROR 4.7; 95% CI: 3.7-5.9; p < 0.0001), heart failure (ROR: 3.5; 95% CI: 3.1-3.8; p < 0.0001), conduction disorders (ROR: 3.5; 95% CI: 2.7-4.6; p < 0.0001), As expected, supraventricular arrhythmias (SVA), predominantly atrial fibrillation were most significantly overreported with ibrutinib (ROR: 23.1; 95% CI: 21.6-24.7; p < 0.0001). This approach also provided chronologic data on the time from initiation of treatment with ibrutinib to onset of an adverse event, with the median time of 2-3 months with SVA, HF and VA, while conduction system disorders appeared to be reported earlier (within the first month of treatment). Cardiac disorders including cardiac ischemia, myocarditis, QT prolongation, and valvular disorders were not overreported in this population, consistent with adverse events from clinical trials.
Vascular side effects, including hypertension, increased risk of bleeding, and central nervous system (CNS) hemorrhagic events, were also observed in clinical trials of ibrutinib98, 99. Hypertension is a commonly noted side effect from ibrutinib treatment, with ibrutinib treatment for CLL resulting in an incident hypertension of 18% and grade ≥ 3 hypertension in 6%105. In a study of 247 patients at 3 institutions treated with ibrutinib at one year post initial exposure, median (range) peak blood pressure (BP) following ibrutinib exposure for the entire cohort was significantly (p<0.001) elevated from baseline 127 (90-182) mmHg systolic and 71 (48-95) mmHg diastolic to post-exposure 153 (105-215) mmHg systolic and 80 (53-121) mmHg diastolic106. Median (range) time to peak BP was 6 (0-35) months, and systolic BP was increased (19% median excursion from baseline) to a greater degree than diastolic BP (11% excursion from baseline). Changes to antihypertensive regimens were common, with 80.4% starting at least 1 new antihypertensive medication and 29.4% increasing the dose of an existing antihypertensive regimen within one year. Vigibase disproportionality analysis corroborated an increased incidence of hypertension (ROR: 1.7; 95% CI: 1.5 to 1.9; p < 0.0001) associated with ibrutinib treatment26.
An increased risk for development of hypertension was identified in patients treated with both first and second generation BTK inhibitors107. In a phase 2 study of treatment of relapsed/refractory CLL with the second generation BTK inhibitor acalabrutinib, 7% of patients developed hypertension101. In the ASPEN study, a randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom’s macroglobulinemia, zanubrutinib treatment resulted in atrial fibrillation in 11% of patients treated with 6% of patient’s treated experiencing grade ≥3 hypertension104. While zanubrutinib did result in hypertension, patients treated with ibrutinib in the ASPEN study developed hypertension at a ≥5% higher incidence. The similarities between first and second generation BTK inhibitors raises the concern that hypertension may be a drug class effect.
Major bleeding events, including subdural hematomas, gastrointestinal bleeding, and hematuria, were noted in early clinical trials with ibrutinib86, 108. These early trials led to the exclusion of patient’s taking vitamin K inhibitors in subsequent trials109. After three-years of follow-up, more than 50% of patients on ibrutinib will experience a bleeding event98. A systematic review and meta-analysis of published trials of patients treated with ibrutinib showed a pooled annual incidence of any bleeding event of 20.8 per 100 patient-years (95%CI: 19.1-22.1) for patients receiving ibrutinib compared to 11.6 per 100 patient years (95%CI: 9.1-14.4) for those receiving alternative treatments110. The relative risk (RR) of any bleeding event with ibrutinib treatment compared to an alternative therapy was found to be 2.72 fold (95%CI: 1.62-4.58); the relative risk (RR) of a major bleeding event was 1.66 (95%CI: 0.96-2.85).
Major bleeding events have been especially associated with BTK inhibitors in the setting of concurrent dual-antiplatelet therapy (DAPT) treatment or systemic anticoagulation111. In a retrospective analysis of 70 patients who received ibrutinib, major bleeding (defined as grade ≥3 bleed using the Common Toxicity Criteria for Adverse Events, CTCAE) occurred in 19% of patients. Risk factors for major bleed included an elevated INR (>1.5) and anemia (hemoglobin < 12 g/dL). The majority of patients who experienced major bleeding were also taking an antiplatelet agent (70%) or an anticoagulant (17%), and the combined use of both an antiplatelet and an anticoagulant greatly increased the risk of a major bleed event (HR 19.2, 95%CI:2.3-166.7, p < 0.01).
Second-generation BTK inhibitors are also associated with bleeding (suggesting a class effect) although recent reports indicate differences in severity with reduction of high-grade bleeding events with second-generation BTK inhibitors compared with ibrutinib. In a phase 1-2 trial of acalabrutinib treatment for 61 patients with relapsed CLL, low-grade bleeding events including petechiae (16%) and contusions (18%) were common, however, no major bleeding events occurred112. In a study of 33 patients with CLL who were intolerant of ibrutinib therapy, including 10 of whom had serious bleeding events, treatment with acalabrutinib resulted in 22 bleeding events, however, all but one of these events was minor (grade 1 or grade 2 per CTCAE)113. The ASPEN study, a randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom’s macroglobulinemia, showed a decreased incidence of any bleeding event in patient’s treated with zanubrutinib compared to ibrutinib (7.0% vs 4.4%) with a decreased risk of major hemorrhage (0.5% vs 0.3%)104. While promising, further studies will be necessary to conclusively determine if second-generation BTK inhibitors are superior to first-generation inhibitors from a bleeding adverse events standpoint.
Recent reports suggest that both ibrutinib and acalabrutinib have the ability to prevent platelet thrombus formation on human atherosclerotic plaque homogenate, and thus BTK inhibitors may play a future role in prevention and treatment of atherosclerotic disease114. Although at this time the side effect profile seen in both first and second generation BTK inhibitors likely makes the risks outweigh the benefits, the development of more specific BTK inhibitors may reverse this equation. These findings also provide an insight how cardio-oncology can benefit patients with atherosclerotic disease in addition to identifying toxic effects of chemotherapeutics115.
BTK inhibitors in basic Cardiac and Vascular biology
While the three FDA approved BTK inhibitors covalently and irreversibly bind BTK, differences in binding mechanisms at the clinical dose of the drug used may contribute to the differential cardiovascular side effects noted in the clinical trials discussed above59, 116, 117. Ibrutinib, acalabrutinib, and zanubrutinib all covalently bind to a cysteine residue (Cys-481) at the rim of the ATP binding pocket of BTK102. However, biochemical assays have shown that acalabrutinib binds Cys-481 through a more specific mechanism that reduces the intrinsic reactivity of acalabrutinib in inhibiting off-target kinases when compared with ibrutinib116, 118. The increased biochemical specificity of acalabrutinib for BTK when compared with ibrutinib may potentially explain the difference in side effects between ibrutinib and second generation BTK inhibitors.
The mechanism by which ibrutinib treatment results in atrial fibrillation is an area of active investigation, although recent evidence suggests that AF results from off-target signaling rather than through a BTK dependent mechanism27. An elegant study utilizing mouse models of chronic treatment with ibrutinib resulted in inducible AF, left atrial enlargement and myocardial fibrosis. This effect was not observed in mice treated acalabrutinib, a second generation and more specific BTK inhibitor, or in mouse lacking expression of BTK. Chemoproteomic profiling with mass spectrometry produced a small list of potential off-target candidate kinases for which inhibition may result in AF. Utilizing pharmacologic and genetic approaches, this list was narrowed to C-terminal Src kinase (CSK) as the most-likely candidate for ibrutinib induced AF. Cardiac specific CSK knockdown in mice resulted in inducible AF, left atrial enlargement, and myocardial fibrosis, thus phenocopying ibrutinib treatment. Vigibase disproportionality analysis was utilized and confirmed an increased reported of AF associated with kinase inhibitors which block CSK vs non-CSK inhibitors, with a reporting odds-ratio of 8.0 (95% CI 7.3-8.7, p<0.001). The downstream signaling pathway by which inhibition of CSK results in AF remain unknown. CSK is a non-receptor tyrosine kinase which serves as a master negative regulator of the Src family tyrosine kinases (SFKs) by phosphorylating and negatively regulating at least 8 downstream SFKs119. Although the downstream kinase signaling is yet to be determined, perturbation of CSK signaling likely represents a novel pathway for the development of AF. As such, this yet-to-be discovered pathway provides both potential therapeutic targets for the prevention and/or treatment of AF as well as a pathway for which interactions must be avoided in future drug development to prevent iatrogenic AF.
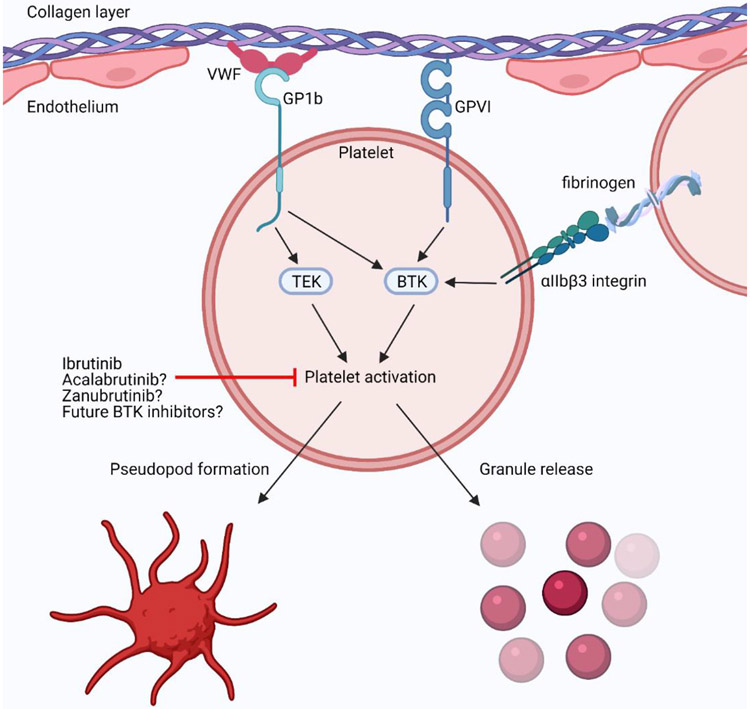
The mechanism by which BTK inhibition results in bleeding has been well studied and occurs though inhibition of collagen-mediated platelet aggregation120. BTK acts downstream of the collagen receptor Glycoprotein VI (GPVI), the Von Willebrand Factor (VWF) binding protein platelet membrane glycoprotein 1b (GP1b), and the platelet specific integrin alpha IIb/beta 3 (αIIbβ3 integrin) to facilitate intracellular signaling during platelet aggregation121-124. BTK and the related tyrosine kinase Tec are both involved in platelet activation through their phosphorylation of phospholipase Cγ2 (PLCγ2)125, 126. Activation of PLCγ2 continues collagen-mediated intracellular signaling cascade leading to platelet aggregation, and thus inhibition of this pathway inhibits platelet aggregation. (Figure 3).
Figure 3:
BTK inhibitor blockage of platelet activation and aggregation. GPVI: collagen receptor Glycoprotein VI; GP1b: platelet membrane glycoprotein 1b; Platelet specific integrin alpha IIb/beta 3, αIIbβ3 integrin; VWF: Von Willebrand Factor.
The increased specificity of second-generation BTK inhibitors for BTK, compared to first-generation BTK inhibitors, may explain the reduced incidence of major bleeding events seen in clinical trials121. Utilizing mouse models, BTK was shown necessary for both collagen/GPVI and VWF/GP1b induced platelet activation127. However, loss-of-function mutations in BTK which result in X-linked agammaglobulinemia have a mildly diminished, but not abolished, collagen activation, thus suggesting compensation of BTK by Tec121, 125, 126. While ibrutinib binds both BTK and Tec with a high affinity, acalabrutinib binds more specifically to BTK than to Tec116, 128. Experimental models have shown that treatment with the second-generation BTK inhibitors acalabrutinib and zanubrutinib result in less anti-platelet effects than the first-generation BTK inhibitor ibrutinib129, 130. However, this may not be clinically relevant to patient’s who experience bleeding events with ibrutinib, as will be discussed in management strategies below121.
The mechanisms by which ibrutinib and other BTK inhibitors lead to hypertension remain to be elucidated. Hypertension is an observed side-effect of a number of other cancer therapies, most notably VEGF inhibitors and the proteasome inhibitor, carfilzomib48. In the case of VEGF inhibitors, both the clinical and pre-clinical observations support an “on-target” mechanism for hypertension. In the case of carfilzomib, the specific mechanisms of hypertension have not been elucidated. Further basic and translational studies will be necessary to identify the pathways by which BTK inhibitor treatment results in hypertension, and it remains unclear if this occurs through an on-target or off-target mechanisms.
Management of cardiac and vascular complications from BTK inhibitor treatment
While the introduction of BTK inhibitors has changed the prognosis of CLL and other lymphoid malignancies, both cardiac and vascular complications must be considered given the early toxicities observed with the therapies. These include management of bleeding, management of anti-platelet therapy, hypertension, atrial fibrillation and other arrhythmia, prevention of cardioembolic events, and peri-operative/peri-procedural management. Managing the above is further complicated given the relatively older age and cardiovascular comorbidities of many patients receiving ibrutinib therapy in addition to the increased risk of spontaneous bleed and traumatic falls in the elderly131. However, there is little high-quality evidence regarding management of complications from BTK inhibitor treatment, with recommendations currently derived from trial data and expert opinions. These data are even less with second-generation BTK inhibitors given their more recent approval and lack of clinical experience. Therefore, the majority of management considerations will focus on treatment with ibrutinib.
Ibrutinib primarily undergoes cytochrome P450 (CYP) 3A4-mediated metabolism with minor contributions from CYP2D6132. Ibrutinib is sensitive to CYP3A4-mediated drug-drug interactions. Avoidance of concurrent treatment with strong CYP3A4 inhibitors (such as ketoconazole) is recommended to avoid increased risk of ibrutinib-mediated side effects, including bleeding133. Dose reductions are recommended for concomitant use of ibrutinib with posaconazole, voriconazole, and moderate CYP3A4 inhibitors132, 133. Grapefruit and grapefruit juice, which contain CYP3A4 inhibitors, should also be avoided with ibrutinib132, 134. Ibrutinib is also a P-glycoprotein inhibitor and co-administration with P-glycoprotein substrates (e.g., digoxin) may result in increase in plasma concentrations. Drug-drug interactions between the second generation BTK inhibitors and the CYP3A subfamily must also be considered. Acalabrutinib is a CYP3A substrate and a weak CYP3A/CYP2C8 inhibitor, and zanubrutinib has shown to decrease the systemic exposure of CYP3A substrates135, 136.
Ibrutinib increases the risk of atrial fibrillation and poses unique challenges in its management due to multiple drug-drug interactions and ibrutinib-related antiplatelet and coagulopathic properties. Such drug-drug interactions must be considered when considering rate and rhythm control agents for treatment of atrial fibrillation. Amiodarone, verapamil and diltiazem (moderate CYP3A inhibitors) should be avoided, due to the risk of elevated ibrutinib levels via CYP3A4 inhibition132, 133, 137, 138. Avoidance of digoxin, a P-glycoprotein substrate, is recommended as concomitant administration of ibrutinib raises digoxin levels and the risk of digoxin toxicity. In clinical practice beta-blockers are often the best rate-controlling strategy in ibrutinib-associated atrial fibrillation. Metoprolol is preferred due to P-glycoprotein inhibiting properties of carvedilol.
For prevention of cardioembolic events in patients who develop atrial fibrillation associated with BTK inhibitors, anticoagulation is recommended following guidance for general population. Although CHA2DS2-VASc and HAS-BLED scores have not been validated in this population they are commonly used in absence of more specific measures. The use of vitamin K antagonists (warfarin) is contraindicated in patients receiving ibrutinib due to high bleeding rates seen in initial clinical trials139. Patients requiring warfarin and vitamin K inhibitors were excluded from later trials and the use of direct-acting oral anticoagulants (DOACs) has been recommended133. Individual DOACs have not been compared head to head in this patient population and drug-drug interactions represent an important consideration. Dabigatran is a P-glycoprotein substrate and avoidance or dose reduction can be considered with ibrutinib. Rivaroxaban, apixaban and edoxaban all undergo CYP3A4-mediated metabolism, however routine dose reduction is not recommended with concomitant use of ibrutinib. At present time there is no evidence to suggest differential safety and efficacy profiles of specific DOACs.
Management of bleeding with BTK inhibitors must be considered with a risk-benefit analysis for patient’s taking either anti-platelet or anti-coagulants. Minor bleeding with BTK inhibitors may be well tolerated; however, counseling should be performed to minimize the risk of bleed. Patients should be cautioned against the use of NSAIDs (excluding for primary or secondary cardiovascular prevention), vitamin E and fish oil140. In patients on anti-coagulation, discontinuing aspirin should be considered. For patients that require percutaneous intervention and stent placement, bare-metal stents can be considered preferential over drug-eluting stents due to the shorter need for dual-antiplatelet therapy. Another group of special interest who require the use of dual antiplatelet therapy (such as post coronary intervention) who develop atrial fibrillation or need anticoagulation for stroke prophylaxis. In this patients, alternative strategies need to be discussed with the oncology team (e.g. venetoclax and obinutuzumab can be considered for CLL)141.
In patients treated with ibrutinib who experience bleeding, switching to a second-generation BTK inhibitor has been proposed to reduce the risk of bleeding; however, data are insufficient to determine if this is an effective strategy. While second generation BTK inhibitors are generally more specific for BTK, with less Tec inhibition when compared to ibrutinib, a recent study has questioned whether switching BTK classes for patient’s experiencing ibrutinib-related bleeding is effective121. Healthy control volunteers were treated with ibrutinib and collection of platelets was performed after BTK inhibitor treatment. Platelet aggregation response to collagen in platelet-rich plasma was performed and characterized by low or high response to ibrutinib as a surrogate for bleeding response. In platelets collected from individuals with a low response and low risk of bleeding from ibrutinib, treatment with acalabrutinib did not inhibit platelet aggregation, whereas those with a high response and a high risk of bleed from ibrutinib, treatment with acalabrutinib did inhibit platelet aggregation. This raises the possibility that certain individuals are more susceptible to platelet dysfunction and bleeding when treated with BTK inhibitors, and that switching classes once a bleeding event has occurred on a first generation BTK inhibitor may not be effective.
While anti-platelet agents remain a mainstay in the treatment of vascular disease, the increased risk of bleeding and plaque rupture must be considered when concurrently treating patients with anti-platelets and BTK inhibitors115. For patients with stroke and symptomatic peripheral artery disease (PAD), AHA/ACC guidelines recommend treatment with an anti-platelet agent (aspirin 75-325 mg daily or clopidogrel 75 mg daily) and high intensity statin therapy142. Given the increased risk of bleeding from anti-platelet therapy, low dose aspirin (81 mg daily) may be preferred. Clopidogrel is metabolized by CYP2C19 to the active metabolite, so CYP mediated drug-drug interactions with BTK inhibitors are not of concern, however, synergistic anti-platelet effects are still of major concern143. In patients with significant bleeding risk who require anti-platelet agents for symptomatic PAD, initiation of second generation BTK inhibitor can be considered in collaboration with the patient’s oncologist. High intensity statins (atorvastatin and rosuvastatin) should be used according to the ACC/AHA guidelines144. Atorvastatin is a substrate of CYP3A4, and thus rosuvastatin may be the preferred agent145.
In patients who experience severe and/or life-threatening bleeding during ibrutinib therapy, the steps in acute management include stopping BTK inhibitor, stopping antiplatelets and anticoagulation, addressing bleeding source and providing supportive care which may include reversal anticoagulation strategies146. After bleeding has resolved and the source is controlled, an individualized discussion with the oncology team needs to weigh the risk of future bleeding, benefit of anticoagulation and alternative therapies. Elevated blood pressure is frequent among patients receiving BTKs and early recognition and management are important. In addition to BP checks in the outpatient office setting, home BP monitoring can be considered in patients with history of hypertension or elevated BP values during office visits. Previous guidelines recommended blood pressure treatment goals in patients with PAD the same way as other established cardiovascular disease, and the treatment target was historically <130/80 mmHg147. Recent post hoc analysis of the ALLHAT (Antihypertensives and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed a higher risk of lower extremity PAD events associated with a SBP < 120 mmHg vs a reference group with SBPs of 120-129 mmHg148. No specific studies have been performed to address what the optimal antihypertensive agents to use first line in the treatment of BTK-induced hypertension. Therefore, physicians should treat hypertension based on recommended blood pressure goals rather than choose between particular antihypertensive medication. Consideration may be given to avoid antihypertensives with CYP3A4 metabolism to avoid potential drug-drug interactions.
Pre-operative and peri-procedural medication management must be considered for patients taking BTK inhibitors. For elective procedures or planned major surgeries, ibrutinib should be held for 7 days prior, as the antiplatelet effects of ibrutinib have been shown to be fully reversed after a week off therapy. Ibrutinib can be restarted 1-3 days postoperatively, as long as serious bleeding has resolved. For urgent, unplanned procedures, platelet transfusion to achieve 50% fresh platelets should be performed.
Conclusion/Future Directions
In the future, better defining the various cardiovascular effects of BTK inhibitors will advance the care and outcomes of patients with B-cell malignancies and cardiovascular comorbidities. Basic and translational studies to elucidate the mechanisms by which these side effects occur, particularly into the biologic processes by which BTK inhibitors result in hypertension, are needed both for treatment of BTK associated hypertension and for discovery of potential novel signaling pathways which may result in hypertension. Additional clinical studies are needed to determine optimal medical strategies for management of BTK associated bleeds, including comparison of class switching first to second generation BTK inhibitors in the setting of major bleeds. Given the increasing incidence and prevalence of hematologic malignancies worldwide, such investigation is of utmost importance149.
Figure 2:
B-cell receptor and downstream tyrosine-kinase signaling in B-cell tumor growth. BCAP: B-cell PI3K adaptor protein; BCR: B-cell receptor; BTK: Bruton’s tyrosine kinase; ITAM: immunoreceptor tyrosine-based activation motif; PI3K: PI3 kinase; SYK: tyrosine protein kinase Syk; PIP2: phosphatidylinositol 4,5-biphosphate; PIP3: phosphatidylinositol-3,4,5-triphoshate.
Acknowledgments:
Figures 1-3 were created using Biorender.
Sources of Funding:
J.A.B. is supported by American Heart Association (18SFRN33960373) and National Institutes of health grants (R01HL131977). M.R.F. is supported by a National Institute of Health post-doctoral training grant (5T32NS007491-19). K.D.J. is supported by an intramural pilot grant from the North Carolina Translational and Clinical Sciences (NC TraCS) Institute (550KR231911) (NC TraCS is funded by CTSA grant UL1TR002489.) J.J.M. is supported by National Institutes of Health grants (R01HL141466, R01HL155990, and R01HL156021). L.X. is supported by an American Heart Association Career Development Award (20CDA35260081).
Abbreviations
- AF
atrial fibrillation
- ATP
adenosine triphosphate
- BCAP
B-cell PI3K adaptor
- BCR
B-cell receptor
- BTK
Bruton tyrosine kinase
- CART
chimeric-antigen receptor
- CHIP
Clonal hematopoiesis of indeterminate potential
- CLL
chronic lymphocytic leukemia
- CML
chronic myelogenous leukemia
- CNS
central nervous system
- CSK
C-serine kinase
- CTLA-4
cytotoxic T lymphocyte antigien-4
- CRS
cytokine release syndrome
- DAPT
dual-antiplatelet therapy
- DOAC
direct-acting oral anticoagulants
- EGFR
epidermal growth factor receptor
- GPVI
collagen receptor Glycoprotein VI
- GP1b
platelet membrane glycoprotein 1b
- HR
hazard ratio
- ICI
immune checkpoint inhibitors
- IMiDs
immunomodulators
- ITAMs
immunoreceptor tyrosine-based activation motifs
- KI
kinase inhibitor
- OS
overall survival
- PAD
peripheral artery disease
- PD-1
programmed death-1 receptor
- PD-L1
programmed death-1 ligand
- PFS
progression-free survival
- PH
plecktein homology domain
- PIP2
phosphatidylinositol 4,5-biphosphate
- PIP3
phosphatidylinositol-3,4,5-triphoshate
- PROTAC
proteolysis-targeting chimera
- SFK
Src family tyrosine kinase
- sFLT1
soluble VEGF receptor 1
- SLL
small lymphocytic lymphoma
- SVA
supraventricular arrhythmias
- TH
TEC homology
- VA
ventricular arrhythmias
- VEGF
vascular endothelial growth factor
- VTE
venous thromboembolism
- VWF
Von Willebrand Factor
- XLA
X-linked agammaglobulinemia
Footnotes
Disclosures: A.B. has served on advisory board of Takeda. J.A.B. has served on the advisory boards of Amgen, Bayer, Janssen, JanOne. J.J.M. has served on advisory boards for Bristol Myers Squibb, Takeda, Regeneron, Audentes, Deciphera, Ipsen, Janssen, ImmunoCore, Boston Biomedical, Amgen, Myovant, Triple Gene/Precigen, Cytokinetics, Pfizer, Pharmacyclics, Boehringer and AstraZeneca.
References:
- 1.Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 2.Moslehi J, Zhang Q and Moore KJ. Crosstalk Between the Heart and Cancer: Beyond Drug Toxicity. Circulation. 2020;142:684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D and Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, Zeiher A, Jaiswal S, Schulz C, Blankstein R, Bolton KL, Steensma D, Levine RL and Ebert BL. Clonal Hematopoiesis: Crossroads of Aging, Cardiovascular Disease, and Cancer: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon AR, van der Vegt B, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Sillje HHW and de Boer RA. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation. 2018;138:678–691. [DOI] [PubMed] [Google Scholar]
- 6.Koelwyn GJ, Newman AAC, Afonso MS, van Solingen C, Corr EM, Brown EJ, Albers KB, Yamaguchi N, Narke D, Schlegel M, Sharma M, Shanley LC, Barrett TJ, Rahman K, Mezzano V, Fisher EA, Park DS, Newman JD, Quail DF, Nelson ER, Caan BJ, Jones LW and Moore KJ. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. 2020;26:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboumsallem JP, Moslehi J and de Boer RA. Reverse Cardio-Oncology: Cancer Development in Patients With Cardiovascular Disease. J Am Heart Assoc. 2020;9:e013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellinger AM, Arteaga CL, Force T, Humphreys BD, Demetri GD, Druker BJ and Moslehi JJ. Cardio-Oncology: How New Targeted Cancer Therapies and Precision Medicine Can Inform Cardiovascular Discovery. Circulation. 2015;132:2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J and Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- 10.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr., Chien KR and Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. [DOI] [PubMed] [Google Scholar]
- 11.Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG and Moslehi J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension. 2018;71:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rana S, Lemoine E, Granger JP and Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res. 2019;124:1094–1112. [DOI] [PubMed] [Google Scholar]
- 13.Manouchehri A, Kanu E, Mauro MJ, Aday AW, Lindner JR and Moslehi J. Tyrosine Kinase Inhibitors in Leukemia and Cardiovascular Events: From Mechanism to Patient Care. Arterioscler Thromb Vasc Biol. 2020;40:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, Reinecke M, Ruprecht B, Petzoldt S, Meng C, Zecha J, Reiter K, Qiao H, Helm D, Koch H, Schoof M, Canevari G, Casale E, Depaolini SR, Feuchtinger A, Wu Z, Schmidt T, Rueckert L, Becker W, Huenges J, Garz AK, Gohlke BO, Zolg DP, Kayser G, Vooder T, Preissner R, Hahne H, Tonisson N, Kramer K, Gotze K, Bassermann F, Schlegl J, Ehrlich HC, Aiche S, Walch A, Greif PA, Schneider S, Felder ER, Ruland J, Medard G, Jeremias I, Spiekermann K and Kuster B. The target landscape of clinical kinase drugs. Science. 2017;358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O'Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ and Investigators R-. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV, Yang G, Cao Y, Xu L, Patterson CJ, Rodig S, Zehnder JL, Aster JC, Harris NL, Kanan S, Ghobrial I, Castillo JJ, Laubach JP, Hunter ZR, Salman Z, Li J, Cheng M, Clow F, Graef T, Palomba ML and Advani RH. Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med. 2015;372:1430–40. [DOI] [PubMed] [Google Scholar]
- 17.Denlinger NM, Epperla N and William BM. Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res. 2018;10:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho SG, Bothos J, Goldberg JD, Enny C, Traina S, Balasubramanian S, Bandyopadhyay N, Sun S, Vermeulen J, Rizo A and Rule S. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–8. [DOI] [PubMed] [Google Scholar]
- 19.Brown JR, Moslehi J, O'Brien S, Ghia P, Hillmen P, Cymbalista F, Shanafelt TD, Fraser G, Rule S, Kipps TJ, Coutre S, Dilhuydy MS, Cramer P, Tedeschi A, Jaeger U, Dreyling M, Byrd JC, Howes A, Todd M, Vermeulen J, James DF, Clow F, Styles L, Valentino R, Wildgust M, Mahler M and Burger JA. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, Banerji V, Jones JA, Walewska R, Savage KJ, Michaud GF, Moslehi JJ and Brown JR. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guha A, Derbala MH, Zhao Q, Wiczer TE, Woyach JA, Byrd JC, Awan FT and Addison D. Ventricular Arrhythmias Following Ibrutinib Initiation for Lymphoid Malignancies. J Am Coll Cardiol. 2018;72:697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng C, Woronow D, Nayernama A, Wroblewski T and Jones SC. Ibrutinib-associated ventricular arrhythmia in the FDA Adverse Event Reporting System. Leuk Lymphoma. 2018;59:3016–3017. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, Guha A, Rogers KA, Bhat S, Byrd JC, Woyach JA, Awan F and Addison D. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JR, Moslehi J, Ewer MS, O'Brien SM, Ghia P, Cymbalista F, Shanafelt TD, Fraser G, Rule S, Coutre SE, Dilhuydy MS, Cramer P, Jaeger U, Dreyling M, Byrd JC, Treon S, Liu EY, Chang S, Bista A, Vempati R, Boornazian L, Valentino R, Reddy V, Mahler M, Yang H, Graef T and Burger JA. Incidence of and risk factors for major haemorrhage in patients treated with ibrutinib: An integrated analysis. Br J Haematol. 2019;184:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, Parikh SA, Coutre S, Hurria A, Brown JR, Lozanski G, Blachly JS, Ozer HG, Major-Elechi B, Fruth B, Nattam S, Larson RA, Erba H, Litzow M, Owen C, Kuzma C, Abramson JS, Little RF, Smith SE, Stone RM, Mandrekar SJ and Byrd JC. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. 2018;379:2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem JE, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, Yang T, Reddy NM, Funck-Brentano C, Brown JR, Roden DM and Moslehi JJ. Cardiovascular Toxicities Associated With Ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, Iwamoto Y, Wojtkiewicz G, Cetinbas M, Schloss MJ, Tedeschi J, Lebrun-Vignes B, Lundby A, Sadreyev RI, Moslehi J, Nahrendorf M, Ellinor PT and Milan DJ. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation. 2020;142:2443–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choueiri TK and Kaelin WG, Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med. 2020;26:1519–1530. [DOI] [PubMed] [Google Scholar]
- 29.Nazer B, Humphreys BD and Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation. 2011;124:1687–91. [DOI] [PubMed] [Google Scholar]
- 30.Bair SM, Choueiri TK and Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc Med. 2013;23:104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravid T and Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen P and Tcherpakov M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell. 2010;143:686–93. [DOI] [PubMed] [Google Scholar]
- 33.Schapira M, Calabrese MF, Bullock AN and Crews CM. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov. 2019;18:949–963. [DOI] [PubMed] [Google Scholar]
- 34.June CH and Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, Lyon AR, Padera RF, Johnson DB and Moslehi J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L and Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, Hartmann SE, Gilman HK, Gong J, Zubiri L, Sullivan RJ, Reynolds KL, Mayrhofer T, Zhang L, Hoffmann U and Neilan TG. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020;142:2299–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, Cohen P, Groarke JD, Herrmann J, Reilly CM and Weintraub NL. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e579–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ and Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. [DOI] [PubMed] [Google Scholar]
- 40.Cronin-Fenton DP, Sondergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, Baron JA and Sorensen HT. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103:947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ and Group CT. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ and Group CT. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 43.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S and Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moslehi J. The cardiovascular perils of cancer survivorship. N Engl J Med. 2013;368:1055–6. [DOI] [PubMed] [Google Scholar]
- 45.Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Grines CL and Marmagkiolis K. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation. 2016;133:1272–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA and Ebert BL. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE and Kaelin WG, Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, Garcia D, Cornell RF, Gailani D, Laubach J, Maglio ME, Richardson PG and Moslehi J. Cardiovascular and Thrombotic Complications of Novel Multiple Myeloma Therapies: A Review. JAMA Oncol. 2017;3:980–988. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Cornell RF, Lenihan D, Slosky D, Jagasia M, Piazza G and Moslehi J. Cardiovascular Complications of Novel Multiple Myeloma Treatments. Circulation. 2016;133:908–12. [DOI] [PubMed] [Google Scholar]
- 50.Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, Carver JR, Cohen AD, Engelhardt BG, Garfall AL, Goodman SA, Harrell SL, Kassim AA, Jadhav T, Jagasia M, Moslehi J, O'Quinn R, Savona MR, Slosky D, Smith A, Stadtmauer EA, Vogl DT, Waxman A and Lenihan D. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J Clin Oncol. 2019;37:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB and Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, Lee DH, Zlotoff DA, Zhang L, Drobni ZD, Hassan MZO, Bassily E, Rhea I, Ismail-Khan R, Mulligan CP, Banerji D, Lazaryan A, Shah BD, Rokicki A, Raje N, Chavez JC, Abramson J, Locke FL and Neilan TG. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol. 2019;74:3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein J, Barrett DM, Weiss SL, Fitzgerald JC, Berg RA, Aplenc R, Callahan C, Rheingold SR, Zheng Z, Rose-John S, White JC, Nazimuddin F, Wertheim G, Levine BL, June CH, Porter DL and Grupp SA. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manning G, Whyte DB, Martinez R, Hunter T and Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. [DOI] [PubMed] [Google Scholar]
- 55.Sharma K, D'Souza RC, Tyanova S, Schaab C, Wisniewski JR, Cox J and Mann M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 2014;8:1583–94. [DOI] [PubMed] [Google Scholar]
- 56.Grimminger F, Schermuly RT and Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov. 2010;9:956–70. [DOI] [PubMed] [Google Scholar]
- 57.Wu P, Nielsen TE and Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36:422–39. [DOI] [PubMed] [Google Scholar]
- 58.Dar AC and Shokat KM. The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annu Rev Biochem. 2011;80:769–95. [DOI] [PubMed] [Google Scholar]
- 59.Roskoski R, Jr. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol Res. 2016;103:26–48. [DOI] [PubMed] [Google Scholar]
- 60.Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH and Gray NS. Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol. 2013;20:146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang T, Hatcher JM, Teng M, Gray NS and Kostic M. Recent Advances in Selective and Irreversible Covalent Ligand Development and Validation. Cell Chem Biol. 2019;26:1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roskoski R Jr. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. 2019;139:395–411. [DOI] [PubMed] [Google Scholar]
- 63.Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O and Moslehi J. Vascular and Metabolic Implications of Novel Targeted Cancer Therapies: Focus on Kinase Inhibitors. J Am Coll Cardiol. 2015;66:1160–78. [DOI] [PubMed] [Google Scholar]
- 64.Munoz L. Non-kinase targets of protein kinase inhibitors. Nat Rev Drug Discov. 2017;16:424–440. [DOI] [PubMed] [Google Scholar]
- 65.Azizi M, Chedid A and Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–7. [DOI] [PubMed] [Google Scholar]
- 66.de Klein A, van Kessel AG, Grosveld G, Bartram CR, Hagemeijer A, Bootsma D, Spurr NK, Heisterkamp N, Groffen J and Stephenson JR. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–7. [DOI] [PubMed] [Google Scholar]
- 67.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ and Investigators I. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moslehi JJ and Deininger M. Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J Clin Oncol. 2015;33:4210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barber MC, Mauro MJ and Moslehi J. Cardiovascular care of patients with chronic myeloid leukemia (CML) on tyrosine kinase inhibitor (TKI) therapy. Hematology Am Soc Hematol Educ Program. 2017;2017:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S, Dorken B and Valent P. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103:1347–8. [DOI] [PubMed] [Google Scholar]
- 71.Groarke JD, Cheng S and Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med. 2013;369:1779–81. [DOI] [PubMed] [Google Scholar]
- 72.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio J, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Muller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah NP, Kantarjian H and Investigators P. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latifi Y, Moccetti F, Wu M, Xie A, Packwood W, Qi Y, Ozawa K, Shentu W, Brown E, Shirai T, McCarty OJ, Ruggeri Z, Moslehi J, Chen J, Druker BJ, Lopez JA and Lindner JR. Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood. 2019;133:1597–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen T, Wang J, Shi Y, Qian H and Liu P. Inhibitors targeting Bruton's tyrosine kinase in cancers: drug development advances. Leukemia. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang CPS, McMullen J and Tam C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. 2018;59:1554–1564. [DOI] [PubMed] [Google Scholar]
- 76.Pal Singh S, Dammeijer F and Hendriks RW. Role of Bruton's tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu W, Tolar P, Song W and Kim TJ. Editorial: BCR Signaling and B Cell Activation. Front Immunol. 2020;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Muller S, Kantor AB, Herzenberg LA and et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–99. [DOI] [PubMed] [Google Scholar]
- 79.O'Rourke LM, Tooze R, Turner M, Sandoval DM, Carter RH, Tybulewicz VL and Fearon DT. CD19 as a membrane-anchored adaptor protein of B lymphocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity. 1998;8:635–45. [DOI] [PubMed] [Google Scholar]
- 80.Fruman DA, Ferl GZ, An SS, Donahue AC, Satterthwaite AB and Witte ON. Phosphoinositide 3-kinase and Bruton's tyrosine kinase regulate overlapping sets of genes in B lymphocytes. Proc Natl Acad Sci U S A. 2002;99:359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith EM, Gregg M, Hashemi F, Schott L and Hughes TK. Corticotropin Releasing Factor (CRF) activation of NF-kappaB-directed transcription in leukocytes. Cell Mol Neurobiol. 2006;26:1021–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Sayed ZA, Abramova I, Aldave JC, Al-Herz W, Bezrodnik L, Boukari R, Bousfiha AA, Cancrini C, Condino-Neto A, Dbaibo G, Derfalvi B, Dogu F, Edgar JDM, Eley B, El-Owaidy RH, Espinosa-Padilla SE, Galal N, Haerynck F, Hanna-Wakim R, Hossny E, Ikinciogullari A, Kamal E, Kanegane H, Kechout N, Lau YL, Morio T, Moschese V, Neves JF, Ouederni M, Paganelli R, Paris K, Pignata C, Plebani A, Qamar FN, Qureshi S, Radhakrishnan N, Rezaei N, Rosario N, Routes J, Sanchez B, Sediva A, Seppanen MR, Serrano EG, Shcherbina A, Singh S, Siniah S, Spadaro G, Tang M, Vinet AM, Volokha A and Sullivan KE. X-linked agammaglobulinemia (XLA):Phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ J. 2019;12:100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S and et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. [DOI] [PubMed] [Google Scholar]
- 84.Thomas JD, Sideras P, Smith CI, Vorechovsky I, Chapman V and Paul WE. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993;261:355–8. [DOI] [PubMed] [Google Scholar]
- 85.Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, Flowers ME, Logan AC, Nakamura R, Blazar BR, Li Y, Chang S, Lal I, Dubovsky J, James DF, Styles L and Jaglowski S. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF and O'Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown JR, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre SE, Tam CS, Mulligan SP, Jaeger U, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Thornton P, Caligaris-Cappio F, Delgado J, Montillo M, DeVos S, Moreno C, Pagel JM, Munir T, Burger JA, Chung D, Lin J, Gau L, Chang B, Cole G, Hsu E, James DF and Byrd JC. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burger JA. Bruton Tyrosine Kinase Inhibitors: Present and Future. Cancer J. 2019;25:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, Bairey O, Hillmen P, Coutre SE, Devereux S, Grosicki S, McCarthy H, Simpson D, Offner F, Moreno C, Dai S, Lal I, Dean JP and Kipps TJ. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res. 2019;144:19–50. [DOI] [PubMed] [Google Scholar]
- 91.Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, Damaj G, Doorduijn J, Lamy T, Morschhauser F, Panizo C, Shah B, Davies A, Eek R, Dupuis J, Jacobsen E, Kater AP, Le Gouill S, Oberic L, Robak T, Covey T, Dua R, Hamdy A, Huang X, Izumi R, Patel P, Rothbaum W, Slatter JG and Jurczak W. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romero D. Acalabrutinib - a new option in CLL. Nat Rev Clin Oncol. 2020;17:390. [DOI] [PubMed] [Google Scholar]
- 93.Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, Ji J, Xu W, Jin J, Lv F, Feng R, Gao S, Guo H, Zhou L, Elstrom R, Huang J, Novotny W, Wei R and Zhu J. Treatment of Patients with Relapsed or Refractory Mantle-Cell Lymphoma with Zanubrutinib, a Selective Inhibitor of Bruton's Tyrosine Kinase. Clin Cancer Res. 2020;26:4216–4224. [DOI] [PubMed] [Google Scholar]
- 94.Dhillon S. Tirabrutinib: First Approval. Drugs. 2020;80:835–840. [DOI] [PubMed] [Google Scholar]
- 95.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O'Brien S, Chiorazzi N and Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E and Fowler NH. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum K, Sharman JP, Wierda W, Zhao W, Heerema NA, Luan Y, Liu EA, Dean JP and O'Brien S. Ibrutinib Treatment for First-Line and Relapsed/Refractory Chronic Lymphocytic Leukemia: Final Analysis of the Pivotal Phase Ib/II PCYC-1102 Study. Clin Cancer Res. 2020;26:3918–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Zhang L, Baher L, Cheng M, Lee D, Beaupre DM and Rule S. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Shaw Y, Bilotti E, Zhou C, James DF and O'Brien S. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, Hillmen P and Investigators R. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, Hillmen P, Martin P, Awan FT, Stephens DM, Ghia P, Barrientos J, Pagel JM, Woyach JA, Burke K, Covey T, Gulrajani M, Hamdy A, Izumi R, Frigault MM, Patel P, Rothbaum W, Wang MH, O'Brien S and Furman RR. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135:1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L and Roberts AW. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laubach JP, Faber EA Jr., Voorhees P, Moslehi J, Varga C, Niculescu L, Anderson KC and Richardson PG. The challenge of cross-trial comparisons using limited data. Haematologica. 2014;99:e145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tam CS, Opat S, D'Sa S, Jurczak W, Lee HP, Cull G, Owen RG, Marlton P, Wahlin BE, Sanz RG, McCarthy H, Mulligan S, Tedeschi A, Castillo JJ, Czyz J, Fernandez de Larrea C, Belada D, Libby E, Matous JV, Motta M, Siddiqi T, Tani M, Trneny M, Minnema MC, Buske C, Leblond V, Trotman J, Chan WY, Schneider J, Ro S, Cohen A, Huang J and Dimopoulos M. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coutre SE, Byrd JC, Hillmen P, Barrientos JC, Barr PM, Devereux S, Robak T, Kipps TJ, Schuh A, Moreno C, Furman RR, Burger JA, O'Dwyer M, Ghia P, Valentino R, Chang S, Dean JP, James DF and O'Brien SM. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roeker LE, Sarraf Yazdy M, Rhodes J, Goodfriend J, Narkhede M, Carver J and Mato A. Hypertension in Patients Treated With Ibrutinib for Chronic Lymphocytic Leukemia. JAMA Netw Open. 2019;2:e1916326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahn IE. Cardiovascular adverse events of ibrutinib. Blood. 2019;134:1881–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang ML, Lee H, Chuang H, Wagner-Bartak N, Hagemeister F, Westin J, Fayad L, Samaniego F, Turturro F, Oki Y, Chen W, Badillo M, Nomie K, DeLa Rosa M, Zhao D, Lam L, Addison A, Zhang H, Young KH, Li S, Santos D, Medeiros LJ, Champlin R, Romaguera J and Zhang L. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17:48–56. [DOI] [PubMed] [Google Scholar]
- 109.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy MS, Pylypenko H, Loscertales J, Avigdor A, Rule S, Villa D, Samoilova O, Panagiotidis P, Goy A, Mato A, Pavlovsky MA, Karlsson C, Mahler M, Salman M, Sun S, Phelps C, Balasubramanian S, Howes A, Hallek M and investigators H. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–211. [DOI] [PubMed] [Google Scholar]
- 110.Caron F, Leong DP, Hillis C, Fraser G and Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1:772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mock J, Kunk PR, Palkimas S, Sen JM, Devitt M, Horton B, Portell CA, Williams ME and Maitland H. Risk of Major Bleeding with Ibrutinib. Clin Lymphoma Myeloma Leuk. 2018;18:755–761. [DOI] [PubMed] [Google Scholar]
- 112.Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR, Hillmen P, Stephens DM, Ghia P, Barrientos JC, Pagel JM, Woyach J, Johnson D, Huang J, Wang X, Kaptein A, Lannutti BJ, Covey T, Fardis M, McGreivy J, Hamdy A, Rothbaum W, Izumi R, Diacovo TG, Johnson AJ and Furman RR. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, Stephens DM, Woyach J, Bibikova E, Charuworn P, Frigault MM, Hamdy A, Izumi R, Linghu B, Patel P, Wang MH and Byrd JC. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Busygina K, Jamasbi J, Seiler T, Deckmyn H, Weber C, Brandl R, Lorenz R and Siess W. Oral Bruton tyrosine kinase inhibitors selectively block atherosclerotic plaque-triggered thrombus formation in humans. Blood. 2018;131:2605–2616. [DOI] [PubMed] [Google Scholar]
- 115.Lindner JR. Btk inhibitors in atherosclerosis. Blood. 2018;131:2601–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, van Hoek M, de Zwart E, Mittag D, Demont D, Verkaik S, Krantz F, Pearson PG, Ulrich R and Kaptein A. Acalabrutinib (ACP-196): A Covalent Bruton Tyrosine Kinase Inhibitor with a Differentiated Selectivity and In Vivo Potency Profile. J Pharmacol Exp Ther. 2017;363:240–252. [DOI] [PubMed] [Google Scholar]
- 117.Ratain MJ, Moslehi JJ and Lichter AS. Ibrutinib's Cardiotoxicity-An Opportunity for Postmarketing Regulation. JAMA Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 118.Kapoor I, Li Y, Sharma A, Zhu H, Bodo J, Xu W, Hsi ED, Hill BT and Almasan A. Resistance to BTK inhibition by ibrutinib can be overcome by preventing FOXO3a nuclear export and PI3K/AKT activation in B-cell lymphoid malignancies. Cell Death Dis. 2019;10:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okada M. Regulation of the SRC family kinases by Csk. Int J Biol Sci. 2012;8:1385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, Cole-Sinclair M, Reynolds J, Filshie R, Schischka S, Khot A, Sandhu S, Keating MJ, Nandurkar H and Tam CS. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783–7. [DOI] [PubMed] [Google Scholar]
- 121.Series J, Garcia C, Levade M, Viaud J, Sie P, Ysebaert L and Payrastre B. Differences and similarities in the effects of ibrutinib and acalabrutinib on platelet functions. Haematologica. 2019;104:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, Bordet JC, Tam C, Sie P, Ysebaert L and Payrastre B. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124:3991–5. [DOI] [PubMed] [Google Scholar]
- 123.Bye AP, Unsworth AJ, Vaiyapuri S, Stainer AR, Fry MJ and Gibbins JM. Ibrutinib Inhibits Platelet Integrin alphaIIbbeta3 Outside-In Signaling and Thrombus Stability But Not Adhesion to Collagen. Arterioscler Thromb Vasc Biol. 2015;35:2326–35. [DOI] [PubMed] [Google Scholar]
- 124.Rigg RA, Aslan JE, Healy LD, Wallisch M, Thierheimer ML, Loren CP, Pang J, Hinds MT, Gruber A and McCarty OJ. Oral administration of Bruton's tyrosine kinase inhibitors impairs GPVI-mediated platelet function. Am J Physiol Cell Physiol. 2016;310:C373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quek LS, Bolen J and Watson SP. A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol. 1998;8:1137–40. [DOI] [PubMed] [Google Scholar]
- 126.Atkinson BT, Ellmeier W and Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102:3592–9. [DOI] [PubMed] [Google Scholar]
- 127.Liu J, Fitzgerald ME, Berndt MC, Jackson CW and Gartner TK. Bruton tyrosine kinase is essential for botrocetin/VWF-induced signaling and GPIb-dependent thrombus formation in vivo. Blood. 2006;108:2596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu J, Zhang M and Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bye AP, Unsworth AJ, Desborough MJ, Hildyard CAT, Appleby N, Bruce D, Kriek N, Nock SH, Sage T, Hughes CE and Gibbins JM. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1:2610–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dobie G, Kuriri FA, Omar MMA, Alanazi F, Gazwani AM, Tang CPS, Sze DM, Handunnetti SM, Tam C and Jackson DE. Ibrutinib, but not zanubrutinib, induces platelet receptor shedding of GPIb-IX-V complex and integrin alphaIIbbeta3 in mice and humans. Blood Adv. 2019;3:4298–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, O'Brien S, Gribben J and Rai K. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janssen Biotech, Inc. Imbruvica (ibrutinib) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205552s030,210563s006lblPI.pdf Revised April 2020. Accessed April 9, 2021. [Google Scholar]
- 133.de Zwart L, Snoeys J, De Jong J, Sukbuntherng J, Mannaert E and Monshouwer M. Ibrutinib Dosing Strategies Based on Interaction Potential of CYP3A4 Perpetrators Using Physiologically Based Pharmacokinetic Modeling. Clin Pharmacol Ther. 2016;100:548–557. [DOI] [PubMed] [Google Scholar]
- 134.de Vries R, Smit JW, Hellemans P, Jiao J, Murphy J, Skee D, Snoeys J, Sukbuntherng J, Vliegen M, de Zwart L, Mannaert E and de Jong J. Stable isotope-labelled intravenous microdose for absolute bioavailability and effect of grapefruit juice on ibrutinib in healthy adults. Br J Clin Pharmacol. 2016;81:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou D, Podoll T, Xu Y, Moorthy G, Vishwanathan K, Ware J, Slatter JG and Al-Huniti N. Evaluation of the Drug-Drug Interaction Potential of Acalabrutinib and Its Active Metabolite, ACP-5862, Using a Physiologically-Based Pharmacokinetic Modeling Approach. CPT Pharmacometrics Syst Pharmacol. 2019;8:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ou YC, Tang Z, Novotny W, Tawashi M, Li TK, Coleman HA and Sahasranaman S. Evaluation of drug interaction potential of zanubrutinib with cocktail probes representative of CYP3A4, CYP2C9, CYP2C19, P-gp and BCRP. Br J Clin Pharmacol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lambert Kuhn E, Leveque D, Lioure B, Gourieux B and Bilbault P. Adverse event potentially due to an interaction between ibrutinib and verapamil: a case report. J Clin Pharm Ther. 2016;41:104–5. [DOI] [PubMed] [Google Scholar]
- 138.Lasica M and Tam CS. Management of Ibrutinib Toxicities: a Practical Guide. Curr Hematol Malig Rep. 2020;15:177–186. [DOI] [PubMed] [Google Scholar]
- 139.Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV and DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Finnes HD, Chaffee KG, Call TG, Ding W, Kenderian SS, Bowen DA, Conte M, McCullough KB, Merten JA, Bartoo GT, Smith MD, Leis J, Chanan-Khan A, Schwager SM, Slager SL, Kay NE, Shanafelt TD and Parikh SA. Pharmacovigilance during ibrutinib therapy for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in routine clinical practice. Leuk Lymphoma. 2017;58:1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, Robrecht S, Warburton S, Humphrey K, Samoylova O, Liberati AM, Pinilla-Ibarz J, Opat S, Sivcheva L, Le Du K, Fogliatto LM, Niemann CU, Weinkove R, Robinson S, Kipps TJ, Boettcher S, Tausch E, Humerickhouse R, Eichhorst B, Wendtner CM, Langerak AW, Kreuzer KA, Ritgen M, Goede V, Stilgenbauer S, Mobasher M and Hallek M. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med. 2019;380:2225–2236. [DOI] [PubMed] [Google Scholar]
- 142.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D and Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR and Clinical Pharmacogenetics Implementation C. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Neuvonen PJ, Niemi M and Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. [DOI] [PubMed] [Google Scholar]
- 145.Bellosta S, Paoletti R and Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109:III50–7. [DOI] [PubMed] [Google Scholar]
- 146.Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, Florido R, Gluckman TJ, Hucker WJ, Mehran R, Messe SR, Perino AC, Rodriguez F, Sarode R, Siegal DM and Wiggins BS. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594–622. [DOI] [PubMed] [Google Scholar]
- 147.Fudim M and Jones WS. New Curveball for Hypertension Guidelines? Circulation. 2018;138:1815–1818. [DOI] [PubMed] [Google Scholar]
- 148.Itoga NK, Tawfik DS, Lee CK, Maruyama S, Leeper NJ and Chang TI. Association of Blood Pressure Measurements With Peripheral Artery Disease Events. Circulation. 2018;138:1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miranda-Filho A, Pineros M, Ferlay J, Soerjomataram I, Monnereau A and Bray F. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol. 2018;5:e14–e24. [DOI] [PubMed] [Google Scholar]