Abstract
In this study, we report a case of catastrophic propeller brain injury with large scalp defect treated with omental flap reconstruction.
A 62-year-old man was accidentally caught in a powered paraglider propeller during maintenance. The rotor blades impacted the left part of his head. On arrival at the hospital, he presented with a Glasgow Coma Scale score of E4V1M4. On some areas on his head, skin was noticeably cut off, and the brain tissue out-slipped through an open skull fracture. Continuous bleeding from the superior sagittal sinus (SSS) and the brain surface was observed during emergency surgery. Massive bleeding from the SSS was controlled using a number of tenting sutures and hemostatic agents. We evacuated the crushed brain tissue and coagulated the severed middle cerebral arteries. Dural plasty using the deep fascia of the thigh was performed. The skin defect was closed using an artificial dermis. The administration of high-dose antibiotics has failed to prevent meningitis. Moreover, the severed skin edges and fasciae were necrotic. Plastic surgeons performed debridement and vacuum-assisted closure therapy to promote wound healing. Follow-up head computed tomography revealed hydrocephalus. Lumbar drainage was performed; however, sinking skin flap syndrome was observed. After removing the lumbar drainage, cerebrospinal fluid leakage occurred. We then performed cranioplasty with a titanium mesh and omental flap on day 31. After the surgery, perfect wound healing and infection control were achieved; however, severe disturbance of consciousness remained. The patient was transferred to a nursing home. Primary hemostasis and infection control are mandatory. An omental flap has been determined to be effective in controlling infection by covering the exposed brain tissue.
Keywords: open head trauma, powered paragliding propeller, omental flap reconstruction
Introduction
Accidents involving powered propellers may cause catastrophic injuries to the human body. Some cases of propeller-induced injuries have been reported; however, most cases are limited to the upper or lower extremities. To the best of our knowledge, there are very few case reports on brain injury with large scalp defect due to a propeller accident.1,2) In this case, we examined a case of catastrophic propeller brain injury with large scalp defect treated with omental flap. We also report our repeated surgical treatment and intensive care, along with a literature review.
Case Report
Informed consent was obtained from the patient's family.
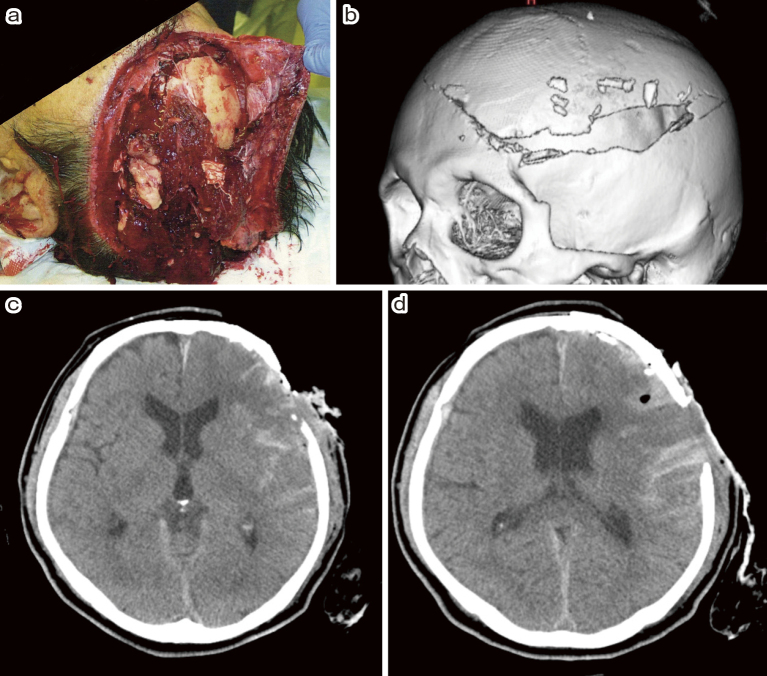
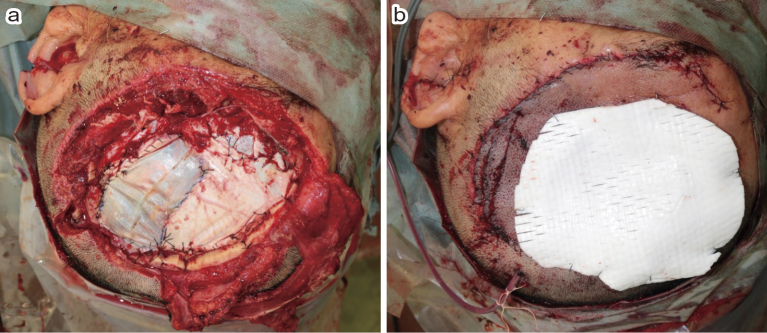
A 62-year-old man was accidentally caught in the propeller of a powered paraglider during maintenance. The rotor blades impacted the left part of this head, resulting in injury. He lost consciousness and was admitted to the emergency department. In the ambulance, the patient experienced hemorrhagic shock and was infused with saline. On arrival, he presented with a Glasgow Coma Scale (GCS) score of E4V1M4. On some parts of his head, skin was cut off, and the brain tissue out-slipped through an open skull fracture (Fig. 1a). No obvious trauma to other body parts was reported, except for a head injury. Computed tomography (CT) of the head showed a widely open skull fracture, brain contusion, and acute subdural hematoma (Fig. 1b-d). We assumed that the left hemisphere was widely damaged by the propeller blade and thus was contaminated. After the assessment, we rushed the patient into surgery to perform hemostasis and immediately evacuated the contaminated brain tissue. After craniotomy, we noted continuous bleeding from the superior sagittal sinus (SSS) and the brain surface. There were some acute subdural hematomas. We made dural tenting sutures along the SSS as much as possible and then sprayed gelatin-human thrombin absorbable hemostatic agent (Floseal, Baxter Healthcare Corporation Fremont, CA, USA). Thereafter, there was massive bleeding from the cutting plane of the middle cerebral artery cortical segment and the widely crushed brain tissue. The arteries were coagulated to stop the bleeding. We minimally stopped the bleeding and irrigated the contaminated brain tissue with 3000 mL of saline. Subsequently, plastic surgeons harvested deep fascia from the right thigh to close the dura mater because the latter was torn into pieces. We then performed dural plasty using fascia and fibrin glue (Fig. 2a). A part of the patient's skin was widely severed and lost. Plastic surgeons closed the skin defect with an artificial dermis (Terdermis, Olympus Terumo Biomaterials Corp., Tokyo, Japan) (Fig. 2b). Given the concerns about contamination, we aborted working on the patient's severed cranial bone and performed epidural drainage. The total blood loss was 2300 mL.
Fig. 1.
(a) The patient on arrival. On some parts of his head, skin was severed, and the brain tissue out-slipped through the open skull fracture.
(b), (c), (d) Head computed tomography (CT) showed widely open skull fracture, brain contusion, and acute subdural hematoma.
Fig. 2.
(a) Dural defect was reconstructed using the deep fascia of the thigh.
(b) Skin defect was reconstructed using an artificial dermis.
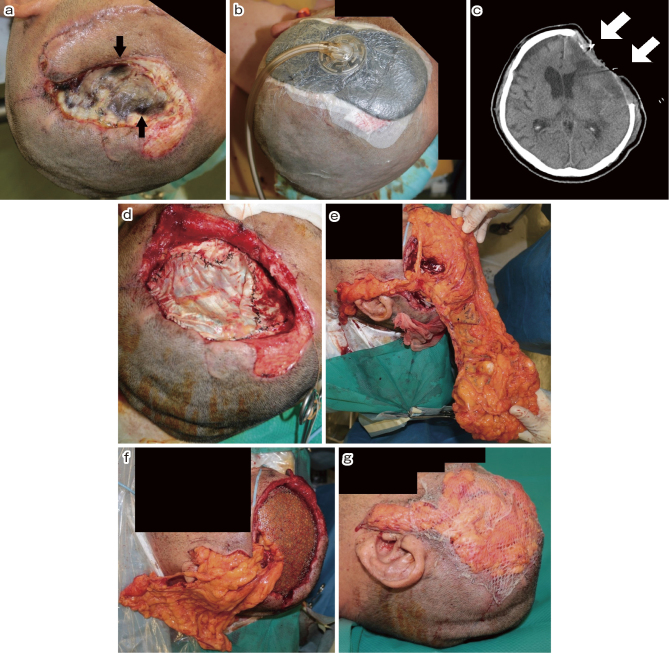
Postoperative head CT showed a small parenchymal hematoma in his left frontal lobe. Although we initiated antibiotic infusion during the surgery, the patient developed meningitis and encephalitis. Methicillin-resistant Staphylococcus lugdunensis was detected by wound and cerebrospinal fluid (CSF) culture. We continued infusion of vancomycin (2 g/day) and meropenem (6 g/day). However, the severed skin edges, fasciae, and the substitution of the dura mater were deemed necrotic (Fig. 3a). On day 22, plastic surgeons performed debridement and vacuum-assisted closure (VAC) therapy to promote wound healing (Fig. 3b). Follow-up head CT on day 16 showed ventriculomegaly and brain swelling at the decompression site, implying hydrocephalus. Lumbar drainage was performed on day 24; however, sinking skin flap syndrome (SSFS) was observed on day 25 (Fig. 3c). Although the lumbar drainage was removed, CSF leakage was noted from the skin defect.
Fig. 3.
(a) The severed skin edges, fasciae, and substitution of the dura mater were necrotic.
(b) After debridement, vacuum-assisted closure (VAC) therapy was performed to promote wound healing.
(c) After lumber drainage for hydrocephalus, sinking skin flap syndrome (SSFS) was observed (arrow).
(d) The dural defect was filled with the deep fascia of the thigh.
(e) The gastro-omental artery and vein were anastomosed to the superficial temporal artery and vein under a microscope, and an omental flap was then created to close the skin defect.
(f) Titanium mesh was wrapped with the omental flap, and extradural dead space was also filled with excessive omental flap.
(g) The surface of omental flap was covered with dermal graft from the thigh.
As a radical treatment for SSFS and CSF leakage, we performed cranioplasty with titanium mesh and omental flap closure.
First, neurosurgeons removed the infected brain tissue under a microscope on day 29. Thereafter, plastic surgeons filled the dural defect with the contralateral deep fascia of the thigh (Fig. 3d). Vancomycin (2 g/day) and meropenem (6 g/day) were administered for 1 month. We then switched from vancomycin to linezolid (1.2 g/day). On day 31, the cranial bone defect was filled with titanium mesh (Ultra Pre-Contoured Mesh, Lorenz Plating System Neuro, Zimmer Biomet, IN, USA). General surgeons harvested the omental flap while preserving the gastro-omental artery and vein. Then, the plastic surgeons anastomosed the gastro-omental artery and vein to the superficial temporal artery and vein under a microscope and created an omental flap to close the skin defect (Fig. 3e). The titanium mesh was wrapped with an omental flap, and the extradural dead space was filled with sufficient omental flap (Fig. 3f). The surface of the omental flap was then covered with a dermal graft from the thigh (Fig. 3g). Lumbar drainage was performed to control hydrocephalus. Coagulase-negative Staphylococcus was detected via brain tissue culture. After prolonged administration of antibiotics, meningitis and encephalitis were well controlled. We performed ventriculoperitoneal shunting on day 64 (Fig. 4a). Linezolid (1.2 g/day) and meropenem (6 g/day) were administered until day 86.
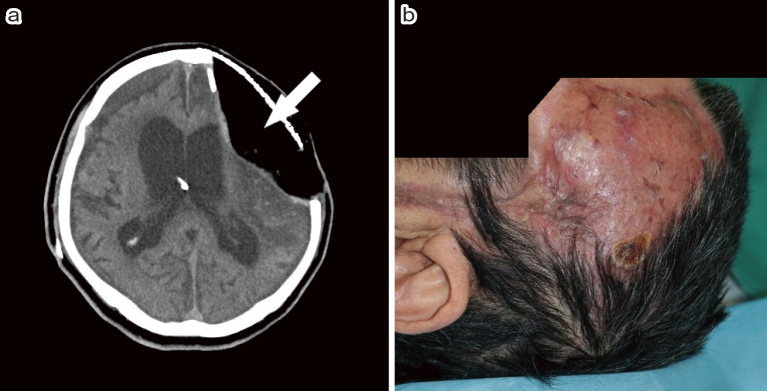
Fig. 4.
(a) The head computed tomography (CT) after ventriculoperitoneal (VP) shunt. Note that the extradural dead space was filled with the omental flap (arrow).
(b) Perfect wound healing was obtained 5 months after the reconstructive surgery.
The postoperative course was uneventful. There was no recurrence of meningitis or skin complication. Perfect wound healing was achieved with omental flap reconstruction. However, severe consciousness disturbance remained due to primary brain damage (Fig. 4b). The patient was transferred to a nursing home with a severe disability on day 156 (mRS = 5).
Discussion
To the best of our knowledge, this is the first case report on a patient who survived open head trauma with large scalp defect after sustaining injuries caused by a powered paraglider propeller. Very few surviving cases of open head trauma due to propeller injury have been reported.1-3) In surviving cases, primary skin closure was performed, although the contaminated skull fragment was removed and external decompression was performed. The biggest problems in our case were severe skin defects and exposure of the brain tissue to open air. Although the skin defect was covered with an artificial skin graft during the first surgery following postoperative high-dose antibiotic administration, the patient still developed meningitis and encephalitis. Isolation from open air was mandatory to control infection; therefore, VAC therapy was performed. The patient then developed hydrocephalus, and VAC therapy has failed to control CSF leakage. Lumbar drainage was performed for CSF leakage; however, the patient had SSFS.4,5) VAC therapy was applied to promote skin closure on other parts of the body; however, the exposed brain tissue was affected by the atmosphere.6) Therefore, VAC therapy, which actually resulted in SSFS, was deemed not a suitable choice in our case. Cranioplasty with hard tissue is mandatory to control SSFS.5,7) After thorough discussions with plastic surgeons, we performed cranioplasty with a titanium mesh and omental flap. Complete closure and perfect isolation of brain tissue from the open air were performed, and infection control was achieved.
Generally, the implantation of artificial instruments is not recommended until full infection control is achieved. According to a previous report, cranioplasty with titanium mesh was performed 3 years after the primary surgery.1) We performed cranioplasty using an artificial instrument before infection control was fully achieved. There are several options for the skin flap method, such as split rib, split calvarial bone, rectus abdominis flap, and omental flap.8) Different from other reconstructive options, the omental flap is known to have an abundant blood supply and fat tissue, which enables the phylactic system. Omental flap is widely used as an effective biomaterial in defect reconstruction. Large osteomyelitis-related cranial defects treated with omental flap and titanium mesh have been reported.9,10) Therefore, we chose an omental flap instead of other reconstruction options.
In our case, plastic surgeons wrapped the titanium plate with an omental flap, and the extradural dead space was filled with sufficient omental flap to control the infection. We believe that almost all parts of the artificial instrument were covered with an omental flap, which enabled infection control.
Five months after the surgery, excellent wound healing was achieved. The patient experienced primary brain damage in the dominant hemisphere; therefore, the final neurological outcome was severe. The patient would have had a better neurological outcome if the primary damage was limited to the minor hemisphere. Our treatment strategy can be applied to other brain injuries with large scalp defect to facilitate wound healing and infection control. However, this strategy cannot be performed without plastic surgeons. Therefore, immediate consultation and cooperation with plastic surgeons are mandatory.
Conclusion
We report a case of catastrophic brain injury with large scalp defect due to a propeller accident. Primary hemostasis and infection control are mandatory. An omental flap is effective in controlling infection as it covers the exposed brain tissue.
Details of the Previous Presentation
The 100th Meeting of the Japan Neurosurgical Society Chubu Division, 2021/9/18, Aichi, Japan, oral presentation.
Abbreviations
CSF; cerebrospinal fluid
CT; computed tomography
GCS; Glasgow Coma Scale
SSFS; sinking skin flap syndrome
SSS; superior sagittal sinus
VAC; vacuum-assisted closure
Funding
This study received no funding or financial support.
Conflicts of Interest Disclosure
The authors declare no conflict of interest. All authors belonging to the Department of Neurosurgery registered online self-reported COI disclosure statement forms through the website of the Japan Neurosurgical Society members.
Acknowledgments
We sincerely appreciate Dr. Takashi Kato and Dr. Takahiro Mori, Department of Plastic Surgery, JCHO Chukyo Hospital, for their cooperation in treating this case.
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1). Hsieh CC, Chan PY, Liou LR, et al. : A case of penetrating head wound due to helicopter rotor blade injury in a 34-year-old naval helicopter pilot who returned to active service 5 years later. Am J Case Rep 22: e933862, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Dhall SS, Lin FJ, Tumialan LM, Mapstone TB: Significant neurologic recovery following a catastrophic open head injury from a motorboat propeller: case illustration. J Trauma 65: 249-250, 2008 [DOI] [PubMed] [Google Scholar]
- 3). Gibbons JR, Orr L, Carr DJ, Gibbons CE: Helicopter main rotor blade injury to the head with survival. J R Army Med Corps 143: 122-123, 1997 [DOI] [PubMed] [Google Scholar]
- 4). Chugh A, Punia P, Gotecha S: Sinking skin flap syndrome following posttraumatic hydrocephalus. Case Rep Neurol Med 2021: 6682310, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Annan M, De Toffol B, Hommet C, Mondon K: Sinking skin flap syndrome (or syndrome of the trephined): a review. Br J Neurosurg 29: 314-318, 2015 [DOI] [PubMed] [Google Scholar]
- 6). Ford CN, Reinhard ER, Yeh D, et al. : Interim analysis of a prospective, randomized trial of vaccum-assisted closure versus the healthpoint system in the manegement of pressure ulcers. Ann Plast Surg 49: 55-61, 2002 [DOI] [PubMed] [Google Scholar]
- 7). Kugisaki A, Kimura T, Yano T, Ichi S: Reconstruction of the temporal line for sinking skin flap syndrome using split rib grafts: a technical note. Br J Neurosurg 28: 1-3, 2021[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8). Sugawara Y, Harii K, Yamada A, et al. : Reconstruction of skull defects with vascularized omentum transfer and split calvarial bone graft: two case report. J Reconstr Microsurg 14: 101-108, 1998 [DOI] [PubMed] [Google Scholar]
- 9). Liebermann-Meffert D: The greater omentum. Anatomy, embryology, and surgical application. Surg Clin Am 80: 275-293, 2000 [DOI] [PubMed] [Google Scholar]
- 10). Asai S, Kamei Y, Torii S: One-stage reconstruction of infected cranial defects using a titanium mesh plate enclosed in an omental flap. Ann Plast Surg 52: 144-147, 2004 [DOI] [PubMed] [Google Scholar]