Abstract
Dietary mono-unsaturated fatty acids (MUFAs) are linked to longevity in several species. But the mechanisms by which MUFAs extend lifespan remain unclear. Here we show that an organelle network involving lipid droplets and peroxisomes is critical for MUFA-induced longevity in Caenorhabditis elegans. MUFAs upregulate the number of lipid droplets in fat storage tissues. Increased lipid droplet number is necessary for MUFA-induced longevity and predicts remaining lifespan. Lipidomics datasets reveal that MUFAs also modify the ratio of membrane lipids and ether lipids—a signature associated with decreased lipid oxidation. In agreement with this, MUFAs decrease lipid oxidation in middle-aged individuals. Intriguingly, MUFAs upregulate not only lipid droplet number but also peroxisome number. A targeted screen identifies genes involved in the co-regulation of lipid droplets and peroxisomes, and reveals that induction of both organelles is optimal for longevity. Our study uncovers an organelle network involved in lipid homeostasis and lifespan regulation, opening new avenues for interventions to delay aging.
Subject terms: Cell biology, Genetics
Papsdorf et al. show that mono-unsaturated fatty acids extend lifespan of C. elegans through induction of peroxisomes and lipid droplets in fat tissues and of a lipid signature predictive of decreased lipid oxidation.
Main
Lifespan is strongly influenced by diet. Although high-fat diets are mostly detrimental to lifespan, specific lipids can be beneficial for health and longevity1–9. Diets rich in mono-unsaturated fatty acids (MUFAs), such as olive oil in the Mediterranean diet, are correlated with longevity in humans8,10 and promote longevity in rodents11. Specific MUFAs (for example, oleic acid and palmitoleic acid) also causally extend the lifespan of invertebrate species such as Caenorhabditis elegans7,12–16. Yet the mechanism by which some lipids promote longevity, while others are detrimental for health, remain unclear.
Attractive candidates for the mechanism of MUFA action are conserved organelles involved in fat storage and metabolism, such as lipid droplets17–19. Although the role of lipid droplets has started to be evaluated during aging, age-related diseases and the response to stressors in different species20–30, it is still unclear whether these organelles are positive or negative regulators of health. Furthermore, the importance and mode of action of lipid droplets in MUFA-mediated longevity remain largely unknown.
Results
MUFA accumulation upregulates the number of intestinal lipid droplets
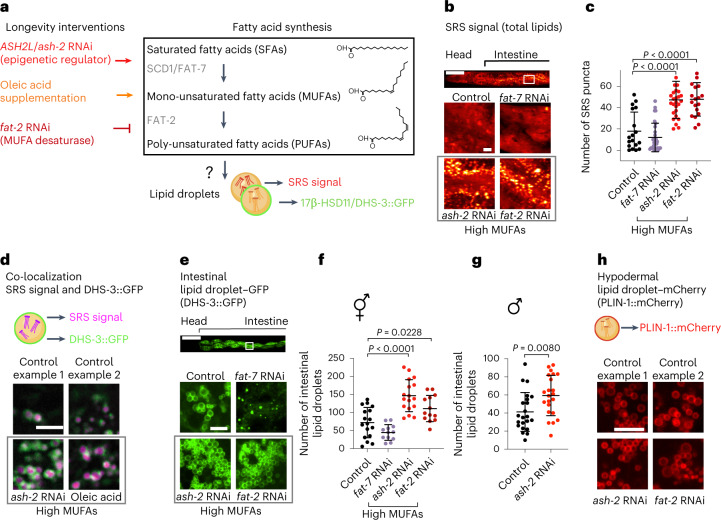
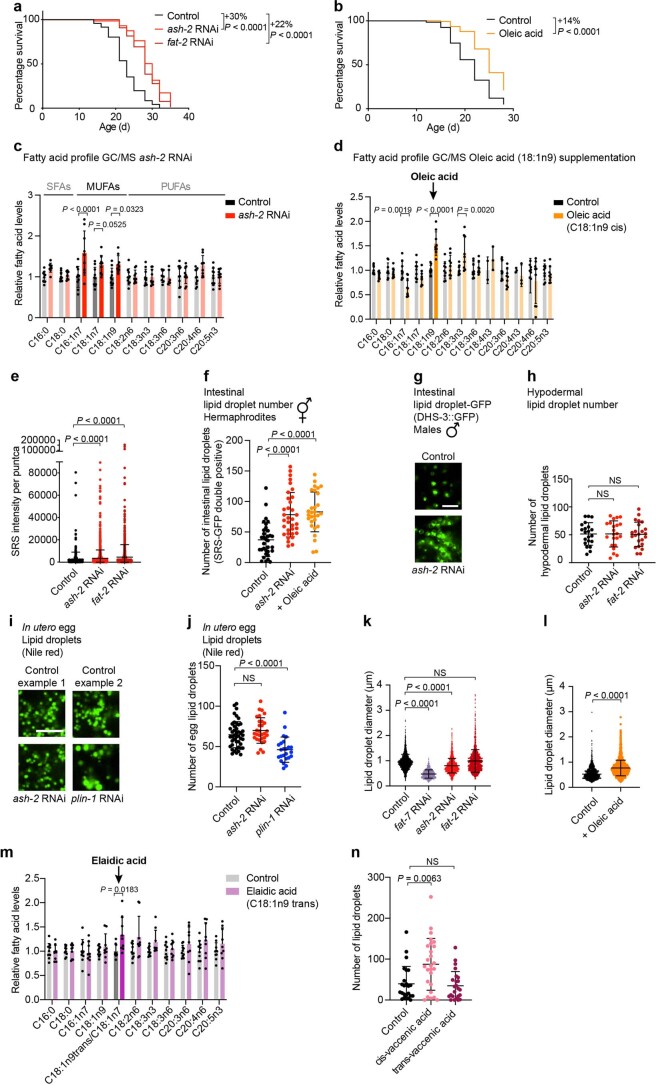
We investigated whether MUFAs influence lipid droplets in C. elegans. To assess the number of lipid droplets, we first used stimulated Raman scattering (SRS)—a spectroscopy method that enables label-free imaging of lipids by visualizing carbon-hydrogen bonds31–35. MUFA accumulation was induced by upregulating SCD1 (FAT-7 in C. elegans), the enzyme that produces MUFAs36,37 (via knockdown of the chromatin regulator ASH2L (ash-2 in C. elegans)13), or downregulating FAT-2, the enzyme that catabolizes MUFAs13,14,37,38 (Fig. 1a). We verified that these manipulations led to lifespan extension and MUFA accumulation (Extended Data Fig. 1a–d)13,14,38,39. Interestingly, MUFA accumulation by ash-2 or fat-2 RNA interference (RNAi) resulted in an increased number of puncta with high SRS intensity in intestinal cells, the main fat storage cells in C. elegans (Fig. 1b,c). These puncta were not only more numerous but also had higher intensity following MUFA accumulation (Extended Data Fig. 1e).
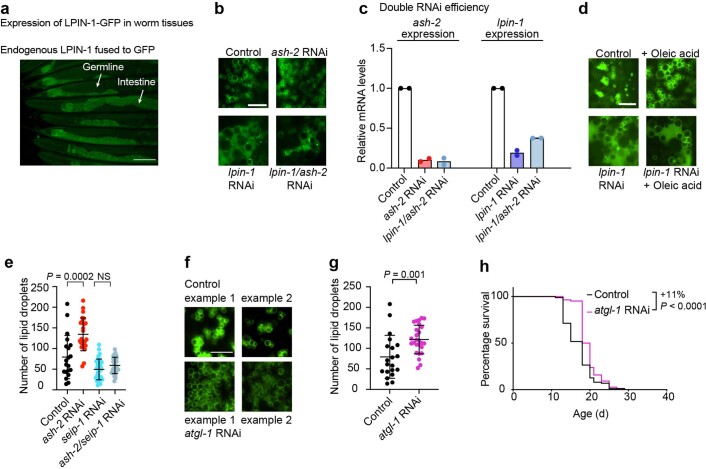
Fig. 1. MUFAs upregulate the number of lipid droplets in the intestine.
a, Schematic of genetic and dietary interventions that lead to MUFA accumulation in C. elegans. Mammalian gene names are indicated first. In all figures and panels, worms are hermaphrodites (female-like), except for Fig. 1g and Extended Data Fig. 1g, where males are used. b,c, Intestinal puncta, assessed by SRS microscopy, following MUFA accumulation. b, SRS image of total lipids in the anterior part of one worm (head and intestine; top). Zoomed-in images of the intestine (bottom). Scale bars, 100 µm (top) and 5 µm (bottom). c, Number of intestinal puncta in n = 18, 30, 27 and 19 worms treated with control, fat-7, ash-2 and fat-2 RNAi, respectively. Puncta intensities are provided in Extended Data Fig. 1e. d, Intestinal lipid droplets, assessed by SRS microscopy, in worms expressing the lipid droplet protein DHS-3 fused to GFP driven by the endogenous dhs-3 promoter (intestinal expression; dhs-3p::dhs-3::GFP) following MUFA accumulation. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet numbers, as assessed by double-positive puncta, are provided in Extended Data Fig. 1f. e,f, Number of intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms following MUFA accumulation. e, Fluorescence image of the anterior part of one worm (head and intestine; top). Zoomed-in fluorescence images of the intestine (bottom). Scale bars, 100 µm (top) and 5 µm (bottom). f, Number of lipid droplets in n = 17, 12, 17 and 13 worms treated with control, fat-7, ash-2 and fat-2 RNAi, respectively. g, Number of intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms (n = 22 male worms for each condition) following MUFA accumulation. h, Hypodermal lipid droplet number—assessed by fluorescence in worms expressing the lipid droplet protein PLIN-1 fused to mCherry driven by the endogenous plin-1 promoter (ubiquitous expression; plin-1p::plin-1::mCherry)—following MUFA accumulation. Zoomed-in images of the hypodermis. Scale bar, 5 µm. Lipid droplet numbers are provided in Extended Data Fig. 1h. Elements of a, d and h are created with BioRender.com. c,f,g, Data are representative of three (c,f) or two (g) independent experiments. Each dot represents the number of puncta in a 26 × 26 µm2 area in the intestine of an individual worm. Data are the mean ± s.d. P values were determined using a two-tailed Mann–Whitney test. Source data are provided.
Extended Data Fig. 1. Perturbations that increase MUFAs and their effect on lipid droplets.
a, ash-2 or fat-2 depletion extends lifespan; n ≥ 92 worms for each condition. Percentage of median lifespan extension and P values are indicated. P values: log-rank Mantel–Cox test. b, Dietary oleic acid supplementation extends lifespan; n ≥ 135 worms for each condition. Analysis as in a. c, Fatty acid profile by GC–MS following ash-2 depletion. Fatty acid levels were normalized to the control condition. Data are the mean ± s.d. of three independent experiments, each with three biological replicates. Significant P values are shown. P values: two-way ANOVA with Bonferroni’s multiple comparison test. d, Fatty acid profile by GC–MS following oleic acid supplementation. Analysis as in c. e, Puncta intensity, quantified by SRS microscopy; n = 420, 1,603 and 927 puncta in ≥18 worms treated with control, ash-2 RNAi and fat-2 RNAi, respectively. Data are the mean ± s.d. Each dot represents the SRS signal intensity of one puncta. Lower segment of the y axis displays values of 0–90,000; upper segment of the y axis of 90,000–200,000. P values: two-tailed Mann–Whitney test. f, Quantification of intestinal lipid droplet number (double-positive puncta), measured by SRS microscopy, in dhs-3p::dhs-3::GFP worms following MUFA accumulation; n = 33, 34 and 29 worms treated with control and ash-2 RNAi, and following oleic acid supplementation, respectively. Data are the mean ± s.d. Each dot represents the lipid droplet number in a 26 × 26 µm2 area in the intestine of an individual worm. P values: two-tailed Mann–Whitney test. Lipid droplets shown in Fig. 1d. g, Intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms (males) following ash-2 depletion. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet number quantified in Fig. 1g. h, Quantification of hypodermal lipid droplet number, measured by fluorescence, in plin-1p::plin-1::mCherry worms following MUFA accumulation; n = 21, 22 and 23 worms treated with control, ash-2 and fat-2 RNAi, respectively. Data are the mean ± s.d. Each dot represents the lipid droplet number in a 15 × 15 µm2 area of an individual worm. Lipid droplets shown in Fig. 1h. P values: two-tailed Mann–Whitney test. i,j, Lipid droplets in eggs (in utero), assessed by Nile red fluorescence, following ash-2 depletion. i, Zoomed-in images of one egg per condition. Scale bar, 5 µm. j, Quantification of lipid droplet number; n = 45, 30 and 24 eggs in ≥15 worms treated with control, ash-2 and plin-1 RNAi, respectively. Data are the mean ± s.d. Each dot represents the lipid droplet number in a 16 × 16 µm2 area of an individual egg. P values: two-tailed Mann–Whitney test. k,l, Lipid droplet size, measured by fluorescence, in dhs-3p::dhs-3::GFP worms following MUFA accumulation. k, Quantification of lipid droplet diameter; n = 1,720, 875, 1,803 and 1,550 lipid droplets in ≥14 worms treated with control, fat-7, ash-2 and fat-2 RNAi, respectively. Data are the mean ± s.d. Each dot represents the diameter of one lipid droplet. P values: two-tailed Mann–Whitney test. l, Quantification of lipid droplet diameter; n = 1,299 and 2,168 lipid droplets in ≥20 worms following control and oleic acid supplementation, respectively. Analysis as in k. m, Fatty acid profile by GC–MS following elaidic acid supplementation. Data are the mean ± s.d of two independent experiments, each with three biological replicates. Analysis as in c. n, Quantification of intestinal lipid droplet number, measured by fluorescence, in dhs-3p::dhs-3::GFP worms following dietary cis- or trans-vaccenic acid supplementation; n = 24, 26 and 24 worms following control, and dietary cis- and trans-vaccenic acid supplementation, respectively. Analysis as in f. a, Representative of three independent experiments. b, Representative of four independent experiments. e,f,h,j,k,l,n, Representative of two independent experiments. Source numerical data of all experiments, replicates and statistics are provided.
We next used confocal microscopy with a transgenic strain that expresses the lipid droplet membrane protein dehydrogenase-3 (DHS-3) fused to green fluorescent protein (GFP), which has been used to assess lipid droplets in C. elegans40–42. The DHS-3 protein is orthologous to 17β-HSD11 in mammals43. We verified that the puncta identified by SRS signal colocalized with GFP fluorescence40 (Fig. 1d), indicating that they are lipid droplets. Quantification of fluorescence in the DHS-3::GFP strain confirmed that MUFA enrichment increased the number of lipid droplets in the intestine, the main fat storage tissue in C. elegans (Fig. 1d–f and Extended Data Fig. 1f).
Both hermaphrodites (female-like) and male C. elegans had increased numbers of lipid droplets in the intestine following MUFA accumulation (Fig. 1g and Extended Data Fig. 1g), showing that the effect of MUFAs on the lipid droplet number in the intestine generalizes across sexes. Although MUFA accumulation increased the number of lipid droplets in the intestine, it did not affect the number of lipid droplets in the hypodermis (skin) or eggs (progeny; Fig. 1h and Extended Data Fig. 1h–j), which indicates tissue specificity for lipid droplet increase following MUFA accumulation. Finally, unlike lipid droplet number, the lipid droplet size was not affected uniformly by MUFA accumulation (Extended Data Fig. 1k,l). Thus, endogenous MUFA accumulation consistently increases the number of lipid droplets—organelles involved in lipid storage and metabolism—in the intestine of C. elegans.
Cis-MUFA supplementation increases lipid droplet number
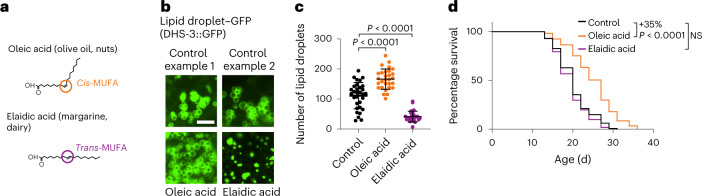
We investigated how dietary supplementation of MUFAs impacts the number of lipid droplets. Dietary supplementation with oleic acid, a cis-MUFA present in olive oil and nuts, upregulated the intestinal lipid droplet number and extended lifespan (Fig. 2a–d). In contrast, dietary supplementation with elaidic acid, a trans-MUFA present in margarine and dairy known to have detrimental effects on human health44, decreased the number of lipid droplets and did not extend lifespan (Fig. 2a–d, Extended Data Fig. 1m). In agreement with this, cis-vaccenic acid (a cis-MUFA that extends lifespan13), but not trans-vaccenic acid, increased the lipid droplet number (Extended Data Fig. 1n). Hence, cis-MUFAs (but not trans-MUFAs) increase the number of lipid droplets, which correlates with lifespan extension.
Fig. 2. Supplementation with cis-MUFA (oleic acid) but not trans-MUFA (elaidic acid) increases lipid droplet number and extends lifespan.
a, Chemical structure of the cis-MUFA oleic acid and the trans-MUFA elaidic acid. b,c, Number of intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms following supplementation with sterically different dietary MUFAs. b, Zoomed-in images of the intestine. Scale bar, 5 µm. c, Number of lipid droplets in n = 31, 29 and 28 worms following control, dietary oleic acid and dietary elaidic acid supplementation, respectively. Data are the mean ± s.d. Each dot represents the number of puncta in a 26 × 26 µm2 area in the intestine of an individual worm. P values were determined using a two-tailed Mann–Whitney test. d, Cis-MUFA (oleic acid), but not trans-MUFA (elaidic acid), extends lifespan; n ≥ 128 worms for each condition. Percentages of the median lifespan extension and P values (log-rank Mantel–Cox test) are indicated; NS, not significant. c,d, Data are representative of three (d) or four (c) independent experiments. Source data are provided.
Increased lipid droplet number is critical for longevity
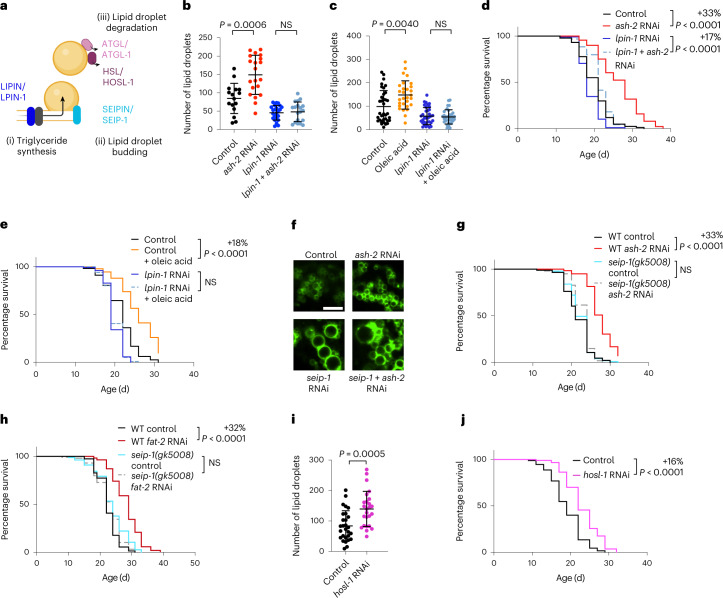
We investigated whether the increase in the number of lipid droplets is necessary for MUFA-induced lifespan extension. Lipid droplet organelles are generated by a process involving conserved proteins (Fig. 3a). We targeted LIPIN1 (lpin-1 in C. elegans), which is important for lipid droplet synthesis45–50 (Fig. 3b), among other functions in lipid homeostasis51–54, and is expressed in the intestine (Extended Data Fig. 2a). lpin-1 RNAi knockdown resulted in fewer lipid droplets in basal conditions and prevented the increase in lipid droplets due to MUFA accumulation (Fig. 3b,c and Extended Data Fig. 2b,d). Interestingly, lpin-1 deficiency blunted longevity by MUFA accumulation due to ash-2 depletion (Fig. 3d) or oleic acid supplementation (Fig. 3e).
Fig. 3. Increased lipid droplet number is necessary for MUFA-induced longevity and sufficient to extend lifespan.
a, Schematic of conserved proteins involved in lipid droplet synthesis and degradation. Mammalian protein names are indicated first. Created with BioRender.com. b,c, Number of intestinal lipid droplets, measured by fluorescence, in dhs-3p::dhs-3::GFP worms following lpin-1 depletion and MUFA accumulation. b, Number of lipid droplets in n = 16, 20, 23 and 19 worms treated with control, ash-2, lpin-1, and ash-2 + lpin-1 RNAi, respectively (Extended Data Fig. 2c for the efficiency of the double knockdown). c, Number of lipid droplets in n = 35, 29, 35 and 32 worms treated with control or dietary oleic acid supplementation in the absence or presence of lpin-1 RNAi, respectively. d, lpin-1 is necessary for longevity following ash-2 depletion; n ≥ 96 worms for each condition. e, lpin-1 is necessary for longevity following dietary supplementation with oleic acid; n ≥ 105 worms for each condition. f, Number of intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms following seip-1 and ash-2 depletion. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet numbers are provided in Extended Data Fig. 2e. g, seip-1(gk5008) is necessary for longevity following ash-2 depletion; n ≥ 64 worms for each condition. h, seip-1(gk5008) is necessary for longevity following fat-2 depletion; n ≥ 90 worms for each condition. i, Number of intestinal lipid droplets, measured by fluorescence, in dhs-3p::dhs-3::GFP worms following hosl-1 depletion; n = 28 and 25 worms treated with control and hosl-1 RNAi, respectively. b,c,i, Each dot represents the number of lipid droplets in a 26 × 26 µm2 area of the intestine of an individual worm. Data are the mean ± s.d. P values were determined using a two-tailed Mann–Whitney test. j, hosl-1 depletion is sufficient to extend lifespan; n ≥ 94 worms for each condition. d,e,g,h,j, Percentages of median lifespan extension and P values are indicated. P values were determined using a log-rank Mantel–Cox test. b–e,g–j, Data are representative of three (b–e,g,i,j) or two (h) independent experiments. NS, not significant; WT, wild-type worms. Source data are provided.
Extended Data Fig. 2. Lipid droplet perturbations that are important for longevity.
a, Endogenous LPIN-1 expression, measured by fluorescence of GFP tagged to the C-terminus of LPIN-1 at the endogenous lpin-1 locus. Fluorescent images of the anterior and posterior parts of young adult lpin-1(wbm76[lpin-1::GFP]) worms. Scale bar, 100 µm. b, Intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP transgenic worms following lpin-1 and ash-2 depletion. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet number is quantified in Fig. 3b. c, RNAi efficiency in conditions using two RNAi constructs. RT–qPCR on RNA extracted from worms treated with control, ash-2, lpin-1 and ash-2 + lpin-1 RNAi. The mRNA levels of target genes relative to act-1 mRNA levels were normalized to the empty vector controls. Data are the mean of two independent experiments, with three replicates each. d, Intestinal lipid droplets, assessed by fluorescence, in dhs-3p::dhs-3::GFP transgenic worms following lpin-1 depletion and oleic acid supplementation. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet number is quantified in Fig. 3c. e, Quantification of intestinal lipid droplet number, measured by fluorescence, in dhs-3p::dhs-3::GFP worms following seip-1 and ash-2 depletion; n = 20, 25, 31 and 28 worms treated with control, ash-2, seip-1, and ash-2 + seip-1 RNAi, respectively. Data are the mean ± s.d. Each dot represents the lipid droplet number in a 26 × 26 µm2 area in the intestine of an individual worm. P values: two-tailed Mann–Whitney test. Lipid droplets shown in Fig. 3f. f,g, Intestinal lipid droplet number, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms following atgl-1 depletion. f, Zoomed-in images of the intestine. Scale bar, 5 µm. g, Quantification of lipid droplet number; n = 20 and 26 worms treated with control and atgl-1 RNAi, respectively. Analysis as in e. h, Atgl-1 knockdown extends lifespan; n ≥ 108 worms for each condition. The percentage of median lifespan extension and P values are indicated. P values: log-rank Mantel–Cox test. a,e,h, Representative of two independent experiments. g, Representative of three independent experiments. Source numerical data of all experiments, replicates and statistics are provided.
We also targeted SEIPIN (seip-1 in nematodes), which is implicated in the early steps of lipid droplet biogenesis55–59. seip-1 knockdown abolished the increase in lipid droplet number in response to MUFA accumulation by ash-2 RNAi (and led to heterogeneous lipid droplets, with a few very large droplets and some small droplets; Fig. 3f and Extended Data Fig. 2e). Consistent with these data, seip-1 mutants no longer exhibited lifespan extension in response to MUFA accumulation by ash-2 or fat-2 RNAi (Fig. 3g,h). Thus, an increase in the number of lipid droplets is necessary for MUFA-induced longevity; however, other lipid droplet characteristics (for example, heterogeneity) or other aspects of lipid metabolism could also contribute.
Conversely, depletion of HSL (hosl-1 in nematodes) or ATGL (atgl-1 in nematodes), which are involved in lipid droplet hydrolysis (Fig. 3a)19,60–62, led to an increase in lipid droplets and a slight but significant lifespan extension (Fig. 3i,j and Extended Data Fig. 2f–h). Hence, an increase in the number of lipid droplets is sufficient to extend lifespan, suggesting a beneficial impact of this organelle on lifespan.
Lipid droplet number predicts remaining lifespan
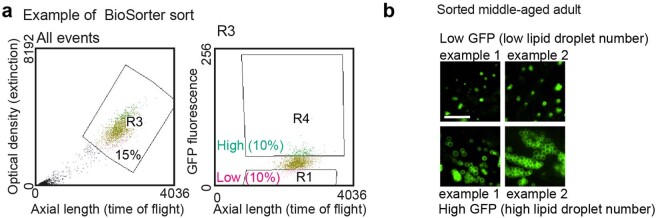
Could the number of lipid droplets in young or middle-aged individuals predict their remaining lifespan? To address this question, we assessed the lifespan of genetically identical individuals in a population of C. elegans with varying amounts of lipid droplets. We used a large-particle BioSorter63,64 to sort two subpopulations of young adult worms expressing high or low levels of fluorescence of lipid droplet marker fused to GFP (Fig. 4a and Extended Data Fig. 3a). We verified that the worms with higher fluorescence had more lipid droplets compared with those with lower fluorescence (Fig. 4b,c). Interestingly, young individuals with more lipid droplets lived slightly but significantly longer than their counterparts with fewer lipid droplets (Fig. 4d). The predictive power of lipid droplet number for longevity was even more evident in middle-aged individuals: middle-aged individuals with more lipid droplets lived 33% longer than their counterparts with fewer lipid droplets (Fig. 4e,f and Extended Data Fig. 3b). Thus, increased numbers of lipid droplets in young or middle-aged individuals predicts their remaining lifespan, corroborating the positive role of lipid droplet number for longevity.
Fig. 4. Increased numbers of lipid droplets in young or middle-aged individuals is predictive of a long life.
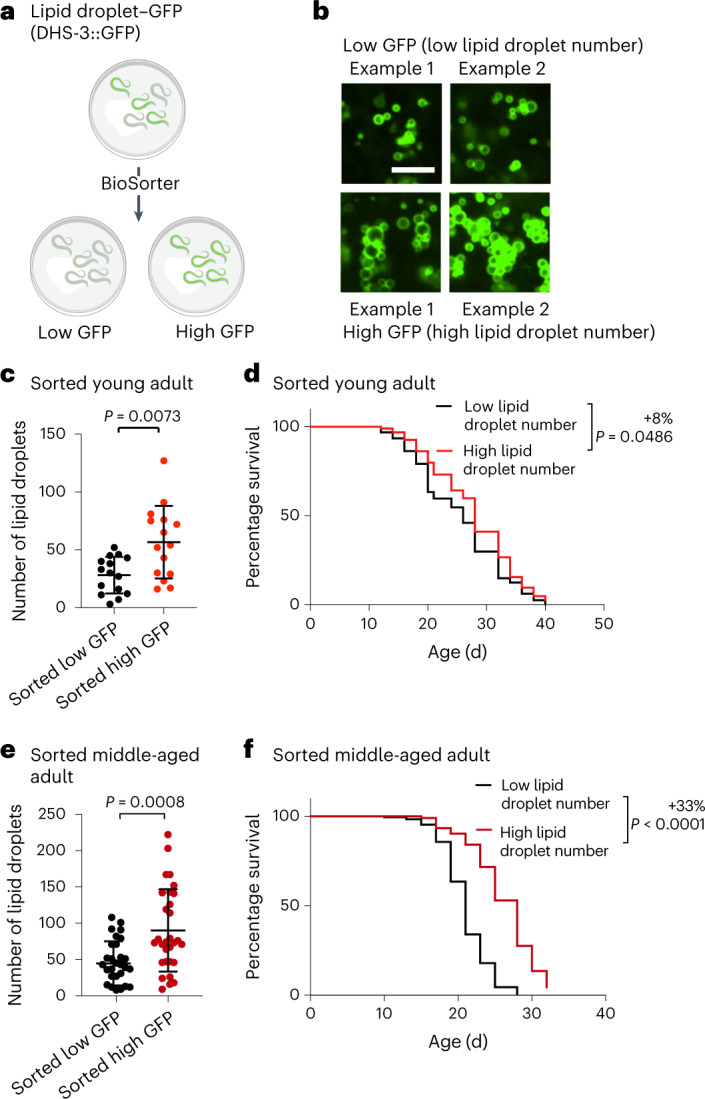
a, Experimental set-up for sorting worms according to the fluorescence intensity of the DHS-3::GFP lipid droplet reporter in dhs-3p::dhs-3::GFP worms using a large-particle BioSorter. Created with BioRender.com. b,c, Number of intestinal lipid droplets, assessed by fluorescence, in a synchronized population of young adult (adult day 1) dhs-3p::dhs-3::GFP worms after sorting using a BioSorter. b, Zoomed-in images of the intestine. Scale bar, 5 µm. c, Number of lipid droplets in n = 15 worms for each condition. d, Worms sorted at young-adult age (adult day 1) with high numbers of lipid droplets live longer than worms with low numbers of lipid droplets; n ≥ 117 worms for each condition. e, Number of intestinal lipid droplets, measured by fluorescence, in a synchronized population of middle-aged (adult day 6) dhs-3p::dhs-3::GFP worms after manual sorting; n = 30 worms for each condition. Zoomed-in images are provided in Extended Data Fig. 3b. c,e, Each dot represents the number of lipid droplets in a 26 × 26 µm2 area of the intestine of an individual worm. Data are the mean ± s.d. P values were determined using a two-tailed Mann–Whitney test. f, Worms sorted at middle age (adult day 6) with high numbers of lipid droplets live longer than worms with low numbers of lipid droplets; n ≥ 195 for each condition. d,f, Percentages of the median lifespan extension and P values, determined using a log-rank Mantel–Cox test, are indicated. c–f, Data are representative of two (c,d) or three (e,f) independent experiments. Source data are provided.
Extended Data Fig. 3. BioSorter gating strategy and sorting example.
a, BioSorter sorting example on a synchronized population of adult dhs-3p::dhs-3::GFP worms according to their GFP fluorescence intensity (as a proxy for lipid droplet number). Optical density (extinction) and axial length (time of flight) of all particles measured by the sorter (left). Gate R3 was used to exclude bacterial debris and eggs and enrich for adult worms (15% of all particles). Individual worms from Gate R3 were sorted by GFP fluorescence intensity to separate the highest 10% fluorescent worms (Gate R4; right) and the lowest 10% fluorescent worms (Gate R1; right). b, Intestinal lipid droplet number, assessed in a synchronized population of middle-aged (adult day 6) dhs-3p::dhs-3::GFP worms, after manual sorting. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet number is quantified in Fig. 4e.
Lipidomics analysis of MUFA-enriched worms
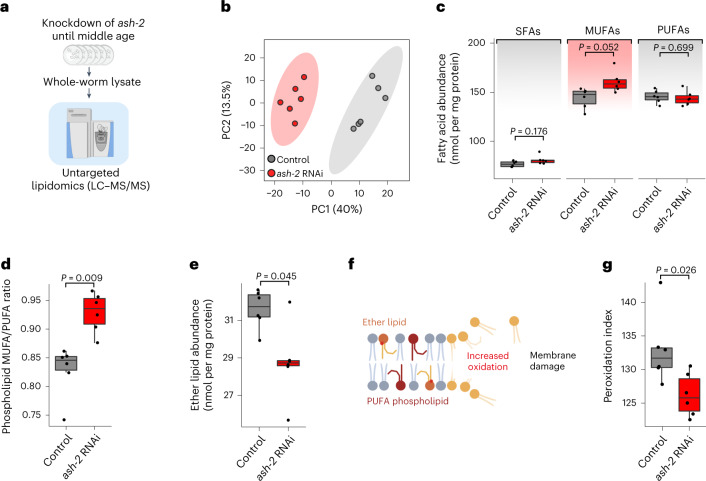
To determine how MUFAs (which increase lipid droplets) affect global lipid profiles, we performed untargeted lipidomics. We isolated all lipids from middle-aged adult worms treated with control or ash-2 RNAi (to induce MUFA accumulation and an increase in the number of lipid droplets) and performed liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS; Fig. 5a). Principal component analysis on all lipid species easily separated samples with MUFA enrichment (ash-2 RNAi) compared with controls (Fig. 5b). As expected, ash-2 depletion led to a global increase in MUFAs and triglycerides (Fig. 5c and Extended Data Fig. 4a).
Fig. 5. Lipidomic datasets of MUFA-enriched worms reveal changes in membrane lipids and predict decreased lipid oxidation.
a, Untargeted lipidomic analysis on whole worms using LC–MS/MS. b, Principal component (PC) analysis of the lipidome separates MUFA-enriched conditions (ash-2 RNAi) from control conditions. c, Fatty acyl chain abundance of saturated fatty acids (SFAs), MUFAs and PUFAs among all lipids in middle-aged worms following ash-2 depletion. d, Lipidomic analysis of MUFAs and PUFAs in membrane lipids (phospholipids) following ash-2 depletion. e, Lipidomics analysis of ether lipids following ash-2 RNAi depletion. f, Schematic indicating that membrane lipid oxidation is generally increased in the presence of PUFA-containing phospholipids and ether lipids, particularly those present in PUFAs. Accumulation of lipid oxidation can lead to membrane damage and loss of membrane integrity. g, Lipidomic analysis of the peroxidation index (probability of lipid oxidation; calculation in Methods) following ash-2 depletion. a and f are created with BioRender.com. b–e,g, Each dot represents a biological replicate; n = 6 independent biological replicates examined in one experiment. c–e,g, Box-and-whisker plots with the median (central line), 25th and 75th percentiles (outer lines), and minimum and maximum within 1.5× the interquartile range (whiskers) indicated. P values were determined using a two-tailed Wilcoxon test with Benjamini–Hochberg test for multiple hypothesis correction. Source data are provided.
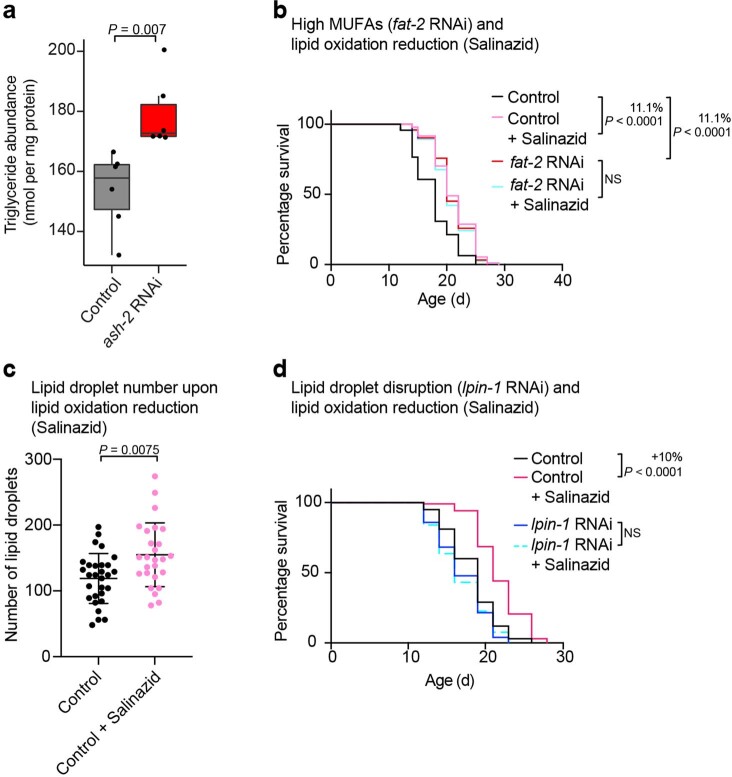
Extended Data Fig. 4. Effect of salinazid treatment on lifespan and number of lipid droplets.
a, Triglyceride abundance among all lipids in middle-aged worms following ash-2 depletion; n = 6 independent biological replicates examined in one experiment. Box-and-whisker plot, with median (central line), 25th and 75th percentile (outer lines) and minimum and maximum within 1.5× the interquartile range (whiskers). Each dot represents one biological replicate. P values: two-tailed Wilcoxon test with Benjamini–Hochberg test for multiple hypothesis correction. b, Salinazid and fat-2 depletion act in the same pathway to extend lifespan; n ≥ 111 worms for each condition. Percentages of median lifespan extension and P values are indicated. P values: log-rank Mantel–Cox test. c, Intestinal lipid droplets, measured by fluorescence, in dhs-3p::dhs-3::GFP worms following Salinazid treatment; n = 29 and 26 worms treated with and without salinazid, respectively. Data are the mean ± s.d. Each dot represents the lipid droplet number in a 26 × 26 µm2 area in the intestine of an individual worm. P values: two-tailed Mann–Whitney test. d, Lpin-1 is necessary for longevity following salinazid treatment; n ≥ 105 worms for each condition. Analysis as in b. b–d, Representative of two independent experiments. Source numerical data of all experiments, replicates and statistics as well as Cox proportional hazard interaction values are provided.
Interestingly, MUFA accumulation by ash-2 RNAi also led to the remodeling of many membrane lipids, with an increase ratio of MUFA/poly-unsaturated fatty acid (PUFA) in membrane lipids (Fig. 5d) and a decrease in specific membrane lipids—ether lipids—which have an ether bond instead of the classical ester bond (Fig. 5e). Membrane lipids with a low MUFA/PUFA ratio and high ether lipids are associated with increased lipid oxidation (Fig. 5f)65–68. In agreement with this, the peroxidation index—a measure of the likelihood of lipid oxidation—was reduced in MUFA accumulation conditions (Fig. 5g). These data raise the possibility that MUFA accumulation could decrease lipid oxidation.
MUFAs decrease lipid oxidation during aging
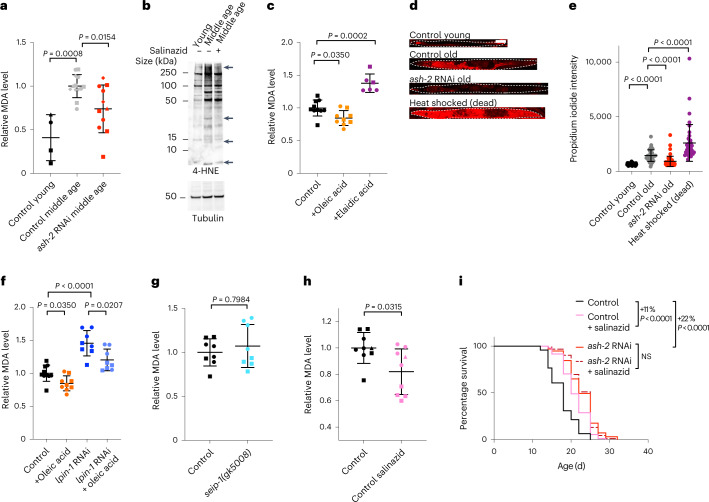
We tested whether lipid oxidation is impacted by MUFA accumulation. To quantify lipid oxidation, we measured the levels of malondialdehyde (MDA; a degradation product of oxidized lipids) and 4-hydroxynonenal (4-HNE; a degradation product of oxidized lipids that modifies proteins). With both methods we observed that middle-aged adults had higher levels of lipid oxidation compared with their younger counterparts (Fig. 6a,b), as previously reported69. We showed that MUFA accumulation by ash-2 RNAi reduced lipid oxidation in middle-aged individuals (Fig. 6a). Dietary supplementation of the cis-MUFA oleic acid (present in olive oil), which upregulates lipid droplets and extends lifespan, also reduced lipid oxidation in middle-aged adults (Fig. 6c). In contrast, dietary supplementation of the trans-MUFA elaidic acid (present in margarine and dairy), which does not upregulate lipid droplets or extend lifespan, increased lipid oxidation (Fig. 6c). These data suggest that MUFAs can counter the age-dependent increase in lipid oxidation.
Fig. 6. MUFAs decrease age-dependent lipid oxidation and preserve cell and membrane integrity.
a, Relative MDA levels, used as a measure of the level of lipid oxidation, during aging and following ash-2 depletion. Data for n = 4, 13 and 11 samples from control young, control middle-aged (control RNAi) and middle-aged worms treated with ash-2 RNAi, respectively, were normalized to the middle-aged control of the corresponding experiment. b, Lipid oxidation assessed via western blotting for 4-HNE levels during aging and following salinazid treatment. The arrows indicate bands that change following salinazid treatment. c, Levels of lipid oxidation, quantified via MDA levels, following supplementation with oleic acid or elaidic acid; n = 10, 9 and 6 samples from middle-aged worms with control, oleic acid and elaidic acid supplementation, respectively. d,e, Cell and membrane integrity, assessed according to the intensity of propidium iodide (PI) staining, during aging and following ash-2 depletion. d, Images of one worm per condition stained with PI. Dashed lines outline the worms. Scale bar, 100 µm. e, Intensity of PI staining for n = 32, 40, 40 and 55 young and old worms treated with control RNAi, old worms treated with ash-2 RNAi and dead worms, respectively. Each dot represents the mean PI signal in one worm. f, Levels of lipid oxidation, quantified via MDA levels, following oleic acid supplementation and lpin-1 depletion; n = 10, 9, 8 and 8 samples from middle-aged worms with control or oleic acid supplementation in the absence or presence of lpin-1 RNAi, respectively. g, Levels of lipid oxidation, quantified via MDA levels, in seip-1 mutant worms; n = 8 samples from middle-aged worms for each condition. h, Levels of lipid oxidation, quantified via MDA levels, following salinazid treatment; n = 9 samples from middle-aged worms for each condition. a,c,f–h, Each dot represents a biological replicate. Each shape represents an independent experiment. a,c,f–h, Data are the mean ± s.d. of four (a), two (c,f,g) or three (h) independent experiments. P values were determined using a two-tailed Mann–Whitney test. i, Salinazid and ash-2 depletion act in the same pathway to extend longevity. Percentages of the median lifespan extension and P values, determined using a log-rank Mantel–Cox test, are indicated; n ≥ 110 worms for each condition. b,e,i, Data are representative of two (b) or three (e,i) independent experiments. Source data are provided.
Lipid oxidation is associated with ferroptosis—an iron-dependent form of cell death in mammalian cells70–76. Although it is unclear whether bona fide ferroptosis occurs in the intestine of C. elegans, we observed that propidium iodide staining—which detects lack of cell and membrane integrity77,78—increases in older individuals (Fig. 6d,e). MUFA accumulation reduced propidium iodide staining in older individuals (Fig. 6d,e), suggesting that MUFAs preserve cell and membrane integrity during aging, perhaps by lowering lipid oxidation.
We investigated whether lipid droplet synthesis is necessary for the decrease in lipid oxidation by MUFAs. Knockdown of lpin-1, which decreases the number of lipid droplets, led to a strong increase in lipid oxidation in middle-aged worms in both basal conditions and oleic acid supplementation (Fig. 6f). However, deficiency in seip-1, which decreases the number of lipid droplets (but increases their heterogeneity, with a few very large and some very small droplets) did not affect lipid oxidation (Fig. 6g). The discrepancy between lpin-1 and seip-1 could be due to different roles in lipid droplet synthesis (Fig. 3a), additional function outside of lipid droplet synthesis or different effects on lipid droplet size/heterogeneity. Together, these data suggest that lpin-1 is critical for maintaining lower levels of lipid oxidation during aging, including in response to MUFAs.
To assess the functional importance of lipid oxidation in MUFA-induced longevity, we used salinazid, a drug that chelates iron and prevents iron-induced lipid oxidation (a characteristic of ferroptosis)69. We verified that salinazid reduced lipid oxidation in C. elegans (Fig. 6b,h). Salinazid treatment extended the lifespan of C. elegans in control conditions (Fig. 6i), as previously shown69. Interestingly, salinazid did not further extend lifespan in conditions of MUFA accumulation (by ash-2 or fat-2 RNAi; Fig. 6i and Extended Data Fig. 4b), suggesting that MUFAs and decreased lipid oxidation are in the same pathway. Salinazid also increased the number of lipid droplets (Extended Data Fig. 4c), and this was required for salinazid to extend lifespan (Extended Data Fig. 4d). Thus, blocking iron-induced lipid oxidation is important for MUFA-induced longevity (although salinazid could also extend lifespan via other iron-dependent processes or its effect on lipid droplet number).
Hence, MUFAs impact lipid homeostasis—with a decrease in ether lipids and lipid oxidation. Maintaining low lipid oxidation could be an important component of the beneficial effects of MUFAs on longevity.
Peroxisomes are critical for MUFA-induced longevity
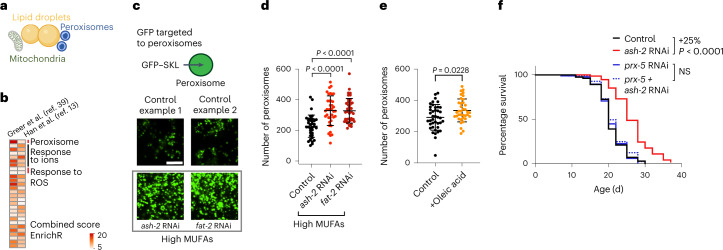
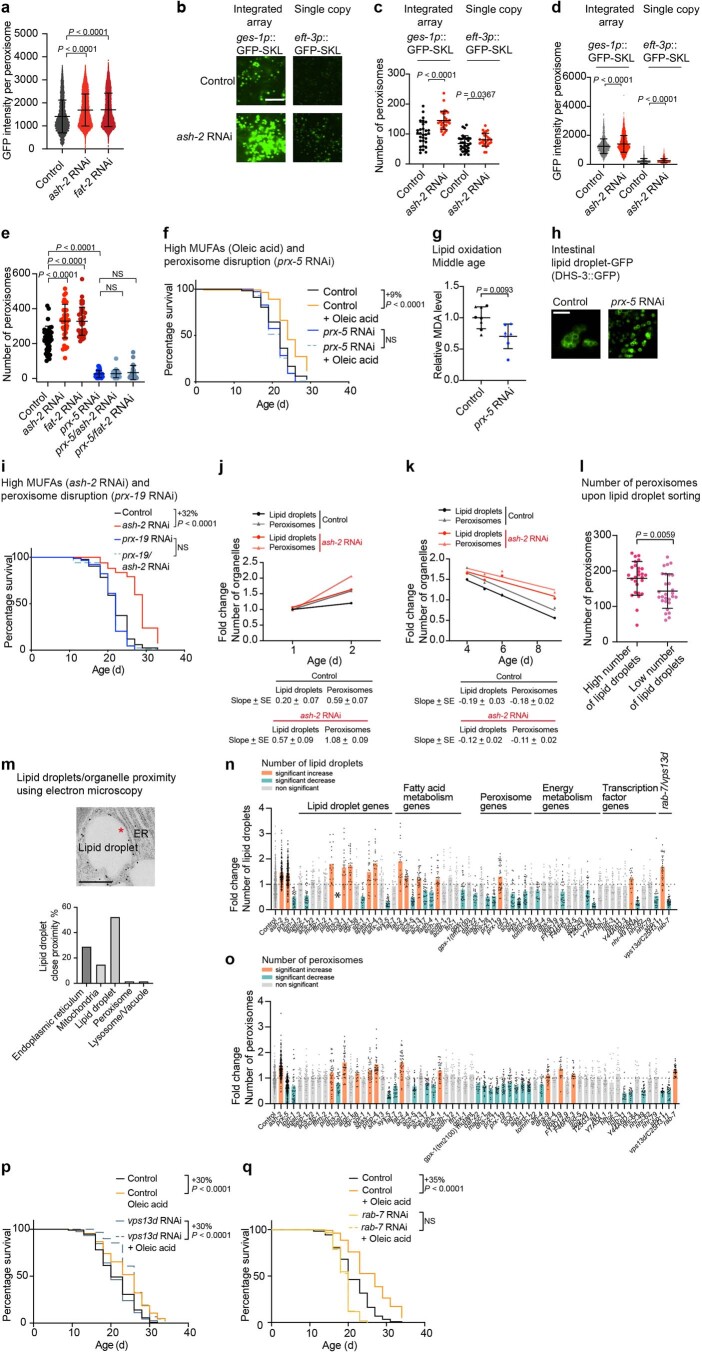
We next investigated whether MUFAs induce other protective mechanisms that could act together with lipid droplets. Our re-analysis of transcriptomic datasets of C. elegans with or without MUFA accumulation13,39 showed peroxisome-related Gene Ontology (GO) terms in conditions of MUFA accumulation (Fig. 7a,b), raising the possibility that peroxisomes may also have a functional role in lifespan extension by MUFAs. Using a transgenic reporter strain that carries GFP fused to a peroxisome import signal79,80, we found that MUFA accumulation due to ash-2 depletion, fat-2 depletion or oleic acid supplementation led to increased intensity of the GFP signal in the intestine, indicative of increased number/function of peroxisomes in this fat storage tissue (Fig. 7c–e and Extended Data Fig. 5a–d,h). Deficiency in PEX5 (prx-5 in nematodes) and PEX19 (prx-19 in nematodes), which are critical for peroxisome function, abolished lifespan extension due to MUFA accumulation (Fig. 7f and Extended Data Fig. 5e,f,i). This effect is unlikely to be via the lowering of lipid oxidation because prx-5 deficiency decreased lipid oxidation (Extended Data Fig. 5g). Thus, MUFAs upregulate not only lipid droplet number but also peroxisome number/function in the intestine, with peroxisome function being necessary for MUFA-induced longevity.
Fig. 7. MUFAs upregulate peroxisome number, which is required for MUFA-induced longevity.
a, Organelles such as mitochondria and peroxisomes are also involved in lipid metabolism. Created with BioRender.com. b, Analysis of existing transcriptomic datasets of worms following MUFA accumulation. Upregulated GO terms that are shared between worms treated with either control or ash-2 RNAi. GO terms analyzed using WormEnrichR. GO terms upregulated in middle-aged individuals (adult day 5, whole worms; left column). GO terms upregulated in young individuals (adult day 1/intestine; right column). GO terms were considered significant if they had a combined score of >5 for the log-transformed P value (Fisher’s exact test) multiplied by the rank-based enrichment z-score. ROS, reactive oxygen species. c–e, Number of intestinal peroxisomes, assessed by fluorescence, following MUFA accumulation in worms expressing a peroxisome-localized GFP (GFP–SKL) driven by the intestinal ges-1 promoter (ges-1p::GFP–SKL). c, Zoomed-in images of the intestine. Scale bar, 5 µm. Intensity of peroxisome-localized GFP in Extended Data Fig. 5a. d, Number of peroxisomes following MUFA accumulation in n = 39, 38 and 40 worms treated with control, ash-2 and fat-2 RNAi, respectively. e, Number of peroxisomes following dietary supplementation with oleic acid; n = 40 and 37 control- and oleic acid-supplemented worms, respectively. d,e, Data are the mean ± s.d. Each dot represents the number of peroxisomes in a 26 × 26 µm2 area in the intestine of an individual worm. P values were determined using a two-tailed Mann–Whitney test. f, Prx-5 is necessary for longevity following ash-2 depletion. Percentages of the median lifespan extension and P values are indicated; P values were determined using a log-rank Mantel–Cox test; NS, not significant; n ≥ 94 worms for each condition. d–f, Data are representative of three independent experiments. Source data are provided.
Extended Data Fig. 5. Interventions that regulate the number of peroxisomes and lipid droplets.
a, Quantification of intestinal peroxisome-localized GFP intensity, measured by fluorescence, in ges-1p::GFP–SKL worms following MUFA accumulation; n = 6,061, 13,446 and 10,598 peroxisomes in ≥35 worms treated with control, ash-2 and fat-2 RNAi, respectively. Data are the mean ± s.d. Each dot represents the GFP intensity of one peroxisome. P values: two-tailed Mann–Whitney test. Peroxisomes shown in Fig. 7c. b–d, Intestinal peroxisomes, assessed by fluorescence, in worms expressing an integrated array of ges-1p::GFP–SKL or a single copy knock-in of a peroxisome-localized GFP (GFP–SKL) driven by the ubiquitous eft-3 promoter (eft-3p::GFP–SKL) following ash-2 depletion. b, Zoomed-in images of the intestine. Scale bar, 5 µm. c, Quantification of peroxisome number; n = 30, 29, 32 and 27 ges-1p::GFP–SKL worms treated with control and ash-2 RNAi, and eft-3p::GFP-SKL worms treated with control and ash-2 RNAi, respectively. Data are the mean ± s.d. Each dot represents the peroxisome number in a 13 × 13 µm2 area in the intestine of an individual worm. P values: two-tailed Mann–Whitney test. d, Quantification of peroxisome-localized GFP intensity; n = 1,922, 2,814, 1,537 and 1,553 peroxisomes in ≥22 ges-1p::GFP–SKL worms treated with control and ash-2 RNAi, and eft-3p::GFP–SKL worms treated with control and ash-2 RNAi, respectively. Analysis in a. e, Quantification of intestinal peroxisomes, measured by fluorescence, in ges-1p::GFP–SKL worms following prx-5 depletion and MUFA accumulation; n = 39, 38, 40, 33, 26 and 22 worms treated with control, ash-2, fat-2, prx-5, ash-2 + prx-5, and fat-2 + prx-5 RNAi, respectively. Data are the mean ± s.d. Each dot represents the peroxisome number in a 26 × 26 µm2 area in the intestine of an individual worm. P values: two-tailed Mann–Whitney test. f, prx-5 is necessary for longevity following oleic acid supplementation; n ≥ 120 worms for each condition. Percentages of median lifespan extension and P values are indicated. P values: log-rank Mantel–Cox test. g, Lipid oxidation quantified via MDA levels following prx-5 depletion; n = 8 and 7 samples from worms treated with control and prx-5 RNAi, respectively. Normalized to the control condition. Data are the mean ± s.d. of two independent experiments. Each dot represents a biological replicate. Each shape represents an independent experiment. P values: two-tailed Mann–Whitney test. h, Intestinal lipid droplet number, assessed by fluorescence, in dhs-3p::dhs-3::GFP worms following prx-5 depletion. Zoomed-in images of the intestine. Scale bar, 5 µm. Lipid droplet number quantified in n. i, prx-19 is necessary for longevity following ash-2 depletion; n ≥ 95 worms for each condition. Analysis as in f. j,k, Intestinal lipid droplet and peroxisome number, measured by fluorescence, in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL worms during aging and following ash-2 depletion; n ≥ 23 worms. Data are the mean ± s.d. Each dot represents the mean organelle number of all worms imaged for this condition normalized to young control (adult day 1) worms. Organelle number increases at younger ages (j) and organelle number decreases at older ages (k). Linear regression fit. l, Intestinal peroxisomes, measured by fluorescence, in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL worms after sorting based on lipid droplet number using a BioSorter; n = 27 and 28 worms sorted based on high and low lipid droplet number, respectively. Analysis as in e. m, Lipid droplets assessed by immunogold labeling (against GFP) using transmission electron microscopy in dhs-3p::dhs-3::GFP worms. The asterisk indicates close proximity/contact between a lipid droplet and the endoplasmic reticulum (top). Scale bar, 500 nm. Quantification of lipid droplet contact/close proximity with other organelles as a percentage (bottom). n, Intestinal lipid droplets, measured by fluorescence, in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL transgenic worms following treatment with 62 different RNAis; n = 19–423 worms. Data are the mean ± s.d. Each dot represents the organelle number in a 26 × 26 µm2 area in the intestine of an individual worm normalized to control worms. Orange, significant increase in organelle number. Turquoise, significant decrease in organelle number. P values: two-tailed Wilcoxon test with Benjamini–Hochberg test for multiple hypothesis correction. Conditions are colored if the adjusted P < 0.05. *dhs-3 RNAi abolishes the GFP signal of the lipid droplet DHS-3::GFP reporter. o, Quantification of intestinal peroxisome measured by fluorescence in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL transgenic worms. Quantification of peroxisome number in worms treated as in n; n = 18–286 worms for each condition. Analysis as in n. p, vps13d depletion does not reduce longevity following oleic acid supplementation; n ≥ 99 for each condition. Analysis as in f. q, rab-7 depletion reduces longevity following oleic acid supplementation; n ≥ 120 for each condition. Analysis as in f. a,e, Representative of three independent experiments. c,d,f,i–m,p,q, Representative of two independent experiments. Source numerical data of all experiments, replicates, exact n values and statistics as well as Cox proportional hazard interaction values are provided.
Lipid droplets and peroxisomes are co-regulated
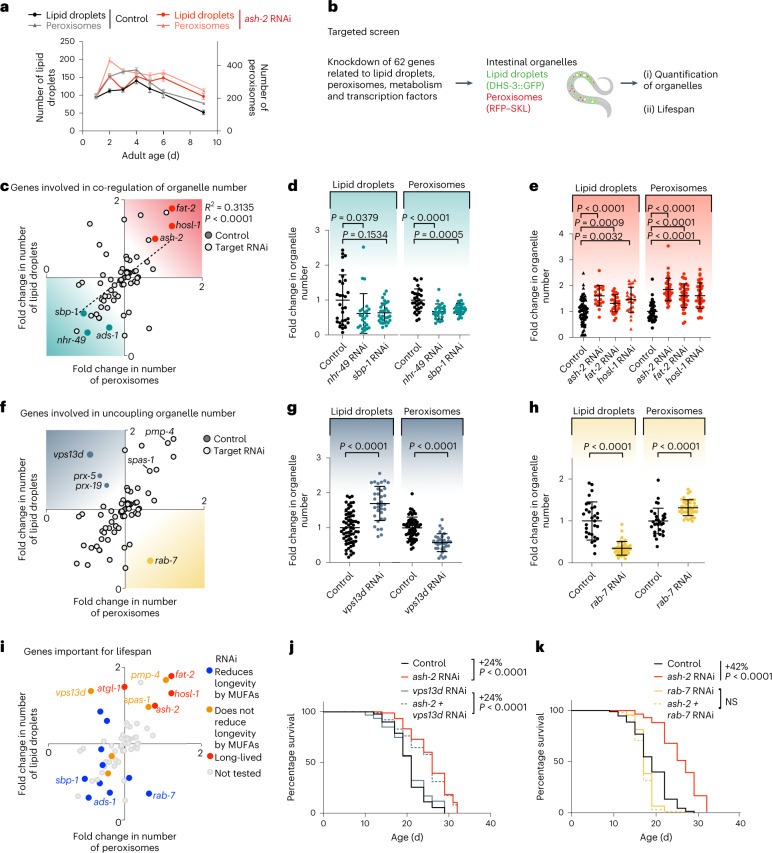
We examined the relationship between lipid droplets and peroxisomes. Both organelles exhibited similar dynamics with age and in response to MUFA accumulation (Fig. 8a), with an increase in young adults, followed by a decrease at middle age (Extended Data Fig. 5j,k). Furthermore, the number of lipid droplets correlated with that of peroxisomes (Extended Data Fig. 5l). Spatially, lipid droplets and peroxisomes were only rarely in close proximity or direct contact with each other, as determined by electron microscopy analysis (Extended Data Fig. 5m). Hence, lipid droplets and peroxisomes are co-regulated in response to MUFAs in the organism and this co-regulation is unlikely to occur via direct contact.
Fig. 8. Targeted screen to identify genes involved in the co-regulation of lipid droplet and peroxisome number.
a, Number of intestinal lipid droplets and peroxisomes, measured by fluorescence, in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL worms during aging and following ash-2 depletion. Data are the mean ± s.e.m. of n = 21–37 worms. Each dot represents the mean organelle number of all worms imaged for this condition. b, Targeted screen design. Created with BioRender.com c, Genes involved in the co-regulation of organelle number. Number of intestinal lipid droplets and peroxisomes, measured by fluorescence, in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL worms following treatment with 62 different RNAis. RNAi of the indicated genes resulted in an increase (red) or decrease (teal) of both organelles; n ≥ 18 worms per condition. d, Number of intestinal lipid droplets and peroxisomes, quantified by fluorescence, in dhs-3p::dhs-3::GFP; vha-6p::mRFP–SKL worms following sbp-1 and nhr-49 transcription factor depletion; n = 24–30 worms. e, Number of intestinal lipid droplets and peroxisomes, quantified as in d, following ash-2, fat-2 or hosl-1 depletion; n = 20–42 worms. f, Genes involved in uncoupling lipid droplet and peroxisome number. RNAi of the indicated genes resulted in decreased numbers of lipid droplets and increased peroxisomes (yellow) or increased numbers of lipid droplets and decreased peroxisomes (blue). g, Number of lipid droplets and peroxisomes, measured as in d, following vps13d depletion; n = 33 or 34 worms. h, Number of lipid droplets and peroxisomes, measured as in d, following rab-7 depletion; n = 29–41 worms. d,e,g,h, Data are the mean ± s.d. Each dot represents the organelle number of an individual worm normalized to control worms. P values were determined using a two-tailed Mann–Whitney test. i, Genes important for lifespan. Perturbations that affect the co-regulation of the number of lipid droplets and peroxisomes, color-coded according to their effect on lifespan and MUFA-induced longevity. c,f,i, Each dot represents the mean organelle number, normalized to control, of all worms imaged for this condition. A two-tailed Pearson’s R2 test was used to analyze correlation; dotted line, nonlinear fit. j, vps13d is not necessary for longevity following ash-2 depletion; n ≥ 71 worms for each condition. k, rab-7 is necessary for longevity following ash-2 depletion; n ≥ 93 worms for each condition. j,k, Percentages of the median lifespan extension and P values, determined using a log-rank Mantel–Cox test, are indicated. a,d,e,g,h,j,k, Data are representative of three (d,g,h,j,k) or two (a,e) independent experiments. Source data are provided.
To identify genes involved in the co-regulation between lipid droplets and peroxisomes, we performed a targeted RNAi screen for genetic perturbations that impact both organelles. We tested 62 genes involved in aspects of lipid metabolism or physically located on lipid droplets or peroxisomes40,41,54,59,79,81–102 (Fig. 8b and Extended Data Fig. 5n,o). As a readout for this targeted screen we used strains expressing fluorescent markers of lipid droplets and peroxisomes. Most gene perturbations impacted the number of lipid droplets and peroxisomes in the same manner (Fig. 8c–e). Perturbations that led to more lipid droplets (ash-2, fat-2 or hosl-1 knockdown) also resulted in an increase in peroxisomes (Fig. 8c,e). Interestingly, perturbations in conserved transcription factors that regulate lipid metabolism (SREBP (sbp-1 in nematodes) and PPAR (nhr-49 in nematodes)) or in the ether lipid synthesis enzyme AGPS (ads-1 in nematodes) decreased the number of both lipid droplets and peroxisomes (Fig. 8c,d). Thus, lipid droplets and peroxisomes are co-regulated via transcription factors and lipid synthesis enzymes, although their mechanisms of action may be indirect.
The outliers of this targeted screen are interesting because they uncover genes that uncouple lipid droplet and peroxisome number and could be involved between these two organelles. Deficiency in VPS13D (C25H3.11/vps13d in nematodes) resulted in high numbers of lipid droplets but low numbers of peroxisomes (Fig. 8f,g). In mammalian cells VPS13D is a lipid transporter101 that regulates peroxisome biogenesis103. Conversely, deficiency in the gene RAB7 (rab-7 in nematodes) led to low lipid droplet and high peroxisome numbers (Fig. 8f,h). In mammalian cells RAB7 is an endosomal protein that can also localize to lipid droplets to regulate lipolysis104, among other processes105. In contrast, deficiency in genes involved in direct contact between lipid droplets and peroxisomes (SPASTIN (spas-1 in nematodes) and ABCD1 (pmp-4 in nematodes))84 did not uncouple organelle number (and increased both organelles concomitantly; Fig. 5f). These results suggest that vps13d and rab-7, which regulate lipid transport and lipolysis, are involved in between lipid droplets and peroxisomes, although their mode of action may be indirect. Hence, lipid droplets and peroxisomes form an organelle network that is influenced by several types of regulators.
Finally, we investigated how regulators of the lipid droplet–peroxisome network impact lifespan. Perturbations that increased both organelles concomitantly (ash-2, fat-2 and hosl-1) extended lifespan (Fig. 8i), whereas perturbations that decreased both organelles concomitantly (sbp-1 and ads-1) blunted MUFA-induced longevity (Fig. 8i)13. Perturbations in genes involved in direct contact between lipid droplets and peroxisomes (spas-1 and pmp-4)84 did not affect lifespan (Fig. 8i). Deficiency in vps13d, which increases the number of lipid droplets but decreases peroxisomes, did not extend lifespan or reduce MUFA-induced longevity (by ash-2 RNAi or oleic acid supplementation; Fig. 8i,j and Extended Data Fig. 5p). In contrast, deficiency in rab-7, which decreases lipid droplets but increases peroxisomes, blunted MUFA-induced longevity (by ash-2 RNAi and oleic acid supplementation; Fig. 8i,k and Extended Data Fig. 5q). Collectively, these data suggest that increased numbers of lipid droplets is more important than increased numbers of peroxisomes to promote longevity, but that a concomitant increase in both lipid droplets and peroxisomes is critical for the full beneficial impact of MUFAs on lifespan.
Discussion
Our study identifies mechanisms by which dietary fatty acids such as MUFAs extend lifespan and uncovers the importance of a lipid droplet–peroxisome organelle network in longevity (Extended Data Fig. 6). We find that cis-MUFAs such as oleic acid (present in olive oil) increase the number of both lipid droplets and peroxisomes and modify lipid homeostasis, and that these processes are critical for MUFA-induced longevity. The magnitude of lifespan extension by MUFAs is approximately 20–30%, which is in the range of other dietary manipulations that impact the lifespan of C. elegans and other species106,107. Interestingly, the concomitant increase in the number of lipid droplets and peroxisomes is optimal for the full beneficial effects of MUFAs on lifespan.
Extended Data Fig. 6. Schematic of the proposed model for how MUFAs extend lifespan.
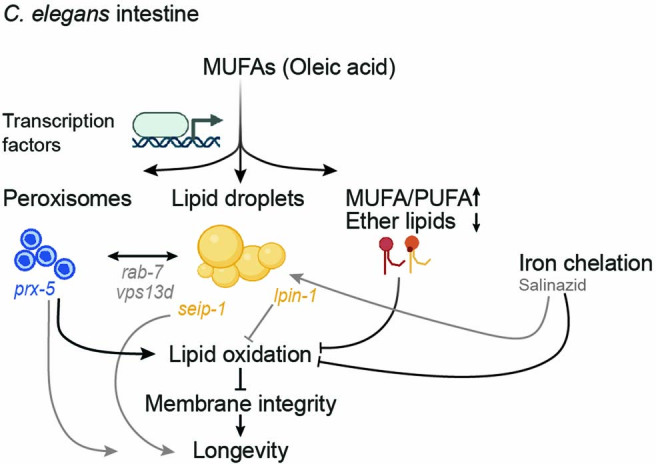
MUFA accumulation (for example, following dietary oleic acid supplementation) increases lipid droplet and peroxisome numbers, and both organelles are required for MUFA-induced lifespan extension. This concomitant increase in lipid droplets and peroxisomes requires shared upstream transcription factors (MUFAs and other lipids could also directly serve as substrates for lipid droplets synthesis). Genes encoding proteins implicated in lipid transport (VPS13D/vps13d) and organelle regulation (RAB7/rab-7) are probably involved between these two organelles (because their deficiency uncouples co-regulation of lipid droplet and peroxisome number). MUFAs increase the MUFA-to-PUFA ratio in membrane lipids and decrease ether lipids—a signature predicted to lower lipid oxidation. MUFAs reduce lipid oxidation and preserve cell and membrane integrity in the organism, and this is one way in which MUFAs extend lifespan. Genes encoding proteins that regulate lipid droplet number can also influence lipid oxidation. For example, LIPIN1/lpin-1 deficiency increases lipid oxidation (but not SEIPIN/seip-1). Both LIPIN1/lpin-1 and SEIPIN/seip-1 deficiency reduce MUFA-induced longevity, suggesting that SEIPIN has other beneficial effects, independently of lipid oxidation, perhaps on the endoplasmic reticulum. Salinazid, an iron-chelator, prevents lipid oxidation and acts in a similar pathway as MUFAs (as these are not additive on lifespan extension). Salinazid also increases lipid droplet number, perhaps indirectly or as part of a feedforward mechanism. Peroxisome function drives lipid oxidation (probably by generating reactive oxygen species), suggesting that their beneficial effect for MUFA-induced longevity is also due to other processes (perhaps production of membrane lipids important for membrane integrity or lipids important for metabolism). Although the increase in lipid droplet number seems to be more important than that of peroxisomes, the concomitant upregulation of lipid droplet and peroxisome number in the organism is optimal for lifespan extension. Black arrows, direct effects. Gray arrows, indirect effects. Created with BioRender.com.
The role of lipid droplets in health has remained unclear, with studies reporting either beneficial or detrimental effects. In Drosophila, overexpression of a protein that tethers lipid droplets is associated with a high median lifespan25 and lipid droplets can protect stem cell niches24. Yet lipid droplets also accumulate during old age and disease108, and they are detrimental in many contexts20–22,26,28,109–111. Our results indicate that a high number of lipid droplets in the intestine is beneficial for longevity. Other lipid droplet features such as size, heterogeneity and content may be associated with detrimental effects. Moreover, as both increased fat storage and lipolysis have been associated with longevity6,7,16,42,112–129, there may also be an optimal level of free MUFAs for lifespan extension. Interestingly, lipid droplet numbers in youth and middle age can predict remaining lifespan. Early life events (for example, levels of reactive oxygen species63) could influence MUFA levels and result in different lifespan trajectories. The predictive potential of lipid droplet number on remaining lifespan is consistent with the observation that among dietary-restricted individuals (in mice)130—the fattest ones live the longest.
Our lipidomics data reveal that MUFA accumulation decreases ether lipids and increases the MUFA-to-PUFA ratio in membrane lipids. It will be interesting to determine whether lipid droplets are directly involved in membrane lipid metabolism (for example, phospholipid metabolism) and, if so, what mechanisms underlie this effect. Ether lipids, which are decreased following the accumulation of MUFAs, are known to modulate lipid oxidation and ferroptosis—a conserved iron-dependent form of cell death131–135. Furthermore, MUFAs provide protection from ferroptosis by displacing PUFAs from membrane lipids in cancer cells136. Although we have not directly tested ferroptosis in C. elegans, we find that MUFA accumulation decreases lipid oxidation and preserves membrane and cell integrity in the intestine of older individuals. Hence, a diet high in MUFAs may change the balance of ether lipids as well as the MUFA-to-PUFA ratio in cells, thereby preventing lipid oxidation and intestinal malfunction in the organism.
Our data also reveal a previously uncharacterized connection between lipid droplets and peroxisomes in longevity. Although physical interactions between both organelles had been identified in mammalian cells84,137, a co-regulation at the organismal level—especially in the context of aging—was not known. Our screen identifies upstream key regulators of this lipid droplet–peroxisome network as well as genes involved between lipid droplets and peroxisomes. It will be interesting to determine whether proteins encoded by these genes act directly or indirectly to regulate the lipid droplet–peroxisome network by examining their subcellular localization during aging and their specific mechanism of action. Lipid droplets have recently been found to regulate endoplasmic reticulum responses in C. elegans138, and this could also contribute to the modulation of lipid droplets and peroxisomes during aging. The lipid droplet–peroxisome network may be critical not only for longevity but also in other biological processes or disrupted in diseases. Given the conservation of lipid metabolism and organelles in other species, our findings open new avenues—including lipid-based strategies—for promoting longevity and health.
Methods
C. elegans and bacteria strains
All C. elegans strains (wild type and mutants) used in this study are listed in Supplementary Table 1. Worms from mutant deletion strains were genotyped by PCR, and the PCR amplicon sizes were used to check for the presence of the deletion. C. elegans were cultured and maintained at 20 °C on standard Nematode Growth Media (NGM) plates seeded with a lawn of OP50-1 (gift from M. -W. Tan). For all experiments, the worms were cultured at 20 °C on RNAi plates seeded with the RNAi strains HT115 (DE3). All experiments were conducted on hermaphrodite worms, apart from the experiments in Fig. 1g and Extended Data Fig. 1g, which were conducted in male worms. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440) and the Mitani Laboratory at the Tokyo Women’s Medical University School of Medicine.
The WBM1177 strain (wbmIs81[eft-3p::3XFLAG::GFP::SKL::unc-54 3′ untranslated region (UTR), *wbmIs65]), which expresses a peroxisome localization sequence (SKL) fused to GFP driven by the eft-3 promoter, was generated by microinjection using clustered regularly interspaced short palindromic repeats (CRISPR) into WBM1140 (wbmIs65 [eft-3p::3XFLAG::dpy-10 crRNA::unc-54 3′ UTR]) using the SKI LODGE system139. This system allows for knock-in of a single copy of the construct downstream of the eft-3 promoter, which drives gene expression in all somatic cells and allows for ubiquitous expression of peroxisome-targeted GFP. The ABR339 strain (lpin-1(wbm76[lpin-1::GFP])), which expresses endogenous LPIN-1 fused to GFP at the endogenous lpin-1 locus, was generated by microinjection using CRISPR into wild-type worms. The CRISPR insertion was performed as described previously140. The following crRNA sequence was used for carboxy-terminal lpin-1 editing: 5′-ATTGTTGCTGGCATCAAAAA-3′. Worms were genotyped by PCR; the PCR amplicon sizes were used to check for the presence of the insertion and the strain was crossed with wild-type worms to remove the dpy-10 coinjection marker.
RNAi
For knockdown by RNAi, worms were fed HT115 (DE3) transformed with vectors expressing double-stranded RNA against the gene of interest. The HT115 strains expressing RNAi targeting the gene of interest were obtained from the Ahringer library (gift from A. Fire). For all genes, the annotated WormBase name was used except for C25H3.11, for which we used vps13d. RNAi clones were confirmed by Sanger sequencing141. All experiments were performed on HT115.
To generate RNAi-expressing bacteria, a single bacterial colony was used to inoculate LB medium containing 100 µg ml−1 ampicillin (Sigma Aldrich). The bacteria were cultured overnight at 37 °C to stationary phase and the expression of the double-stranded RNAi was induced with 0.4 mM isopropylthiogalactoside (IPTG; Thermo Fisher) for 4 h at 37 °C. The cultures were centrifuged and the bacterial pellet resuspended in LBAmp/IPTG (LB with 100 µg ml−1 ampicillin and 0.4 mM IPTG) at 1:30 of the initial volume. This bacterial resuspension was stored at 4 °C for no longer than 2 weeks. Concentrated bacteria were added to 6-cm or 10-cm NGM plates containing 100 µg ml−1 ampicillin and 0.4 mM IPTG (RNAi plates). For the knockdown experiments, worms were kept on 6-cm plates seeded with RNAi-expressing bacteria. Unless indicated otherwise, worms were fed the RNAi from egg lay onwards. For lpin-1 and fat-2 depletion, the RNAi treatment was initiated in young adults (adult day 1) to minimize the effects on development. For a negative control, worms were fed the empty vector (L4440) bacterial strain.
When two genes were knocked down simultaneously, the optical density at 600 nm (OD600) of the bacteria was adjusted to the same value (OD600 = 50, stationary phase) and the RNAi-expressing bacteria were mixed in a 1:1 ratio. To assess the gene knockdown efficiency in the context of single versus double RNAi, we performed reverse transcription, followed by real-time quantitative PCR (RT–qPCR) using primers to each gene of interest (Extended Data Fig. 2c) as described in the following section.
RT–qPCR
To test the efficiency of gene knockdown in the context of single versus double RNAi, RT–qPCR was performed as described before13. Briefly, 300 wild-type worms were subjected to RNAi-expressing bacteria (or control bacteria) on 6-cm RNAi plates from egg lay to adult day 1. For experiments with lpin-1 RNAi, knockdown for all conditions was initiated at the young adult age and worms were harvested at adult day 2. The worms were washed three times with M9 buffer (22 mM KH2PO4, 34 mM K2HPO4, 86 mM NaCl and 1 mM MgSO4) to remove residual bacteria in the worm pellet. To isolate total RNA, the worm pellets were resuspended in 500 µl TRIzol Reagent (Invitrogen) and subjected to six freeze–thaw cycles in a dry ice–ethanol bath. RNA was extracted according to the standard TRIzol procedure, resuspended in 30 µl of RNase- and DNase-free water, and quantified using a Nanodrop (Thermo Fisher). The RNA was treated with DNase (Thermo Fisher, 18068015), followed by reverse transcription using Oligo (dT)20 primers (Invitrogen, 18418020) and SuperScript IV Reverse Transcriptase (Invitrogen,18090010). iTaq Universal SYBR Green super mix (Bio-Rad,1725124) was used in a 20-µl reaction volume for the RT–qPCR reaction. Primers were designed to span exon–exon junctions and used at a final concentration of 250 nM. All primers are listed in Supplementary Table 2. RT–qPCR was performed using a C1000 thermal cycler (Bio-Rad). Melt curves were examined to ensure the specificity of the primers. Results were analyzed using the ΔΔCt method. For each biological replicate, the median Ct value of three technical replicates was analyzed. act-1 served as the internal reference gene. Bar plots were generated using Prism 9. Experiments were performed twice independently, with three replicates each.
SRS for lipid droplet quantification
To visualize all lipids in a label-free way, we used SRS microscopy31,142. Middle-aged (adult day 6) hermaphrodites, treated with control or RNAi-expressing HT115 bacteria, were mounted on a 2% agar pad, anesthetized with 50 mM sodium azide and covered with a glass coverslide for SRS imaging. The worms were imaged directly after mounting to avoid confounds from starvation on the microscope slide. For each experiment, approximately 18 worms were imaged per condition. The experimental set-up was built on an inverted microscope (IX81, Olympus). For SRS microscopy, spatially and temporally overlapped pulsed Pump (tunable from 720 to 990 nm, 7 ps, 80 MHz repetition rate) and Stokes (1064 nm, 5–6 ps, 80 MHz repetition rate, modulated at 8 MHz) beams provided by picoEMERALD (Applied Physics & Electronics) were coupled into an inverted laser-scanning microscope (FV1000 MPE, Olympus) optimized for near-infrared throughput. A ×20 air objective (0.75 numerical aperture (N.A.); UPlanSAPO, Olympus) and a ×60 water objective (1.2 N.A.; UPlanAPO/IR, Olympus) were used for imaging. After passing through the sample, the forward-travelling Pump and Stokes beams were collected in transmission by an air condenser (0.9 N.A.; Olympus) for the ×20 magnification and an oil condenser (1.4 N.A.; Olympus) for the ×60 magnification. A high OD bandpass filter (890/220; Chroma) was used to block the Stokes beam completely and transmit only the Pump beam onto a large area Si photodiode (FDS1010, Thorlabs) to detect the stimulated Raman loss signal. The output current from the photodiode was terminated, filtered and demodulated by a lock-in amplifier (HF2LI; Zurich Instruments) at 8 MHz to ensure shot noise-limited detection sensitivity. The laser power of the infrared laser and the optical parametric oscillator were set at 600 mW. For lipid imaging, CH2 signals from lipid droplets were imaged at 2,845 cm−1 in the SRS channel. These settings were used to visualize all lipids, as lipids are rich in CH2 bonds. For imaging with the SRS signal alone, two replicates were recorded at ×60 and the triplicate was recorded using a ×20 objective. Within one experiment, the same threshold was applied to all images and lipid droplets were quantified in a 26 × 26 µm2 area using the analyze particle function in Fiji version 2.0.0 (ref. 143). The experiment was carried out three times independently. For lipid droplet imaging with SRS and GFP (DHS-3::GFP, see the next section) together, two replicates were recorded at ×60 and the double-positive puncta (red SRS puncta surrounded by green GFP signal) in a 26 × 26 µm2 area were quantified manually. The experiment was carried out twice independently. The investigators were not blinded to allocation during experiments and outcome assessment. The lipid droplet numbers and intensities were plotted in Prism 9 and statistically significant differences between samples were assessed using the two-tailed unpaired nonparametric Mann–Whitney test.
Confocal microscopy for lipid droplet quantification
To visualize lipid droplets by confocal microscopy, we used a reporter strain expressing the lipid droplet protein DHS-3 fused to GFP, which has been used as a marker of lipid droplets in several studies in C. elegans96,144–146. The DHS-3 protein is orthologous to 17β-HSD11 in mammals43. It is one of the most abundant proteins on lipid droplets found by mass spectrometry in C. elegans40,41 and its localization at the lipid droplet membrane was verified by fluorescence microscopy40. Transgenic dhs-3p::dhs-3::GFP (LIU1) hermaphrodites were imaged at middle age (adult day 6), unless noted otherwise. For each experiment, approximately 20 worms were imaged per condition. The worms were mounted on a 2% agar pad, anesthetized with 50 mM sodium azide and covered with a glass coverslide for imaging. The worms were imaged using a Nikon Eclipse Ti confocal microscope with a Zyla sCMOS camera (Andor) and the NIS-Elements software (AR 4.30.02, 64 bit) using the ×100 oil objective (Plan Apo, ×100; Nikon) and a 50-µm pinhole (0.8 µm optical section thickness) or a Zeiss confocal microscope (LSM900, Axio Observer) using the ×63 oil objective (Plan-Apochromat ×63/1.40 oil DIC M27), a 1 Airy Unit/45 µm pinhole (0.5 µm optical section thickness) and the Zen software (3.0, blue). The mid-intestinal region was imaged over 0.8 µm (five slices each, 0.2 µm interval for the Nikon confocal) and 0.92 µm (five slices each, 0.23 µm interval for the Zeiss confocal). The images on each confocal were taken using the same exposure time/laser power. Lipid droplet numbers were analyzed in Fiji version 2.0.0 (ref. 143) by generating z-stack projections of the individual slices, applying the same threshold to all images and manually counting the lipid droplets in a 26 × 26 µm2 area. The lipid droplet diameters were analyzed in Fiji version 2.0.0 (Ref. 143) by generating z-stack projections of five slices, applying the same threshold to all images, and manually measuring the diameter of all lipid droplets in focus.
To measure lipid droplets in males, transgenic dhs-3p::dhs-3::GFP (LIU1) males were maintained by crossing with hermaphrodites. For experiments, male worms were processed similarly as described earlier.
To measure hypodermal lipid droplets, transgenic plin-1p::plin-1::mCherry (LIU2) hermaphrodites were imaged at middle age (adult day 6). This reporter strain has been used to visualize hypodermal and intestinal lipid droplets40. For each experiment, approximately 20 worms were imaged per condition. The worms were processed similarly as described above with the following differences. To analyze hypodermal lipid droplets, the head hypodermis was imaged to avoid imaging intestinal lipid droplets. As the width of the worms is smaller in the head, a smaller area (15 × 15 µm2) was selected to count the number of lipid droplets.
To visualize lipid droplets in eggs in utero, Nile red staining was performed as described previously41. Briefly, young adult (adult day 1) wild-type hermaphrodites were fixed in 40% isopropanol for 3 min and stained with 8 µM Nile red (MP Biomedicals, 0215174450) for 2 h in the dark. For each experiment, approximately 20 worms were imaged per condition. The worms were mounted and imaged as described above. One or two fertilized eggs per worm were imaged in utero with the settings described above. The number of lipid droplets was counted in a 16 × 16 µm2 area using the Squassh plugin147 with the following settings: the background was subtracted with a rolling-ball radius of 10 pixels and the segmentation parameters were set at 0.05 regularization with a minimum object intensity of 0.15.
All experiments and lipid droplet analyses were performed in a blinded manner, unless noted otherwise. Key experiments were carried out three times independently but all experiments were carried out at least two times independently. The numbers and intensities of lipid droplets were plotted in Prism 9 and statistically significant differences between samples were assessed using the two-tailed unpaired nonparametric Mann–Whitney test.
MUFA supplementation
To increase the level of specific fatty acids in C. elegans, we performed dietary supplementation experiments13. Briefly, fatty acid sodium derivatives (Nu-check prep) of oleic acid (C18:1n9 cis), elaidic acid (C18:1n9 trans), cis-vaccenic acid (18:1n7 cis) and trans-vaccenic acid (18:1n7 trans) were dissolved in water at 100 mM. To dissolve elaidic acid and trans-vaccenic acid, the solution was heated in a water bath at 50 °C for no more than 10 min. We verified by mass spectrometry that heated elaidic acid is still taken up (Extended Data Fig. 1m) and heated oleic acid retains its beneficial effect to increase lipid droplets and lifespan (Source Data for Fig. 2c and Extended Data Fig. 1b). The detergent Tergitol (NP-40, Sigma Aldrich) was added at a concentration of 0.001% to RNAi agar prior to autoclaving for all fatty acid (cis and trans) and control supplementation plates. After autoclaving, the agar media was cooled to approximately 60 °C and the fatty acid solutions were added to a final concentration of 0.8 mM. The agar media was stirred for 2 min after addition of the fatty acids to ensure even distribution. The plates were dried overnight in a dark ventilated space, stored at 4 °C and used within a month. Live bacteria were seeded on fatty acid-containing plates 24 h before worms were transferred to the plates. Although using fixed—metabolically inactive148—bacteria would be helpful for dietary MUFA supplementation, many experiments using MUFAs are also RNAi experiments, which require live bacteria.
For supplementation experiments, worms were kept on fatty acid-containing plates from egg lay onwards. If not noted otherwise, worms were treated with control (empty vector) RNAi. For experiments including lpin-1 knockdown, RNAi treatment and fatty acid supplementation were started simultaneously at adult day 1 to minimize the negative effects of lpin-1 knockdown on development.
Lifespan assays
All C. elegans lifespan assays were performed at 20 °C on RNAi plates. Hermaphrodites were age-synchronized with a short 4-h egg lay using synchronized young adult (adult day 1) parents. The hermaphrodites were transferred to new plates and scored at least every other day to avoid the presence of confounding progeny. Each lifespan assay was performed with three plates of approximately 30 worms per 6-cm RNAi plates (approximately 90 worms in total). Worms were scored as censored if they crawled off the media or died following vulval rupture/internal hatching. Worms were scored as dead when they failed to move following gentle prodding with a platinum wire pick (90% Pt, 10% Ir). For lifespan curves, censored worms were included until the day of censorship. All lifespan experiments were performed in a blinded and randomized manner unless noted otherwise. Key experiments were carried out three times independently and all experiments were performed at least twice independently, often with independent investigators (as indicated in the source data). Kaplan–Meier survival curves were plotted in Prism 9. For pairwise comparison, the log-rank Mantel–Cox statistical test in Prism was used. To test if two interventions significantly interact with each other, the Cox proportional hazard test was applied using R (version 3.6.3). All lifespan statistics are provided in the source data.
GC–MS analysis of fatty acid profiles
Targeted gas chromatography coupled with mass spectrometry (GC–MS) was performed as described before13 to ensure that fatty acid profiles change following ash-2 RNAi, and oleic acid and elaidic acid supplementation. Briefly, for each condition, approximately 500 age-synchronized young adult (adult day 1) animals were collected in M9 buffer and washed three times to remove residual bacteria in the worm pellets. For fatty acid supplementation experiments, worms were transferred to empty (no food) RNAi plates for 20 min to clear the gut of residual bacteria13. Worm pellets were lysed by sonication and the protein concentration of the lysate was determined using a Pierce BCA protein assay kit (Thermo Scientific, 23227). The fatty acid C13:0 (NuChek Prep; dissolved in methanol) was added to each sample to serve as the internal reference control for variations introduced during the derivatization and extraction steps. Fatty acids were derivatized into their respective fatty acid methyl esters (FAMEs) by incubation in 2% H2SO4 (Sigma Aldrich) in methanol (Fisher) at 55 °C overnight. The reaction was stopped by the addition of 1.5 ml water (Fisher; MS grade). The FAMEs were extracted in 300 µl hexane (Sigma Aldrich) by vigorous vortexing and centrifugation at 188g for 1 min. The hexane layer containing the FAMEs was transferred into an amber GC vial (Agilent). FAME analysis was performed using an Agilent 7890A gas chromatograph equipped with an HP-5MS column and the MassHunter software (version 10.0.368). Each FAME peak was identified based on its retention time and unique fragmentation ions, and quantified using a serial dilution standard curve. The peaks and ion fragmentation patterns derived from elaidic acid and cis-vaccenic acid overlapped. These two fatty acids could not be uniquely identified with our current protocol, and we report both fatty acids together (Extended Data Fig. 1m).
The FAME abundance measured by GC–MS was normalized to the internal C13:0 reference control of each sample. For each sample, the FAME concentration (µg ml−1) was normalized to protein concentration (mg ml−1) as microgram of fatty acid detected per milligram of protein (µg mg−1). The fatty acid concentration of the interventions was normalized to the fatty acid concentration of the empty vector control. The final ratio is expressed as relative fatty acid levels in the graph. Each experiment was carried out at least three times independently. Relative fatty acid abundances were plotted using Prism 9 and statistically significant differences between samples were assessed using a two-way analysis of variance with Bonferroni’s multiple comparison test.
Separating fluorescent worms using the BioSorter
To sort worms according to their lipid droplet number at young age (adult day 1), we separated hermaphrodites expressing the lipid droplet marker DHS-3 fused to GFP (dhs-3p::dhs-3::GFP, LIU1) according to their fluorescence intensity using the large particle BioSorter (Union Biometrica). To retrieve a large number of age-synchronized worms for the sorting procedure, approximately 5,000 eggs were laid by age-synchronized adult day 1 parent worms. After approximately 72 h at 20 °C, the eggs developed into adult worms that were collected in M9 buffer and sorted on a large-particle flow cytometer BioSorter (Union Biometrica) with a 6.5 psi sample cup pressure, 2.00 psi diverter pressure, 50% sheath flow rate, rotary valve, 8.0 ms drop width and 10.0 ms sort delay using the FlowPilot III software. These settings allowed for accurate sorting of single worms. The worms with the 10% highest and lowest fluorescence in the population were sorted using the 488-nm laser (Extended Data Fig. 3a). The worms were directly sorted onto 6-cm RNAi plates containing a bacterial lawn (HT115, empty vector). We confirmed that the sorting reflects the lipid droplet number by fluorescence confocal microscopy (Fig. 4b,c). For this, worms were mounted, imaged and lipid droplets quantified as described earlier. Each experiment was performed at least twice independently. BioSorter graphs were plotted using the FlowPilot III software and lipid droplet quantifications were plotted in Prism 9. Statistically significant differences between samples were assessed using the two-tailed unpaired nonparametric Mann–Whitney test.
Separating fluorescent worms through manual sorting
To sort worms according to their lipid droplet number at middle age (adult day 6), we manually separated hermaphrodites expressing the lipid droplet marker DHS-3 fused to GFP (dhs-3p::dhs-3::GFP, LIU1) according to their fluorescence intensity on a fluorescence dissecting microscope. To retrieve a large number of age-synchronized worms for the sorting procedure, approximately 5,000 eggs were laid by age-synchronized adult day 1 parent worms. After approximately 72 h at 20 °C, the eggs developed into adult worms. The adult worms were washed each day during their reproductive period to separate adult worms from larvae/eggs. For this, worms were collected in M9 buffer in a 15-ml tube and allowed to settle to the bottom of the tube. The supernatant was removed, and adult worms were resuspended in 15 ml of fresh M9. This washing procedure was repeated five times and the adult worms were transferred to 10-cm RNAi plates containing a bacterial lawn (HT115, empty vector). Middle-aged worms (adult day 6) were sorted under a fluorescence dissecting microscope (Leica M165 FC) equipped with a Sola light engine (Leica) onto 6-cm RNAi plates containing a bacterial lawn (HT115, empty vector). We confirmed that the sorting reflects the lipid droplet number by fluorescence confocal microscopy (Fig. 4e). For this, worms were mounted, imaged and lipid droplets quantified as described earlier. The experiment was performed three times independently. Graphs were plotted in Prism 9. Statistically significant differences between samples were assessed using the two-tailed unpaired nonparametric Mann–Whitney test.
Sample preparation for lipidomics
To analyze lipid composition using mass spectrometry, wild-type hermaphrodites were treated with control (empty vector) and ash-2 RNAi until middle age (adult day 6). Each condition consists of six biological replicates. To retrieve a large number of age-synchronized worms, approximately 500 eggs were laid by age-synchronized adult day 1 parent worms per replicate plate. After approximately 72 h at 20 °C, the eggs developed into adult worms. The adult worms were washed each day during their reproductive period to separate adult worms from larvae/eggs. For this, worms were collected in M9 buffer in a 15-ml tube and allowed to settle to the bottom of the tube. The supernatant was removed, and adult worms were resuspended in 15 ml of fresh M9. This washing procedure was repeated six times and the adult worms were transferred to 6-cm RNAi plates containing a bacterial lawn (HT115, empty vector or ash-2 RNAi). Middle-aged (adult day 6) worms were transferred to empty RNAi plates without any bacteria for 15 min to clear the gut of residual bacteria. The worms were collected in 200 µl M9 in Protein LoBind tubes (Eppendorf, 13-698-794). The worms were lysed using a pre-chilled stainless steel homogenizer (Wheaton, 357572) with 15 plunger strokes and the protein concentration of the lysate was determined using a Pierce BCA protein assay kit (Thermo Scientific, 23227). The lysate (from approximately 500 worms) was frozen on dry ice and stored at −80 °C.
Lipid isolation for mass spectrometry
Lipids from whole-worm lysates were extracted using a biphasic separation with methyl tert-butyl ether, methanol and water149. LC–MS/MS grade reagents were used for lipidomics. Briefly, 298 μl of ice-cold methanol and 2 μl of internal standard (equiSPLASH, Avanti Polar Lipids, 330731) were added to 50 μl of worm lysate. The mixture was vortexed for 20 s and 1,000 μl of ice-cold methyl tert-butyl ether was added. The mixture was incubated under agitation for 30 min at 4 °C. After the addition of 250 μl water, the samples were vortexed for 1 min and centrifuged at 14,000g for 10 min at room temperature. The upper phase containing the lipids was collected and dried under nitrogen. The dry extracts were reconstituted with 300 μl of 9:1 methanol:toluene (Fisher Scientific) with 10 mM ammonium acetate (Sigma Aldrich) and centrifuged at 14,000g for 5 min. Water extracted using the same protocol was used as a blank control. Samples were randomized during lipid extraction.
LC–MS/MS
To identify complex lipids, isolated lipids were analyzed with untargeted lipidomics using liquid chromatography coupled to a Q Exactive mass spectrometer (Thermo Fisher; LC–MS/MS). Lipids were separated using an Accucore C30 column 2.1 mm × 150 mm, 2.6 μm (Thermo Scientific, 27826-152130). The mobile phase solvents consisted of 1 mM ammonium formate and 0.1% formic acid in 60/40 acetonitrile/water (A), and 1 mM ammonium formate and 0.1% formic acid in 90/10 isopropanol/acetonitrile (B). The gradient profile used was 30% B for 3 min, 30–43% B over 5 min, 43–50% B over 1 min, 55–90% B over 9 min, 90–99% B over 9 min and 99% B for 5 min. Lipids were eluted from the column at 0.2 ml min−1, the oven temperature was set at 30 °C and the injection volume was 15 μl. The autosampler temperature was set at 15 °C to prevent lipid aggregation. The Q Exactive plus was equipped with a HESI-II probe and operated in full MS scan mode for all samples. MS/MS spectra were acquired in a data-dependent acquisition mode on pooled samples. The source conditions were as follows: spray voltage = 3.5 kV (ESI positive mode), vaporizer = 200 °C, capillary temperature = 375 °C, S-lens = 55.0%, SheathGas = 40, auxiliary gas = 8 and SweepGas = 0. The following acquisition settings were used: AGC (MS) = 3 × 106, AGC (MS2) = 1 × 105, maximum injection time (MS) = 200 ms, maximum injection time (MS2) = 50 ms, mass range = 260–1,900 Da, resolution MS = 70,000 (FWHM at m/z 200), resolution MS2 = 35,000 (FWHM at m/z 200), MS2 spectra were acquired in the top-10 ions in each cycle, isolation window = 1.0 m/z, dynamic exclusion = 12 s and normalized collision energy = 25–30. External calibration was performed using an infusion of Pierce LTQ Velos ESI positive ion calibration solution.
Analysis of mass spectrometry results
Spectra were acquired in both positive and negative mode in a data-dependent manner. Lipid species were identified by matching the precursor ion mass to a database and the experimental MS/MS spectra to a spectral library containing theoretical fragmentation spectra using the LipidSearch software (version 4.1; Thermo Scientific)150. LipidSearch identifies phospholipids with fatty acid compositions due to the alignment of positive- and negative-mode liquid chromatography runs. Peaks corresponding to individual species were identified in both modes obtaining information of the polar head in positive mode and information of the fatty acyl chain in negative mode, similar to as described before151. The output of the LipidSearch software is available in GitHub (https://github.com/brunetlab). Further data processing was done using an in-house analysis pipeline written in R (version 3.6.3; available in GitHub at https://github.com/brunetlab). Briefly, processing for samples and spike-in standards were done in the same way. All ions for one lipid were aggregated and lipids with a signal <0 were discarded from further analysis. Lipid species were quantified using the corresponding internal standard (equiSPLASH, Avanti Polar Lipids, 330731) for each lipid class. Lipids with signals lower than 3× the blank signal were discarded. Lipids with more than 50% of missing values were discarded, and for the remaining missing values, imputation was performed. For this, a value was randomly assigned based on the bottom 5% for the corresponding lipid. Lipids were filtered for a coefficient of variance <0.5. Each sample was divided by its corresponding protein concentration to correct for sample-input variations. To calculate normalized abundance, each lipid within a sample was divided by the sample median, followed by multiplication with the global median. This resulted in a total of 499 filtered and normalized lipids belonging to 16 lipid classes. For a complete list of all lipidomic data, see Supplementary Table 3. Changes in the most abundant lipid classes—triacylglycerol (TG), phosphatidylethanolamine (PE), phosphatidylcholine (PC) and phosphatidylinositol (PI)—as well as differences in fatty acyl chain abundances are provided in the source data. The peroxidation index was used as a measure of the likelihood of lipid oxidation and was calculated using the following formula: 0.025 × (percentage of monoenoics) + 1 × (percentage of dienoics) + 2 × (percentage of trienoics) + 4 × (percentage of tetraenoics) + 6 × (percentage of pentaenoics) + 8 × (percentage of hexaenoics)152. Box plots were plotted using R (Version 3.6.3). Statistically significant differences between samples were assessed using the two-tailed Wilcoxon test with the Benjamini–Hochberg test for multiple hypothesis correction.
MDA levels
To quantify lipid oxidation, we measured MDA, a product of the degradation of oxidized lipids. A lipid peroxidation kit (Abcam, ab118970), which uses a colorimetric reaction of MDA with thiobarbituric acid, was used to quantify the MDA levels in whole-worm lysates. Worms were treated with control (empty vector) and ash-2 RNAi until middle age (adult day 5). Each condition consisted of several (2–4) biological replicates. Preparation of worms was carried out as described in ‘Lipid isolation for mass spectrometry’. The worm lysates were adjusted to the same protein concentration (100 µg µl−1) and MDA levels were measured according to the manufacturer’s instructions with the following exception: 50 µl lysate together with 150 µl TBA substrate solution were used, which allowed downscaling of sample sizes while still being in the range of the standard curve. Absorbance/fluorescence was measured using a Varioskan LUX spectrophotometer (Thermo Scientific). The investigators were not blinded to allocation during experiments and outcome assessment. Each experiment was performed at least twice independently. The MDA levels of samples were normalized to control (empty vector) RNAi conditions of middle-aged worms (adult day 5). Graphs from all independent experiments were plotted together using Prism 9. Each dot shape represents an independent experiment. Statistically significant differences between samples were assessed using the two-tailed unpaired nonparametric Mann–Whitney test.
4-HNE measurement by western blot
To measure lipid oxidation, we quantified the levels of 4-HNE, which is a product of the degradation of oxidized lipids and can be used as a proxy for lipid oxidation. 4-HNE reacts with cellular proteins and can be visualized using western blots of whole-worm lysates69. Briefly, a large number of worms was generated as described in ‘Lipid isolation for mass spectrometry’, with the following exceptions: worms were aged until adult days 1 and 8 and lysed by sonication for 30 s at 15 W on a Virsonic 600 Ultrasonic Homogenizer (Virtis Virsonic) to homogenize the tissues. The protein concentration of the lysate was determined using a Pierce BCA protein assay kit (Thermo Scientific, 23227). The protein (20–25 µg per sample) was loaded on a NuPAGE 4–12% bis-Tris PAGE gel and transferred onto a polyvinylidene fluoride membrane (0.45 µm). Ponceau staining was used to verify even transfer across the whole blot. The blots were blocked with 5% skim milk in PBS containing 0.1% Tween (PBST) and incubated using the following antibodies and dilutions: anti-4-HNE (1:2,000; AB5605, Millipore Sigma), anti-α-tubulin (1:10,000; T6074, Millipore Sigma), anti-goat-HRP (1:10,000; 401515, Calbiochem) or anti-mouse-HRP (1:10,000; 401215, Calbiochem) and visualized using enhanced chemiluminescence detection reagent (Amersham ECL, GE Healthcare). Each experiment was performed twice independently.
Propidium iodide staining
To test for cell and membrane integrity, wild-type worms were stained with propidium iodide. Propidium iodide is a fluorescent molecule that only stains cells that have lost membrane integrity78. Hermaphrodites were age-synchronized with a short 4-h egg lay using synchronized young adult (adult day 1) wild-type parents. The worms were transferred to new plates every other day until adult day 11 to avoid the presence of confounding progeny. For each experiment, approximately 50 worms were imaged per condition. As a positive control for death, worms were heat shocked at 39 °C for 30 min. Propidium iodide staining was performed as described before69. Briefly, plates were prepared by mixing 2.5 µl of 0.5 mg ml−1 propidium iodide (BioLegend, 421301) with 50 µl of HT115 bacteria and adding this mix onto RNAi plates. Propidium iodide plates were prepared no more than 24 h before the staining and kept in the dark. Worms were stained with propidium iodide for 24 h. Live worms were prepared for imaging (except for the heat-shock control, in which dead worms were prepared for imaging) by mounting and anesthetizing them as described above. Images were taken using a Zeiss confocal microscope (LSM900, Axio Observer) with a ×10 air objective (×10: Pln Apo ×10/0.45 DIC II), a 23-µm pinhole (optical section thickness of 15.9 µm) and the Zen software (3.0, blue). Whole worms were imaged over 8 µm (three slices with a 4.02 µm interval). The same laser power was used within one experiment. The propidium iodide signal per worm was analyzed in Fiji version 2.0.0 (ref. 143) by generating z-stack projections and measuring the mean fluorescence intensity of each worm. The investigators were not blinded to allocation during experiments and outcome assessment. Each experiment was performed three times independently. The mean propidium iodide signal per worm was plotted in Prism 9 and statistically significant differences between samples were tested using the two-tailed nonparametric Mann–Whitney test.
Prevention of lipid oxidation by iron chelation via salinazid
To inhibit lipid oxidation, we exposed worms to salinazid (LGC Standards, DRE-C16904350), a lipophilic compound that scavenges intracellular iron, thereby inhibiting iron-triggered lipid peroxidation that induces ferroptosis69,153. We used published protocols and concentrations for salinazid treatment69. Briefly, salinazid was dissolved in dimethylsulfoxide and added to autoclaved RNAi media before solidification to a final concentration of 250 µM. The plates were dried overnight in a dark ventilated space, stored at 4 °C and used within a month. Worms were transferred to salinazid and control (dimethylsulfoxide) plates at adult day 1. Lifespan and lipid oxidation were measured as described earlier.
Re-analysis of gene expression datasets
We re-analyzed two independent gene expression datasets that were generated previously in the laboratory to test for shared GO term enrichment in conditions that lead to MUFA accumulation. First, we analyzed an RNA-sequencing dataset from the intestine of young adult (adult day 1) worms treated with ash-2 RNAi13. We selected genes that had a log2-transformed fold enrichment larger than one (42 genes; adjusted P < 0.05) and analyzed enrichment for GO terms using WormEnrichR154,155. All categories of GO terms were included in the subsequent analyses. Second, we analyzed a microarray dataset from the entire body of middle-aged (adult day 5) worms treated with ash-2 RNAi39. We selected genes that had a log2-transformed fold enrichment larger than one (304 genes; adjusted P < 0.05) and analyzed enrichment for GO terms using WormEnrichR. All categories of GO terms were included in the subsequent analyses. Significantly enriched GO terms (combined score > 5)154–156 that were shared between the two datasets were plotted using R (Version 3.6.3). The combined score was calculated by multiplying the P value retrieved from the Fisher’s Exact test with the z-score. For a detailed list of all GO terms, see the source data.
Confocal microscopy for peroxisome quantification
Peroxisomes were visualized by confocal microscopy using worm strains that express a fluorophore fused to a peroxisome localization sequence (SKL). The fluorophore was either GFP for ges-1p::GFP–SKL (VS15) and eft-3p::GFP–SKL (WBM1177) or mRFP for vha-6p::mRFP–SKL; dsh-3p::dhs-3::GFP (ABR161). Transgenic worms were imaged at middle age (adult day 6), unless noted otherwise. For each experiment, approximately 20 hermaphrodites were imaged per condition. The last intestinal cells were imaged80 because they retain homogenous fluorophore expression with age. Worms were mounted as described earlier for lipid droplet quantification. The last intestinal cells (int9R, int9L or both) were imaged using the settings described for lipid droplet quantification. Images were processed in Fiji version 2.0.0 (ref. 143) by generating z-stack projections of five slices, applying the same threshold to all images and analyzing peroxisomes in a 26 × 26 µm2 area. For experiments with eft-3p::GFP–SKL (WBM1177), a smaller area of 13 × 13 µm2 was analyzed to exclude hypodermal peroxisomes. The Squassh plugin147 was used to quantify peroxisomes using the following settings: the background was subtracted with a rolling-ball radius of ten and the segmentation parameters were set at 0.075 regularization with a minimum object intensity of 0.05. Peroxisomes usually appeared as fluorescent puncta. On the rare occasion when the fluorophore was only cytosolic and failed to localize to peroxisomes, the plugin did not perform correctly, and these quantifications were excluded from further analysis. All experiments and analyses were performed in a blinded manner unless noted otherwise in the source data. Most experiments were performed three times independently but at least twice independently. Peroxisome number was plotted in Prism 9 and statistically significant differences between samples were tested using the two-tailed unpaired nonparametric Mann–Whitney test.
Lipid droplet and peroxisome number as a function of age
To visualize lipid droplet and peroxisome dynamics as a function of age, we imaged worms during their adult life. Lipid droplets and peroxisomes were visualized at different ages and quantified as described in the previous section. For each experiment, approximately 24 hermaphrodites were imaged per condition. Peroxisomes were visualized by mRFP and lipid droplets by GFP in the vha-6p::mRFP–SKL; dsh-3p::dhs-3::GFP (ABR161) double-marker strain in the last intestinal cells as described above. Lipid droplet and peroxisome number was quantified using the same region of interest per worm. The investigators were not blinded to allocation during experiments and outcome assessment. Experiments were carried out twice independently. Mean organelle number and regression lines were plotted using Prism 9.
Targeted lipid droplet and peroxisome screen
To identify regulators of the lipid droplet–peroxisome network, we performed a targeted RNAi screen. We selected genes to target for the screen based on proteins identified by mass spectrometry at the surface of lipid droplets40,41 and based on annotated protein function in lipid metabolism, lipid droplet biology, peroxisome biology, lipid transport and transcription factors involved in lipid metabolism. As a positive control, we used ash-2 RNAi and prx-5 RNAi. We measured the effect of RNAi knockdown of these genes on lipid droplet and peroxisome number at middle age (adult day 6) in approximately 30 hermaphrodites per condition. As the worms were imaged at day 6, it is possible that some of the effects we observed were due to compensatory mechanisms. Lipid droplets and peroxisomes were visualized and quantified as described earlier. The screen was performed in eight groups. Each group contained control (empty vector), ash-2 and prx-5 RNAi. Peroxisomes were visualized by mRFP (except for the first group) and lipid droplets by GFP in the vha-6p::mRFP–SKL; dsh-3p::dhs-3::GFP (ABR161) double-marker strain. For the first group, peroxisomes were measured using the single marker strain ges-1p::GFP–SKL (VS15) and the corresponding lipid droplet number was from an independent experiment. For all other groups, lipid droplets and peroxisomes were quantified using the same 26 × 26 µm2 region of interest per worm in the last intestinal cells. Organelles were quantified as described above and normalized to the control RNAi within the corresponding group. All experiments and analyses were performed in a blinded manner. To assess correlation, the mean number of organelles normalized to the control RNAi was plotted using Prism 9 and statistically significant correlation was tested using the two-tailed Pearson’s R2 test. Manhattan graphs were plotted using Prism 9 and statistical significance was tested using a two-tailed Wilcoxon test with Benjamini–Hochberg correction for multiple hypothesis correction.
Transmission electron microscopy
We performed transmission electron microscopy to visualize cellular organelles. To ensure we identified the organelles correctly, we immunostained lipid droplets with gold antibody (see the representative image in Extended Data Fig. 5m). To this end, middle-aged (adult day 6) vha-6p::mRFP–SKL; dsh-3p::dhs-3::GFP (ABR161) hermaphrodites were fixed using a Leica ICE high pressure freezer (Leica) by placing them in a Type A, 200 µm deep, 6 mm diameter, gold-coated specimen carrier (Leica, 6770181) and covered with a Type B, 300 µm deep, 6 mm diameter aluminum specimen carrier (Leica, 16770127). The worms were picked along with enough bacteria to fill the carrier. Once frozen, carriers with worms were placed into cryo-vials with frozen fixative (1% uranyl acetate and 1% glutaraldehyde in acetone). For embedding the worms in resin, the samples were placed into a paused, pre-cooled/programmed Leica AFS unit at −50 °C, washed with acetone and then stepwise infiltrated with Lowicryl HM20 (EMSdiasum, 14345), followed by exposure to only Lowicryl HM20 (EMSdiasum, 14345). Individual specimen carriers were placed into cooled flat-bottomed TAAB plastic capsules (EMSdiasum, 70021), filled with cold Lowicryl HM20 (EMSdiasum, 14345), covered and exposed to ultraviolet light to polymerize the resin. Next, 80-nm sections were prepared using an UC7 ultramicrotome (Leica) and transferred to nickel grids. The samples were rehydrated in PBST, blocked in 0.5% ovalbumin, followed by an incubation in antibody to GFP (1:100; Abcam, ab6556) and by protein A gold (20 nm; 1:50; Sigma Aldrich, P6855). The samples were fixed in 8% glutaraldehyde, contrast stained for 40 s in 3.5% uranyl acetate in 50% acetone, followed by Sato’s lead citrate for 2 min. The sections were observed using a JEOL JEM-1400 120 kV (Jeol USA) microscope and images were taken using a Gatan OneView 4 k × 4 k digital camera (Gatan). For quantification of organelle proximity, we also used images of non-immunostained wild-type worms. To this end, middle-aged (adult day 6) wild-type hermaphrodites were fixed and sectioned157. The experiment was performed twice independently. Organelles in close proximity/direct organelle contacts were identified manually and plotted as bar graphs using Prism 9.
Statistics and reproducibility
Parameters such as the minimum n value, mean ± s.d. and significant P values are reported in the figures, figure legends and source data. Significance was defined as P < 0.05. Pairwise comparisons were made using the Mann–Whitney test. When more than five comparisons were made, Benjamini–Hochberg correction was used for multiple hypothesis correction. When testing for correlation, Pearson’s correlation test was used, and normality distribution was confirmed using the Kolmogorov–Smirnov test. For pairwise comparison of lifespan data, the log-rank Mantel–Cox statistical test was used. To test if two lifespan interventions significantly interact with each other, the Cox proportional hazard test was applied using R (version 3.6.3). All statistics are reported in the source data. Statistical analyses were performed using Prism 9 or R (Version 3.6.3).
No statistical method was used to pre-determine sample size. No data were excluded from analysis, except for the peroxisome imaging, as described earlier. The lifespan and lipidomics assays were randomized, all other experiments were not randomized. The investigators were blinded to allocation during experiments and outcome assessment for most experiments (detailed list in the source data). Quantification and statistics were performed within a single experiment, except for GC–MS fatty acid, relative messenger RNA and MDA quantifications, which were analyzed across all replicates. Most assays were performed in three independent experiments unless specified in the corresponding method section and source data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41556-023-01136-6.
Supplementary information
Supplementary Tables 1–3.
Acknowledgements
We thank the Stanford Mass Spectrometry facility (Vincent Coates Foundation Mass Spectrometry Laboratory) and A. Chien for GC–MS use and support, the Stanford High Throughput BioCenter and D. E. Solow-Cordero for BioSorter use and support (NIH S10OD025004), and the Cell Science Imaging Facility and J. Perrino for use and support (NIH S10OD028536). We thank the Brunet Laboratory—in particular C. Bedbrook, L. N. Booth, J. Chen, P. Navarro and X. Zhao—for feedback and reading of the manuscript. We thank A. S. McKay and X. Zhao for independently checking scripts and B. Michael for his help uploading the lipidomics data to the public data repository. We thank J. Long for insightful discussion and critical reading of the manuscript. This work was supported by NIH R01AG054201 (A.B. and W.B.M.), NIH R01AT01023204 (M.P.S.), NIH RM1HG00773509 (M.P.S.), NIH DP1DK113644 (M.C.W.), the Howard Hughes Medical Institute (M.C.W.), a Deutsche Forschungsgemeinschaft (DFG) postdoctoral fellowship (K.P.) and a Hillblom Foundation postdoctoral fellowship (J.W.M.). Elements of Figs. 1,3–5,7,8 and Extended Data Fig. 6 were created with BioRender.com.
Extended data
Source data
Statistical source data Fig. 1.
Statistical source data Fig. 2.
Statistical source data Fig. 3.
Statistical source data Fig. 4.
Statistical source data Fig. 5.
Statistical source data Fig. 6.
Western blots Fig. 6.
Statistical source data Fig. 7.
Statistical source data Fig. 8.
Statistical source data Extended Data Fig. 1.
Statistical source data Extended Data Fig. 2.
Statistical source data Extended Data Fig. 4.
Statistical source data Extended Data Fig. 5.
Author contributions
K.P. and A.B. planned the study. K.P. performed and analyzed all experiments, except for those indicated below. J.W.M. performed the peroxisome sorting experiment and participated in the planning and analysis of the study. A.H. performed and analyzed the peroxisome quantification, targeted screen, independent repeats of lifespan experiments, salinazid experiments and western blots. M.C. ran lipidomics samples supervised by M.P.S. C.S.M. performed and analyzed subsets of the targeted screen supervised by W.B.M. M.S. and Y.Y. performed and analyzed the SRS experiments supervised by M.C.W. C.G.S.G. generated several C. elegans lines supervised by W.B.M. N.R.H. performed the propidium iodide staining and independent replicates of organelle number and lifespan experiments. L.M.M. and E.d.L. helped K.P. with data collection on lipid droplet and peroxisome number, respectively. P.Y. generated the WBM1177 line, supervised by W.B.M. C.G.S.G., W.B.M and S.J.D. had intellectual input in the study. K.P. and A.B. wrote the manuscript, and all authors provided comments.
Peer review
Peer review information
Nature Cell Biology thanks Alexander Mendenhall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Raw lipidomic files are available at Metabolomics Workbench (https://www.metabolomicsworkbench.org/) under the study ID ST002504. All other lipidomic files are available in the GitHub repository for this paper (https://github.com/brunetlab). For genes used in this study, the annotated WormBase name was used (www.wormbase.org). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
The code used to analyze lipidomic data in this study is available in the GitHub repository for this paper (https://github.com/brunetlab).
Competing interests
A.B. is a scientific advisor of Calico. M.P.S. is a co-founder and scientific advisor of Personalis, SensOmics, Qbio, January AI, Fodsel, Filtricine, Protos, RTHM, Iollo, Marble Therapeutics, Crosshair Therapeutics and Mirvie. He is a scientific advisor of Jupiter, Neuvivo, Swaza and Mitrix. S.J.D. is a co-founder of Prothegen Inc, a scientific advisor of Hillstream BioPharma and holds patents related to ferroptosis. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/11/2023
A Correction to this paper has been published: 10.1038/s41556-023-01220-x
Extended data
is available for this paper at 10.1038/s41556-023-01136-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41556-023-01136-6.
References
- 1.Mutlu AS, Duffy J, Wang MC. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell. 2021;56:1394–1407. doi: 10.1016/j.devcel.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bustos V, Partridge L. Good ol’ fat: links between lipid signaling and longevity. Trends Biochem. Sci. 2017;42:812–823. doi: 10.1016/j.tibs.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder EA, Brunet A. Lipid profiles and signals for long life. Trends Endocrinol. Metab. 2015;26:589–592. doi: 10.1016/j.tem.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puca AA, Chatgilialoglu C, Ferreri C. Lipid metabolism and diet: possible mechanisms of slow aging. Int. J. Biochem. Cell Biol. 2008;40:324–333. doi: 10.1016/j.biocel.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Qi W, et al. The ω-3 fatty acid α-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARα and oxidation to oxylipins. Aging Cell. 2017;16:1125–1135. doi: 10.1111/acel.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folick A, et al. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science. 2015;347:83–86. doi: 10.1126/science.1258857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosti V, Bertozzi B, Fontana L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J. Gerontol. A. 2018;73:318–326. doi: 10.1093/gerona/glx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi LS, Shi C, Ashraf J, Sohrabi S, Murphy CT. Oleic acid protects Caenorhabditis mothers from mating-induced death and the cost of reproduction. Front. Cell Dev. Biol. 2021;9:690373. doi: 10.3389/fcell.2021.690373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez MA, Newman AB. Can a Mediterranean diet pattern slow aging? J. Gerontol. A. 2018;73:315–317. doi: 10.1093/gerona/gly003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez-Tortosa CL, et al. Longevity and cause of death in male wistar rats fed lifelong diets based on virgin olive oil, sunflower oil, or fish oil. J. Gerontol. A. 2020;75:442–451. doi: 10.1093/gerona/glz091. [DOI] [PubMed] [Google Scholar]
- 12.Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S, et al. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature. 2017;544:185–190. doi: 10.1038/nature21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, et al. Enhanced energy metabolism contributes to the extended life span of calorie-restricted Caenorhabditis elegans. J. Biol. Chem. 2012;287:31414–31426. doi: 10.1074/jbc.M112.377275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudeau J, et al. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol. 2011;9:e1000599. doi: 10.1371/journal.pbio.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imanikia S, Sheng M, Castro C, Griffin JL, Taylor RC. XBP-1 remodels lipid metabolism to extend longevity. Cell Rep. 2019;28:581–589. doi: 10.1016/j.celrep.2019.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Goodman JM. The lipid droplet—a well-connected organelle. Front. Cell Dev. Biol. 2015;3:49. doi: 10.3389/fcell.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marschallinger J, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020;23:194–208. doi: 10.1038/s41593-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluchowski NL, Becuwe M, Walther TC, Farese RV., Jr Lipid droplets and liver disease: from basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017;14:343–355. doi: 10.1038/nrgastro.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geltinger F, et al. The transfer of specific mitochondrial lipids and proteins to lipid droplets contributes to proteostasis upon stress and aging in the eukaryotic model system Saccharomyces cerevisiae. Geroscience. 2020;42:19–38. doi: 10.1007/s11357-019-00103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey AP, et al. Antioxidant role for lipid droplets in a stem cell niche of Drosophila. Cell. 2015;163:340–353. doi: 10.1016/j.cell.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ugrankar R, et al. Drosophila Snazarus regulates a lipid droplet population at plasma membrane-droplet contacts in adipocytes. Dev. Cell. 2019;50:557–572. doi: 10.1016/j.devcel.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krahmer N, Farese RV, Jr, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol. Med. 2013;5:973–983. doi: 10.1002/emmm.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mashek DG. Hepatic lipid droplets: a balancing act between energy storage and metabolic dysfunction in NAFLD. Mol. Metab. 2021;50:101115. doi: 10.1016/j.molmet.2020.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralhan I, Chang CL, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. J. Cell Biol. 2021;220:e20212136. doi: 10.1083/jcb.202102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najt CP, et al. Lipid droplet-derived monounsaturated fatty acids traffic via PLIN5 to allosterically activate SIRT1. Mol. Cell. 2020;77:810–824. doi: 10.1016/j.molcel.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch M, et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. 2020;370:eaay8085. doi: 10.1126/science.aay8085. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran PV, Mutlu AS, Wang MC. Label-free biomedical imaging of lipids by stimulated Raman scattering microscopy. Curr. Protoc. Mol. Biol. 2015;109:30.33.31–30.33.17. doi: 10.1002/0471142727.mb3003s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, Yavuz A, Wang MC. Dissecting lipid droplet biology with coherent Raman scattering microscopy. J. Cell Sci. 2022;135:jcs252353. doi: 10.1242/jcs.252353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutlu AS, Chen T, Deng D, Wang MC. Label-free imaging of lipid storage dynamics in Caenorhabditis elegans using stimulated Raman scattering microscopy. J. Vis. Exp. 2021;171:e61870. doi: 10.3791/61870. [DOI] [PubMed] [Google Scholar]
- 34.Chen WW, et al. Spectroscopic coherent Raman imaging of Caenorhabditis elegans reveals lipid particle diversity. Nat. Chem. Biol. 2020;16:1087–1095. doi: 10.1038/s41589-020-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barros AG, Liu J, Lemieux GA, Mullaney BC, Ashrafi K. Analyses of C. elegans fat metabolic pathways. Methods Cell. Biol. 2012;107:383–407. doi: 10.1016/B978-0-12-394620-1.00013-8. [DOI] [PubMed] [Google Scholar]
- 36.Brock TJ, Browse J, Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 2007;176:865–875. doi: 10.1534/genetics.107.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol. Metab. 2009;20:58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greer EL, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Na H, et al. Identification of lipid droplet structure-like/resident proteins in Caenorhabditis elegans. Biochim. Biophys. Acta. 2015;1853:2481–2491. doi: 10.1016/j.bbamcr.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Vrablik TL, Petyuk VA, Larson EM, Smith RD, Watts JL. Lipidomic and proteomic analysis of Caenorhabditis elegans lipid droplets and identification of ACS-4 as a lipid droplet-associated protein. Biochim. Biophys. Acta. 2015;1851:1337–1345. doi: 10.1016/j.bbalip.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniele JR, et al. UPRER promotes lipophagy independent of chaperones to extend life span. Sci. Adv. 2020;6:eaaz1441. doi: 10.1126/sciadv.aaz1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, et al. Hydroxysteroid dehydrogenase family proteins on lipid droplets through bacteria, C. elegans, and mammals. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2018;1863:881–894. doi: 10.1016/j.bbalip.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Ganguly R, Pierce GN. Trans fat involvement in cardiovascular disease. Mol. Nutr. Food Res. 2012;56:1090–1096. doi: 10.1002/mnfr.201100700. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P, Reue K. Lipin proteins and glycerolipid metabolism: roles at the ER membrane and beyond. Biochim. Biophys. Acta Biomembr. 2017;1859:1583–1595. doi: 10.1016/j.bbamem.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 47.Adeyo O, et al. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 2011;192:1043–1055. doi: 10.1083/jcb.201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, et al. Lipin-1γ isoform is a novel lipid droplet-associated protein highly expressed in the brain. FEBS Lett. 2011;585:1979–1984. doi: 10.1016/j.febslet.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sellers RS, Mahmood SR, Perumal GS, Macaluso FP, Kurland IJ. Phenotypic modulation of skeletal muscle fibers in LPIN1-deficient lipodystrophic (fld) mice. Vet. Pathol. 2019;56:322–331. doi: 10.1177/0300985818809126. [DOI] [PubMed] [Google Scholar]
- 50.Jung Y, et al. Caenorhabditis elegans Lipin 1 moderates the lifespan-shortening effects of dietary glucose by maintaining ω-6 polyunsaturated fatty acids. Aging Cell. 2020;19:e13150. doi: 10.1111/acel.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smulan LJ, et al. Cholesterol-independent SREBP-1 maturation is linked to ARF1 inactivation. Cell Rep. 2016;16:9–18. doi: 10.1016/j.celrep.2016.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterson TR, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Rui BB, Tang LY, Hu CM. Lipin family proteins—key regulators in lipid metabolism. Ann. Nutr. Metab. 2015;66:10–18. doi: 10.1159/000368661. [DOI] [PubMed] [Google Scholar]
- 54.Golden A, Liu J, Cohen-Fix O. Inactivation of the C. elegans lipin homolog leads to ER disorganization and to defects in the breakdown and reassembly of the nuclear envelope. J. Cell Sci. 2009;122:1970–1978. doi: 10.1242/jcs.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nettebrock NT, Bohnert M. Born this way-biogenesis of lipid droplets from specialized ER subdomains. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158448. doi: 10.1016/j.bbalip.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Szymanski KM, et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl Acad. Sci. USA. 2007;104:20890–20895. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cartwright BR, et al. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology. Mol. Biol. Cell. 2015;26:726–739. doi: 10.1091/mbc.E14-08-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, et al. Seipin is required for converting nascent to mature lipid droplets. eLife. 2016;5:e16582. doi: 10.7554/eLife.16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao Z, et al. Dietary fatty acids promote lipid droplet diversity through seipin enrichment in an ER subdomain. Nat. Commun. 2019;10:2902. doi: 10.1038/s41467-019-10835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 61.Yang L, et al. Neuronal lipolysis participates in PUFA-mediated neural function and neurodegeneration. EMBO Rep. 2020;21:e50214. doi: 10.15252/embr.202050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sathyanarayan A, Mashek MT, Mashek DG. ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism. Cell Rep. 2017;19:1–9. doi: 10.1016/j.celrep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bazopoulou D, et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature. 2019;576:301–305. doi: 10.1038/s41586-019-1814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Booth LN, Maures TJ, Yeo RW, Tantilert C, Brunet A. Self-sperm induce resistance to the detrimental effects of sexual encounters with males in hermaphroditic nematodes. eLife. 2019;8:e46418. doi: 10.7554/eLife.46418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 66.Doll S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui W, Liu D, Gu W, Chu B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021;28:2536–2551. doi: 10.1038/s41418-021-00769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou Y, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenkins NL, et al. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. eLife. 2020;9:e56580. doi: 10.7554/eLife.56580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kagan VE, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang WS, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magtanong L, et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem. Biol. 2022;29:1409–1418. doi: 10.1016/j.chembiol.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bersuker K, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dierge E, et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021;33:1701–1715. doi: 10.1016/j.cmet.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Watterson A, et al. Intracellular lipid surveillance by small G protein geranylgeranylation. Nature. 2022;605:736–740. doi: 10.1038/s41586-022-04729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Los FC, et al. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe. 2011;9:147–157. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang SO, et al. Genetic and dietary regulation of lipid droplet expansion in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2010;107:4640–4645. doi: 10.1073/pnas.0912308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weir HJ, et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 2017;26:884–896. doi: 10.1016/j.cmet.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Narayan V, et al. Deep proteome analysis identifies age-related processes in C. elegans. Cell Syst. 2016;3:144–159. doi: 10.1016/j.cels.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bersuker K, et al. A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Dev. Cell. 2018;44:97–112. doi: 10.1016/j.devcel.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watts JL, Ristow M. Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics. 2017;207:413–446. doi: 10.1534/genetics.117.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang CL, et al. Spastin tethers lipid droplets to peroxisomes and directs fatty acid trafficking through ESCRT-III. J. Cell Biol. 2019;218:2583–2599. doi: 10.1083/jcb.201902061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boström P, et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 2007;9:1286–1293. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- 86.Chung J, et al. LDAF1 and Seipin form a lipid droplet assembly complex. Dev. Cell. 2019;51:551–563. doi: 10.1016/j.devcel.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walther TC, Chung J, Farese RV., Jr Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 2017;33:491–510. doi: 10.1146/annurev-cellbio-100616-060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coppa A, et al. The peroxisomal fatty acid transporter ABCD1/PMP-4 is required in the C. elegans hypodermis for axonal maintenance: a worm model for adrenoleukodystrophy. Free Radic. Biol. Med. 2020;152:797–809. doi: 10.1016/j.freeradbiomed.2020.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thieringer H, Moellers B, Dodt G, Kunau WH, Driscoll M. Modeling human peroxisome biogenesis disorders in the nematode Caenorhabditis elegans. J. Cell Sci. 2003;116:1797–1804. doi: 10.1242/jcs.00380. [DOI] [PubMed] [Google Scholar]
- 90.Choudhary V, Ojha N, Golden A, Prinz WA. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J. Cell Biol. 2015;211:261–271. doi: 10.1083/jcb.201505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi X, et al. A Caenorhabditis elegans model for ether lipid biosynthesis and function. J. Lipid Res. 2016;57:265–275. doi: 10.1194/jlr.M064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen TB, Olzmann JA. Getting a handle on lipid droplets: insights into ER–lipid droplet tethering. J. Cell Biol. 2019;218:1089–1091. doi: 10.1083/jcb.201902160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joshi AS, Cohen S. Lipid droplet and peroxisome biogenesis: do they go hand-in-hand. Front. Cell Dev. Biol. 2019;7:92. doi: 10.3389/fcell.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joshi AS, et al. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat. Commun. 2018;9:2940. doi: 10.1038/s41467-018-05277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006;20:1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng L, et al. Nuclear receptors NHR-49 and NHR-79 promote peroxisome proliferation to compensate for aldehyde dehydrogenase deficiency in C. elegans. PLoS Genet. 2021;17:e1009635. doi: 10.1371/journal.pgen.1009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang F, et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442:700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 98.Heestand BN, et al. Dietary restriction induced longevity is mediated by nuclear receptor NHR-62 in Caenorhabditis elegans. PLoS Genet. 2013;9:e1003651. doi: 10.1371/journal.pgen.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liang B, Ferguson K, Kadyk L, Watts JL. The role of nuclear receptor NHR-64 in fat storage regulation in Caenorhabditis elegans. PLoS ONE. 2010;5:e9869. doi: 10.1371/journal.pone.0009869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y, Ding W, Li CY, Liu Y. HLH-11 modulates lipid metabolism in response to nutrient availability. Nat. Commun. 2020;11:5959. doi: 10.1038/s41467-020-19754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J, et al. An ESCRT-dependent step in fatty acid transfer from lipid droplets to mitochondria through VPS13D–TSG101 interactions. Nat. Commun. 2021;12:1252. doi: 10.1038/s41467-021-21525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mejhert N, et al. The Lipid Droplet Knowledge Portal: A resource for systematic analyses of lipid droplet biology. Dev. Cell. 2022;57:387–397. doi: 10.1016/j.devcel.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baldwin HA, et al. VPS13D promotes peroxisome biogenesis. J. Cell Biol. 2021;220:e202001188. doi: 10.1083/jcb.202001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schroeder B, et al. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61:1896–1907. doi: 10.1002/hep.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guerra F, Bucci C. Multiple roles of the small GTPase Rab7. Cells. 2016;5:34. doi: 10.3390/cells5030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen D, et al. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep. 2013;5:1600–1610. doi: 10.1016/j.celrep.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Geltinger F, et al. Friend or foe: lipid droplets as organelles for protein and lipid storage in cellular stress response, aging and disease. Molecules. 2020;25:5053. doi: 10.3390/molecules25215053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ioannou MS, et al. Neuron–astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Shimabukuro MK, et al. Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes. Sci. Rep. 2016;6:23795. doi: 10.1038/srep23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosquera JV, Bacher MC, Priess JR. Nuclear lipid droplets and nuclear damage in Caenorhabditis elegans. PLoS Genet. 2021;17:e1009602. doi: 10.1371/journal.pgen.1009602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lapierre LR, Gelino S, Meléndez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr. Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seah NE, et al. Autophagy-mediated longevity is modulated by lipoprotein biogenesis. Autophagy. 2016;12:261–272. doi: 10.1080/15548627.2015.1127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee G, et al. Post-transcriptional regulation of de novo lipogenesis by mTORC1–S6K1–SRPK2 signaling. Cell. 2017;171:1545–1558. doi: 10.1016/j.cell.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steinbaugh MJ, et al. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife. 2015;4:e07836. doi: 10.7554/eLife.07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lynn DA, et al. Omega-3 and -6 fatty acids allocate somatic and germline lipids to ensure fitness during nutrient and oxidative stress in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2015;112:15378–15383. doi: 10.1073/pnas.1514012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lapierre LR, Meléndez A, Hansen M. Autophagy links lipid metabolism to longevity in C. elegans. Autophagy. 2012;8:144–146. doi: 10.4161/auto.8.1.18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim HE, et al. Lipid biosynthesis coordinates a mitochondrial-to-cytosolic stress response. Cell. 2016;166:1539–1552. doi: 10.1016/j.cell.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Higuchi-Sanabria R, et al. Divergent nodes of non-autonomous UPRER signaling through serotonergic and dopaminergic neurons. Cell Rep. 2020;33:108489. doi: 10.1016/j.celrep.2020.108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goh GYS, et al. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell. 2018;17:e12743. doi: 10.1111/acel.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Webster CM, et al. Genome-wide RNAi screen for fat regulatory genes in C. elegans identifies a proteostasis–AMPK axis critical for starvation survival. Cell Rep. 2017;20:627–640. doi: 10.1016/j.celrep.2017.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heimbucher T, Hog J, Gupta P, Murphy CT. PQM-1 controls hypoxic survival via regulation of lipid metabolism. Nat. Commun. 2020;11:4627. doi: 10.1038/s41467-020-18369-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang P, Judy M, Lee SJ, Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Littlejohn NK, Seban N, Liu CC, Srinivasan S. A feedback loop governs the relationship between lipid metabolism and longevity. eLife. 2020;9:e58815. doi: 10.7554/eLife.58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang MC, O’Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen AL, et al. Pharmacological convergence reveals a lipid pathway that regulates C. elegans lifespan. Nat. Chem. Biol. 2019;15:453–462. doi: 10.1038/s41589-019-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee D, et al. MDT-15/MED15 permits longevity at low temperature via enhancing lipidostasis and proteostasis. PLoS Biol. 2019;17:e3000415. doi: 10.1371/journal.pbio.3000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amrit FR, et al. DAF-16 and TCER-1 facilitate adaptation to germline loss by restoring lipid homeostasis and repressing reproductive physiology in C. elegans. PLoS Genet. 2016;12:e1005788. doi: 10.1371/journal.pgen.1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liao CY, et al. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi: 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perez MA, Magtanong L, Dixon SJ, Watts JL. Dietary lipids induce ferroptosis in Caenorhabditis elegans and human cancer cells. Dev. Cell. 2020;54:447–454. doi: 10.1016/j.devcel.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perez MA, et al. Ether lipid deficiency disrupts lipid homeostasis leading to ferroptosis sensitivity. PLoS Genet. 2022;18:e1010436. doi: 10.1371/journal.pgen.1010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magtanong L, et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 2019;26:420–432. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kong J, et al. Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nat. Commun. 2020;11:578. doi: 10.1038/s41467-019-14176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castillo-Quan JI, et al. An antisteatosis response regulated by oleic acid through lipid droplet-mediated ERAD enhancement. Sci. Adv. 2023;9:eadc8917. doi: 10.1126/sciadv.adc8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silva-García CG, et al. Single-copy knock-in loci for defined gene expression in Caenorhabditis elegans. G3. 2019;9:2195–2198. doi: 10.1534/g3.119.400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paix A, Folkmann A, Rasoloson D, Seydoux G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR–Cas9 ribonucleoprotein complexes. Genetics. 2015;201:47–54. doi: 10.1534/genetics.115.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 142.Yu Y, Mutlu AS, Liu H, Wang MC. High-throughput screens using photo-highlighting discover BMP signaling in mitochondrial lipid oxidation. Nat. Commun. 2017;8:865. doi: 10.1038/s41467-017-00944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie K, et al. MDT-28/PLIN-1 mediates lipid droplet-microtubule interaction via DLC-1 in Caenorhabditis elegans. Sci. Rep. 2019;9:14902. doi: 10.1038/s41598-019-51399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu X, Liu Y, Zhang H, Liu P. Whole-genome RNAi screen identifies methylation-related genes influencing lipid metabolism in Caenorhabditis elegans. J. Genet. Genomics. 2018;45:259–272. doi: 10.1016/j.jgg.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 146.Zhang P, et al. Proteomic study and marker protein identification of Caenorhabditis elegans lipid droplets. Mol. Cell Proteom. 2012;11:317–328. doi: 10.1074/mcp.M111.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rizk A, et al. Segmentation and quantification of subcellular structures in fluorescence microscopy images using Squassh. Nat. Protoc. 2014;9:586–596. doi: 10.1038/nprot.2014.037. [DOI] [PubMed] [Google Scholar]
- 148.Beydoun S, et al. An alternative food source for metabolism and longevity studies in Caenorhabditis elegans. Commun. Biol. 2021;4:258. doi: 10.1038/s42003-021-01764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taguchi R, Ishikawa M. Precise and global identification of phospholipid molecular species by an Orbitrap mass spectrometer and automated search engine Lipid Search. J. Chromatogr. A. 2010;1217:4229–4239. doi: 10.1016/j.chroma.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 151.Yamada T, et al. Development of a lipid profiling system using reverse-phase liquid chromatography coupled to high-resolution mass spectrometry with rapid polarity switching and an automated lipid identification software. J. Chromatogr. A. 2013;1292:211–218. doi: 10.1016/j.chroma.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 152.Witting LA, Horwitt MK. Effect of degree of fatty acid unsaturation in tocopherol deficiency-induced creatinuria. J. Nutr. 1964;82:19–33. doi: 10.1093/jn/82.1.19. [DOI] [PubMed] [Google Scholar]
- 153.Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharm. Rev. 2005;57:547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 154.Chen EY, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuleshov MV, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuleshov MV, et al. modEnrichr: a suite of gene set enrichment analysis tools for model organisms. Nucleic Acids Res. 2019;47:W183–W190. doi: 10.1093/nar/gkz347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Richardson CE, et al. PTRN-1, a microtubule minus end-binding CAMSAP homolog, promotes microtubule function in Caenorhabditis elegans neurons. eLife. 2014;3:e01498. doi: 10.7554/eLife.01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–3.
Data Availability Statement
Raw lipidomic files are available at Metabolomics Workbench (https://www.metabolomicsworkbench.org/) under the study ID ST002504. All other lipidomic files are available in the GitHub repository for this paper (https://github.com/brunetlab). For genes used in this study, the annotated WormBase name was used (www.wormbase.org). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
The code used to analyze lipidomic data in this study is available in the GitHub repository for this paper (https://github.com/brunetlab).