Abstract
Background
Respiratory failure is the primary cause of death in patients with COVID-19, whereas coagulopathy is associated with excessive inflammation and multiorgan failure. Neutrophil extracellular traps (NETs) may exacerbate inflammation and provide a scaffold for thrombus formation.
Objectives
The goal of this study was to determine whether degradation of NETs by recombinant human DNase-I (rhDNase), a safe, Food and Drug Administration–approved drug, reduces excessive inflammation, reverses aberrant coagulation, and improves pulmonary perfusion after experimental acute respiratory distress syndrome (ARDS).
Methods
Intranasal poly(I:C), a synthetic double-stranded RNA, was administered to adult mice for 3 consecutive days to simulate a viral infection, and these subjects were randomized to treatment arms, which received either an intravenous placebo or rhDNase. The effects of rhDNase on immune activation, platelet aggregation, and coagulation were assessed in mice and donor human blood.
Results
NETs were observed in bronchoalveolar lavage fluid and within regions of hypoxic lung tissue after experimental ARDS. The administration of rhDNase mitigated peribronchiolar, perivascular, and interstitial inflammation induced by poly(I:C). In parallel, rhDNase degraded NETs, attenuated platelet-NET aggregates, reduced platelet activation, and normalized the clotting time to improve regional perfusion, as observed using gross morphology, histology, and microcomputed tomographic imaging in mice. Similarly, rhDNase reduced NETs and attenuated platelet activation in human blood.
Conclusion
NETs exacerbate inflammation and promote aberrant coagulation by providing a scaffold for aggregated platelets after experimental ARDS. Intravenous administration of rhDNase degrades NETs and attenuates coagulopathy in ARDS, providing a promising translational approach to improve pulmonary structure and function after ARDS.
Keywords: coagulation, immunothrombosis, inflammation, platelets, thrombosis
1. Introduction
Acute respiratory distress syndrome (ARDS), a common sequala following pneumonia, nonpulmonary sepsis, aspiration of gastric contents, or severe trauma [1], is characterized by shortness of breath, tachypnea, hypoxemia, respiratory failure, and increased mortality [2,3]. Among the numerous causes, viral pneumonia frequently induces ARDS. Respiratory viruses, such as influenza virus and coronaviruses, are associated with an increased prevalence of ARDS [4], as evidenced by the recent COVID-19 pandemic. Unfortunately, the clinical management of ARDS is complicated by a lack of efficacious therapeutic strategies.
Respiratory failure is the primary cause of death in patients with COVID-19, whereas aberrant coagulopathy associated with excessive inflammation frequently contributes to multiorgan failure and patient deterioration. Patients with SARS-CoV-2 infection exhibit prolonged prothrombin time, increased fibrin degradation products, and disseminated intravascular coagulopathy, which is noted in a majority of COVID-19–associated deaths [5]. Coagulopathy is associated with tissue hypoxia, venous thromboembolism, acute coronary syndrome, and cerebral infarction [6,7], and anticoagulant therapy with low-molecular–weight heparin appeared to be associated with lower mortality in a subpopulation that met sepsis-induced coagulopathy criteria or that with markedly elevated D-dimer levels in a study of 449 patients with severe COVID-19 [5,8]. Coagulation and inflammation are tightly linked processes that exhibit reciprocal cross-talk via a process termed as thromboinflammation [9]. Systemic inflammation increases tissue factor–mediated thrombin production and limits endogenous fibrinolysis to enhance coagulation, whereas increased coagulation perpetuates inflammatory activation. Thus, elucidation of the interplay between the immune system and coagulation may identify efficacious therapeutics to proactively address the complications of SARS-CoV-2 infection.
Mobilization of circulating neutrophils establishes a proinflammatory milieu after hemorrhage- or endotoxemia-induced acute lung injury, providing the first line of host defense against pathogens [10]. Circulating neutrophils may also initiate endothelial injury and fluid leakage into the alveoli, contributing to decreased lung compliance and hypoxemia in patients with ARDS. Elevated neutrophil-to-lymphocyte ratio is an independent risk factor for disease severity and mortality in hospitalized patients with COVID-19, whereas extensive neutrophil infiltration is associated with pulmonary fibrin deposition and vascular lesions [[11], [12], [13]]. Neutrophil depletion prevents endotoxin-induced lung vascular permeability in sheep [14]; however, given the critical role of neutrophils in host defense, global depletion strategies are not a clinically feasible approach to improve outcomes after ARDS. Thus, alternative, targeted approaches are needed to minimize the detrimental effect of neutrophil activation after acute lung injuries.
In addition to engulfing pathogens into phagosomes, activated neutrophils extrude a meshwork of chromatin fibers to form cloud-like neutrophil extracellular traps (NETs). While primarily implicated in extracellular pathogen trapping and host defense, NETs increase endothelial permeability and exacerbate inflammatory activation to potentiate tissue injury during viral pneumonitis and ARDS [15,16] and provide a scaffold for thrombus formation [17,18]. The extent of neutrophil priming and NET formation is correlated with disease severity and mortality in patients with ARDS [19]. Moreover, neutrophil hyperactivation and increased NET formation is correlated with an elevated risk of venous thromboembolism in hospitalized patients with COVID-19 compared with that in healthy controls [19]. Thus, NETs may provide a therapeutic target to proactively reduce tissue injury associated with coagulopathy following SARS-CoV-2 infection.
In the present study, we identified NETs as scaffolds for aggregated platelets, which promoted thrombus formation in an experimental ARDS model. We further showed the therapeutic potential of recombinant human DNase-I (rhDNase; Pulmozyme [dornase-α]), a safe, Food and Drug Administration–approved drug under investigation for the management of COVID-19–induced ARDS [20], to degrade NETs and improve pulmonary structure and function.
2. Methods
2.1. Experimental design
A double-blinded, randomized study design was utilized. Mice were the research subjects in controlled laboratory experiments. Donor human blood was also studied ex vivo. The primary study endpoints were quantification of NETs and platelet activation. Parallel studies were used to measure the pulmonary vascular structure. Mice were randomized to study arms using a random number generator. Power analyses were performed a priori to determine sample sizes using an α value of 0.05 and β value of 0.10. Investigators (A.J., N.S.M., Y.L., M.E.A., H.K., E.L.S.) performed data acquisition of predetermined outcome measures. Following final data acquisition, the mice were decoded and final analyses were performed. No subjects were removed from the final analyses. The Institutional Animal Care and Use Committee at Augusta University approved all animal studies, in compliance with the National Institutes of Health guidelines.
2.2. ARDS model
Adult (10-12 weeks old), mixed-sex C57BL/6J mice (Jackson Laboratories, Stock #000664) were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited, pathogen-free vivarium. The mice were anesthetized using 3% isoflurane and maintained with 1.5% to 2% isoflurane throughout all procedures. Sham controls received 50 μL of intranasal sterile phosphate-buffered saline (PBS) for 3 consecutive days. Experimental groups received intranasal poly(I:C) (200 μg in 50 μL of sterile PBS; Sigma Aldrich), a synthetic double-stranded RNA that mimics viral infection, for 3 consecutive days, per our group [21,22]. Experimental mice were treated with either an intravenous (i.v.) injection of placebo (PBS) or 5-mg/kg rhDNase via the tail vein on days 2 and 3 after the first poly(I:C) administration. The body temperature was maintained at 37 °C using a small animal temperature controller throughout all procedures. Food and water were provided ad libitum.
2.3. Tissue collection
On experimental day 5, a subset of randomly selected mice from each group was sacrificed, blood was collected via cardiac puncture, and bronchoalveolar lavage fluid (BALF) was collected using standard protocols [23]. Thereafter, the lungs were harvested for analytic flow cytometry. On experimental day 6, the remaining mice from each group were sacrificed for gross and histologic examinations or randomly assigned to undergo microcomputed tomography (microCT) imaging.
2.4. Analytic flow cytometry
Single-cell suspensions were sieved through a 100-μm cell strainer, centrifuged (252× g, 10 minutes), and stained with fluorescent antibodies to quantify neutrophils, macrophages, lymphocytes, and cytokine expression. Cells were stained with anti-Ly6G (Cat#127625; BioLegend), anti-F4/80 (Cat#123116; BioLegend), anti-CD3 (Cat#100214; BioLegend), anti-CD4 (Cat#100432; BioLegend), anti-CD8 (Cat#140416; BioLegend), anti-CD19 (Cat#551001; BD Bioscience), anti-CD45 (Cat#103138; BioLegend), anti-NE (Cat#bs-6982R; Bioss), or anti-MPO (Cat#PA5-16672; Invitrogen). Cells were then fixed, permeabilized, and stained for the intracellular cytokines IL-6 (Cat#504602; BioLegend), TNF-alpha (Cat#506344; BioLegend), IL-2 (Cat#517605; BioLegend), IL-17 (Cat#506907; BioLegend), IL-10 (Cat#505006; BioLegend), and IFN-gamma (Cat#505850; BioLegend) per our laboratory protocol [[24], [25], [26]]. Platelets were stained based on the functional mode of resting or activation. Resting platelets, which retain their discoid shape, were identified using anti-CD41 (mouse: Cat#133932; BioLegend) or anti-CD42b (human: Cat#303903; BioLegend). Activated platelets, which exhibit a more amorphous form, were stained with anti-CD62P (mouse: Cat#148305; BioLegend and human: Cat#304942; BioLegend). Macroaggregates of platelet-NETs were identified using 2 analytical live gates that were set during the acquisition process. While the first gate was based on the morphologic characteristics of platelets (forward scatter/side scatter), the second gate was based on the expression of CD62P antigen on platelets already expressing CD41. These platelets were further analyzed based on the association and attachment level of NET markers (eg, extracellular MPO/Cit-H3/chromatin), providing a quantitative assessment of platelet-NET macroaggregates. Human blood was obtained from mixed-sex, healthy donors and randomized to undergo a 24-hour incubation with a vehicle, 200 μg of poly(I:C), or 200 μg of poly(I:C) + 200 μg of rhDNase. Platelets were mechanophenotyped as either resting or activated, as above. Samples were gated based on forward and side scatter properties as well as marker combinations using the 4-Laser LSR II flow cytometer. All acquired flow cytometry data were analyzed using the FlowJo software (version 10; Becton Dickinson).
2.5. Histology
Mice were transcardially perfused with chilled PBS, followed by 10% neutral buffered formalin. The lungs were removed and 5-μm midcoronal sections were stained with hematoxylin and eosin to visualize tissue structure. Random views were analyzed by an investigators (A.J., Y.L.) using bright-field microscopy. For quantification of hypoxia, hypoxyprobe-1 (Cat #HP1) was administered via the tail vein 1.5 hours prior to sacrifice per our laboratory protocol [24]. Coronal sections were stained with mouse anti-hypoxyprobe-1 antibody overnight, followed by incubation with secondary Alexa Flour-488–tagged IgG (Cat#A21202; Invitrogen). In parallel, neutrophils and NETs were visualized using anti-mouse Ly6G antibody (Cat#127608; BioLegend) and anti-histone H3 (citrulline R26) (Cit-H3) antibody (Cat#ab19847; Abcam) per our group [24]. Optical images were captured by an investigators (A.J., Y.L.) using the Zeiss AxioImager2 microscope. Fluorescence intensity was quantified using color intensity measurement using the Zeiss ZEN blue software (version 3.2) as previously detailed [25].
2.6. MicroCT analysis
Anesthetized mice were transcardially perfused with PBS, formalin, and BriteVu contrast agent, which has a high radiodensity and penetrates to the capillary level. The BriteVu solution was freshly prepared by dissolving 25 g of BriteVu powder in 200 mL of distilled water plus 500 μL of BriteVu Enhancer. Following perfusion, the mice were placed on ice for 2 hours, and then, the lungs were harvested, fixed in 4% paraformaldehyde for 72 hours, and washed with 70% ethanol. The lungs were scanned by an investigators (M.E.A., A.J.) using the Bruker Skyscan 1272 microCT system with scanning parameters of 2k (2452 × 1640) resolution, 9.3-μm image pixel size, 0.5-mm aluminum filter, and 0.4 rotation step. Three-dimensional reconstruction was created using the NRecon software (Bruker Corporation), followed by analysis using the CTan software, which uses high-order rendering algorithms to create volume-rendering and maximum-intensity projection images of complex vascular networks within the lungs. Identical parameters were used for all samples during scanning, reconstruction, and analysis.
2.7. Clotting time
On day 5, 100 μL of whole blood was placed on a glass slide. Blood was gently pricked with a needle tip every 30 seconds until a fibrin thread was observed. The time from blood withdrawal to clot formation was recorded using a stopwatch [27].
2.8. Western blotting
On experimental day 5, a subset of randomly selected mice from each group was sacrificed, and blood collected via cardiac puncture was centrifuged to collect plasma. Plasma protein concentrations were quantified using the Pierce Rapid Gold BCA protein assay kit. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Mini-PROTEAN TGX Stain-Free Precast Gel, Bio-Rad) and transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% bovine serum albumin for 1 hour and incubated overnight at 4 °C with mouse anti-DNase-I antibody (1:200; clone B-4, sc-376207; Santa Cruz Biotechnology), followed by 1-hour incubation at room temperature with a goat anti-mouse Alexa Fluor 750-tagged secondary antibody (1:3000; A21037; Life technologies). Total protein loading was visualized and normalized using Ponceau S staining. Blots were visualized using the Bio-Rad ChemiDoc MP imaging system, and densitometry analysis was performed using the ImageJ software, version 1.53.
2.9. Statistical analysis
Data were analyzed using the GraphPad Prism 9 software. Two group comparisons were analyzed using the Student’s t-test. Multigroup comparisons were made using 1-way analysis of variance with adjustments for multiple comparisons, followed by the Tukey post hoc test. Results are expressed as mean ± SD. A p value of <.05 was considered to be statistically significant.
3. Results
3.1. rhDNase reversed proinflammatory activation after experimental ARDS
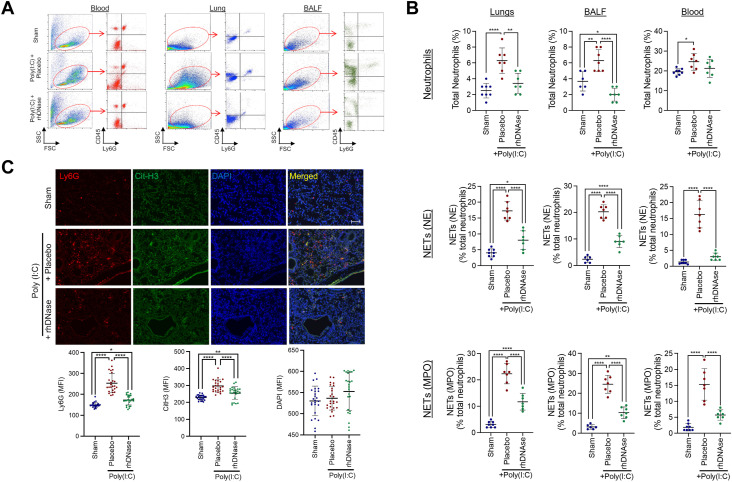
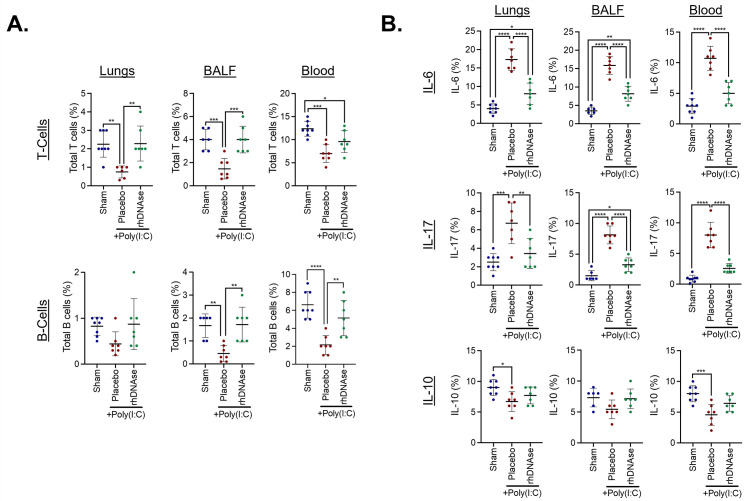
Intranasal poly(I:C) administration induced a robust innate immune response, characterized by neutrophil activation in the lungs and BALF (Figure 1A–C). These changes were paralleled by a lesser, but statistically significant, increase in the number of circulating neutrophils. Further analysis revealed the presence of NETs in the lungs, BALF, and blood (Figure 1B, C). The administration of rhDNase reduced the number of infiltrated neutrophils in the lungs and BALF following poly(I:C) administration, whereas the number of circulating neutrophils was not statistically different from that in the placebo-treated mice (Figure 1A–C). rhDNase also decreased the number of NETs in the lungs, BALF, and blood (Figure 1B, C). In parallel with these data, intranasal poly(I:C) administration significantly reduced the number of circulating T and B cells in the blood, compared with that in the saline-treated control mice (Supplementary Figure S1), to produce an elevated neutrophil-to-lymphocyte ratio. The administration of rhDNase, which decreased total neutrophils and NETs after poly(I:C), increased the total number of T and B cells in the lungs, BALF, and blood (Supplementary Figure S1). Moreover, poly(I:C) administration increased the expression of IL-6 and IL-17 in the lungs, BALF, and blood (Supplementary Figure S1). The expression of these proinflammatory cytokines was completely reversed by rhDNase administration. Conversely, the anti-inflammatory cytokine IL-10 was suppressed by poly(I:C) administration in the lungs and blood, although no significant changes were observed in BALF (Supplementary Figure S1). Increased plasma expression of DNase-I was observed in poly(I:C) mice treated with i.v. administration of 5 mg/kg of rhDNase compared with that in the sham mice or poly(I:C) mice treated with saline (placebo) (Supplementary Figure S2).
Figure 1.
Increased neutrophil infiltration and neutrophil extracellular trap (NET) formation in the lung and bronchoalveolar lavage fluid (BALF) after experimental acute respiratory distress syndrome. (A) Representative flow cytometry plots showing activation of CD45+ Ly6G+ neutrophils in the blood, lung tissue, or BALF on experimental day 5. Poly(I:C) mice were treated with either saline (placebo) or 5-mg/kg recombinant human DNase-I (rhDNase) (intravenous). Data are representative of 5 to 7 mice per group. (B) Quantification of poly(I:C)-induced neutrophil activation and NET formation in the lung, BALF, and blood after administration of placebo or rhDNase. Scatterplots represent 5 to 7 mice per group. Data were compared using 1-way analysis of variance, followed by the Tukey post hoc test. ∗p < .05, ∗∗p < .01, ∗∗∗∗p < .0001. (C) Immunohistochemistry of Ly6G+ Cit-H3+ NETs in lung tissue after administration of placebo or rhDNase. 4′,6-diamidino-2-phenylindole was used as a counterstain. Scale bar = 100 μm. Mean fluorescence intensity from 25 random fields from 5 mice per group was quantified and presented as mean ± SD. Data were compared using 1-way analysis of variance, followed by the Tukey post hoc test. ∗p < .05, ∗∗p < .01, ∗∗∗∗p < .0001. DAPI, 4′,6-diamidino-2-phenylindole; FSC, forward scatter; MFI, mean fluorescence intensity; SSC, side scatter.
3.2. rhDNase improves pulmonary structure after administration of intranasal poly(I:C)
Consistent with increased inflammation, gross analysis of the lungs after poly(I:C) administration revealed regions of hyperemia as well as pale tissue indicative of regional hypoperfusion and tissue hypoxia (Figure 2A). The administration of rhDNase reduced the relative occurrence of poly(I:C)-induced gross tissue injury compared with that in the placebo-treated control mice (Figure 2A). Histologic analysis of lung tissue revealed hypercellularity, indicating immune cell infiltration (peribronchiolar, perivascular, and interstitial inflammation) and diffuse alveolar damage after poly(I:C) administration (Figure 2B). These changes induced by poly(I:C) were partially reduced by rhDNase compared with that in the placebo-treated mice (Figure 2B). Immunofluorescence staining showed a distinct spatial and temporal overlap between infiltrated, Ly6G+ neutrophils and hypoxic lung tissue after poly(I:C) administration (Figure 2C). The administration of rhDNase reduced neutrophil infiltration in parallel with decreased pulmonary tissue hypoxia, which returned to sham levels (Figure 2C).
Figure 2.
Recombinant human DNase-I (rhDNase) reduces neutrophil infiltration and improves lung morphology after experimental acute respiratory distress syndrome. (A) Gross images of lung tissue on experimental day 6. Poly(I:C) mice were treated with intravenous administration of either saline (placebo) or 5-mg/kg rhDNase. Bilateral regions of hyperemia are denoted by black dotted lines. Regions of pale tissue, indicative of hypoperfusion and regional hypoxia, are labeled with blue dotted lines. Lung injury was observed across the lung lobes from placebo-treated mice, with less pronounced effects observed after rhDNase treatment. Scale bar = 5 mm. (B) Histologic assessment of lung tissues. Data are representative of 6 mice per group. Top panel: Note that the increased cellularity observed in the poly(I:C) group, consistent with inflammatory activation, was attenuated following rhDNase treatment. Scale bar = 200 μm. Middle panel (second and third rows): Peribronchiolar and perivascular inflammation was observed in the poly(I:C) group. These changes were reduced with rhDNase treatment. Scale bar = 50 μm. Bottom panel: Interstitial inflammation and diffuse alveolar damage, a feature associated with early stages of acute respiratory distress syndrome, were seen in the poly(I:C) group. rhDNase treatment mitigated interstitial inflammation. Scale bar = 50 μm. (C) Confocal microscope images of poly(I:C)-treated lung tissue on experimental day 6. Ly6G+ infiltrating neutrophils localized in regions of tissue hypoxia, as indicated by increased hypoxyprobe-1 fluorescence. Scale bar = 100 μm. The mean fluorescence intensity was quantified from 25 random fields from 5 mice per group. Scatterplots were compared using 1-way analysis of variance, followed by the Tukey post hoc test. ∗∗∗p < .001, ∗∗∗∗p < .0001. DAPI, 4′,6-diamidino-2-phenylindole; MFI, mean fluorescence intensity.
We next utilized microCT imaging to assess pulmonary function. Using volume-rendering images, we observed perfusion of the contrast dye in large, medium, and small pulmonary vessels of the control mice. Following poly(I:C) administration, the contrast dye was largely confined to larger vessels, with less detection in smaller vessels. Treatment with rhDNase partially reversed the deficits observed after poly(I:C) administration, with some smaller vessels exhibiting perfusion (Figure 3A). A similar pattern was observed using maximum-intensity projection images. In the control mice, the contrast dye was observed in both larger and smaller vessels, whereas poly(I:C) administration revealed a higher concentration of the dye in larger vessels only (Figure 3A). Three-dimensional reconstructions are provided in Supplementary Figures S3–S5. Quantitative analysis revealed no changes in blood vessel lumen thickness or separation between the groups (Figure 3B). In contrast, poly(I:C) administration reduced the vascular volume, capillary network, and number of blood vessels. Treatment with rhDNase completely reversed the effect of poly(I:C), returning values to levels observed in the sham-treated control mice (Figure 3B).
Figure 3.
Recombinant human DNase-I (rhDNase) improves pulmonary perfusion after experimental acute respiratory distress syndrome. (A) Ex vivo microcomputed tomography imaging of the lungs on experimental day 6. Poly(I:C) mice were treated with intravenous administration of either sterile phosphate-buffered saline or 5-mg/kg rhDNase. Representative images depict volume-rendering imaging and maximal intensity project. For volume-rendering imaging, every voxel is assigned an emission color and opacity, which indicates the intensity of the contrast dye within the vasculature. All lungs were scanned using identical parameters, and the same log-scaled histogram was used to color map the lung vasculature. Red color represents lower-intensity structures (vessels with lower contrast in their lumen), blue color represents higher-intensity structures (vessels with higher contrast in their lumen), and green is the range in between those 2. Dorsal, ventral, side, and transverse views are depicted and show perfusion deficits in the poly(I:C)-treated mice. Following rhDNase treatment, perfusion of smaller-caliber blood vessels was observed. (B) Quantification of vascular volume, capillary network, number of blood vessels (BVs), BV lumen thickness, and BV separation, as assessed using microcomputed tomography. rhDNase improved vascular volume, capillary network, and the number of BVs to sham-injured levels. Quantified data are from 6 mice per group and were analyzed using 1-way analysis of variance, followed by the Tukey post hoc test. ∗p < .05, ∗∗p < .01. MIP, maximum-intensity projection; VRI, volume-rendering imaging.
3.3. rhDNase reduces platelet activation and platelet-neutrophil aggregates after intranasal poly(I:C) administration
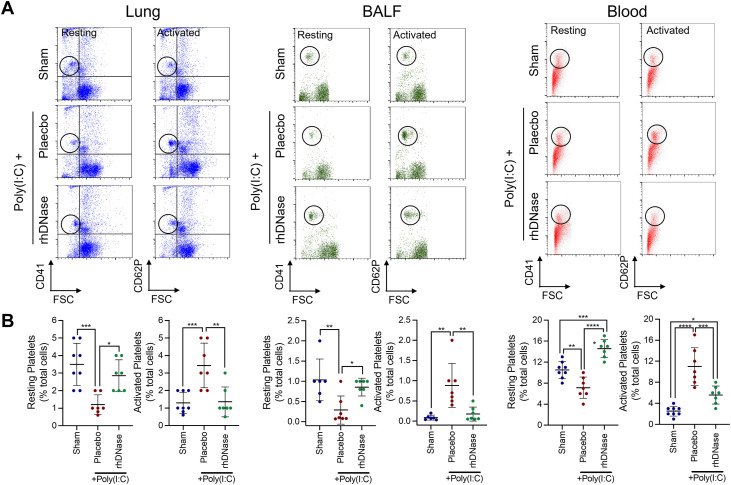
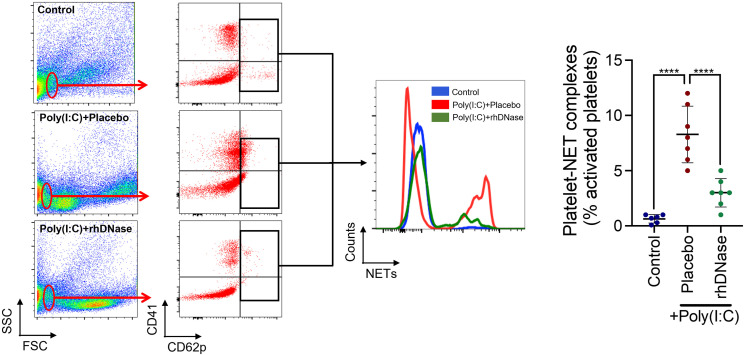
We observed disruption of vascular networks and an association between pulmonary hypoxia and infiltrated neutrophils/NETs. NETs provide a scaffold for platelet aggregation and thrombus formation; thus, we next explored whether rhDNase affected platelet activation. Poly(I:C) administration reduced the number of resting platelets and increased the number of activated platelets in the blood, lung tissue, and BALF on day 5 (Figure 4 ). The administration of rhDNase significantly increased the number of resting platelets in all groups following the administration of poly(I:C) while simultaneously suppressing the number of activated platelets in both blood and lung tissue; however, no significant effect of rhDNase on activated platelets was observed in BALF (Figure 4). In line with these data, rhDNase reduced the number of activated platelet-NET aggregates in lung tissue compared with that in the placebo-treated mice (Figure 5 ).
Figure 4.
Recombinant human DNase-I reduces platelet activation in blood and lung tissue after experimental acute respiratory syndrome. (A) Quantification of platelet activation in blood, lung tissue, or bronchoalveolar lavage fluid on experimental day 5. Poly(I:C) mice were treated with intravenous administration of either sterile phosphate-buffered saline or 5-mg/kg recombinant human DNase-I. Top panels depict flow cytometry plots to differentiate CD41+ (resting) and CD62P+ (activated) platelets. (B) Quantification of platelet activation data from 7 mice per group was analyzed using 1-way analysis of variance, followed by the Tukey post hoc test. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001, ∗∗∗∗p < .0001. BALF, bronchoalveolar lavage fluid; FSC, forward scatter.
Figure 5.
Recombinant human DNase-I attenuates activated platelet-neutrophil extracellular trap (NET) macroaggregates in lung tissue after experimental acute respiratory distress syndrome. Association of activated platelets (CD41+ CD62p+) with NETs in lung tissue on experimental day 5, as assessed using flow cytometry. Poly(I:C) mice were treated with intravenous administration of either sterile phosphate-buffered saline or 5-mg/kg recombinant human DNase-I. Quantification of platelet-NET aggregates from 7 mice per group was analyzed using 1-way analysis of variance, followed by the Tukey post hoc test. ∗∗∗∗p < .0001. FSC, forward scatter; SSC, side scatter.
3.4. rhDNase normalizes clotting time after experimental ARDS
Given the increased number of activated platelets following intranasal poly(I:C) administration, we explored whether these changes reduced clotting times in a mixed-sex cohort of mice. A significant reduction in clotting time was observed in whole blood collected from the placebo-treated poly(I:C) mice compared with that in untreated control mice. Furthermore, i.v. administration of rhDNase reversed the effect of poly(I:C), normalizing the clotting time to control levels (Figure 6A). As male sex was associated with a 3-fold higher risk of admission to the intensive care unit and an increased risk of death after SARS-CoV-2 infection [28], we further assessed whether the observed differences in clotting were sex-dependent after poly(I:C) administration. Of note, we found that the effect of poly(I:C) was lost in females compared with that in males, suggesting that male sex was associated with elevated coagulation (Figure 6B). Finally, poly(I:C) treatment of whole blood from healthy human donors increased NETs (Figure 6C), reduced the number of resting platelets, and increased the number of activated platelets (Figure 6D), mirroring the response in mice after experimental ARDS (Figure 4). The addition of rhDNase completely reversed the number of both NETs and activated platelets to control levels, with a simultaneous increase in the number of resting platelets (Figure 6C, D).
Figure 6.
Recombinant human DNase-I (rhDNase) normalizes the clotting time after experimental acute respiratory distress syndrome. (A) Ex vivo quantification of the clotting time of whole blood collected from mixed-sex mice on experimental day 5. Administration of poly(I:C) reduced the clotting time in placebo-treated mice, whereas rhDNase treatment normalized the clotting time to control levels. Data were compared using 1-way analysis of variance (ANOVA), followed by the Tukey post hoc test. ∗∗p < .01. (B) Sex-dependent changes in clotting time were observed following experimental acute respiratory distress syndrome, with males exhibiting a more robust response to poly(I:C)-induced clotting compared with female mice. Data were compared using 1-way ANOVA, followed by the Tukey post hoc test. ∗∗p < .01, ∗∗∗p < .001. Quantification of (C) neutrophil extracellular traps and (D) platelet activation in healthy donor human blood following poly(I:C) treatment ex vivo. Poly(I:C) increased the number of activated platelets, with a concomitant reduction in resting platelets. Treatment with rhDNase normalized platelet activity to untreated control levels. Data were analyzed using 1-way ANOVA, followed by the Tukey post hoc test. ∗∗p < .01, ∗∗∗p < .001, ∗∗∗∗p < .0001. FSC, forward scatter; NET, neutrophil extracellular trap; SSC, side scatter.
4. Discussion
Throughout the recent pandemic, critically ill patients overwhelmed hospitals and intensive care units worldwide, with many patients requiring mechanical ventilation. While rapid vaccine development reduced the number of hospitalized patients, identification of safe, efficacious therapeutics is needed to limit the deleterious consequences of pulmonary inflammation and/or structural injury following viral infection. In this study, we provide experimental evidence to support the clinical repurposing of rhDNase, a Food and Drug Administration–approved therapy with an excellent patient safety record, to reduce inflammatory activation, degrade NETs, and improve pulmonary structure after experimental ARDS.
Individuals infected with SARS-CoV-2 exhibit early alveolar damage, followed by a robust immune reaction, platelet hyperactivation, hypercoagulation, and microvascular pulmonary thrombosis [29]. A progressive endothelial thromboinflammatory syndrome, termed as “microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome” was observed in a study of 850 patients with COVID-19 and bilateral pneumonia [29,30]; however, the underlying mechanisms are undefined. Neutrophils derived from patients with COVID-19 exhibited excessive NET formation, whereas NETs contributed to immunothrombosis, vascular occlusion, and poor clinical outcomes in patients with COVID-19 [12,19,31,32]. Impaired clearance of NETs, which occurs in patients with ARDS [33], is clinically associated with acute thrombotic microangiopathies [34], whereas the presence of citrullinated histone H3, a component of NETs in retrieved thrombi, was independently associated with mortality in patients with acute ischemic stroke [35]. In addition, we reported that NET formation was associated with microvascular occlusion and cerebral hypoperfusion after brain injury in mice and humans [24]. Notably, patients with COVID-19 requiring intensive care frequently exhibit ≥1 comorbid conditions, including obesity and hypertension, which exacerbate NET formation [15,18,36]. In this study, we did not observe a change in plasma DNase-I levels following poly(I:C) administration, suggesting that experimental ARDS is associated with elevated NET formation rather than impaired degradation. Thus, targeted degradation of NETs may reduce microthrombus formation, improve tissue perfusion, and enhance gas exchange in patients with COVID-19, while circumventing the associated risks of global neutrophil depletion [12,31].
Platelets contribute to tissue damage in experimental models of acute lung injury, at least in part, via the release of inflammatory mediators and neutrophil activation at sites of endothelial injury [37,38]. Consistent with the aforementioned clinical findings, an increased presence of neutrophils and NETs was observed in the blood, lung tissue, and BALF after poly(I:C) administration, paralleling increases in platelet activation. In particular, we observed a spatial overlap between infiltrated neutrophils and NETs in regions of insufficient perfusion and pulmonary hypoxia after experimental ARDS, consistent with a purported role of NETs in microthrombus formation. In line with our observation of increased activated platelet-NET complexes after experimental ARDS, rolling neutrophils extract large fragments from dying platelets to generate neutrophil macroaggregates, resulting in widespread occlusion of pulmonary arteries, veins, and microvasculature after experimental gut ischemia [39]. Although international case-control studies revealed that elevated levels of platelet activation markers and dysregulated coagulation in BALF were associated with increased mortality in patients with ARDS [40], administration of antiplatelet drugs produced inconsistent improvements in patients with ARDS [37], suggesting a need for alternative therapeutic approaches. That rhDNase reduced platelet-neutrophil complexes in parallel with improved lung perfusion indicates a potential mechanism of action to support therapeutic potential in patients with ARDS.
DNase-I is an endonuclease that catalyzes the hydrolysis of extracellular DNA. Aerosolized rhDNase, a monomeric, 260-amino–acid glycoprotein produced in Chinese hamster ovary cells [41], has been used for >3 decades to reduce high-molecular–weight DNA to smaller fragments to reduce mucus viscosity and improve airway function in patients with cystic fibrosis [42]. Given its clinical safety and efficacy, the use of rhDNase has extended to patients with prolonged mechanical ventilation, chronic sinusitis, emphysema, and pediatric pulmonary diseases [[43], [44], [45], [46]]. Moreover, rhDNase has been shown by our laboratory and others to efficiently degrade NETs, which comprise, in part, extracellular DNA that is expelled from activated neutrophils. While the precise relationship between NETs and pulmonary hypoxia is undetermined, the administration of rhDNase degraded extracellular NETs, decreased lung inflammation, and improved pulmonary perfusion after poly(I:C) treatment. Coupled with data from our laboratory and others who showed that rhDNase improved blood flow and outcomes after experimental stroke and traumatic brain injury [24,47], rhDNase may provide a safe, feasible, and clinically efficacious treatment option to improve lung function during ARDS.
Despite the translational potential of rhDNase, several study limitations warrant consideration. In the present study, i.v. rhDNase administration was utilized to preferentially target circulating NETs following experimental ARDS; however, the short plasma half-life of DNase-I may provide a potential translational limitation [48]. While aerosolized rhDNase may partially overcome this limitation, the recent development of recombinant DNase-I–coated polydopamine-polyethylene glycol nanoparticles may provide longer-acting enzymatic activity, with delayed excretion [49]. Of note, long-acting DNase-I reduced the number of peritoneal neutrophils and reduced mortality after experimental sepsis compared with that in control or DNase-I-treated mice [49]. Thus, comparison of the routes of administration (inhalational vs. i.v.) and different formulations may establish preclinical support prior to clinical translation.
In addition to extracellular DNA, NETs contain histones and granular proteins (eg, MPO and NE) that may directly contribute to tissue damage. While not explored in this study, the addition of antihistone antibodies, polysialic acid (binds histones), or MPO (dihydrolipoic acid) reduced NET-mediated cytotoxicity in A549 human lung adenocarcinoma cells [50]. Additionally, rhDNase reduced thrombus formation via a NET-independent mechanism by hydrolyzing adenosine triphosphate and adenosine diphosphate to adenosine, which in turn inhibited platelet aggregation and neutrophil activation after laser-induced injury [51]. Thus, adjunct therapies that target other aspects of NETs may provide further protection after ARDS. In support of this possibility, a phase 2 multicenter, double-blind, randomized, placebo-controlled trial concluded that i.v. administration of α-1 antitrypsin, a serine protease inhibitor that limits NE activity, was safe and well tolerated and decreased inflammation in patients with moderate-to-severe ARDS secondary to SARS-CoV-2 infection [52]. As such, combination therapy with rhDNase and α-1 antitrypsin may provide maximal protection against the detrimental effects of NETs.
Finally, intranasal poly(I:C) recapitulates many aspects of viral infection, including systemic and local inflammation as well as pulmonary injury; however, this sterile inflammation model may not fully replicate infectious ARDS. Importantly, poly(I:C) is a synthetic analog of double-stranded RNA, which is a molecular pattern associated with viral infections. Along these lines, poly(I:C) binds to TLR3, a pattern recognition receptor that recognizes RNA viruses, including influenza A, coronavirus, and rhinovirus. Future studies will be expanded to infectious disease models to better determine the clinical reach of our findings. An i.v. bolus of rhDNase (5 mg/kg) was chosen based on reports showing maximal NET degradation [24]. Future translational work will also optimize the dose and therapeutic window to maximize efficacy after ARDS. We postulate that rhDNase degrades NETs to improve outcomes; however, we cannot exclude the possibility that degradation of other extracellular DNA contributes to the observed benefits. Extracellular DNA does not typically circulate under physiological conditions, supporting the rationale and safety of repurposing rhDNase for ARDS; however, elevated levels of circulating mitochondrial DNA predicted poor outcomes in hospitalized patients with COVID-19 [53]. As mitochondrial DNA can potentiate inflammatory activation and NET formation via activation of TLR9 [54], rhDNase may reduce pulmonary inflammation and improve function via multiple mechanisms of action. Additionally, medium-term treatment (8 days) with rhDNase reduced NET-dependent thrombosis in a murine breast cancer model, whereas long-term treatment (18 days) reduced overall survival, an effect that was ameliorated by a combination of rhDNase with ertapenem [55]. In our study, short-to-medium-term treatment (3 days) with rhDNase diminished NETs, reduced platelet activation, and limited activated platelet-NETs macroaggregates, which in turn normalized the clotting time after experimental ARDS. The effects of long-term rhDNase treatment require further investigation along with studies of concomitant rhDNase treatment with antibiotics for those developing sepsis. While a reduction in NETs could heighten the risk of infection in critically ill patients, PAD4−/− mice, which cannot form NETs [56], exhibited decreased organ dysfunction and improved survival following hemorrhagic shock and sepsis [57]. Moreover, elevated NETs are positively correlated with the severity of sepsis in pediatric patients, whereas treatment with rhDNase or a PAD4 inhibitor ameliorated sepsis in infant mice, which exhibit significantly more NETs than adult mice [58]. Thus, the suitability of rhDNase in the management of pediatric ARDS requires further exploration. Taken together, our findings suggest that rhDNase is a low-risk, cost-effective, and efficacious candidate for drug repurposing to target NETs in the context of ARDS.
Acknowledgments
Author contributions
A.J., N.S.M., Y.L., K.V., B.B., and K.M.D. conceived the study and analyzed the data. A.J. and K.V. performed acute respiratory distress syndrome modeling. A.J. and M.E.A. performed microcomputed tomography studies. H.K. and E.S.L. performed flow cytometry studies. K.M.D. oversaw the project, had full access to all the data in the study, and takes responsibility for its integrity and the data analysis. All authors provided input regarding experimental design, data analysis, and manuscript preparation.
Declaration of competing interests
There are no competing interests to disclose.
Footnotes
Funding information Grant Number: R01NS110378 (to K.M.D. and B.B.), R01NS117565 (to K.M.D.), and R01NS114560 (to K.V.) from the National Institutes of Health.
Manuscript handled by: Christophe Dubois
Final decision: Christophe Dubois, 28 April 2023
The online version contains supplementary material available at https://doi.org/10.1016/j.jtha.2023.04.044
Supplemental Data
Supplemental Figure 1.
rhDNase suppresses inflammatory activation after experimental ARDS. (A) Quantification of lymphocyte activation in lung tissue, BALF, or blood at experimental d5. Poly(I:C) mice were treated with intravenous injections of either sterile PBS or 5 mg/kg rhDNase. Data were analyzed using flow cytometry and are representative of n=3-6 mice/group. (B) Quantification of poly(I:C)-induced inflammatory cytokine (IL-6, IL-17, IL-10) expression in lung, BALF, and blood after administration of placebo or rhDNase. Data were analyzed using flow cytometry and scatterplots represent n=6-8 mice/group. For all panels, data were compared by one-way ANOVA followed by Tukey’s post hoc test. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001, ∗∗∗∗p<0.0001.
Supplemental Figure 2.
Plasma DNase-I levels are increased at day 5 following rhDNase intravenous administration. (A) Plasma was collected from randomly selected mice from each group at experimental day 5. Western blots were probed for DNase-I. (B) Total protein loading was visualized by Ponceau S staining. (C) DNase-I expression was normalized to total protein. Densitometry analysis revealed the increased expression of plasma DNase-I in poly(I:C) mice treated with intravenous 5mg/kg rhDNase, as compared to sham and poly(I:C) mice treated with saline (placebo). Data are representative of n=3 mice/group and were compared by one-way ANOVA followed by Tukey’s post hoc test. ∗∗p<0.01.
Three-dimensional reconstruction of the lung in placebo-treated sham mice.
Three-dimensional reconstruction of the lung in placebo-treated mice following intranasal administration of poly(I:C).
Three-dimensional reconstruction of the lung in rhDNase-treated mice following intranasal administration of poly(I:C).
References
- 1.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., Van Haren F., Larsson A., McAuley D.F., Ranieri M., Rubenfeld G., Thompson B.T., Wrigge H., Slutsky A.S., Pesenti A., LUNG SAFE Investigators; ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Luyt C.E., Combes A., Trouillet J.L., Nieszkowska A., Chastre J. Virus-induced acute respiratory distress syndrome: epidemiology, management and outcome. Presse Med. 2011;40:e561. doi: 10.1016/j.lpm.2011.05.027. –8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta N., Zhao Y.Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M., van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E., Carmody A., Shenkar R., Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., Luo M., Chen L., Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heflin A.C., Brigham K.L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981;68:1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S., Park D.W., Tadie J.M., Gregoire M., Deshane J., Pittet J.F., Abraham E., Zmijewski J.W. Human resistin promotes neutrophil proinflammatory activation and neutrophil extracellular trap formation and increases severity of acute lung injury. J Immunol. 2014;192:4795–4803. doi: 10.4049/jimmunol.1302764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., Phoon M.C., van Rooijen N., Chow V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Jr., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S., Hofbauer T.M., Ondracek A.S., Chausheva S., Alimohammadi A., Artner T., Panzenboeck A., Rinderer J., Shafran I., Mangold A., Winker R., Wohlschläger-Krenn E., Moser B., Taghavi S., Klepetko W., Preissner K.T., Lang I.M. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood. 2021;137:1104–1116. doi: 10.1182/blood.2020005861. [DOI] [PubMed] [Google Scholar]
- 19.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earhart A.P., Holliday Z.M., Hofmann H.V., Schrum A.G. Consideration of dornase alfa for the treatment of severe COVID-19 acute respiratory distress syndrome. New Microbes New Infect. 2020;35 doi: 10.1016/j.nmni.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salles E.L., Khodadadi H., Jarrahi A., Ahluwalia M., Paffaro V.A., Jr., Costigliola V., Yu J.C., Hess D.C., Dhandapani K.M., Baban B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J Cell Mol Med. 2020;24:12869–12872. doi: 10.1111/jcmm.15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khodadadi H., Salles E.L., Jarrahi A., Chibane F., Costigliola V., Yu J.C., Vaibhav K., Hess D.C., Dhandapani K.M., Baban B. Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA. Cannabis Cannabinoid Res. 2020;5:197–201. doi: 10.1089/can.2020.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun F., Xiao G., Qu Z. Murine bronchoalveolar lavage. Bio Protoc. 2017;7:e2287. doi: 10.21769/BioProtoc.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaibhav K., Braun M., Alverson K., Khodadadi H., Kutiyanawalla A., Ward A., Banerjee C., Sparks T., Malik A., Rashid M.H., Khan M.B., Waters M.F., Hess D.C., Arbab A.S., Vender J.R., Hoda N., Baban B., Dhandapani K.M. Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun M., Vaibhav K., Saad N., Fatima S., Brann D.W., Vender J.R., Wang L.P., Hoda M.N., Baban B., Dhandapani K.M. Activation of myeloid TLR4 mediates T lymphocyte polarization after traumatic brain injury. J Immunol. 2017;198:3615–3626. doi: 10.4049/jimmunol.1601948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaibhav K., Braun M., Khan M.B., Fatima S., Saad N., Shankar A., Khan Z.T., Harris R.B.S., Yang Q., Huo Y., Arbab A.S., Giri S., Alleyne C.H., Jr., Vender J.R., Hess D.C., Baban B., Hoda M.N., Dhandapani K.M. Remote ischemic post-conditioning promotes hematoma resolution via AMPK-dependent immune regulation. J Exp Med. 2018;215:2636–2654. doi: 10.1084/jem.20171905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Hao J., Gao W., Liu Z., Wu S., Jing S. Study on hemostatic activities of the rhizome of Paris bashanensis. Pharm Biol. 2013;51:1321–1325. doi: 10.3109/13880209.2013.790065. [DOI] [PubMed] [Google Scholar]
- 28.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., Rosser E.C., Webb K., Deakin C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D'Angelo A., De Cobelli F., Rovere-Querini P., Tresoldi M., Dagna L., Zangrillo A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renzi S., Landoni G., Zangrillo A., Ciceri F. MicroCLOTS pathophysiology in COVID 19. Korean J Intern Med. 2020 doi: 10.3904/kjim.2020.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Stürzl M., Staats L., Mahajan A., Schauer C., Kremer A.N., Völkl S., Amann K., Evert K., Falkeis C., Wehrfritz A., Rieker R.J., Hartmann A., Kremer A.E., Neurath M.F., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grégoire M., Uhel F., Lesouhaitier M., Gacouin A., Guirriec M., Mourcin F., Dumontet E., Chalin A., Samson M., Berthelot L.L., Tissot A., Kerjouan M., Jouneau S., Le Tulzo Y., Tarte K., Zmijewski J.W., Tadié J.M. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur Respir J. 2018;52 doi: 10.1183/13993003.02590-2017. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Alcazar M., Napirei M., Panda R., Köhler E.C., Hovinga J.K., Mannherz H.G., Peine S., Renne T., Lammle B., Fuchs T.A. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost. 2015;13:732–742. doi: 10.1111/jth.12796. [DOI] [PubMed] [Google Scholar]
- 35.Laridan E., Denorme F., Desender L., Francois O., Andersson T., Deckmyn H., Vanhoorelbeke K., De Meyer S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 36.Chrysanthopoulou A., Gkaliagkousi E., Lazaridis A., Arelaki S., Pateinakis P., Ntinopoulou M., Mitsios A., Antoniadou C., Argyriou C., Georgiadis G.S., Papadopoulos V., Giatromanolaki A., Ritis K., Skendros P. Angiotensin II triggers neutrophil extracellular traps release linking thromboinflammation with essential hypertension. JCI Insight. 2021;6 doi: 10.1172/jci.insight.148668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav H., Kor D.J. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L915–L923. doi: 10.1152/ajplung.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarbock A., Singbartl K., Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Y., Alwis I., Wu M.C.L., Kaplan Z., Ashworth K., Bark D., Jr., Pham A., Mcfadyen J., Schoenwaelder S.M., Josefsson E.C., Kile B.T., Jackson S.P. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam5861. [DOI] [PubMed] [Google Scholar]
- 40.Wang T., Liu Z., Wang Z., Duan M., Li G., Wang S., Li W., Zhu Z., Wei Y., Christiani D.C., Li A., Zhu X. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shak S. Aerosolized recombinant human DNase I for the treatment of cystic fibrosis. Chest. 1995;107:65S–70S. doi: 10.1378/chest.107.2_supplement.65s. [DOI] [PubMed] [Google Scholar]
- 42.Konstan M.W., Ratjen F. Effect of dornase alfa on inflammation and lung function: potential role in the early treatment of cystic fibrosis. J Cyst Fibros. 2012;11:78–83. doi: 10.1016/j.jcf.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cimmino M., Nardone M., Cavaliere M., Plantulli A., Sepe A., Esposito V., Mazzarella G., Raia V. Dornase alfa as postoperative therapy in cystic fibrosis sinonasal disease. Arch Otolaryngol Head Neck Surg. 2005;131:1097–1101. doi: 10.1001/archotol.131.12.1097. [DOI] [PubMed] [Google Scholar]
- 44.Rahman N.M., Maskell N.A., West A., Teoh R., Arnold A., Mackinlay C., Peckham D., Davies C.W., Ali N., Kinnear W., Bentley A., Kahan B.C., Wrightson J.M., Davies H.E., Hooper C.E., Lee Y.C., Hedley E.L., Crosthwaite N., Choo L., Helm E.J. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365:518–526. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 45.Scala M., Hoy D., Bautista M., Palafoutas J.J., Abubakar K. Pilot study of dornase alfa (Pulmozyme) therapy for acquired ventilator-associated infection in preterm infants. Pediatr Pulmonol. 2017;52:787–791. doi: 10.1002/ppul.23656. [DOI] [PubMed] [Google Scholar]
- 46.Simpson G., Roomes D., Reeves B. Successful treatment of empyema thoracis with human recombinant deoxyribonuclease. Thorax. 2003;58:365–366. doi: 10.1136/thorax.58.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peña-Martínez C., Durán-Laforet V., García-Culebras A., Ostos F., Hernández-Jiménez M., Bravo-Ferrer I., Pérez-Ruiz A., Ballenilla F., Díaz-Guzmán J., Pradillo J.M., Lizasoain I., Moro M.A. Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tPA (tissue-type plasminogen activator) resistance. Stroke. 2019;50:3228–3237. doi: 10.1161/STROKEAHA.119.026848. [DOI] [PubMed] [Google Scholar]
- 48.Prince W.S., Baker D.L., Dodge A.H., Ahmed A.E., Chestnut R.W., Sinicropi D.V. Pharmacodynamics of recombinant human DNase I in serum. Clin Exp Immunol. 1998;113:289–296. doi: 10.1046/j.1365-2249.1998.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y.Y., Park H.H., Park W., Kim H., Jang J.G., Hong K.S., Lee J.Y., Seo H.S., Na D.H., Kim T.H., Choy Y.B., Ahn J.H., Lee W., Park C.G. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials. 2021;267 doi: 10.1016/j.biomaterials.2020.120389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., Lohmeyer J., Preissner K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carminita E., Crescence L., Brouilly N., Altie A., Panicot-Dubois L., Dubois C. DNAse-dependent, NET-independent pathway of thrombus formation in vivo. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2100561118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McElvaney O.J., McEvoy N.L., Boland F., McElvaney O.F., Hogan G., Donnelly K., Friel O., Browne E., Fraughen D.D., Murphy M.P., Clarke J., Choileáin O.N., O'Connor E., McGuinness R., Boylan M., Kelly A., Hayden J.C., Collins A.M., Cullen A., Hyland D., et al. A randomized, double-blind, placebo-controlled trial of intravenous alpha-1 antitrypsin for ARDS secondary to COVID-19. Med. 2022;3:233–248. doi: 10.1016/j.medj.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scozzi D., Cano M., Ma L., Zhou D., Zhu J.H., O'Halloran J.A., Goss C., Rauseo A.M., Liu Z., Sahu S.K., Peritore V., Rocco M., Ricci A., Amodeo R., Aimati L., Ibrahim M., Hachem R., Kreisel D., Mudd P.A., Kulkarni H.S., et al. Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19. JCI Insight. 2021;6 doi: 10.1172/jci.insight.143299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varady C.B.S., Oliveira A.C., Monteiro R.Q., Gomes T. Recombinant human DNase I for the treatment of cancer-associated thrombosis: a pre-clinical study. Thromb Res. 2021;203:131–137. doi: 10.1016/j.thromres.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 56.Martinod K., Fuchs T.A., Zitomersky N.L., Wong S.L., Demers M., Gallant M., Wang Y., Wagner D.D. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biron B.M., Chung C.S., Chen Y., Wilson Z., Fallon E.A., Reichner J.S., Ayala A. PAD4 deficiency leads to decreased organ dysfunction and improved survival in a dual insult model of hemorrhagic shock and sepsis. J Immunol. 2018;200:1817–1828. doi: 10.4049/jimmunol.1700639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colón D.F., Wanderley C.W., Franchin M., Silva C.M., Hiroki C.H., Castanheira F.V.S., Donate P.B., Lopes A.H., Volpon L.C., Kavaguti S.K., Borges V.F., Speck-Hernandez C.A., Ramalho F., Carlotti A.P., Carmona F., Alves-Filho J.C., Liew F.Y., Cunha F.Q. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit Care. 2019;23:113. doi: 10.1186/s13054-019-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-dimensional reconstruction of the lung in placebo-treated sham mice.
Three-dimensional reconstruction of the lung in placebo-treated mice following intranasal administration of poly(I:C).
Three-dimensional reconstruction of the lung in rhDNase-treated mice following intranasal administration of poly(I:C).