Abstract
Background
Detecting antibody responses following infection with SARS-CoV-2 is necessary for sero-epidemiological studies and assessing the role of specific antibodies in disease, but serum or plasma sampling is not always viable due to logistical challenges. Dried blood spot sampling (DBS) is a cheaper, simpler alternative and samples can be self-collected and returned by post, reducing risk for SARS-CoV-2 exposure from direct patient contact. The value of large-scale DBS sampling for the assessment of serological responses to SARS-CoV-2 has not been assessed in depth and provides a model for examining the logistics of using this approach to other infectious diseases. The ability to measure specific antigens is attractive for remote outbreak situations where testing may be limited or for patients who require sampling after remote consultation.
Methods
We compared the performance of SARS-CoV-2 anti-spike and anti-nucleocapsid antibody detection from DBS samples with matched serum collected by venepuncture in a large population of asymptomatic young adults (N = 1070) living and working in congregate settings (military recruits, N = 625); university students, N = 445). We also compared the effect of self-sampling (ssDBS) with investigator-collected samples (labDBS) on assay performance, and the quantitative measurement of total IgA, IgG and IgM between DBS eluates and serum.
Results
Baseline seropositivity for anti-spike IgGAM antibody was significantly higher among university students than military recruits. Strong correlations were observed between matched DBS and serum samples in both university students and recruits for the anti-spike IgGAM assay. Minimal differences were found in results by ssDBS and labDBS and serum by Bland Altman and Cohen kappa analyses. LabDBS achieved 82.0% sensitivity and 98.2% specificity and ssDBS samples 86.1% sensitivity and 96.7% specificity for detecting anti-spike IgGAM antibodies relative to serum samples. For anti-SARS-CoV-2 nucleocapsid IgG there was qualitatively 100% agreement between serum and DBS samples and weak correlation in ratio measurements. Strong correlations were observed between serum and DBS-derived total IgG, IgA, and IgM.
Conclusions
This is the largest validation of DBS against paired serum for SARS-CoV-2 specific antibody measurement and we have shown that DBS retains performance from prior smaller studies. There were no significant differences regarding DBS collection methods, suggesting that self-collected samples are a viable sampling collection method. These data offer confidence that DBS can be employed more widely as an alternative to classical serology.
Keywords: Dried blood spot, Antibody responses, SARS-CoV-2, ELISA
1. Introduction
The SARS-CoV-2 pandemic has resulted in unprecedented levels of testing globally, to both diagnose current viral infection but also to detect evidence of prior infection or measure response to vaccination through antibody testing. This is particularly important for seroprevalence studies where there is limited ability to test for infection by serology, as was the case in the spring of 2020 at the beginning of the COVID-19 pandemic (Long et al., 2020; Shields et al., 2020; Thevis et al., 2020). The scale of testing undertaken during this pandemic has exposed limitations in the traditional model of in-person attendance for a blood test and subsequent processing and analysis in a laboratory. Patient travel and exposure to a health care setting can increase infection risk, and transportation to the laboratory can cause delays and deterioration of the sample. Increased use of remote healthcare consultation during the pandemic also necessitates travel for a blood sampling appointment.
Dried blood spot (DBS) offers a potential alternative method to venepuncture, and is comparatively simple, inexpensive, and can be self-collected and securely posted to dedicated laboratories for analysis (Page et al., 2019). Commercially available DBS kits have been used to detect antibodies of various infections, with prolonged stability demonstrated post-collection (Behets et al., 1992; Condorelli et al., 1994; Vázquez-Morón et al., 2019). SARS-CoV-2 antibody testing using DBS sampling has been previously undertaken (Parry et al., 2021a; Parry et al., 2021b; Parry et al., 2022; Parry et al., 2021c; Shields et al., 2022a; Shields et al., 2022b; Talaei et al., 2022); however, the accuracy of DBS testing for detecting SARS-CoV-2 antibodies conducted at scale in asymptomatic individuals, and the impact of DBS self-sampling on test performance, are unknown. Such data will inform the clinical utility of DBS as a sampling for routine clinical service.
This study evaluated the performance of SARS-CoV-2 anti-spike and anti-nucleocapsid antibody detection from DBS samples compared with matched serum collected by venepuncture in a large population of asymptomatic young adults living and working in congregate settings (military recruits and university students). This study also compared total IgA, IgG and IgM concentrations between DBS eluates and serum using a high throughput analyser, to determine the use of DBS sampling for a routine quantitative protein assay.
2. Methods
Samples were collected between 7 October 2020 and 14 June 2021 from university students at the campus of Leeds Beckett University, Leeds, and military recruits at the Infantry Training Centre, Catterick. University student participants performed self-sampling after receiving face-to-face or video tuition by an investigator at the point of DBS collection (ssDBS). In contrast, DBS sampling from military recruits was performed by a single investigator experienced in the technique (labDBS). Capillary blood samples (50 μL blood per spot) were obtained using finger-prick lancets and collected onto forensic-grade 226 DBS cards (Ahlstrom-Munksjo, https://www.ahlstrom-munksjo.com). DBS cards were stored at room temperature in individual sample bags with desiccant prior to processing. Venous blood samples were concurrently collected by venepuncture.
Serum was separated by centrifugation at 10,600 ×g for 10 min at room temperature and aliquots were stored at -20 °C until use. DBS samples were processed by the Clinical Immunology Service at the University of Birmingham as previously described (Cook et al., 2021; Morley et al., 2020). Briefly, individual 12 mm diameter pre-perforated blood spots were isolated using a sterile pipette tip and placed into a universal tube at a ratio of one spot to 250 μL 0.05% PBS-Tween-20. Blood spots were eluted overnight, aliquoted and stored at 4 °C prior to processing.
Serum samples and DBS eluates were tested for anti-SARS-CoV-2 spike glycoprotein antibodies at a dilution of 1:40 using a commercially available combined IgGAM ELISA (MK654, The Binding Site (TBS), Birmingham, UK) (Watanabe et al., 2020). A ratio value ≥1.0 was classified as positive. In a subset of matched serum and DBS samples, SARS-CoV-2 anti-nucleocapsid IgG was measured by ELISA, according to the manufacturer's instructions (EuroImmun US, NJ., USA). Ratios <0.8 were considered negative, ≥0.8 to <1.1 borderline and ≥ 1.1 positive. In a subset of randomly selected samples we also measured total IgG, IgA, and IgM antibody concentrations by turbidimetry using a COBAS c311/501 analyser, according to the manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany). Normal ranges for healthy adults are: total IgG = 6.0–16.0 g/L, IgA = 0.8–4.0 g/L and IgM = 0.5–2.0 g/L (Ward et al., 1986).
All statistical analyses were performed using Prism (GraphPad Prism 9.0, GraphPad Software, San Diego, California). Performance between the DBS and serum assay was determined by calculating the comparative sensitivity, specificity, and positive and negative predictive values, with 95% confidence intervals (CIs). Agreement between matched serum and DBS ELISA results was assessed by Spearman's r, the Cohen k coefficient and Bland-Altman mean-difference (with standard deviation [SD] and 95% limits of agreement [LoA]). Proportional bias was assessed visually in Bland–Altman plots and by simple regression analysis.
Ethical approval was obtained from Leeds Beckett University (Reference 73,520) and the UK Ministry of Defence Research Ethics Committee (1070/MODREC/20). The study design complied with the principles of the Helsinki Declaration. Clinical Trial Registration number was NCT04476680.
3. Results
Samples were collected over 35 weeks from a total of 1070 volunteers consisting of 445 university students (ssDBS) and 625 military recruits (labDBS) (Table 1 ). Both cohorts contained young adults of a similar age, but the labDBS group comprised of significantly more men (p = 0.0001), reflecting the typical demographic of military recruits.
Table 1.
Characteristics of study cohort. Serum was collected by venepuncture as the gold standard while DBS samples were either collected by a study investigator (military recruits, labDBS) or self-sampling (university students, ssDBS). DBS, dried blood spot.
| All participants | Recruits (labDBS) | Students (ssDBS) | p | ||
|---|---|---|---|---|---|
| Number of participants | 1070 | 625 | 455 | ||
| Age (years) | 21.3 ± 3.6 | 20.9 ± 3.6 | 21.7 ± 3.4 | ||
| Gender (%) | Men | 76.0 | 98.4 | 45.2 | 0.0001 |
| Women | 24.0 | 1.6 | 54.8 | – | |
| Seropositivity for SARS-CoV-2 (%) | Positive | 175 (16.4) | 82 (13.1) | 93 (20.9) | 0.0008 |
| Negative | 895 (83.6) | 543 (86.9) | 352 (79.1) | – | |
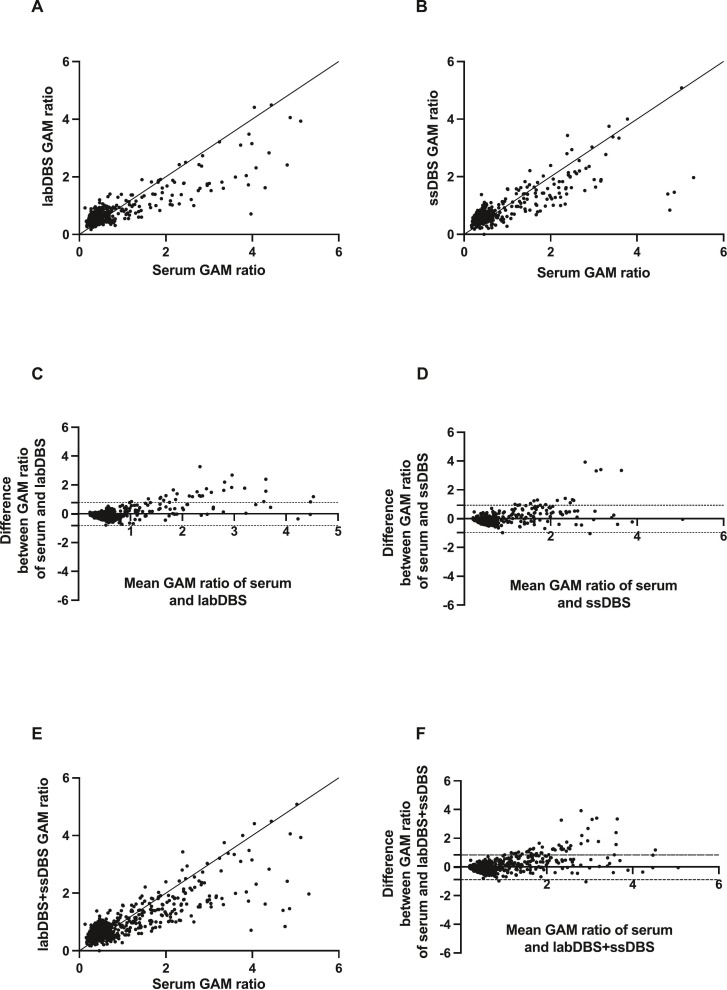
Baseline seropositivity for SARS-CoV-2, determined as a positive IgGAM ratio to the spike glycoprotein antigen using serum collected by venepuncture, was significantly higher among the students (20.9%) than the recruits (13.2%) (p < 0.001). We observed strong, significant correlations between matched serum and DBS samples for both the labDBS (r = 0.63 [95% CI 0.58–0.67]; p < 0.0001) and ssDBS sampling (r = 0.67 [95% CI 0.61–0.71]; p < 0.0001) (Fig. 1 , panels A and B, respectively). Similarly, there were minimal differences between the labDBS and ssDBS sampling and serum results (Bland-Altman bias: labDBS = −0.03 [SD ± 0.41, 95% LoA -0.83-0.77]; ssDBS = −0.02 [SD ± 0.48, 95% LoA -0.97-0.93]) (Fig. 1, panels C and D, respectively). Relative to serum samples, labDBS achieved 82.0% sensitivity and 98.2% specificity and ssDBS samples 86.1% sensitivity and 96.7% specificity for detecting spike glycoprotein IgGAM antibodies (Table 2 ). Discordance occurred between 28/625 matched labDBS and 27/445 matched ssDBS samples (κ = 0.79 and 0.81, respectively).
Fig. 1.
Effectiveness of DBS sampling for the detection of SARS-CoV-2 anti-spike glycoprotein IgGAM. Correlation between matched DBS eluate and serum IgGAM ratios for military recruits (A, labDBS, n = 625) and university students (B, ssDBS, n = 445). Bland-Altman mean-difference comparisons of DBS eluate and serum IgGAM ratios for labDBS (C) and ssDBS (D). Pooled analyses (labDBS+ssDBS, n = 1070) showing correlation between matched DBS eluate and serum IgGAM ratios (E) and Bland-Altman mean-difference comparisons (F). DBS, dried blood spot; dashed lines indicate 95% limits of agreement.
Table 2.
Sensitivity and specificity of DBS eluate anti-SARS-CoV-2 IgGAM ratios relative to matched serum samples. DBS, dried blood spot; PPV, positive predictive value; NPV, negative predictive value.
| Sensitivity, % (95% CI) | Specificity, % (95% CI) |
PPV, % (95% CI) |
NPV, % (95% CI) |
Accuracy, % (95% CI) |
Cohen's kappa coefficient | |
|---|---|---|---|---|---|---|
|
Total cohort n = 1070 |
81.1 (74.6–86.7) |
97.5 (96.3–98.5) |
86.6 (80.9–90.8) |
96.4 (95.1–97.3) |
94.9 (93.4–96.1) |
0.81 |
|
labDBS n = 625 |
82.0 (73.1–89.0) |
98.2 (96.7–99.1) |
87.3 (78.6–92.7) |
97.3 (96.0–98.2) |
– | 0.79 |
|
ssDBS n = 445 |
86.1 (78.1–92.0) |
96.7 (94.3–98.3) |
84.7 (76.0–90.7) |
97.0 (95.4–98.1) |
– | 0.81 |
Overall, both DBS sampling techniques and matched serum samples (n = 1070) showed a strong correlation (r = 0.65 [95% CI 0.61–0.69]; p < 0.0001) with minimal differences in results (Bland-Altman bias: -0.03, [SD ± 0.44, 95% LoA -0.89-0.84] ((Fig. 1, panels E and F, respectively), an overall sensitivity of 81.1%, specificity of 97.5% and test accuracy of 94.9% (κ = 0.81) for spike IgGAM antibodies.
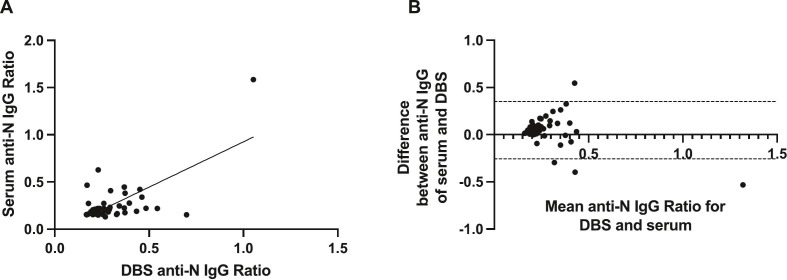
To corroborate these findings we then used a different assay to measure anti-SARS-CoV-2 nucleocapsid IgG in a subset of 53 paired serum and labDBS samples. There was total agreement between matched serum and DBS samples and a weak significant correlation (r = 0.38 [95% CI 0.11–0.59]; p = 0.005) and minimal differences in results from DBS and serum samples were observed (Bland-Altman bias: -0.047 [SD ± 0.15, 95% LoA -0.35-0.26]) (Fig. 2 , panels A and B, respectively).
Fig. 2.
Effectiveness of DBS sampling for the detection of SARS-CoV-2 anti-nucleocapsid IgG. (A). Correlation between matched DBS eluate and serum anti-N IgG ratios among 53 matched pairs (n = 27 week 0 and n = 26 week 16 matched pairs). (B). Corresponding Bland-Altman mean-difference comparisons. DBS, dried blood spot; dashed lines indicate 95% limits of agreement.
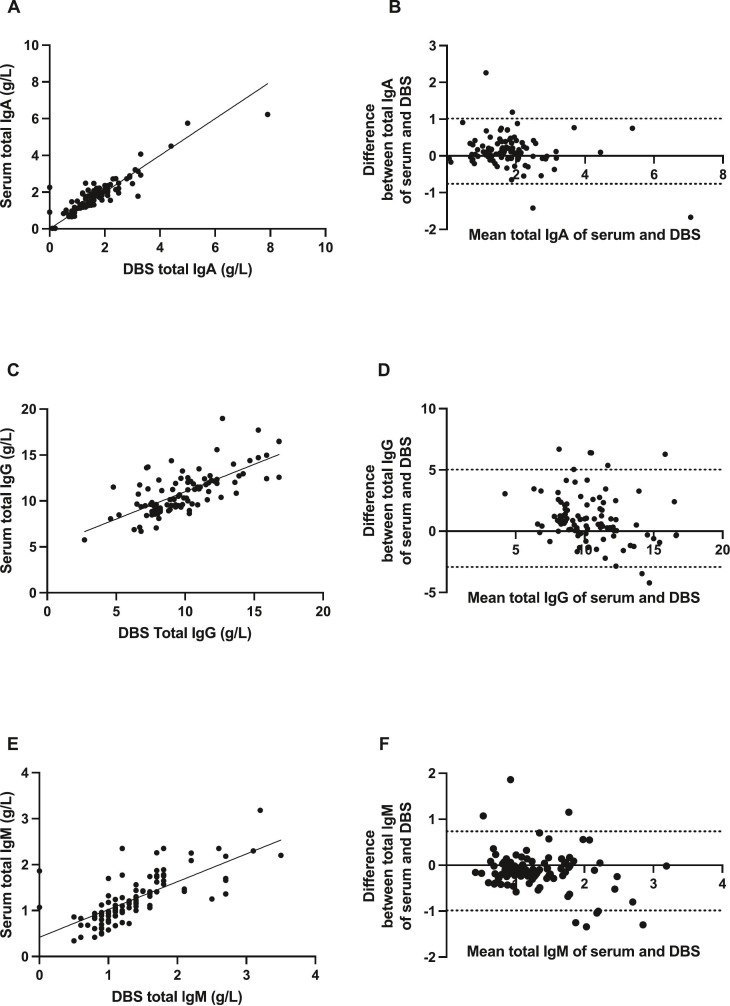
Finally, the quantification of total IgG from 97 matched labDBS and serum samples, and total IgA and IgM from 100 matched samples, was measured and compared between different sampling methods. Total IgA showed the most significant and strongest correlation between matched serum and DBS samples (r = 0.84 [95% CI 0.77–0.89]; p < 0.0001), followed by total IgM (r = 0.79 [95% CI 0.69–0.86]; p < 0.0001) and total IgG (r = 0.68 [95% CI 0.55–0.78]; p < 0.0001) (Fig. 3 , panels A, C, E). Again, minimal differences in results by different sampling methods were observed for IgA and IgM (Bland-Altman bias: IgA = 0.13 [SD ± 0.45, 95% LoA -0.76-1.01]; IgM = −0.13 [SD ± 0.44, 95% LoA -0.99-0.74]), however this was slightly higher for IgG = 1.05 [SD ± 2.03, 95% LoA -2.92-5.02]) (Fig. 3, panels B, D, F).
Fig. 3.
Effectiveness of DBS sampling for the quantification of total immunoglobulins. (A). Correlation between total IgA from 100 matched DBS eluate and serum pairs (n = 50 week 0 and n = 50 week 16 matched pairs). (B). Bland-Altman mean-difference comparisons for total IgA. (C). Correlation between total IgG from 97 matched DBS eluate and serum pairs (n = 50 week 0 and n = 47 week 16 matched pairs). (D). Bland-Altman mean-difference comparisons for total IgG. (E). Correlation between total IgM from 100 matched DBS eluate and serum pairs (n = 50 week 0 and n = 50 week 16 matched pairs). (F). Bland-Altman mean-difference comparisons for total IgM. DBS, dried blood spot; dashed lines indicate 95% limits of agreement.
4. Discussion
To our knowledge this study of over 1000 participants is the largest validation of DBS against paired serum for measuring serological status. We show that DBS retains the diagnostic performance of smaller studies (Cook et al., 2021) with good correlation for two different semi-quantitative specific antibody assays and quantitative total immunoglobulin measurement. Minimal differences in performance, irrespective of collection modality, demonstrates that self-collected samples are a viable sampling strategy, which have previously been shown to have high levels of acceptability and confidence in users (Valentine-Graves et al., 2020).
DBS is inexpensive and provides not only the convenience of self-collection at home, but also protects vulnerable patients and healthcare workers from exposure to infection. This method facilitates sampling in largescale sero-epidemiologic studies and widens access to serologic platforms in countries where transport of samples and access to laboratory assays may be limited. Furthermore, as highlighted by the recent global shortage of blood tubes (Rimmer, 2021), alternative and innovative approaches to traditional venepuncture may be required to overcome logistical challenges. DBS cards also require minimal storage space and are stable for long periods which allows archiving of samples for subsequent analysis.
Overall, relative to serum samples, DBS samples achieved a sensitivity of 81.1% and specificity of 97.5% for anti-spike IgGAM antibodies. The sensitivity is lower than previously reported, which may reflect differences in size, demographic, clinical features and seroprevalence of participants (Morley et al., 2020). Uniquely, this study enrolled young adults with no preceding diagnosis or recent symptoms of COVID-19, allowing the assessment of undiagnosed prior infection among individuals likely exposed to SARS-CoV-2 in congregate settings. Assessment of such a large population with a lower seroprevalence will reduce DBS sample test performance, as previously demonstrated in other contexts (Brown et al., 2021; Mulchandani et al., 2021). However, such populations are important to assess and the preserved high negative predictive values with DBS samples indicate that this method of determining serostatus would be effective in identifying immune-naïve individuals among large, real-world populations with modest rates of seropositivity. The ability to use DBS in both semi-quantitative and quantitative assays with good agreement highlights the robustness of this sampling technique that is likely to be suitable for most antibody assays.
The SARS-CoV-2 pandemic has required significant innovation in healthcare. The global effort to develop, translate and implement new vaccines, drugs and tests has been remarkable. An important part of the testing pathway is access to sampling, and we report DBS to be an easy to perform technique resulting in a stable sample for transport and equivalent to serum across different antibody assays.
Acknowledgements
This study was funded by Strategic Command (Director Resources & Policy) and Army Health Branch. We are grateful for the support of: Warrant Officer Beth Hoddy, Staff Sergeant Natasha Sinclair, Flight Sergeant Lia Spark, Professor Neil Walsh, Dr. Kate Donnan, Captain Richard Cruttenden, Major Nat Taylor, Surgeon Commander Ade Mellor.
Data availability
Data will be made available on request.
References
- Behets F., Kashamuka M., Pappaioanou M., Green T.A., Ryder R.W., Batter V., et al. Stability of human immunodeficiency virus type 1 antibodies in whole blood dried on filter paper and stored under various tropical conditions in Kinshasa, Zaire. J. Clin. Microbiol. 1992;30(5):1179–1182. doi: 10.1128/jcm.30.5.1179-1182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Byrne R.L., Fraser A., Owen S.I., Cubas-Atienzar A.I., Williams C.T., et al. Self-sampling of capillary blood for SARS-CoV-2 serology. Sci. Rep. 2021;11(1):7754. doi: 10.1038/s41598-021-86008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli F., Scalia G., Stivala A., Gallo R., Marino A., Battaglini C.M., et al. Detection of immunoglobulin G to measles virus, rubella virus, and mumps virus in serum samples and in microquantities of whole blood dried on filter paper. J. Virol. Methods. 1994;49(1):25–36. doi: 10.1016/0166-0934(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Cook A.M., Faustini S.E., Williams L.J., Cunningham A.F., Drayson M.T., Shields A.M., et al. Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients. J. Immunol. Methods. 2021;494 doi: 10.1016/j.jim.2021.113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Morley G.L., Taylor S., Jossi S., Perez-Toledo M., Faustini S.E., Marcial-Juarez E., et al. Sensitive detection of SARS-CoV-2-specific antibodies in dried blood spot samples. Emerg. Infect. Dis. 2020;26(12):2970–2973. doi: 10.3201/eid2612.203309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulchandani R., Brown B., Brooks T., Semper A., Machin N., Linley E., et al. Use of dried blood spot samples for SARS-CoV-2 antibody detection using the Roche Elecsys ® high throughput immunoassay. J. Clin. Virol. 2021;136:104739. doi: 10.1016/j.jcv.2021.104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M., Atabani S.F., Wood M., Smit E., Wilson S., Atherton C., et al. Dried blood spot and mini-tube blood sample collection kits for postal HIV testing services: a comparative review of successes in a real-world setting. Sex. Transm. Infect. 2019;95(1):43–45. doi: 10.1136/sextrans-2018-053567. [DOI] [PubMed] [Google Scholar]
- Parry H., Bruton R., Tut G., Ali M., Stephens C., Greenwood D., et al. Immunogenicity of single vaccination with BNT162b2 or ChAdOx1 nCoV-19 at 5-6 weeks post vaccine in participants aged 80 years or older: an exploratory analysis. Lancet Healthy Longev. 2021;2(9) doi: 10.1016/S2666-7568(21)00169-0. e554-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry H., McIlroy G., Bruton R., Ali M., Stephens C., Damery S., et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. doi: 10.1038/s41408-021-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry H., Tut G., Bruton R., Faustini S., Stephens C., Saunders P., et al. mRNA vaccination in people over 80 years of age induces strong humoral immune responses against SARS-CoV-2 with cross neutralization of P.1 Brazilian variant. Elife. 2021:10. doi: 10.7554/eLife.69375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry H., McIlroy G., Bruton R., Damery S., Tyson G., Logan N., et al. Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J. Hematol. Oncol. 2022;15(1):3. doi: 10.1186/s13045-021-01219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer A. What has caused the NHS blood tube shortage, and how is it affecting doctors and patients? BMJ. 2021;374 doi: 10.1136/bmj.n2174. [DOI] [PubMed] [Google Scholar]
- Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D., et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A.M., Faustini S.E., Hill H.J., Al-Taei S., Tanner C., Ashford F., et al. 2022. SARS-CoV-2 Vaccine Responses in Individuals with Antibody Deficiency: Findings from the COV-AD Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A.M., Faustini S.E., Hill H.J., Al-Taei S., Tanner C., Ashford F., et al. 2022. Increased Seroprevalence and Improved Antibody Responses Following Third Primary SARS-CoV-2 Immunisation: An Update from the COV-AD Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaei M., Faustini S., Holt H., Jolliffe D.A., Vivaldi G., Greenig M., et al. Determinants of pre-vaccination antibody responses to SARS-CoV-2: a population-based longitudinal study (COVIDENCE UK) BMC Med. 2022;20(1):87. doi: 10.1186/s12916-022-02286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevis M., Knoop A., Schaefer M.S., Dufaux B., Schrader Y., Thomas A., et al. Can dried blood spots (DBS) contribute to conducting comprehensive SARS-CoV-2 antibody tests? Drug Test Anal. 2020;12(7):994–997. doi: 10.1002/dta.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine-Graves M., Hall E., Guest J.L., Adam E., Valencia R., Shinn K., et al. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: post-collection acceptability of specimen collection process and patient confidence in specimens. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0236775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Morón S., Ardizone Jiménez B., Jiménez-Sousa M.A., Bellón J.M., Ryan P., Resino S. Evaluation of the diagnostic accuracy of laboratory-based screening for hepatitis C in dried blood spot samples: a systematic review and meta-analysis. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-41139-8. 7316- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A.M., Riches P.G., Williams P. PRU publications; 1986. PRU Handbook of Clinical Immunochemistry. [Google Scholar]
- Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(6501):330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.