Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA) is an antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, mostly affecting small-sized arteries and usually occurring in patients with an allergic background. Eosinophils seem to play a significant role in the pathogenesis of the disease and, therefore, biologics targeting interleukin 5 (IL5), a cytokine tightly linked to eosinophils, have emerged as a promising therapeutic tool. A systematic review of Medline was conducted from 2007 to 2022 to search for data regarding the use of anti-IL5 therapies in patients with EGPA. Ongoing or unpublished trials were also searched in ClinicalTrials.gov and the World Health Organization trials portal. The efficacy and safety of mepolizumab, an anti-IL5 monoclonal antibody (mAb), was confirmed by a randomized controlled trial (RCT), although a significant proportion of patients did not respond to this treatment. Other studies showed that both doses of 100 mg and 300 mg of mepolizumab are almost equally effective in EGPA. Benralizumab, an anti-IL5 receptor mAb has preliminary promising results and an RCT is planned to be conducted. Apart from their clinical efficacy in EGPA, anti-IL5 therapies may have steroid-sparing properties. Anti-IL5 therapies seem to be effective and safe in patients with refractory/relapsing EGPA and can be used as a steroid-sparing treatment. Nevertheless, more research is needed to clarify the pathophysiology of the disease; this may potentially lead to the identification of biomarkers to pinpoint patients most likely to respond to anti-IL5-blockade.
Keywords: Eosinophilic granulomatosis with polyangiitis, Egpa, Mepolizumab, Benralizumab, Anti-neutrophil cytoplasmic antibodies, ANCA
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), previously known as Churg-Strauss syndrome, is a systemic vasculitis classified within Anti-Neutrophil Cytoplasmatic Antibody (ANCA)-associated vasculitides, alongside granulomatosis with polyangiitis and microscopic polyangiitis [1]. EGPA mainly affects the small-sized arteries leading to clinical manifestations from many organs, such as lungs, skin, nervous system, kidneys, gastrointestinal and cardiovascular systems. The pathogenesis of the disease is not yet clear, but like many other autoimmune diseases there is strong evidence of environmental and genetic involvement [2]. Although it is an ANCA vasculitis, there is strong evidence that eosinophils play a crucial role in the development of the disease. All patients with EGPA have an allergic background; asthma and/or allergic sinusitis appear in 96–100% of them, with the respiratory system being the most affected system by EGPA [3, 4]. Eosinophilic inflammation is one of the cardinal features in EGPA [2]. The cornerstone therapy of EGPA includes oral corticosteroids and other immunosuppressive agents such as cyclophosphamide, rituximab, mycophenolate, azathioprine and methotrexate. Taking into account that IL5 is the master cytokine controlling eosinophil function [3], mAbs against IL5 or IL5 Receptor, such as mepolizumab and benralizumab, respectively, have emerged as promising therapeutic tools in patients with relapsing/refractory disease [3, 5–7]. Mepolizumab has proven efficacy and is included in the 2021 Guidelines of the American College of Rheumatology/Vasculitis Foundation as a treatment option for relapsing EGPA [8]. In this systematic review we focus on exploring the efficacy and safety of IL5 therapies in patients with EGPA. Moreover, we provide a short overview of eosinophil function as well as their role in EGPA pathogenesis.
Overview of eosinophil physiology
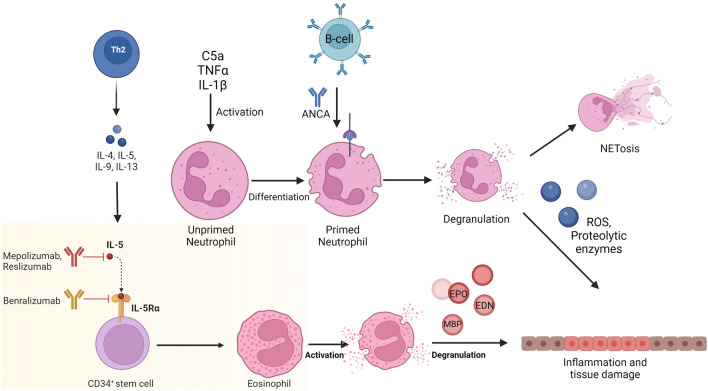
Eosinophils are white blood cells originating from the bone marrow [9] and the average total number in the bloodstream is normally < 500 mm3 cells. Eosinophilia is defined as eosinophil cell count > 500 mm3 and hypereosinophilia > 1500 mm3 [9]. Eosinophils are engaged in a broad spectrum of processes from battling parasitic, bacterial, viral infections and taking part in allergic reactions to hypereosinophilic syndromes and certain types of cancer [9, 10]. In the bone marrow granulocytes, under the effect of specific cytokines such as IL5, IL3 and Granulocyte–macrophage colony-stimulating factor (GM-CSF), differentiate into eosinophils and basophils [11]. Eosinophils are activated by IL5 to proliferate, migrate to the damaged tissue and enhance the inflammation process. IL5 is mostly produced by Th2 cells, Innate Lymphoid Cells-2 (ILC-2), CD34+ progenitor cells, Natural Killer (NK) cells, mast cells as well as the eosinophil itself in an auto/paracrine manner. Eosinophils possess IL5 receptors and IL5 can bind to and activate them. Chemokines, especially CCL11, CCL24 and CCL26, play a crucial role in the migration and recruitment of eosinophils in the inflamed tissue/organ. These chemokines are attached to CCR3 receptors on eosinophils and as a result eosinophils are transferred to the damaged tissue [7]. Eosinophils have the ability to phagocyte, secrete harmful proteins such as major basic protein (MBP), eosinophil peroxidase (EPO), eosinophil-derived neurotoxin (EDN) and also cytokines such as IL4, IL5, IL13, IL25 to activate the immune system and maintain inflammation [2].
Pathogenesis of EGPA. The role of eosinophils
The main histological feature in EGPA is a necrotizing vasculitis with extravascular granulomas that occur mostly in patients with asthma and tissue eosinophilia [2, 12]. In EGPA, granulomas consist of aggregated macrophages and eosinophils in the damaged tissue, mainly locating in the upper/or lower respiratory tract [5, 13]. According to the prevailing pathogenetic model, circulating unprimed neutrophils are activated and attracted to the inflamed region by alternative complement pathway and proinflammatory cytokines such as TNFα and IL1β. Primed neutrophils, under the effect of ANCAs, excrete Reactive Oxygen Species (ROS) and proteolytic enzymes [5]. Furthermore, IL5-activated eosinophils degranulate harmful enzymes which cause endothelium damage; it is a well-known fact that neutrophils produce neutrophil extracellular traps (NETs), but it was only recently discovered that eosinophils are also able to form extracellular traps, contributing to inflammation and thrombogenesis [11]. Increased number of eosinophils is a hallmark of EGPA; the disease is strongly associated with IL5, which promotes the maturation of eosinophils. Patients with EGPA have high serum levels of IL5; on the other hand, IL10, which is an anti-inflammatory and immunoregulatory cytokine, is decreased [14, 15].
Patients with EGPA can be genetically distinguished into 2 groups; the first group characterized by HLA-DQA1 polymorphisms has positive MPO-ANCAs, whereas the second group has no association with HLA-DQA1 and is MPO-ANCA negative [16, 17]. Although EGPA is classified as an ANCA-associated vasculitis, the percentage of patients with ANCA (mostly MPO/p-ANCA) varies among several studies and fluctuates between 30 and 60% [7, 16, 18]. Nevertheless, all patients, regardless of their ANCA status, have eosinophilia and allergic background. The majority of patients, irrespective of ANCA status, are diagnosed with ear/nose/throat manifestations and lung involvement. ANCA-positive patients frequently have peripheral neuropathy and kidney involvement, in contrast to ANCA-negative patients who mainly have pulmonary infiltrates and may develop cardiovascular involvement [7, 19–22]. Response rates with rituximab in EGPA are significantly higher in ANCA ( +) patients compared to ANCA (−) patients (80% versus 38%, respectively). On the other hand, anti-eosinophilic therapies, such as mepolizumab, may have better response rates in ANCA (−) patients [21, 23]. Figure 1 depicts the current pathogenetic model of EGPA and highlights the role of eosinophils.
Fig. 1.
The main pathogenetic pathways in EGPA. ANCA-mediated vascular inflammation and eosinophilic driven pathology
Methods
We performed an electronic search on Medline using the keywords: EGPA, mepolizumab, benralizumab, IL5 and ANCA status in the following combinations: EGPA mepolizumab (n = 115), EGPA benralizumab (n = 32), EGPA anti-IL5 (n = 22), EGPA ANCA status (n = 33) and limited our search to papers published during the last 15 years. Our research was carried out from September 2022 to December 2022. Ongoing or unpublished trials were also searched in ClinicalTrials.gov and the World Health Organization trials portal. In total, 202 articles were retrieved. We excluded 13 duplicate records and finally, 189 articles were screened. Our inclusion criteria were: (i) studies in the English language and (ii) studies reporting data of anti-IL5 therapies in humans with confirmed EGPA. Finally, 58 articles were included in the analysis. A detailed flowchart depicting the methodology used is shown in Fig. 2.
Fig. 2.
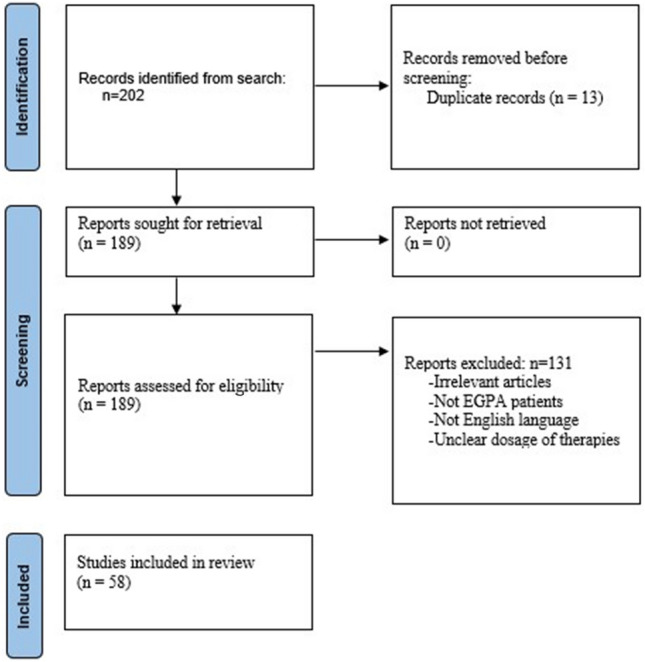
Flowchart of a search strategy
Results
Anti-IL5 therapies in EGPA
Mepolizumab
The first clinical evidence of the successful use of mepolizumab in EGPA was reported in 2010 in a case report [24]. However, the efficacy of anti-IL5 agents in EGPA was strongly supported by data derived from an RCT in 2017. This double-blind, multicenter, phase 3 trial recruited patients with relapsing or refractory EGPA who were treated for at least 4 weeks with a standard of care and aimed to evaluate the efficacy and safety of mepolizumab compared to placebo. In this trial-(MIRRA trial) 136 patients were recruited and divided randomly into two groups; 68 patients were treated with mepolizumab, and the other 68 patients received placebo. All patients had a history of asthma associated with eosinophilia and most of them had a history of abnormalities in the sinonasal cavities. The first group of patients started mepolizumab 300 mg subcutaneously every 4 weeks in addition to steroids for a period of 52 weeks. Patients treated with mepolizumab experienced a longer time of remission compared to patients in the placebo group (28% versus 3%). At weeks 36 and 48 the remission rate in the mepolizumab group was 32%, compared to only 3% in the placebo group. The steroid doses in patients treated with mepolizumab were lower compared to the placebo group (44% of the mepolizumab group were able to taper the prednisone dose to 4 mg or less per day, as compared with 7% receiving placebo).
No major safety signals occurred. The most common adverse effect in the mepolizumab group was headache followed by arthralgia, sinusitis, upper respiratory tract infection (URI) and nasopharyngitis. The least common but most severe adverse event was the worsening of asthma.
It is noteworthy that a total of 47% of the patients in the mepolizumab group did not experience remission during the trial. It is unknown why these patients did not respond to mepolizumab. This fact could be explained by the heterogenous profile of EGPA which means that probably a subgroup of patients did not have an eosinophil-associated disease but mostly had vasculitic manifestations not responding to anti-eosinophilic therapies [23]. Moreover, another consideration is that mepolizumab may not be able to infiltrate inflamed tissues to inhibit and deplete eosinophils, although it can reduce the total number of eosinophils in the bloodstream; it is well known that eosinophils may survive due to other cytokines, except IL5, such as GM-CSF [9].
In conclusion, this study showed that mepolizumab was effective and safe in patients with refractory/relapsing EGPA as an additional therapy on top of steroids and has a significant steroid-sparing effect. However, we should underline that almost half of patients treated with mepolizumab did not respond and, therefore, further research is needed [23]. This study included less than 10% ANCA-positive patients and therefore conclusions regarding treatment response according to ANCA status cannot be drawn [23].
Condreay et al. explored if there is an association between polymorphisms associated with the disease and response to mepolizumab therapy. They sequenced 13 genes (IL5, AKT1, FOS, GSK3B, JUN, MAPK1, MAPK3, STAT1, STAT5A, IL13, IL2, IL4, and IL6) from 61 patients of the mepolizumab group who completed the MIRRA trial. The study concluded that these genetic polymorphisms did not influence mepolizumab-treatment efficacy, although this study did not check rare polymorphisms due to the small proportion of participants [25].
Although EGPA is uncommon in childhood, there has been a documented pediatric case of relapsing EGPA successfully treated with 300 mg of mepolizumab administered subcutaneously every 4 weeks. The 13-year-old girl had improvement in her respiratory and skin symptoms and she was able to reduce the intake of steroids [26]. In another case of an 18-year-old male patient with severe EGPA, the co-administration of mepolizumab and rituximab was effective in achieving remission of his symptoms in contrast to glucocorticoids and cyclophosphamide [27].
There are several studies showing the efficacy of low-dose mepolizumab (100 mg). According to the 2022 retrospective European Multicenter Study, which included 203 patients with EGPA during 2015–2020, it was explored whether a lower dose of mepolizumab (100 mg every 4 weeks) was safe and efficient for these patients. From 191 patients, 158 received 100 mg and 33 received 300 mg of mepolizumab every 4 weeks. Only 20% of the total patients were ANCA-positive, but the group who were ANCA-negative had better response rates to mepolizumab. Adverse events in the high-dose group were more common compared to the low-dose group. Both doses were almost equally effective and proved to reduce the intake of steroids and the number of eosinophils in the bloodstream [28].
Another study in 18 patients showed that 100 mg of mepolizumab was effective in controlling the symptoms of EGPA. The majority of patients (77.7%) were able to reduce their daily steroid dose and the remission rate after 12 months was 94.4%, but there was no correlation between the ANCA status and response to therapy [29]. The efficacy of 100 mg of mepolizumab has been shown in several other studies [30–33]. Mepolizumab has been successfully used in two other cases of refractory EGPA in treating pulmonary and ear lesions [34].
Ueno et al. compared the efficacy of mepolizumab in EGPA versus intravenous cyclophosphamide and found that mepolizumab was more effective in the reduction of oral steroid doses after 3 months [35]. Anti-IL5 therapy with mepolizumab in severe EGPA succeeded in the maintenance of remission, in reducing relapses, in reducing the steroid dose and in controlling active asthma in patients with severe EGPA [36, 37]. It was also found that mepolizumab may be effective in patients with peripheral neuropathy, especially with the co-administration of intravenous immunoglobulin (IVIG) [38–42].
Benralizumab
Benralizumab is a mAb against the IL5a receptor and it is able to induce antibody-dependent cell-mediated cytotoxicity to cells bearing this receptor, such as eosinophils. It is an antibody that has the ability to rapidly decrease the number of eosinophils both in the bloodstream and in affected tissues. Hence, it can reduce the total number of circulating eosinophils almost 100% and does not promote eosinophil degranulation, avoiding further tissue damage [43]. It is noteworthy that benralizumab has been approved since 2017 for the treatment of severe eosinophil-associated asthma [44].
So far, the largest study exploring the efficacy of benralizumab in EGPA recruited 41 patients. All patients reduced the total amount of daily prednisone below 10 mg, and 80% to less than 5 mg. Notably, 40% of the patients were able to discontinue steroids [43].
Limited evidence based on case reports suggests that benralizumab may be effective in mepolizumab-refractory EGPA [45].
According to other studies, benralizumab resulted in significant tapering and even in discontinuation of steroids especially in EGPA patients with severe asthma. The association between ANCA status and response to treatment with benralizumab remains unclear [46–48]. There is still little evidence regarding the efficacy of benralizumab in reducing ANCA titers in patients with MPO-ANCA positivity [49–51]. Moreover, benralizumab was also effective in an ANCA-negative patient with EGPA [52].
Several case reports have explored the potential role of benralizumab in EGPA [53–56]. Of interest, early administration of benralizumab in a patient with EGPA associated—biopsy proven—eosinophilic myocarditis, led to a significant increase in ejection fraction from 40 to 60%, prior to and following treatment, respectively [43, 57–59]. It has been reported that mepolizumab was unable to improve a patient with neuropathy, but when the therapy switched to benralizumab, the patient recovered [43, 57, 60]. Benralizumab was also effective in a 39-year-old patient with otitis media and sinusitis associated with EGPA [61]. As mentioned above, these are case reports, which do not prove the efficacy and safety of benralizumab in EGPA and may only trigger further research.
Finally, there is an ongoing randomized, double-blinded, active-controlled, parallel group, multicenter 52-week Phase 3 study that compares the efficacy and safety of benralizumab 30 mg versus mepolizumab 300 mg administered by subcutaneous (SC) injection in patients with relapsing or refractory EGPA on corticosteroid therapy with or without stable immunosuppressive therapy (MANDARA trial—NCT04157348).
Reslizumab
Reslizumab is an anti-IL5 agent that has been approved by the FDA in 2016 for severe eosinophilic asthma [62]. There has been only one study reporting data regarding reslizumab in EGPA. In this pilot study, reslizumab has been administrated intravenously at a dose of 3 mg/kg in 10 EGPA patients. Reslizumab proved to be safe and effective and most of the patients achieved remission of their symptoms as well as reduction of their daily oral steroids [63]. Further and larger studies should be conducted to evaluate the efficacy of reslizumab in EGPA.
Discussion
Recent research advances have highlighted the role of eosinophils in homeostasis and in several diseases [9]. Our knowledge of EGPA pathophysiology has expanded but the exact mechanisms are yet to be determined. Even though EGPA is classified as an ANCA-associated vasculitis, it is worthwhile exploring whether it could be also considered as an eosinophilic-driven disease. Eosinophils may drive both inflammatory and vasculitic processes and therefore may be key cells in pathogenesis. IL5 may be a significant cytokine in the pathogenesis of EGPA, by attracting and activating eosinophils in the inflamed tissues. Furthermore, some severe clinical manifestations including cardiomyopathy and neuropathy could come as a result of the overlapping influence of eosinophilic infiltration and vasculitis.
The correlation between blood and tissue eosinophilia and their clinical relevance is still unclear. Genetic factors, such as polymorphisms in the HLA-DQA1 gene, may have an impact on the prognosis and clinical outcome of the disease, but more research is needed, as mentioned above [25]. It is still uncertain whether ANCA status plays a role in anti-IL5 therapy response but some evidence points to the direction that ANCA-negative patients may have a more eosinophilic-driven pathophysiology and, therefore may better respond to anti-eosinophilic therapies. However, we should emphasize that clinical data to support this are still limited.
There is no doubt that oral steroids remain the cornerstone therapy of EGPA. Nevertheless, steroid therapy is characterized by chronic and severe adverse events and many cases of EGPA patients are steroid-refractory. Other immunosuppressive agents, such as cyclophosphamide and rituximab, have shown good results in achieving remission for a long period of time and are considered as first-line treatments, according to recent recommendations [8]. However, many patients appear to be refractory to these treatments and thus, new therapies, especially against IL5 (mepolizumab) or IL5 receptor (benralizumab) appear as attractive alternative therapeutic options. Anti-eosinophilic therapies seem to be safe and effective for the management of these patients by reducing clinical manifestations in active and severe EGPA, inducing remission and minimizing the daily administration of oral steroids. Mepolizumab and benralizumab have also shown efficacy in patients with refractory EGPA to other medications. The fact that many patients do not respond to anti-IL5 treatment may suggest that there are more complex inflammatory pathways and targeting only one mechanism may not be enough.
In conclusion, anti-eosinophilic therapies appear as attractive therapeutic options for patients with EGPA with a good efficacy and safety profile. For patients with severe disease, current recommendations indicate steroids alongside RTX or CYC as first-line treatments. Data regarding anti-eosinophilic therapies in patients with severe disease are limited since these patients are usually excluded from clinical trials. However, in patients with active, non-severe disease, steroids and mepolizumab are recommended as first-line treatments. It is noteworthy that anti-eosinophilic therapies have shown efficacy in patients with severe disease refractory to standard therapy and as our clinical experience with the use of these agents is expanding, their place in the treatment algorithm may change. For the time being, we have three main therapeutic options namely, CYC, RTX and anti-eosinophilic therapies apart from steroids which are used universally in all patients. Up until now, we lack reliable biomarkers that could predict response to treatment and this should be an area of research in the near future. We certainly need biomarkers that could pinpoint patients with a prominent vasculitic pathophysiology versus patients with a predominant eosinophilic-driven disease; the former patients may respond better to RTX or CYC whereas the latter may respond better to anti-eosinophilic therapies. Taking into account that vasculitis and eosinophilic-driven pathology overlap in patients with EGPA, combination treatment targeting both pathogenetic arms could also be explored in patients with severe/refractory disease.
Author contributions
IK and AA performed the literature search, analyzed the data and drafted the manuscript. DD conceived the idea of the review, supervised the project and assisted in data analysis and manuscript drafting. All authors take full responsibility for the integrity and accuracy of all aspects of the work.
Funding
Open access funding provided by HEAL-Link Greece. This study was not funded by any organization/institution/university.
Data availability
All data are presented in the current manuscript.
Declarations
Conflict of interest
DD reports honorarium for participation in an advisory from Astra-Zeneca. IK and AA declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ioannis Kouverianos and Andreas Angelopoulos have contributed equally.
Contributor Information
Ioannis Kouverianos, Email: kouv.john@gmail.com.
Andreas Angelopoulos, Email: andaggel@hotmail.com.
Dimitrios Daoussis, Email: jimdaoussis@hotmail.com.
References
- 1.Geetha D, Jefferson JA. ANCA-associated vasculitis: core curriculum 2020. Am J Kidney Dis. 2020;75(1):124–137. doi: 10.1053/j.ajkd.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68:430–436. doi: 10.1016/j.alit.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Isozaki T, Homma T, Sagara H, Kasama T. Role of cytokines in egpa and the possibility of treatment with an anti-il-5 antibody. J Clin Med. 2020;9:1–11. doi: 10.3390/jcm9123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivieri B, Tinazzi E, Caminati M, Lunardi C. Biologics for the treatment of allergic conditions: eosinophil disorders. Immunol Allergy Clin North Am. 2020;40:649–665. doi: 10.1016/j.iac.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Fagni F, Bello F, Emmi G. Eosinophilic granulomatosis with polyangiitis: dissecting the pathophysiology. Front Med. 2021 doi: 10.3389/fmed.2021.627776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caminati M, Menzella F, Guidolin L, Senna G. Targeting eosinophils: severe asthma and beyond. Drugs Context. 2019 doi: 10.7573/dic.212587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen EA, Jackson DJ, Heffler E, Mathur SK, Bredenoord AJ, Pavord ID, et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu Rev Immunol. 2021 doi: 10.1146/annurev-immunol-093019-125918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, et al. 2021 American college of rheumatology/vasculitis foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken) 2021;73(8):1088–1105. doi: 10.1002/acr.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bochner BS. The eosinophil: for better or worse, in sickness and in health. Ann Allergy Asthma Immunol. 2018;121:150–155. doi: 10.1016/j.anai.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wechsler ME, Munitz A, Ackerman SJ, Drake MG, Jackson DJ, Wardlaw AJ, et al. Eosinophils in health and disease: a state-of-the-art review. Mayo Clin Proc. 2021;96(10):2694–2707. doi: 10.1016/j.mayocp.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez GA, Yacoub MR, Ripa M, Mannina D, Cariddi A, Saporiti N, et al. Eosinophils from physiology to disease: a comprehensive review. BioMed Res Int. 2018 doi: 10.1155/2018/9095275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greco A, Rizzo MI, de Virgilio A, Gallo A, Fusconi M, Ruoppolo G, et al. Churg-Strauss syndrome. Autoimmun Rev. 2015;14(4):341–348. doi: 10.1016/j.autrev.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Arnold S, Klapa S, Holl-Ulrich K, Müller A, Kerstein-Stähle A, Lamprecht P. Granulomatous vasculitides and vasculitides with extravascular granulomatosis. Z Rheumatol. 2022;81(7):558–566. doi: 10.1007/s00393-022-01249-7. [DOI] [PubMed] [Google Scholar]
- 14.Tsurikisawa N, Saito H, Tsuburai T, Oshikata C, Ono E, Mitomi H, et al. Differences in regulatory T cells between Churg-Strauss syndrome and chronic eosinophilic pneumonia with asthma. J Allergy Clin Immunol. 2008;122(3):610–616. doi: 10.1016/j.jaci.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Wieczorek S, Hellmich B, Arning L, Moosig F, Lamprecht P, Gross WL, et al. Functionally relevant variations of the interleukin-10 gene associated with antineutrophil cytoplasmic antibody-negative Churg-Strauss syndrome, but not with Wegener’s granulomatosis. Arthritis Rheum. 2008;58(6):1839–1848. doi: 10.1002/art.23496. [DOI] [PubMed] [Google Scholar]
- 16.Uzzo M, Regola F, Trezzi B, Toniati P, Franceschini F, Sinico RA. Novel targets for drug use in eosinophilic granulomatosis with polyangiitis. Front Med. 2021 doi: 10.3389/fmed.2021.754434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayrak Durmaz MS, Çelebi Sözener Z, Bavbek S. Eosinophilic granulomatosis with polyangitis: a new target for biological. Tuberk Toraks. 2022;70(1):93–101. doi: 10.5578/tt.20229911. [DOI] [PubMed] [Google Scholar]
- 18.Milne ME, Kimball J, Tarrant TK, Al-Rohil RN, Leverenz DL. The role of T helper type 2 (Th2) cytokines in the pathogenesis of eosinophilic granulomatosis with polyangiitis (eGPA): an illustrative case and discussion. Curr Allergy Asthma Rep. 2022 doi: 10.1007/s11882-022-01039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf J, Bergner R, Mutallib S, Buggle F, Grau AJ. Neurologic complications of Churg-Strauss syndrome–a prospective monocentric study. Eur J Neurol. 2010;17(4):582–588. doi: 10.1111/j.1468-1331.2009.02902.x. [DOI] [PubMed] [Google Scholar]
- 20.Mavrogeni S, Karabela G, Gialafos E, Stavropoulos E, Spiliotis G, Katsifis G, et al. Cardiac involvement in ANCA (+) and ANCA (-) Churg-Strauss syndrome evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets. 2013;12(5):322–327. doi: 10.2174/18715281113129990054. [DOI] [PubMed] [Google Scholar]
- 21.Lyons PA, Peters JE, Alberici F, Liley J, Coulson RMR, Astle W, et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun. 2019 doi: 10.1038/s41467-019-12515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokołowska B, Szczeklik W, Włudarczyk A, Kuczia P, Jakieła B, Gasior J, et al. ANCA-positive and ANCA-negative phenotypes of eosinophilic granulomatosis with polyangiitis (EGPA): outcome and long-term follow-up of 50 patients from a single polish centre. Clin Exp Rheumatol. 2014;32(SUPPL.82):S41–S47. [PubMed] [Google Scholar]
- 23.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn JE, Grandpeix-Guyodo C, Marroun I, Catherinot E, Mellot F, Roufosse F, et al. Sustained response to mepolizumab in refractory Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125(1):267–270. doi: 10.1016/j.jaci.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Condreay LD, Parham LR, Qu XA, Steinfeld J, Wechsler ME, Raby BA, et al. Pharmacogenetic investigation of efficacy response to mepolizumab in eosinophilic granulomatosis with polyangiitis. Rheumatol Int. 2020;40(8):1301–1307. doi: 10.1007/s00296-020-04523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nara M, Saito M, Abe F, Komatsuda A, Wakui H, Takahashi N. A pediatric case of relapsing eosinophilic granulomatosis with polyangiitis successfully treated with mepolizumab. Intern Med. 2019;58(24):3583–3587. doi: 10.2169/internalmedicine.3406-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsioulos G, Kounatidis D, Vallianou NG, Koufopoulos N, Katsimbri P, Antoniadou A. Severe eosinophilic granulomatosis with polyangiitis responding to a combination of rituximab and mepolizumab. Am J Med Sci. 2022 doi: 10.1016/j.amjms.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Bettiol A, Urban ML, Dagna L, Cottin V, Franceschini F, del Giacco S, et al. Mepolizumab for eosinophilic granulomatosis with polyangiitis: a European multicenter observational study. Arthritis Rheumatol. 2022;74(2):295–306. doi: 10.1002/art.41943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vultaggio A, Nencini F, Bormioli S, Vivarelli E, Dies L, Rossi O, et al. Low-dose mepolizumab effectiveness in patients suffering from eosinophilic granulomatosis with polyangiitis. Allergy Asthma Immunol Res. 2020;12(5):885–893. doi: 10.4168/aair.2020.12.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detoraki A, Tremante E, Poto R, Morelli E, Quaremba G, Granata F, et al. Real-life evidence of low-dose mepolizumab efficacy in EGPA: a case series. Respir Res. 2021;22:185. doi: 10.1186/s12931-021-01775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Özdel Öztürk B, Yavuz Z, Aydın Ö, Mungan D, Sin BA, Demirel YS, et al. Effectiveness of low-dose mepolizumab in the treatment of eosinophilic granulomatosis with polyangiitis (EGPA): a real-life experience. Int Arch Allergy Immunol. 2022;183:1281–1290. doi: 10.1159/000526410. [DOI] [PubMed] [Google Scholar]
- 32.Vergles M, Matković Z, Lalić K, Trkanjec JT, Tudorić N. Mepolizumab as a glucocorticoid-sparing agent in eosinophilic granulomatosis with polyangiitis (EGPA): is a lower dose sufficient? J Asthma. 2021;58(12):1675–1679. doi: 10.1080/02770903.2020.1827417. [DOI] [PubMed] [Google Scholar]
- 33.Canzian A, Venhoff N, Urban ML, Sartorelli S, Ruppert AM, Groh M, et al. Use of biologics to treat relapsing and/or refractory eosinophilic granulomatosis with polyangiitis: data from a European collaborative study. Arthritis Rheumatol. 2021;73(3):498–503. doi: 10.1002/art.41534. [DOI] [PubMed] [Google Scholar]
- 34.Ushio Y, Wakiya R, Kato M, Kameda T, Nakashima S, Shimada H, et al. Two cases of refractory eosinophilic granulomatosis with polyangiitis wherein mepolizumab was effective against pulmonary and ear lesions. Mod Rheumatol Case Rep. 2021;5(2):327–332. doi: 10.1080/24725625.2021.1881205. [DOI] [PubMed] [Google Scholar]
- 35.Ueno M, Miyagawa I, Aritomi T, Kimura K, Iwata S, Hanami K, et al. Safety and effectiveness of mepolizumab therapy in remission induction therapy for eosinophilic granulomatosis with polyangiitis: a retrospective study. Arthritis Res Ther. 2022;24(1):1–9. doi: 10.1186/s13075-022-02845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno M, Miyagawa I, Nakano K, Iwata S, Hanami K, Fukuyo S, et al. Effectiveness and safety of mepolizumab in combination with corticosteroids in patients with eosinophilic granulomatosis with polyangiitis. Arthritis Res Ther. 2021;23(1):1–9. doi: 10.1186/s13075-021-02462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ríos-Garcés R, Prieto-González S, Hernández-Rodríguez J, Arismendi E, Alobid I, Penatti AE, et al. Response to mepolizumab according to disease manifestations in patients with eosinophilic granulomatosis with polyangiitis. Eur J Intern Med. 2022;95:61–66. doi: 10.1016/j.ejim.2021.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda T, Komatsu T, Yokoyama K, Takahashi K, Kawakami T. Early add-on administration of mepolizumab and intravenous immunoglobulin effective in treating eosinophilic granulomatosis with polyangiitis. J Dermatol. 2021;48(4):529–532. doi: 10.1111/1346-8138.15709. [DOI] [PubMed] [Google Scholar]
- 39.Kai Y, Yoshikawa M, Matsuda M, Suzuki K, Ohara H, Iguchi N, et al. Improvement of peripheral neuropathy in a patient with antineutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis by additional mepolizumab. Allergy Asthma Clin Immunol. 2022;18(1):1–5. doi: 10.1186/s13223-022-00653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura Y, Fukutomi Y, Sekiya K, Kajiwara K, Kawasaki Y, Fujita N, et al. Low-dose mepolizumab is effective as an add-on therapy for treating long-lasting peripheral neuropathy in patients with eosinophilic granulomatosis with polyangiitis. Mod Rheumatol. 2022;32(2):387–395. doi: 10.1093/mr/roab005. [DOI] [PubMed] [Google Scholar]
- 41.Nishihara M, Hamaguchi M, Ikumi N, Nishiwaki A, Sugiyama K, Nagasawa Y, et al. Successful early introduction of mepolizumab for peripheral neuropathy with a peripheral circulatory disorder in a patient with myeloperoxidase anti-neutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis. Mod Rheumatol Case Rep. 2021;5(2):354–359. doi: 10.1080/24725625.2021.1916159. [DOI] [PubMed] [Google Scholar]
- 42.Kitamura N, Hamaguchi M, Nishihara M, Ikumi N, Sugiyama K, Nagasawa Y, et al. The effects of mepolizumab on peripheral circulation and neurological symptoms in eosinophilic granulomatosis with polyangiitis (EGPA) patients. Allergol Int. 2021;70(1):148–149. doi: 10.1016/j.alit.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Koga Y, Aoki-Saito H, Kamide Y, Sato M, Tsurumaki H, Yatomi M, et al. Perspectives on the efficacy of benralizumab for treatment of eosinophilic granulomatosis with polyangiitis. Front Pharmacol. 2022;13:765. doi: 10.3389/fphar.2022.865318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 45.Menzella F, Galeone C, Ghidoni G, Ruggiero P, Capobelli S, Simonazzi A, et al. Successful treatment with benralizumab in a patient with eosinophilic granulomatosis with polyangiitis refractory to mepolizumab. Multidiscip Respir Med. 2021 doi: 10.4081/mrm.2021.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guntur VP, Manka LA, Denson JL, Dunn RM, Dollin YT, Gill M, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol: In Practice. 2021;9(3):1186–1193.e1. doi: 10.1016/j.jaip.2020.09.054. [DOI] [PubMed] [Google Scholar]
- 47.Nanzer AM, Dhariwal J, Kavanagh J, Hearn A, Fernandes M, Thomson L, et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020;6(4):00451–2020. doi: 10.1183/23120541.00451-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padoan R, Chieco Bianchi F, Marchi MR, Cazzador D, Felicetti M, Emanuelli E, et al. Benralizumab as a glucocorticoid-sparing treatment option for severe asthma in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol: In Practice. 2020;8(9):3225–3227.e2. doi: 10.1016/j.jaip.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Takenaka K, Minami T, Yoshihashi Y, Hirata S, Kimura Y, Kono H. Decrease in MPO-ANCA after administration of benralizumab in eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68:539–540. doi: 10.1016/j.alit.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Coppola A, Flores KR, de Filippis F. Rapid onset of effect of benralizumab on respiratory symptoms in a patient with eosinophilic granulomatosis with polyangiitis. Respir Med Case Rep. 2020;1:30. doi: 10.1016/j.rmcr.2020.101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyata Y, Inoue H, Homma T, Tanaka A, Sagara H. Efficacy of benralizumab and clinical course of igg4 in eosinophilic granulomatosis with polyangiitis. J Investig Allergol Clin Immunol. 2021;31:346–348. doi: 10.18176/jiaci.0648. [DOI] [PubMed] [Google Scholar]
- 52.Ricciardi L, Soler DG, Bennici A, Brunetto S, Pioggia G, Gangemi S. Case report: Severe eosinophilic asthma associated with ANCA-negative EGPA in a young adult successfully treated with benralizumab. Front Pharmacol. 2022 doi: 10.3389/fphar.2022.858344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y, Hojjati M. Benralizumab as a potential adjunctive therapy in eosinophilic granulomatosis with polyangiitis. J Clin Rheumatol. 2021;27(8S):S401–S402. doi: 10.1097/RHU.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 54.Chica-Guzmán MV, Morillo-Guerrero R, Carrón-Herrero A, González-de-Olano D, Almonacid-Sánchez C. Eosinophilic granulomatosis with polyangiitis successfully treated with benralizumab. Ann Allergy Asthma Immunol. 2020;125(2):228–230. doi: 10.1016/j.anai.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Martínez-Rivera C, Garcia-Olivé I, Urrutia-Royo B, Basagaña-Torrento M, Rosell A, Abad J. Rapid effect of benralizumab in exacerbation of severe eosinophilic asthma associated with eosinophilic granulomatosis with polyangiitis. BMC Pulm Med. 2021;21(1):35. doi: 10.1186/s12890-021-01397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bormioli S, Vultaggio A, Nencini F, Comin CE, Bercich L, Bezzi M, et al. Benralizumab: resolution of eosinophilic pulmonary vasculitis in a patient with EGPA. J Investig Allergol Clin Immunol. 2021;31(6):519–521. doi: 10.18176/jiaci.0689. [DOI] [PubMed] [Google Scholar]
- 57.Kolios AGA, Lutterotti A, Kulcsar Z, Renner T, Rudiger A, Nilsson J. Benralizumab in eosinophilic granulomatosis with polyangiitis complicated by Staphylococcus aureus sepsis. Clin Immunol. 2021;1:222. doi: 10.1016/j.clim.2020.108574. [DOI] [PubMed] [Google Scholar]
- 58.Belfeki N, Abroug S, Ghriss N, Chouchane I, Hamrouni S, Strazzulla A, et al. Successful benralizumab for eosinophilic myocarditis in eosinophilic granulomatosis with polyangiitis. Clin Exp Rheumatol. 2021;40(4):834–837. doi: 10.55563/clinexprheumatol/ahyqld. [DOI] [PubMed] [Google Scholar]
- 59.Colantuono S, Pellicano C, Leodori G, Cilia F, Francone M, Visentini M. Early benralizumab for eosinophilic myocarditis in eosinophilic granulomatosis with polyangiitis. Allergol Int. 2020;69:483–484. doi: 10.1016/j.alit.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Koga Y, Yoshimi S, Harada T, Suzuki S, Ohtsuka T, Dobashi K, et al. Long-term Safety and efficacy of benralizumab for eosinophilic granulomatosis with polyangiitis complicated with severe neuropathy. Intern Med. 2022 doi: 10.2169/internalmedicine.0613-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugiyama T, Yoshida S, Kikuchi S, Iino Y. Successful treatment for otitis media and sinusitis associated with eosinophilic granulomatosis with polyangiitis using anti-IL-5 receptor monoclonal antibody benralizumab. Arerugi. 2022;71(3):242–247. doi: 10.15036/arerugi.71.242. [DOI] [PubMed] [Google Scholar]
- 62.FDA approves Cinqair to treat severe asthma | FDA [Internet]. [cited 2022 Dec 21]. https://www.fda.gov/news-events/press-announcements/fda-approves-cinqair-treat-severe-asthma
- 63.Manka LA, Guntur VP, Denson JL, Dunn RM, Dollin YT, Strand MJ, et al. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann Allergy Asthma Immunol. 2021;126(6):696–701.e1. doi: 10.1016/j.anai.2021.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the current manuscript.