Abstract
Aggressive breast cancers portend a poor prognosis, but current polygenic risk scores (PRSs) for breast cancer do not reliably predict aggressive cancers. Aggressiveness can be effectively recapitulated using tumor gene expression profiling. Thus, we sought to develop a PRS for the risk of recurrence score weighted on proliferation (ROR-P), an established prognostic signature. Using 2363 breast cancers with tumor gene expression data and single nucleotide polymorphism (SNP) genotypes, we examined the associations between ROR-P and known breast cancer susceptibility SNPs using linear regression models. We constructed PRSs based on varying p-value thresholds and selected the optimal PRS based on model r2 in 5-fold cross-validation. We then used Cox proportional hazards regression to test the ROR-P PRS’s association with breast cancer-specific survival in two independent cohorts totaling 10,196 breast cancers and 785 events. In meta-analysis of these cohorts, higher ROR-P PRS was associated with worse survival, HR per SD = 1.13 (95% CI 1.06–1.21, p = 4.0 × 10–4). The ROR-P PRS had a similar magnitude of effect on survival as a comparator PRS for estrogen receptor (ER)-negative versus positive cancer risk (PRSER-/ER+). Furthermore, its effect was minimally attenuated when adjusted for PRSER-/ER+, suggesting that the ROR-P PRS provides additional prognostic information beyond ER status. In summary, we used integrated analysis of germline SNP and tumor gene expression data to construct a PRS associated with aggressive tumor biology and worse survival. These findings could potentially enhance risk stratification for breast cancer screening and prevention.
Subject terms: Cancer epidemiology, Breast cancer, Cancer genetics
Introduction
Polygenic risk scores (PRSs) have emerged as promising tools for breast cancer risk prediction. Over 200 single nucleotide polymorphisms (SNPs) associated with breast cancer risk have been identified1. Though the effects of individual SNPs are weak, PRSs representing the cumulative effects of multiple SNPs can stratify breast cancer risk on a population level2 and improve the performance of clinical breast cancer risk prediction models3,4. Ongoing prospective trials are testing the ability of the PRS to inform decision-making around breast cancer screening and prevention5–7.
Current breast cancer PRSs have limited ability to account for the biological heterogeneity of breast cancer. This is a critical limitation since breast cancer encompasses a variety of subtypes ranging from indolent to aggressive, with the latter defined as having increased proliferation or metastatic potential and poor prognosis8. However, case-only analyses have found that PRSs for overall breast cancer risk are associated with more favorable clinicopathologic9 and prognostic features10, as well as lower risk of interval versus screen-detected cancer11,12. Efforts to fit PRSs to subtypes of breast cancer have focused on estrogen receptor (ER) status given that ER-negative breast cancers tend to be more proliferative and are associated with earlier risk of relapse2,13. However, aggressive cancers can encompass ER-negative and ER-positive subtypes. For instance, ER-positive cancers are commonly divided into luminal A (low-grade) and B (high-grade) subtypes with the latter having worse prognosis14.
Beyond ER status, aggressiveness can be measured using tumor prognostic signatures, which integrate the expression levels of multiple genes to calculate prognostic scores that guide treatment decision-making8,15,16. In this analysis, we selected the risk of recurrence score weighted on proliferation (ROR-P), which is based on the expression of 50 genes included in the Prediction Analysis of Microarray 50 (PAM50) signature. PAM50 classifies tumors by intrinsic subtype (luminal A, luminal B, HER2-enriched, basal-like, and normal-like). ROR-P is calculated by adding the subtype-centroid correlation coefficients for each subtype, weighted by their association with recurrence, to the weighted expression levels of 11 proliferation genes17,18. ROR-P is continuous (though categorical cutoffs are used for clinical decision-making) and has stronger prognostic value than traditional markers such as ER status, grade, and Ki-6717,19.
Prior studies have examined the associations between germline genetics and ROR-P using a transcriptome-wide association study approach20. However, no studies have attempted to develop a PRS for ROR-P or other gene expression-based signature of aggressiveness. We hypothesized that some known breast cancer susceptibility SNPs are positively correlated with ROR-P, whereas others are negatively correlated, and these differential associations can be used to construct a PRS for ROR-P. We therefore sought to construct the ROR-P PRS in datasets with germline SNP genotypes and tumor gene expression. To evaluate whether the ROR-P PRS was associated with aggressive tumors with worse prognosis, we examined its association with breast cancer-specific survival in two independent cohorts.
Results
Study characteristics
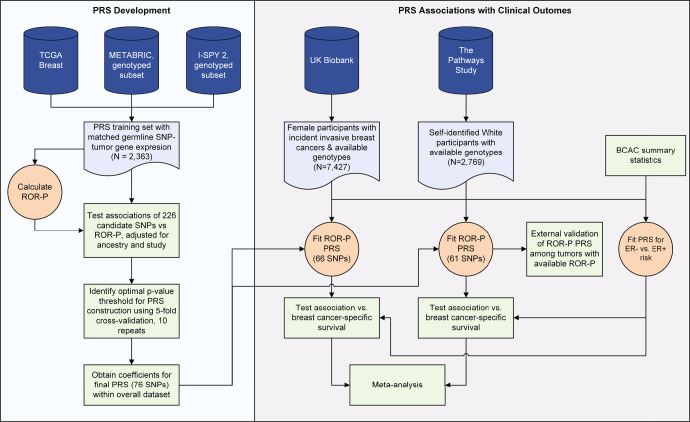
We developed the ROR-P PRS using 2363 breast cancers from three studies: The Cancer Genome Atlas (TCGA)21, Molecular Taxonomy of Breast Cancer International Consortium (METABRIC)22, and Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And molecular analysis 2 (I-SPY 2 TRIAL)23 (Fig. 1, Supplementary Table 1, Supplementary Fig. 1). Most of the participants in these studies were Non-Hispanic White; METABRIC did not report race or ethnicity but predominantly included White participants because recruitment occurred in the United Kingdom and Canada22 (Table 1). Cancers from I-SPY 2 were diagnosed at younger ages and more likely to be ROR-P High and classified as Basal intrinsic subtype. This reflects the trial’s inclusion criteria, which is limited to locally advanced, molecularly high-risk cancers as defined by gene expression profiling and/or clinicopathologic features23.
Fig. 1. Study design.
We developed a polygenic risk score (PRS) for the risk of recurrence score weighted on proliferation (ROR-P) using pooled data from three studies: The Cancer Genome Atlas (TCGA), Molecular Taxonomy of Breast Cancer International Consortium (METABRIC), and Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And molecular analysis 2 (I-SPY 2 TRIAL). We used these datasets to evaluate the performance of PRS constructed using varying p-value thresholds and to calculate the effect sizes of the SNPs included in the PRS with optimal performance (cross-validated r2). We then calculated the ROR-P PRS in breast cancer patients from two independent datasets, the UK Biobank and the Pathways Study. In Pathways, we performed external validation of the ROR-P PRS by examining its association with measured ROR-P in tumors with available gene expression profiling data. We then tested the associations between the ROR-P PRS and breast cancer-specific survival in UK Biobank and the Pathways Study and performed meta-analysis of the results. In parallel, we generated a PRS for the case-case risk of estrogen receptor (ER)-negative versus ER-positive breast cancer using summary statistics from the Breast Cancer Association Consortium (BCAC) and evaluated its association with breast cancer-specific survival.
Table 1.
Characteristics of studies used in development of ROR-P PRS.
| TCGA | METABRIC | I-SPY 2 TRIAL | |
|---|---|---|---|
| Characteristic | N = 953 | N = 496 | N = 914 |
| Age at diagnosis in years, median (IQR) | 58 (49, 68) | 62 (52, 73) | 49 (41, 57) |
| Race, n (%)a | |||
| White | 652 (75%) | 733 (80%) | |
| Black/African-American | 160 (18%) | 102 (11%) | |
| Asian | 55 (6.3%) | 63 (6.9%) | |
| Other | 1 (0.1%) | 16 (1.8%) | |
| Unknown | 85 | ||
| Ethnicity, n (%)a | |||
| Hispanic/Latina | 32 (4.0%) | 112 (12%) | |
| Non-Hispanic/Latina | 764 (96%) | 802 (88%) | |
| Unknown | 157 | ||
| Estrogen receptor status, n (%)b | |||
| Negative | 224 (24%) | 93 (19%) | 410 (45%) |
| Positive | 729 (76%) | 403 (81%) | 504 (55%) |
| HER2 status, n (%)b | |||
| Negative | 512 (78%) | 112 (78%) | 680 (74%) |
| Positive | 148 (22%) | 31 (22%) | 234 (26%) |
| Unknownc | 293 | 353 | |
| Intrinsic subtype call, n (%) | |||
| Basal | 172 (18%) | 57 (11%) | 374 (41%) |
| Her2 | 72 (7.6%) | 74 (15%) | 136 (15%) |
| LumA | 493 (52%) | 132 (27%) | 171 (19%) |
| LumB | 180 (19%) | 147 (30%) | 211 (23%) |
| Normal | 36 (3.8%) | 86 (17%) | 22 (2.4%) |
| ROR-P, median (IQR) | 32 (8, 50) | 41 (29, 51) | 46 (34, 58) |
| ROR-P Group, n (%) | |||
| Low | 266 (28%) | 47 (9.5%) | 42 (4.6%) |
| Medium | 488 (51%) | 340 (69%) | 550 (60%) |
| High | 199 (21%) | 109 (22%) | 322 (35%) |
HER2 human epidermal growth factor receptor 2, IQR interquartile range, I-SPY 2 TRIAL Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And molecular analysis 2, METABRIC Molecular Taxonomy of Breast Cancer International Consortium, ROR-P risk of recurrence score weighted on proliferation, TCGA The Cancer Genome Atlas.
aDistributions by race and ethnicity are not available for METABRIC.
bDetermined by immunohistochemistry.
cIncludes cases with indeterminate or equivocal HER2 status on immunohistochemistry.
We calculated the ROR-P PRS and tested its associations with survival and tumor characteristics in two studies containing prospective follow-up of breast cancer patients, the UK Biobank and the Pathways Study (Fig. 1, Supplementary Table 2). Limited breast cancer characteristics were available for UK Biobank, but age at diagnosis and event rate were comparable across studies, with the Pathways Study having a longer duration of follow-up (Supplementary Table 3). Our analysis of UK Biobank included 7427 breast cancer cases and 544 breast cancer-specific deaths during a median follow-up time of 6.4 (interquartile range 3.7–9.1) years. The Pathways Study included 2769 cancers with 241 breast cancer-specific deaths during a median follow-up time of 10.7 (interquartile range 8.2–12.3) years. Tumors in Pathways were predominantly ER-positive, human epidermal growth factor receptor 2 (HER2)-negative, and Grade 1 or 2.
Development of the ROR-P PRS
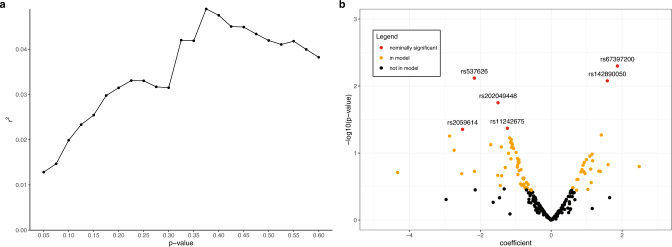
Using pooled data from TCGA, METABRIC, and the I-SPY 2 TRIAL, we evaluated the case-case associations of 226 breast cancer susceptibility SNPs and ROR-P (Supplementary Table 4). Based on these associations, we constructed PRSs using varying p-value thresholds and identified a 76-SNP PRS as having the best performance, with a model r2 of 0.049 in 5-fold cross-validation (Fig. 2a, Supplementary Table 5). For 51 of 76 SNPs in the ROR-P PRS, the breast cancer risk allele, as annotated by the original GWAS, was associated with lower ROR-P (Supplementary Table 6, Fig. 2b), which is consistent with the observation that the PRS for overall breast cancer risk is associated with better survival10. Six of 76 SNPs in our final ROR-P PRS had nominally significant associations with ROR-P, though none met significance after Bonferroni correction. The nominally significant SNP with the strongest positive association with ROR-P, rs67397200 (ABHD8 in 19p13.11), was discovered in GWAS for ER-negative cancer13 and is associated with increased case-case risk of luminal B and triple negative intrinsic-like subtypes, and decreased risk of luminal A subtype24. The nominally significant SNP with the strongest negative association, rs537626 (LINC01488 in 11q13.3), was discovered in GWAS for early onset breast cancer25, although the region has also been implicated in increased risk of ER-positive cancer in an admixture mapping study of African-American women26.
Fig. 2. Development of the polygenic risk score for the risk of recurrence score weighted on proliferation (ROR-P PRS).
a We performed 5-fold cross-validation to identify the optimal p-value threshold for including single nucleotide polymorphisms (SNPs) in the ROR-P PRS. The r2 is shown for each p-value threshold tested. Our final PRS used a p-value cutoff of 0.375. b Volcano plot of associations of 226 SNPs with ROR-P, adjusted for genetic ancestry (principal components 1–10) and study. SNPs included in the final 76-SNP PRS score are indicated in red (nominally significant association with ROR-P) and orange (not nominally significant but included in model).
External validation of ROR-P PRS
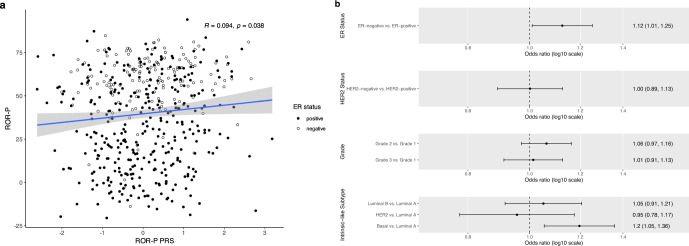
We performed external validation of the ROR-P PRS in a nested subset of 484 tumors from the Pathways Study that had undergone gene expression profiling27 (Supplementary Fig. 2). The ROR-P PRS was weakly correlated with measured ROR-P, with a Pearson correlation coefficient of 0.094 (p = 0.038) (Fig. 3a). We also examined associations between the ROR-P PRS and tumor clinicopathologic features (Fig. 3b). Higher ROR-P PRS was associated with ER-negative status, odds ratio per standard deviation increment (OR per S.D.) of 1.12 (95% CI 1.01–1.25, p = 0.034), but not HER2 status or grade. Higher ROR-P PRS was also associated with increased odds of basal versus luminal A intrinsic-like subtype, as defined by receptor status and grade (OR per S.D. = 1.20, 95% CI 1.05–1.36, p = 0.006).
Fig. 3. Validation of a polygenic risk score for risk of recurrence score weighted on proliferation (ROR-P PRS) in the Pathways Study.
a Scatter plot of the normalized ROR-P PRS and tumor ROR-P in a subset of 484 tumors with both available measures. The Pearson correlation coefficient and p-value are shown. Points are color-coded by estrogen receptor (ER) status. b The associations between ROR-P PRS and tumor features in the Pathways Study were evaluated using logistic regression (for estrogen receptor and human epidermal growth factor 2 status) and multinomial logistic regression (for histologic grade and intrinsic-like subtype). Models were adjusted for genetic ancestry (principal components 1–10). Point estimates and 95% confidence intervals for the association between ROR-P PRS and respective tumor feature are shown.
Association of ROR-P PRS with breast cancer-specific survival
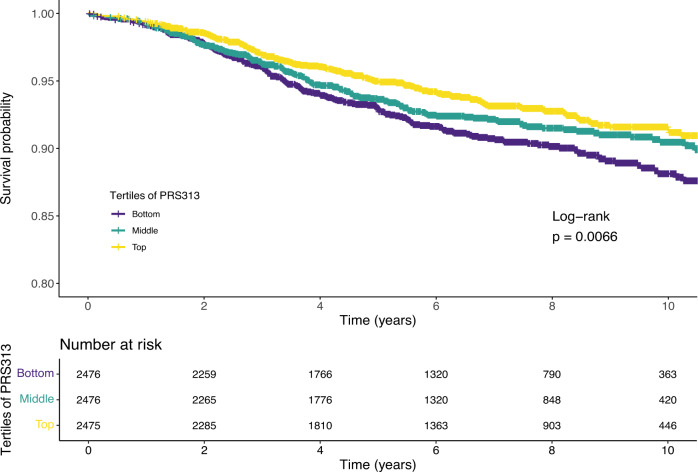
In UK Biobank, we first evaluated the association between a PRS for overall breast cancer risk (PRSoverallBC) and breast cancer-specific survival. We hypothesized that since prior studies have shown that the overall breast cancer PRS is associated with favorable characteristics in breast cancer patients10, then higher overall breast cancer PRS among cases would be associated with more favorable survival. As expected, PRSoverallBC was inversely associated with breast cancer mortality, with a hazard ratio per standard deviation (HR per S.D.) of 0.86 (95% CI 0.78–0.94, p = 0.019). In Kaplan–Meier analyses, the bottom tertile of the PRS, corresponding with the lowest risk of developing breast cancer, was associated with worst survival (Fig. 4).
Fig. 4. Association between a polygenic risk score (PRSoverallBC) for overall invasive breast cancer risk and breast cancer-specific survival.
Kaplan–Meier survival analysis of tertiles of a 274-SNP PRS for overall breast cancer risk in the UK Biobank. p-values for the log-rank test are shown.
We next calculated the ROR-P PRS in the UK Biobank and the Pathways Study (Fig. S3) and examined the respective associations with breast cancer-specific survival in each study. We expected higher ROR-P PRS to be associated with more aggressive cancers and thus shorter breast cancer-specific survival. In UK Biobank, 66 of 76 SNPs were available for inclusion in the ROR-P PRS. In a Cox proportional hazards regression model adjusted for genetic ancestry, higher ROR-P PRS was associated with worse breast cancer-specific survival (HR per S.D. = 1.13, 95% CI 1.04–1.23, p = 0.005) (Fig. 5, Supplementary Table 8). Global calibration of the ancestry-adjusted ROR-P PRS in the UK Biobank was acceptable (Gronnesby-Borgan test statistic = 6.17, p = 0.72) (Supplementary Fig. 4).
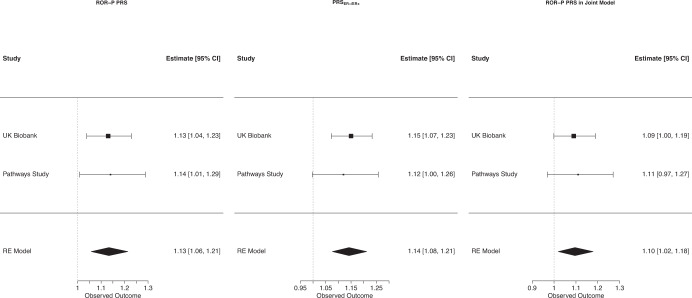
Fig. 5. Associations between polygenic risk scores and breast cancer-specific survival.
Cox proportional hazards models were constructed for: the polygenic risk score weighted on risk of recurrence (ROR-P PRS), the polygenic risk score for risk of estrogen-negative versus positive breast cancer (ROR-P PRSER-/ER+), and ROR-P PRS adjusted for the effects of PRSER-/ER+ in a joint model. All models were adjusted for genetic ancestry (principal components 1–10). Hazard ratios per standard deviation are shown for UK Biobank, the Pathways Study, and the combined studies using random effects meta-analysis.
In Pathways, 61 of 76 SNPs were available for the PRS. Higher ROR-P PRS was similarly associated with worse survival (HR per S.D. = 1.14, 95% CI 1.01–1.29, p = 0.04) (Fig. 5, Supplementary Table 8). In a random-effects meta-analysis of the results from UK Biobank and Pathways, the ROR-P PRS was associated with worse survival (summary HR per S.D. = 1.13, 95% CI 1.06–1.21, p = 4.0 × 10−4). No evidence of heterogeneity was found between studies.
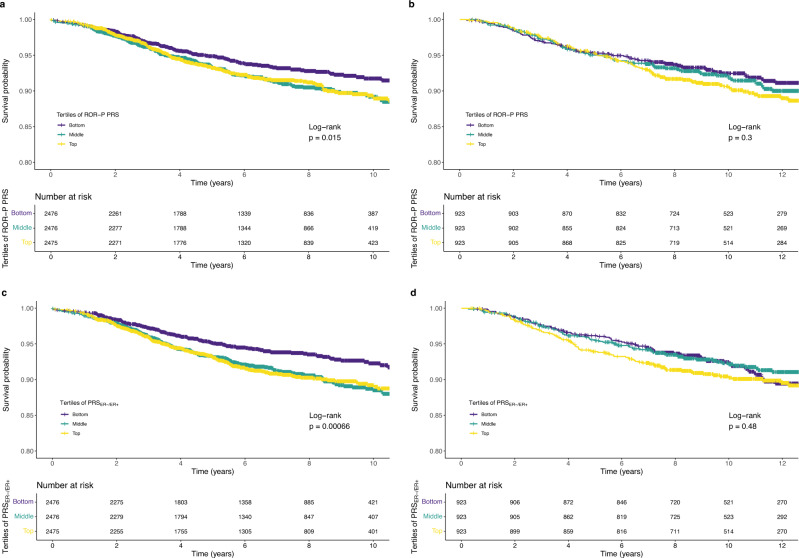
Similarly, in Kaplan–Meier analysis of UK Biobank data, the bottom tertile of the ROR-P PRS (corresponding to the lowest predicted ROR-P) was associated with better survival compared with the top and middle tertiles (log-rank chi-squared test statistic = 8.4, p = 0.015) (Fig. 6a). In contrast, the difference in survival between tertiles in the Pathways Study did not reach statistical significance (log-rank chi-squared = 2.4, p = 0.3) (Fig. 6b).
Fig. 6. Associations between polygenic risk scores for the risk of recurrence score weighted on proliferation (ROR-P PRS) and risk of estrogen-negative versus positive breast cancer (PRSER-/ER+) versus breast cancer-specific survival.
Kaplan–Meier plots of tertiles of the ROR-P PRS in a the UK Biobank and b the Pathways Study. Kaplan–Meier plots of tertiles of the PRSER-/ER+ in c the UK Biobank and d the Pathways Study. p-values for the log-rank test are shown.
In the Pathways Study, we constructed additional models adjusting for patient-level, tumor, and treatment covariates (Supplementary Table 7). The effect size of the ROR-P PRS did not change with adjustment for age at diagnosis and body mass index. However, its effect was attenuated after including stage at diagnosis (HR per S.D. = 1.10, 95% CI 0.97–1.25, p = 0.13). Including treatment covariates in addition to stage did not substantively change the results. As expected, including measured ROR-P and the ROR-P PRS in the same model attenuated the latter’s effect. Similar results were seen when invasive breast cancer recurrence was used as the outcome in Cox proportional hazards models.
Evaluation of joint effects with ER-negative PRS and differential effects by ER status
Given the observed association between the ROR-P PRS and ER-negative status as well as the inclusion of ER signaling pathway genes in the ROR-P gene set, we considered the possibility that our ROR-P PRS was recapitulating ER status. Thus, we constructed a comparable PRS for the case-case risk of ER-negative versus ER-positive cancer (PRSER-/ER+) and tested for collinearity and overlapping effects with the ROR-P PRS. In Pathways, we confirmed that the PRSER-/ER+ was associated with increased risk of ER-negative versus ER-positive cancer (OR per S.D. = 1.38, 95% CI 1.26–1.52, p = 3.1 × 10−11) (Supplementary Fig. 5).
In the UK Biobank, higher PRSER-/ER+ was associated with worse breast cancer-specific survival (HR per S.D. = 1.15, 95% CI 1.07–1.23, p = 6.8 × 10–5) (Fig. 5). Kaplan–Meier analysis showed that the bottom tertile of PRSER-/ER+, corresponding to lowest relative risk of ER-negative versus ER-positive cancer, was associated with more favorable survival (Fig. 6c). In Pathways, there was a similar directional association that did not reach statistical significance (HR per S.D. = 1.12, 95% CI 1.00–1.26, p = 0.057) (Figs. 5, 6d, Supplementary Table 7). Meta-analysis of the UK Biobank and Pathways results showed comparable effect sizes between the PRSER-/ER+ and ROR-P PRS (Fig. 5, Supplementary Table 8).
We then examined the correlation and joint effects between ROR-P PRS and PRSER-/ER+. There was a modest correlation between the ROR-P PRS and PRSER-/ER+ in UK Biobank and Pathways, Pearson correlation coefficient 0.27, p < 2.2 × 10−16, and 0.34, p < 2.2 × 10−16, respectively (Supplementary Fig. 6). In joint models including both ROR-P PRS and PRSER-/ER+, the effect size of the ROR-P PRS was mildly attenuated (Supplementary Table 8, Fig. 5). However, the effect of the ROR-P PRS remained statistically significant in meta-analysis of results from both studies (HR per S.D. = 1.10, 95% CI 1.02–1.18, p = 0.014).
To confirm that our ROR-P PRS was not solely recapitulating ER status, we performed additional analyses in the Pathways Study accounting for tumor ER status. Adjusting the ROR-P PRS for ER status led to a mild attenuation of the ROR-P PRS’s effect similar in magnitude to what was observed for the PRSER-/ER+ (Supplementary Table 7). Slight differences in the distributions of the ROR-P PRS were seen in ER-positive versus ER-negative cancers (Supplementary Fig. 7, Panel A), with the ROR-P PRS having a stronger effect in ER-positive cancers compared with ER-negative cancers (HR per S.D. = 1.23, 95% CI 1.06–1.43, p = 0.006 versus HR per S.D. = 0.87, 95% CI 0.67–1.12, p = 0.27) (Supplementary Fig. 7, Panel B). Taken together, these results strongly suggest the ROR-P PRS contains largely independent information from ER status.
Discussion
We used associations between breast cancer susceptibility SNPs and tumor gene expression to construct a case-only PRS for ROR-P. The ROR-P PRS was modestly predictive of ROR-P in our development dataset and in an external dataset comprised of tumors from the Pathways Study with measured ROR-P. In survival analysis, higher ROR-P PRS was associated with worse breast cancer-specific survival, with nearly identical effects observed in the UK Biobank and Pathways Study and HR per S.D. of 1.13 (95% CI 1.06–1.21) in meta-analysis. In contrast, higher PRSoverallBC was associated with better survival in the UK Biobank, which is consistent with the results of a large study including nearly 100,000 women with breast cancer10. Thus, the associations of the ROR-P PRS and PRSER-/ER+ with worse survival suggest that it is possible to reconfigure PRS to predict aggressive tumors with worse prognosis.
Our findings begin to address an important limitation of current breast cancer PRSs: their preferential associations with less aggressive phenotypes. We decided to fit our PRS to ROR-P, a gene expression-based phenotype, for several reasons. First, standard clinicopathologic markers such as ER status are imperfect proxies for aggressiveness18,28. ER-positive cancers display heterogeneous biology and can be divided into luminal A and B subtypes representing low-grade (more indolent) and high-grade (more aggressive) disease, respectively. In addition, among molecularly high-risk hormone receptor-positive/HER2- tumors, up to a third are classified as basal29. Second, traditional subtyping schemes do not reflect the continuous nature of traits such as receptor expression levels, proliferation, or metastatic potential. Third, aggressiveness is determined by the effects of multiple signaling pathways. For these reasons, continuous, multi-gene signatures such as ROR-P may better recapitulate complex, multidimensional traits such as aggressiveness. Prior studies have shown that ROR-P has greater prognostic value than receptor status, grade, and proliferation markers such as Ki6717. Fourth, ROR-P has been shown to be heritable20, making it an attractive candidate for fitting PRS.
Our targeted approach to PRS building is novel and represents a proof of concept that known breast cancer susceptibility SNPs can be used to fit PRSs to breast cancer aggressiveness. We focused the selection of SNPs for our PRS on variants already known to be associated with breast cancer due to limited power in our datasets for agnostic testing of genome-wide associations with ROR-P and other prognostic features. However, genetic susceptibility to aggressiveness may be influenced by variants outside of those discovered in GWAS for overall breast cancer risk. Prior studies have estimated the heritability of ROR-P to be 13–21% in White women20, though it remains to be seen how much of the heritability is explained by known breast cancer susceptibility SNPs versus other common variants. As larger datasets containing germline SNPs and tumor gene expression become increasingly available, it should become feasible to examine a larger pool of candidate SNPs and additional prognostic signatures. With increasing sample size, the use of alternative methods for PRS building such as lasso or elastic net regression2,30,31 may also improve prediction. Prognosis may be determined by host factors such as tumor immune microenvironment, and such features could also be used to fit PRS given the role of the germline in shaping immune response32.
We also note that a PRS representing the case-case risk of ER-negative versus ER-positive cancer was associated with survival. We included the PRSER-/ER+ as a “positive control” given that ER status has been consistently shown to be prognostic. Whereas the ROR-P PRS and PRSER-/ER+ had comparable magnitudes of association with survival, the more notable finding was the minimal attenuation of the ROR-P PRS’s effects when included in a joint model with PRSER-/ER+. Moreover, the ROR-P PRS had differential associations by ER status with survival, with a stronger effect seen in ER-positive versus ER-negative cancers. This result requires further validation but may reflect the greater heterogeneity in proliferation in ER-positive as opposed to ER-negative cancers, which tend to be more uniformly high-grade. Taken together, our findings suggest that ROR-P PRS captures largely independent prognostic information from ER status, mirroring the composition of the PAM50/ROR-P gene set which includes genes from the estrogen receptor pathway, in addition to genes from multiple others.
A secondary goal of our study was to characterize associations between known breast cancer susceptibility SNPs and ROR-P. The finding that some SNPs are associated with ROR-P is consistent with those of prior studies showing that many of the breast cancer susceptibility SNPs are differentially associated with intrinsic-like subtypes24,33. Intrinsic-like subtyping uses immunohistochemical ER, PR and HER2 status plus histologic grade to recapitulate the intrinsic subtypes defined by PAM50, from which ROR-P is derived18. Given the moderate correlation between immunohistochemical and expression-based subtyping34, we expected to see associations between individual breast cancer SNPs and ROR-P. Our finding of an inverse association between the risk allele and ROR-P for most SNPs adds to the evidence that SNPs discovered in overall breast cancer GWAS are preferentially associated with less aggressive biology12.
One strength of this study is our ability to leverage several datasets containing paired germline SNP-tumor gene expression. In particular, the inclusion of samples from the I-SPY 2 TRIAL, which is restricted to molecularly high-risk cancers, allowed us to enrich our PRS development set for aggressive tumors. ROR-P is a widely available signature with strong prognostic value; in one head-to-head comparison, the risk of recurrence (ROR) score was among the highest performing signatures for early and late distant recurrence35. Another strength of our study is the use of two independent cohorts to demonstrate the association between ROR-P PRS and survival. We also performed extensive analyses to account for confounding of the ROR-P PRS effect by ER status.
There are several limitations to our study. First, tumor gene expression signatures do not account for spatial or temporal heterogeneity and have imperfect prognostic performance. ROR-P has moderate discrimination for early and late recurrence, with c-statistics of 0.76 and 0.64, respectively35. It is possible that fitting the PRS to different prognostic signatures may yield PRSs with stronger associations with prognosis, though these differences would likely be small given the relatively high concordance (approximately 80%) between prognostic signatures36. Second, the sample size of our PRS development dataset limited the precision of our effect size estimates for SNPs in our PRS. Thus, the ROR-P PRS may contain some SNPs representing “noise.” Further refinement of the ROR-P PRS in larger datasets is needed, though we are encouraged that the ROR-P PRS displayed consistent effects in both independent validation datasets. Third, our validation was done in breast cancer cases from the UK Biobank and Pathways Study, two studies with fundamentally different designs. Whereas UK Biobank is a population-wide biobanking initiative, Pathways is a breast cancer-specific study that recruited consecutive cases from a single healthcare system in the U.S. The contrasting study designs, settings, and enrollment strategies provide unique strengths and limitations. The robustness of our findings is strengthened by the observation of similar effects across these datasets (considering the smaller sample size of Pathways). However, the UK Biobank did not contain information on breast cancer stage, pathologic features, or treatment, thus limiting our ability to examine the contributions of other determinants of survival and replicate the associations we observed in Pathways between the ROR-P PRS and tumor characteristics. Though we adjusted for confounding by ancestry, it is difficult to rule out residual confounding from other factors, given that we observed a mild attenuation of the ROR-P PRS’s effect in Pathways after accounting for stage and treatment. Fourth, there was limited diversity in the datasets used for ROR-P PRS development and testing. The UK Biobank, despite containing large numbers of breast cancer cases and events, had limited racial and ethnic diversity. Consequently, we restricted Pathways to self-identified White women (representing ~70% of the study population) for comparability with UK Biobank. Given the disproportionate burden of aggressive cancers in Black/African American women37 and Latinas38, further work is needed to evaluate and optimize the performance of the ROR-P PRS in diverse populations.
We believe that the potential clinical utility of the ROR-P PRS could lie in enhancing risk stratification for screening and prevention. Ongoing trials of risk-based screening are using models for overall breast cancer risk to assign screening recommendations5, but these models do not account for the risk of developing aggressive cancer. Aggressive cancers are over-represented in younger women, including those younger than the starting age for initiating screening recommended by current clinical guidelines. Thus, the ROR-P PRS, PRSER-/ER+, and similar PRS could be tested as modifiers to established risk models, particularly to identify women who should be offered screening at an earlier age, at shorter intervals, or using high-sensitivity modalities such as magnetic resonance imaging. Women at elevated risk of aggressive cancers may be ideal candidates for prevention and interception trials. In addition, it has been suggested that PRS predictive of cancer-related death may be more valuable than PRS for overall risk in selecting individuals with the greatest net benefit from screening—particularly for cancers (such as breast and prostate) where overdiagnosis is common39.
Our work represents a first step toward the prediction of phenotype-specific breast cancer risk. Future studies should seek to refine the methods used to construct PRS for aggressiveness and evaluate the performance of these PRSs in diverse populations with larger numbers of non-White individuals. Since PRS development and testing were done using a case-only design, further work is needed to examine case-control associations and to test the performance (e.g., discrimination, calibration, net reclassification improvement) of PRSs in predicting aggressive, poor-prognosis cancers on a population basis. Concurrent efforts should seek to refine the understanding of how the germline contributes to gene expression and other somatic features, and how these relationships shape tumor aggressiveness. Such analyses should become increasingly feasible with the growing availability of integrated datasets containing germline and somatic genomic data.
Methods
Study population
The PRS development phase of our study included invasive breast cancers from three datasets with paired germline SNP-tumor gene expression data: the Cancer Genome Atlas (TCGA)21, a publicly available pan-cancer atlas; Molecular Taxonomy of Breast Cancer International Consortium (METABRIC)22, a breast cancer genomic profiling study, and the Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And moLecular analysis 2 (I-SPY 2) TRIAL (NCT01042379)23 (Supplementary Table 1). The I-SPY 2 TRIAL is an ongoing clinical trial comparing multiple novel therapeutic agents for neoadjuvant treatment of locally advanced breast cancer. All cancers in I-SPY 2 must be considered molecularly high-risk according to clinicopathologic data or results of the MammaPrint prognostic signature;40 as a result, all tumors undergo gene expression profiling. Cases were included in our analysis based on the following criteria: In TCGA, we included invasive breast cancers (n = 953); samples corresponding to the primary tumor were included while those corresponding to recurrences or normal tissue were excluded. In METABRIC, we included cases with available SNP genotyping data (n = 496). In I-SPY 2, genotyping has been completed for the first 1400 cases; of these, a subset had available ROR-P calls (n = 914).
To test the association between ROR-P PRS and clinical outcomes, we analyzed participants from two studies containing longitudinal follow-up of women diagnosed with breast cancer, the UK Biobank and the Pathways Study (Supplementary Table 2). UK Biobank is a population-based cohort that enrolled individuals aged 40–69 years across the UK between 2006 and 2010, with cancer diagnoses and deaths ascertained from national registries41. To identify women with breast cancer, we used International Classification of Diseases (ICD) codes: ICD-9 (175, 1740, 1741, 1742, 1743, 1744, 1745, 1746, 1748, 1749, and 2330) and ICD-10 (C500, C501, C502, C503, C504, C505, C506, C508, C509, D050, D051, D057, and D059). We converted all codes to ICD-10-CM, then ICD-0-3, using Surveillance, Epidemiology, and Endpoints Registry (SEER) conversion tables. We then linked these ICD codes to SEER site recodes (2008). The SEER site recode 26000 was used for breast cancer. We defined breast cancer-related deaths as those for which breast cancer was indicated as contributing to the death (ICD-10 code C509). For non-censored individuals, the date of last follow-up was December 31, 2019. Given the ICD codes used to identify breast cancer patients included those pertaining to invasive and in situ disease, we created indicator variables for these categories. We restricted our analysis to self-identified British White women with incident invasive breast cancer (n = 7427) to mitigate potential biases related to survivorship and temporal treatment trends. Incident cancers were those with a diagnosis date occurring after the date of UK Biobank enrollment.
The Pathways Study is a longitudinal cohort of women diagnosed with breast cancer at Kaiser Permanente Northern California. Participants included women aged 21 years and older with a first diagnosis of invasive breast cancer between 2006 and 201342. Data on treatment, recurrence, and death were ascertained from the Kaiser Permanente Northern California Cancer Registry, as well as electronic medical records. Imputed genotype data was available for 3973 of 4377 total participants. For comparability with UK Biobank, we included participants with compete clinical and genetic data who were of self-reported White race (n = 2769)43.
The pooled analysis described in this manuscript was approved by the Biomedical Research Alliance of New York.
Genotyping
We performed SNP genotyping using array-based methods and imputed genotypes to population-based references (Supplementary Tables 1, 2). We estimated genetic ancestry by generating the first 10 principal components (PCs) based on genotyped markers using Plink (version 1.9). SNPs with >5% missingness were excluded. SNPs with <5% missingness were randomly assigned a genotype weighted on the distribution of genotypes calculated by the Hardy–Weinberg equation. This calculation used allele frequencies for the respective SNP among individuals without missing genotypes.
Tumor gene expression
Tumor gene expression was measured on the platforms detailed in Supplementary Table 1. ROR-P is the composite of ROR-S, the linear combination of PAM50 subtype-centroid correlations, and a proliferation score calculated from an 11-gene subset of the PAM50 gene set. To generate ROR-P calls, we first performed batch correction of raw gene expression levels (R package comBAT)44. For genes with multiple probes, we collapsed expression levels to the mean across all probes for the gene. Performing PAM50/ROR-P calls on a batch of tumors requires the target dataset to have a similar distribution of ER-positive and ER-negative cases to the original PAM50 training set. To address this “population assumption,” we created a subsample including all ER-negative cancers plus an equal number of randomly selected ER-positive cases. We repeated the subsampling procedure 1000 times and calculated for each repetition the median expression of each PAM50 gene. For each gene, we calculated the median of the 1000 medians and subtracted it from the collapsed expression levels. We then used these normalized expression levels to calculate ROR-P as previously described18. Briefly, the Spearman rank correlation between the individual genes in the PAM50 set and each subtype centroid was calculated for each sample with the subtype assignment based on the highest subtype-centroid correlation. The subtype-centroid correlations were then used to calculate ROR-P using Eq. 1:
| 1 |
where Prolif represents the average normalized expression estimates of an 11-gene proliferation index.
Construction of PRS for ROR-P
We tested 226 candidate SNPs with genome-wide significant associations (p < 5 × 10−8) in prior genome-wide association studies (GWAS) of overall breast cancer susceptibility1,45,46, or a related phenotype such as ER-negative13,47 or intrinsic-like subtype33, age of onset25, or prognosis/survival48–52 (Supplementary Table 4). We identified candidate SNPs and obtained summary statistics from the Breast Cancer Association Consortium (BCAC)1, accessed at https://bcac.ccge.medschl.cam.ac.uk/bcacdata/oncoarray/oncoarray-and-combined-summary-result/gwas-summary-results-breast-cancer-risk-2017, and the GWAS Catalog53. We started with 271 SNPs and performed linkage disequilibrium (LD) clumping using LDLink (R package LDlinkR)54. Within pairs of SNPs in LD (r2 ≥ 0.2 in European populations), we kept the SNP with the lower published p-value for association with breast cancer susceptibility. After LD pruning, 226 SNPs remained.
To address potential confounding, we constructed the linear regression model in Eq. 2 within pooled TCGA, METABRIC, and I-SPY 2 data:
| 2 |
We then regressed the model residual against each individual SNP and obtained the β coefficients and p-values for each association.
To identify the best-performing model for ROR-P, we constructed PRSs according to varying p-value thresholds (0.1–0.6) for SNP inclusion. We used 5-fold cross-validation (R package caret)55 to estimate the r2 of each PRS against the residual in the leave-out subset. We repeated this process 10 times. After identifying the p-value threshold with the highest r2, we included in our PRS all SNPs with a p-value below this threshold. Finally, we obtained within the overall dataset the coefficients of SNP associations with ROR-P, adjusted for genetic ancestry and an indicator variable for study.
To calculate the ROR-P PRS in UK Biobank and Pathways, we applied the SNP coefficients derived from our PRS development set to the genotype data, coded as risk allele dosage. Specifically, the PRS was calculated as shown in Eq. 3:
| 3 |
where βk is the per-allele linear regression coefficient for ROR-P associated with SNP k, xk is the genotype coded as number of risk alleles present, and n is the total number of SNPs in the PRS.
Construction of PRS for overall and ER-specific cancer risk
We generated two comparator PRSs and tested their associations with breast cancer-specific survival: (1) a 274-SNP PRS for overall risk of breast cancer (UK Biobank only), PRSoverallBC; and (2) a 205-SNP PRS representing the case-case risk of developing ER-negative versus ER-positive disease, PRSER-/ER+. PRSoverallBC was derived from a published 313-SNP PRS for overall breast cancer2. In UK Biobank, we were able to retrieve genotypes for 274 of the 313 SNPs in the PRS. The candidate SNPs for the PRSER-/ER+ were the same 271 SNPs considered for inclusion in the ROR-P PRS. The effect sizes of the SNPs were taken from summary statistics for associations with ER-negative and ER-positive breast cancer, as reported in the BCAC iCOGs and OncoArray studies (https://gwas.mrcieu.ac.uk/, ieu-a-1127 and ieu-a-1128, respectively)56. We used the β coefficients calculated from meta-analysis of these studies. Given the β coefficients for ER-negative and ER-positive cancers were derived from case-control comparisons with the same control group, we subtracted the β coefficient for ER-positive cancer from the β coefficient for ER-negative cancer2 to obtain the case-case β coefficient for ER-negative versus ER-positive risk. Summary statistics were available for 237 SNPs. After LD pruning, 205 SNPs remained, of which 193 had available genotypes in UK Biobank and 189 had available genotypes in the Pathways Study. We calculated the PRS using a previously described method3,57. In this calculation, the PRS represents the product of the likelihood ratios across each SNP in the PRS, with the likelihood ratio calculated based on the effect size of the risk allele and the risk allele frequency. For PRSoverallBC, the effect sizes were the published odds ratios from the meta-analyzed BCAC iCOGs and OncoArray studies described above. For PRSER-/ER+, the effect size was the exponent of the case-case β coefficient for ER-negative versus ER-positive risk. We used risk allele frequencies from the European (EUR) population in 1000 Genomes.
Statistical analysis
As a form of external validation, we examined associations between ROR-P PRS and tumor features such as ER status, HER2 status, histologic grade, and intrinsic-like subtype in the Pathways Study. For binary features such as ER and HER status, we used t-tests to compare mean ROR-P PRS between categories. We also constructed logistic regression models adjusted for genetic ancestry principal components 1–10 (PC1-PC10). For categorical outcomes with three or more categories, such as grade and intrinsic-like subtype, we used analysis of variance (ANOVA) tests and constructed multinomial logistic regression models adjusted for genetic ancestry. We also estimated the correlation between ROR-P PRS and actual ROR-P using the Pearson correlation coefficient in the subset of tumors that had undergone gene expression profiling.
To confirm past findings that a PRS representing overall breast cancer risk was associated with improved survival, we first performed survival analysis of PRSoverallBC in the UK Biobank. We constructed a Cox proportional hazards regression model with PRSoverallBC as the predictor and genetic ancestry PC1-PC10 as covariates. We normalized PRSoverallBC to the mean and standard deviation among cases. We also used Kaplan–Meier survival analysis (R packages survival and survminer)58 to examine the association between tertiles of PRSoverallBC and breast cancer-specific survival and tested for differences between tertiles using log-rank tests.
To examine the respective associations between ROR-P PRS and PRSER-/ER+ and breast cancer-specific survival in UK Biobank and Pathways, we constructed Cox proportional hazards models for each PRS, with adjustment for genetic ancestry as above. We normalized the ROR-P PRS to the mean and log-normalized the PRSER-/ER+. We also performed Kaplan–Meier survival analysis using tertiles of the ROR-P PRS and PRSER-/ER+. To examine joint effects between ROR-P PRS and PRSER-/ER+, we calculated the Pearson correlation coefficient between the two PRSs. We also constructed Cox proportional hazards models including terms for ROR-P PRS and PRSER-/ER+. To synthesize the results of Cox proportional hazards models from UK Biobank and Pathways, we performed random-effects meta-analysis using a restricted maximum likelihood estimator (R package metafor)59. We evaluated for heterogeneity between studies using Cochran’s Q test and calculated the I2 index.
We evaluated calibration of the Cox model containing ancestry-adjusted ROR-P PRS using the UK Biobank data. We obtained bias-corrected estimates of predicted versus observed breast cancer-specific survival at 5 years using boostrapping with 200 repeats (R package rms). We compared the predicted survival probabilities for 10 evenly divided strata of risk versus the Kaplan–Meier “observed” estimates for each stratum. We also performed the Gronnesby-Borgan goodness-of-fit test for the Cox model (R package survMisc).
In Pathways, we built additional nested ROR-P PRS models adjusted for the following combinations of covariates: age at diagnosis and body mass index (Model 2); age at diagnosis, body mass index, and stage at diagnosis (Model 3); age at diagnosis, body mass index, stage at diagnosis, and binary variables corresponding to receipt of the following treatments: radiation therapy, chemotherapy, trastuzumab, and hormone therapy (Model 4); measured ROR-P (Model 5); and ER status (Model 6). Lastly, we constructed nested models containing ROR-P PRS and the same combinations of covariates as Models 2–6 but using invasive breast cancer recurrence as the outcome. We also examined differential effects of the ROR-P PRS by ER status by constructing separate Cox proportional hazards models for ER-positive and ER-negative cancers.
All statistical tests were two-sided with α = 0.05. Analyses were done using R version 4.1.2 (R Foundation, Vienna, Austria).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by funding from the National Cancer Institute (K08 CA237829, K24 CA169004, U01 CA196406, P01 CA210961, R01 CA105274, U01 CA195565, R01 CA129059) and the National Human Genome Research Institute (X01 HG008335). The I-SPY 2 Study was also supported by the Quantum Leap Healthcare Collaborative and the Foundation for the National Institutes of Health. The results shown here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Author contributions
Study conception and design: Y.S. and E.Z. Data abstraction and acquisition: Y.S., C.Y., D.M.W., G.L.H., L.B.S., S.H., J.L.N., P.M., L.K., Q.Z., and S.Y. Statistical analysis: Y.S., J.M.R., C.Y., D.M.W., D.H., R.S.H., and Yu.S. Interpretation of data: Y.S., C.Y., D.M.W., L.H.K., and E.Z. Manuscript preparation: Y.S. Manuscript editing and critical review: all authors. Financial support, resources, and study supervision: Y.S., M.L.K., B.J.C., J.S.W., L.H.K., L.V.V., L.J.E., and E.Z.
Data availability
The Cancer Genome Atlas data are publicly available and were accessed through the Broad GDAC Firehose (https://gdac.broadinstitute.org/). METABRIC data are available through application to the METABRIC Data Access Committee and finalization of a Data Access Agreement. For this study, METABRIC data was accessed through the European Genome-Phenome Archive (https://ega-archive.org) under dataset IDs EGAD00010000266 (genotype) and EGAD00010000268 (gene expression). I-SPY 2 data are available upon application to the I-SPY 2 TRIAL Data Access Committee and finalization of a Data Use Agreement. The UK Biobank data are available to approved researchers registered with the UK Biobank. The research was conducted with approved access to UK Biobank data under application number 14105. The Pathways Study genotype data are available on The database of Genotypes and Phenotypes (dbGaP) under study accession phs001534.v1.p1. Clinical and outcomes data are available upon application to the Pathways Study Steering Committee and require an IRB-approved collaboration.
Code availability
Statistical code used to conduct the analyses can be found at https://github.com/shiehy/code-rorp-prs.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41698-023-00382-z.
References
- 1.Michailidou K, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavaddat N, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am. J. Hum. Genet. 2019;104:21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shieh Y, et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res. Treat. 2016;159:513–525. doi: 10.1007/s10549-016-3953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuzick J, et al. Impact of a panel of 88 single nucleotide polymorphisms on the risk of breast cancer in high-risk women: results from two randomized tamoxifen prevention trials. J. Clin. Oncol. 2017;35:743–750. doi: 10.1200/JCO.2016.69.8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shieh Y, et al. Breast cancer screening in the precision medicine era: risk-based screening in a population-based trial. JNCI: J. Natl Cancer Inst. 2017;109:djw290–djw290. doi: 10.1093/jnci/djw290. [DOI] [PubMed] [Google Scholar]
- 6.Roux A, et al. Study protocol comparing the ethical, psychological and socio-economic impact of personalised breast cancer screening to that of standard screening in the “My Personal Breast Screening” (MyPeBS) randomised clinical trial. BMC Cancer. 2022;22:507. doi: 10.1186/s12885-022-09484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, J. D. et al. Personalized risk assessment for prevention and early detection of breast cancer: integration and implementation (PERSPECTIVE I&I). J. Pers. Med.11, 10.3390/jpm11060511 (2021). [DOI] [PMC free article] [PubMed]
- 8.van de Vijver MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 9.Holm J, et al. Associations of breast cancer risk prediction tools with tumor characteristics and metastasis. J. Clin. Oncol. 2016;34:251–258. doi: 10.1200/JCO.2015.63.0624. [DOI] [PubMed] [Google Scholar]
- 10.Lopes Cardozo JMN, et al. Associations of a breast cancer polygenic risk score with tumor characteristics and survival. J. Clin. Oncol. 2023 doi: 10.1200/JCO.22.01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, et al. Breast cancer genetic risk profile is differentially associated with interval and screen-detected breast cancers. Ann. Oncol. 2016;27:1181. doi: 10.1093/annonc/mdw096. [DOI] [PubMed] [Google Scholar]
- 12.Grassmann F, et al. Interval breast cancer is associated with other types of tumors. Nat. Commun. 2019;10:4648. doi: 10.1038/s41467-019-12652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milne RL, et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat. Genet. 2017;49:1767–1778. doi: 10.1038/ng.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prat A, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Sørlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen TO, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin. Cancer Res. 2010;16:5222–5232. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohnstad HO, et al. Prognostic value of PAM50 and risk of recurrence score in patients with early-stage breast cancer with long-term follow-up. Breast Cancer Res. 2017;19:120. doi: 10.1186/s13058-017-0911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A, et al. Gene-level germline contributions to clinical risk of recurrence scores in black and white patients with breast cancer. Cancer Res. 2022;82:25–35. doi: 10.1158/0008-5472.CAN-21-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koboldt DC, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker AD, et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin. Pharmacol. Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 24.Ahearn TU, et al. Common variants in breast cancer risk loci predispose to distinct tumor subtypes. Breast Cancer Res. 2022;24:2. doi: 10.1186/s13058-021-01484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahsan H, et al. A genome-wide association study of early-onset breast cancer identifies PFKM as a novel breast cancer gene and supports a common genetic spectrum for breast cancer at any age. Cancer Epidemiol. Biomark. Prev. 2014;23:658–669. doi: 10.1158/1055-9965.EPI-13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Narváez, E. A. et al. Admixture mapping of African–American women in the AMBER consortium identifies new loci for breast cancer and estrogen-receptor subtypes. Front. Genet.7, 10.3389/fgene.2016.00170 (2016). [DOI] [PMC free article] [PubMed]
- 27.Caan BJ, et al. Intrinsic subtypes from the PAM50 gene expression assay in a population-based breast cancer survivor cohort: prognostication of short- and long-term outcomes. Cancer Epidemiol. Biomark. Prev. 2014;23:725–734. doi: 10.1158/1055-9965.EPI-13-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loi S, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J. Clin. Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 29.Huppert LA, et al. Pathologic complete response (pCR) rates for HR+/HER2- breast cancer by molecular subtype in the I-SPY2 Trial. J. Clin. Oncol. 2022;40:504–504. doi: 10.1200/JCO.2022.40.16_suppl.504. [DOI] [Google Scholar]
- 30.Thomas M, et al. Genome-wide modeling of polygenic risk score in colorectal cancer risk. Am. J. Hum. Genet. 2020;107:432–444. doi: 10.1016/j.ajhg.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattee J, Pan W. Penalized regression and model selection methods for polygenic scores on summary statistics. PLOS Comput. Biol. 2020;16:e1008271. doi: 10.1371/journal.pcbi.1008271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayaman RW, et al. Germline genetic contribution to the immune landscape of cancer. Immunity. 2021;54:367–386.e368. doi: 10.1016/j.immuni.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 2020;52:572–581. doi: 10.1038/s41588-020-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastien RR, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sestak I, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:545–553. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan C, et al. Concordance among gene-expression-based predictors for breast cancer. N. Engl. J. Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 37.Huo D, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J. Clin. Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marker KM, et al. Human epidermal growth factor receptor 2-positive breast cancer is associated with indigenous American ancestry in Latin American women. Cancer Res. 2020;80:1893–1901. doi: 10.1158/0008-5472.CAN-19-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vickers AJ, Sud A, Bernstein J, Houlston R. Polygenic risk scores to stratify cancer screening should predict mortality not incidence. npj Precis. Oncol. 2022;6:32. doi: 10.1038/s41698-022-00280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van ‘t Veer LJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 41.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwan ML, et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–1076. doi: 10.1007/s10552-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Q, et al. UACA locus is associated with breast cancer chemoresistance and survival. NPJ Breast Cancer. 2022;8:39. doi: 10.1038/s41523-022-00401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michailidou K, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet. 2013;45:353–361. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michailidou K, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat. Genet. 2015;47:373–380. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purrington KS, et al. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014;35:1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shu XO, et al. Novel genetic markers of breast cancer survival identified by a genome-wide association study. Cancer Res. 2012;72:1182–1189. doi: 10.1158/0008-5472.CAN-11-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rafiq S, et al. Identification of inherited genetic variations influencing prognosis in early-onset breast cancer. Cancer Res. 2013;73:1883–1891. doi: 10.1158/0008-5472.CAN-12-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafiq S, et al. A genome wide meta-analysis study for identification of common variation associated with breast cancer prognosis. PLoS ONE. 2014;9:e101488. doi: 10.1371/journal.pone.0101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo, Q. et al. Identification of novel genetic markers of breast cancer survival. J. Natl Cancer Inst.107, 10.1093/jnci/djv081 (2015). [DOI] [PMC free article] [PubMed]
- 52.Song N, et al. Prediction of breast cancer survival using clinical and genetic markers by tumor subtypes. PLoS ONE. 2015;10:e0122413. doi: 10.1371/journal.pone.0122413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buniello A, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–d1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuhn M. Building predictive models in R using the caret package. J. Stat. Softw. 2008;28:1–26. doi: 10.18637/jss.v028.i05. [DOI] [Google Scholar]
- 56.Elsworth, B. et al. The MRC IEU OpenGWAS data infrastructure. Preprint at bioRxiv10.1101/2020.08.10.244293 (2020).
- 57.Ziv E, et al. Using breast cancer risk associated polymorphisms to identify women for breast cancer chemoprevention. PLoS ONE. 2017;12:e0168601. doi: 10.1371/journal.pone.0168601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Therneau, T. M. G. & Patricia, M. Modeling Survival Data: Extending the Cox Model (Springer, 2000).
- 59.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Cancer Genome Atlas data are publicly available and were accessed through the Broad GDAC Firehose (https://gdac.broadinstitute.org/). METABRIC data are available through application to the METABRIC Data Access Committee and finalization of a Data Access Agreement. For this study, METABRIC data was accessed through the European Genome-Phenome Archive (https://ega-archive.org) under dataset IDs EGAD00010000266 (genotype) and EGAD00010000268 (gene expression). I-SPY 2 data are available upon application to the I-SPY 2 TRIAL Data Access Committee and finalization of a Data Use Agreement. The UK Biobank data are available to approved researchers registered with the UK Biobank. The research was conducted with approved access to UK Biobank data under application number 14105. The Pathways Study genotype data are available on The database of Genotypes and Phenotypes (dbGaP) under study accession phs001534.v1.p1. Clinical and outcomes data are available upon application to the Pathways Study Steering Committee and require an IRB-approved collaboration.
Statistical code used to conduct the analyses can be found at https://github.com/shiehy/code-rorp-prs.