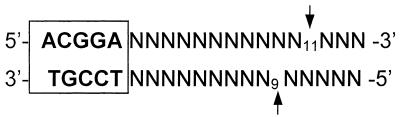Abstract
A novel prototype class-IIS restriction endonuclease, TspGWI, was isolated from the thermophilic bacterium Thermus sp. GW. The recognition sequence and cleavage positions have been established: TspGWI recognizes the non-palindromic 5-bp sequence 5′-ACGGA-3′ and cleaves the DNA 11 and 9 nt downstream in the top and bottom strand, respectively. In addition, an accompanying endonuclease, TspGWII, an isoschizomer of Pst I, was found in Thermus sp. GW cells.
RESULTS AND DISCUSSION
The general characteristics of type II restriction endonucleases are that they require Mg2+ ions as the only obligatory co-factor and recognize a palindromic 4–8-bp cognate site, where cleavage takes place at precisely defined positions within the recognition site (1). The type II enzymes are higly heterogeneous, with a distinct subclass, IIS, which differs from the class-II paradigm by its ability to recognize asymmetric sequences and cleave further downstream at defined distances of 1–20 nt (2). Currently there are 73 known prototypes of endonucleases that recognize asymmetric sites (1). However, only 43 of them meet the criteria of class-IIS. The remaining 30 enzymes either (i) cleave within asymmetric sites, (ii) have not yet had their cleavage positions determined or (iii) cleave on both sides outside their recognition sites (1).
Thermus sp. GW was cultivated aerobically at 60°C in a modified Luria broth (0.5% tryptose, 0.3% yeast extract, 0.2% NaCl, pH 7.2) supplemented with trace elements. Since class-IIS restriction endonuclease TspGWI activity is only present in minute quantities in the host strain, an extensive purification was required to obtain a sufficient amount of enzyme for further analysis. The purification procedure included (i) 0.4% polyethyleneimine removal of nucleic acids from the crude extract in the presence of 100 mM NaCl, (ii) ammonium sulfate 30–50% saturation cut, (iii) phosphocellulose chromatography, (iv) heparin–agarose chromatography, (v) Cibacron blue–agarose chromatography and (vi) DEAE–Sephadex chromatography. The purified preparation was free from non-specific nucleolytic activities. In the course of the purification of TspGWI an accompanying restriction endonuclease, TspGWII, a thermophilic isoschizomer of PstI, was found. TspGWII recognizes 5′-CTGCAG-3′ and cleaves after the A residue, leaving 4 nt 3′-protruding ends (not shown).
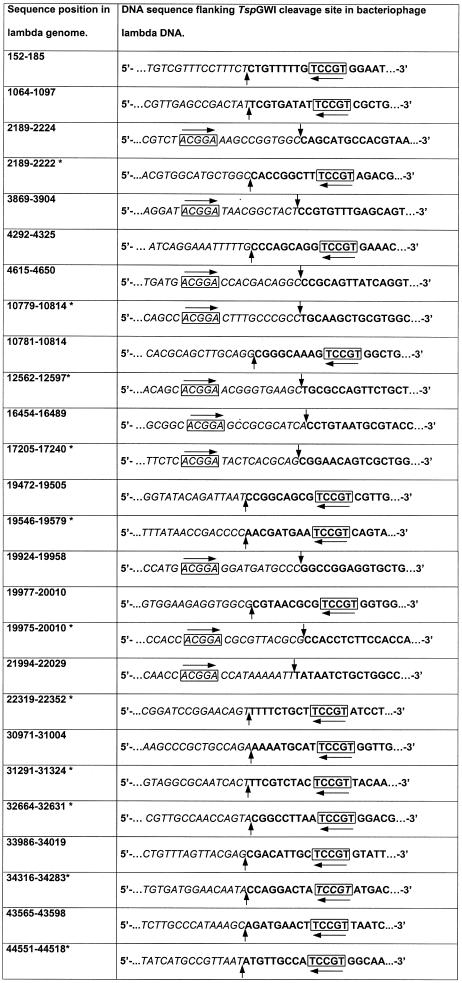
The TspGWI recognition site was established using two procedures: (i) assessment of the digestion pattern on pUC19, pACYC184 and pBR322 DNAs and (ii) cloning and sequencing of TspGWI restriction fragments of pBR322 and bacteriophage lambda DNAs (Table 1). The analysis of the cleavage pattern and the mapping of TspGWI sites present in pUC19 (Fig. 1A and B) suggested that this recognition site is 5′-ACGGA-3′. There are two such sites in pUC19, four in pACYC184 and five in pBR322. Cleavage of pUC19 with TspGWI resulted in a complete digestion. However, cleavage of pBR322 and pACYC184 DNAs showed a stable partial digestion pattern, where all sites except one are efficiently cleaved (Fig. 1C–E). The refractory sites, cleaved at a substantially slower rate, were mapped and they turned out to be located within the tetracycline resistance gene, present in both plasmids. One of the possible explanations for the phenomenon of refractory cleavage might be that it is an effect of the immediate sequence segment surrounding the TspGWI site. The neighboring bases, which are present on both sides of the TspGWI recognition sequences in pBR322 and pACYC184, are shown in bold: 5′-AACGGAT-3′. The putative TspGWI cognate site was further investigated through the digestion of pBR322 DNA and lambda DNA with TspGWI, followed by repair of the TspGWI restriction fragment termini with T4 DNA polymerase/dNTPs (3) and by cloning the resulting restriction fragments into the SmaI site of a modified pTZ18u vector (4). The vector-insert junctions of the resulting clones were sequenced using the ABI Prism 310 automated sequencer with ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit and then analyzed using ABI Sequencing Analysis 3.4.1 software (Applied Biosystems, Foster City, CA) and Hitachi DNASIS 2.5 software (Hitachi Software Engineering Co., Yokohamie, Japan). A total of 58 junction sequences were compared. Twenty-five junctions derived from bacteriophage lambda cloned restriction fragments are exemplified in Table 1, where eight junctions are for the top strand of the 5′-ACGGA-3′ and 17 for the bottom strand of the 5′-TCCGT-3′ reverse complement orientation. Comparison of the junction sequences confirmed the presence of the 5′-ACGGA-3′ site at either defined distances near the ends of a cloned restriction fragment or in pBR322/lambda DNA regions which, prior to TspGWI cleavage, were continuous with their corresponding cloned fragment. The sequences presented in Table 1 were selected to show all found combinations of the neighboring 1 bp, flanking both sides of the TspGWI recognition sequences. The computer prediction revealed 107 5′-ACGGA-3′ sites in lambda. Four out of the 16 possible combinations of 1-bp neighborhoods are under-represented in the lambda genome and they were also not detected during the junction sequence analysis (marked in bold): 5′-AACGGAT-3′ (5/107), 5′-AACGGAC-3′ (6/107), 5′-TACGGAC-3′ (1/107) and 5′-TACGGAG-3′ (0/107). Since the 5′-AACGGAT-3′ site present in the tetracycline resistance gene is slowly cleaved this may apply to lambda as well. This would decrease the cloning efficiency of 5′-AACGGAT-3′ fragments. Whether the absence of 5′-AACGGAC-3′ and 5′-TACGGAC-3′ sequences amongst the analyzed junctions is also caused by diminished reaction efficiency remains to be determined.
Table 1. Determination of the TspGWI recognition sequence and cleavage positions by shot-gun cloning and sequencing of TspGWI restriction fragments.
Base numbering refers to the conventional orientation of the lambda genome. Bold, a terminal portion of TspGWI-cut restriction fragment, T4 DNA polymerase repaired and cloned into pTZ18u derivative; italic, uncloned bacteriophage lambda DNA sequence adjacent to cloned TspGWI-derived restriction fragment; box with horizontal arrow, TspGWI recognition sequence; vertical arrows, TspGWI cleavage positions.
*Sequence obtained from TspGWI restriction fragments cloned in reverse complement orientation.
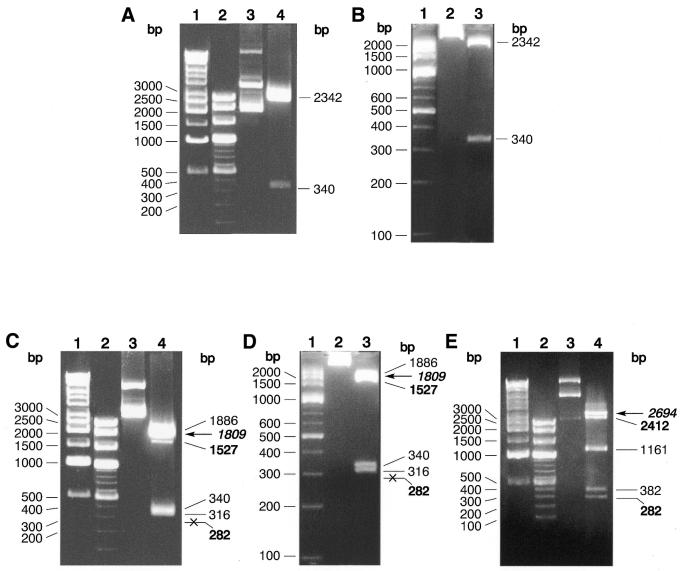
Figure 1.
Digestion pattern and site preference of TspGWI on pUC19, pBR322 and pACYC184 plasmid DNAs. (A) TspGWI cleavage of pUC19 DNA, 1% agarose/TAE. Lane 1, 1 kb ladder; lane 2, 100 bp ladder; lane 3, untreated pUC19 DNA; lane 4, TspGWI-cut pUC19 DNA. (B) TspGWI cleavage of pUC19 DNA, 3.5% NuSieve GTG agarose/TAE. Lane 1, 100 bp ladder; lane 2, untreated pUC19 DNA; lane 3, TspGWI-cut pUC19 DNA. (C) cleavage of pBR322 DNA, 1% agarose/TAE. Lane 1, 1 kb ladder; lane 2, 100 bp ladder; lane 3, untreated pBR322 DNA; lane 4, TspGWI-cut pBR322 DNA. Out of five TspGWI sites in pBR322, one 5′-AACGGAT-3′ variant is cleaved inefficiently, resulting in fragments of 1527 bp (faint band, marked in bold) and 282 bp (not visible on reproduced picture, marked in bold and crossed). The partial digestion band of 1809 is indicated by bold italics and a horizontal arrow. (D) TspGWI cleavage of pBR322 DNA, 3.5% NuSieve GTG agarose/TAE. Lane 1, 100 bp ladder; lane 2, untreated pBR322 DNA; lane 3, TspGWI-cut pBR322 DNA. (E) TspGWI cleavage of pACYC184 DNA, 1% agarose/TAE. Lane 1, 1 kb ladder; lane 2, 100 bp ladder; lane 3, untreated pACYC184 DNA; lane 4, TspGWI-cut pACYC184 DNA. Bands of 2412 and 282 bp, where intensity is decreased due to slow cleavage rate of TspGWI site variant 5′-AACGGAT-3′, are marked in bold. Partial digestion band of 2694 bp is marked in bold italics and a horizontal arrow. Selected band sizes of marker DNAs are shown on the left of each panel.
The TspGWI recognition sites from the sequenced junctions appear in four possible configurations: as top or bottom strand in either an insert or an uncloned segment of adjacent pBR322/lambda DNA regions (Table 1). The comparison of various clone configurations allowed the deduction of the cleavage positions, which are located further downstream from the 5′-ACGGA-3′ sequence, at the 11 and the 9 nucleotide in the top and bottom strands (vertical arrows), respectively, leaving 2 nt 3′-overhangs:
The recognition sequence and cleavage points of TspGWI show similarities to those of the two known Thermus-derived restriction endonucleases of type-IIS: TaqII from Thermus aquaticus, GACCGA(N11/9)-3′ or CACCCA(N11/9)-3′ (5), and Tth111II from Thermus thermophilus 111, CAARCA(N11/9)-3′ (6). The conservation of cleavage positions can be observed, as well as of the first and the last A of the TspGWI recognition site. This may indicate a common evolutionary origin for all these endonucleases.
The optimum reaction conditions for TspGWI are 50 mM Tris–HCl pH 8.5 at 25°C, 10 mM MgCl2, 10 mM DTT and 3 mM spermidine. The enzyme is active between 42 and 85°C, with a maximum at 65 and 75°C. The enzyme can be inactivated by 20 min incubation at 89°C.
Acknowledgments
ACKNOWLEDGEMENTS
The helpful suggestions regarding the manuscript by Prof. Józef Kur and Jakub Zaleski as well as figure preparation by Barbara Skowron-Zaleska are appreciated. The help in isolation of bacteria by Izabela Jaworowska and Robert Atras are acknowledged. This project was supported by EURx Ltd funds available to P.M.S.
REFERENCES
- 1.Roberts R.J. and Macelis,D. (2001) REBASE—restriction enzymes and methylases. Nucleic Acids Res., 29, 268–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szybalski W., Kim,S.C., Hasan,N. and Podhajska,A.J. (1991) Class-IIS restriction enzymes—a review. Gene, 100, 13–26. [DOI] [PubMed] [Google Scholar]
- 3.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 4.Mead D.A., Szczesna-Skorupa,E. and Kemper,B. (1986) Single-stranded DNA ‘blue’ T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng., 1, 67–74. [DOI] [PubMed] [Google Scholar]
- 5.Barker D., Hoff,M., Oliphant,A. and White,R. (1984) A second type II restriction endonuclease from Thermus aquaticus with unusual sequence specificity. Nucleic Acids Res., 12, 5567–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinomiya T., Kobayashi,M. and Sato,S. (1980) A second site-specific endonuclease from Thermus thermophilus 111, Tth111II. Nucleic Acids Res., 8, 3275–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]