Abstract
Background:
Childhood trauma (CT) has been linked to increased risk for mental illness in adulthood. Although work in experimental animals has shown that early life stressors can affect inhibitory and excitatory neurotransmission in adult rodents, with possible excitotoxic effects on local grey matter volumes (GMV), the neurobiological mechanisms that mediate this relationship in humans remain poorly understood.
Aim:
To examine glutamate and gamma-aminobutyric acid (GABA) metabolite concentrations and potential excitotoxic effects on GMV, in adults who experienced CT.
Methods:
Fifty-six young adults (Mage = 20.41) were assigned to High CT (n = 29) and Low CT (n = 27) groups (by using the CT questionnaire) and underwent magnetic resonance spectroscopy (1H-MRS) to measure temporal lobe metabolite concentrations and volumetric imaging to measure GMV.
Results:
Glutamate concentrations did not differ between groups; however, relative to the Low CT group, participants in the High CT group had reduced GABA concentrations in the left superior temporal gyrus (STG) voxel. Furthermore, logistic regression showed that participants with low left STG GABA concentrations and low left STG volumes were significantly more likely to be in the high CT group.
Conclusions:
This study provides the first evidence that both low GABA concentrations and its interaction with GMV in the left STG are associated with high levels of CT and suggest that altered inhibitory neurotransmission/metabolism may be linked to a lower GMV in the left STG in adults who experienced CT. Future studies are warranted to establish if utilizing these measures can stratify clinical high-risk and predict future clinical outcomes in high CT individuals.
Keywords: GABA, glutamate, spectroscopy, childhood trauma, grey matter, early life adversity
Introduction
Childhood trauma (CT) is an established antecedent for 29.8% of all disorders worldwide (Kessler et al., 2010), and it is a well-established risk factor for psychiatric disorders, including depression, anxiety, psychosis and psychosis-like experiences and schizophrenia (Aas et al., 2011; Arseneault et al., 2011; Green et al., 2010; Janssen et al., 2004; Paus et al., 2008; Read et al., 2005; Varese et al., 2012; Whitfield et al., 2005). A possible mechanism for this relationship between CT and psychiatric disorders is stress sensitivity, and research in experimental animals has shown that young rodents exposed to adverse events and environments demonstrate a range of aberrant behaviour and physiology in adulthood (e.g. Bonapersona et al., 2019; Smith & Pollak, 2020 for reviews). For example, rodents exposed to abusive maternal behaviours or maternal separation as pups show decreased dendritic arborization, altered synaptic signalling and epigenetic changes throughout the prefrontal cortex, hippocampus and amygdala, as well as anxiety- and depressive-like behaviours in adulthood (Bagot et al., 2009; Danielewicz and Hess, 2014; Lee et al., 2007; Malter Cohen et al., 2013; Monroy et al., 2010; Romeo et al., 2003).
Recently a number of neuroimaging studies in humans have also investigated the effects of CT on adult brain structure. The most robust finding in adult CT populations is lower grey matter volume (GMV) in temporal lobe and prefrontal regions, an effect seen in both psychiatric and non-psychiatric CT populations (e.g. Paquola et al., 2016; Teicher et al., 2018 for reviews). Interestingly, one of the most robust neuroimaging findings in depression, anxiety and psychosis populations, many of whom will have experienced CT (Green et al., 2010; Paus et al., 2008; Read et al., 2005; Varese et al., 2012), is reduced superior temporal gyrus (STG) GMV (Allen et al., 2019; Arnone et al., 2016; Honea et al., 2005; Keshavan et al., 2020; Madonna et al., 2019; Scheepens et al., 2020). Moreover, reduced GMV may be linked to excitotoxicity due to imbalances in local inhibitory and excitatory neurotransmission (Dong et al., 2009; Schobel et al., 2013; Théberge et al., 2007; Zhou & Danbolt, 2014).
In particular, work in experimental animals has shown that early life stressor can lead to a heighted stress response and can affect cortical gamma-aminobutyric acid (GABA)ergic and glutamatergic interneurons and therefore excitatory/inhibitory (E/I) balance (Gomes et al., 2016). For instance, in a neurodevelopmental disruption model, the administration of methylazoxymethanol acetate (MAM) to pregnant rats on gestational day 17 perturbates neurodevelopment and induces histological, anatomical, neurophysiological, pharmacological and cognitive/behavioural alterations on developing paralimbic, frontal and temporal cortices (Gomes et al., 2016; Lodge & Grace, 2011). Of specific importance, MAM rats show a selective loss of parvalbumin-containing interneurons (that contain and release GABA) in both temporal and frontal cortices (Lodge et al., 2009) and the MAM model posits dysregulation of glutamate neurotransmission occurring in the temporal lobe (Lodge & Grace, 2007, 2011; Moore et al., 2006). Additionally, the administration of corticosterone to rats leads to a decrease of mRNA for GAD67 (an enzyme that synthesizes GABA) (Deslauriers et al., 2013; Giovanoli et al., 2013; Stone et al., 2001).
However, there are very few magnetic resonance spectroscopy (1H-MRS) studies that have examined brain metabolite concentrations in adult humans that have experienced CT. In the small number of studies that have been conducted, results show that CT is associated with glutamatergic alterations, including lower levels of glutamate, Glx (=glutamate + glutamine), and NAA (N-acetylaspartateglutamate)/Glx ratio in frontal cortex areas (Duncan et al., 2015; Ousdal et al., 2018, 2019; Sonmez et al., 2021) and Glx in the temporal lobe, although only in clinically depressed patients (Poletti et al., 2016). On the other hand, although the association between CT and GABA transmission has not been studied in humans, lower levels of frontal GABA concentrations have been observed in response to other types of adversity in adult populations, including post-traumatic stress disorder (PTSD), trauma exposure and threat-of-shock (Hasler et al., 2010; Sheth et al., 2019).
To date however, no studies have examined the relationship between E/I balance (GABA and glutamate metabolite concentrations) and GMV in an adult CT population. In the current study, because CT is a major risk factor for depression, anxiety and psychosis (Green et al., 2010; Paus et al., 2008; Varese et al., 2012), we chose to examine glutamate and GABA concentrations in the left STG as an altered function, perfusion or structure in this region is one of the most robust neuroimaging findings in these populations (see Allen et al., 2019; Arnone et al., 2016; Madonna et al., 2019; Scheepens et al., 2020), and changes in temporal lobe metabolite concentrations have been reported in these populations (Hjelmervik et al., 2022; Hugdahl et al., 2015; Trzesniak et al., 2008; Venkatraman et al., 2009). In the current study, we aimed to compare left STG GABA and glutamate, as well as whole-brain and left STG GMV in young adults with high and low levels of CT. We predicted that, relative to participants with low CT, a high CT group would show reduced glutamate and GABA concentrations and GMV in the left STG. We further predicted that reduced STG metabolite concentrations and STG GMV would interact to predict high levels of CT. We also conducted an exploratory analysis to examine relationships between STG GMV, metabolite concentrations and clinical measures.
Method
Participants
Two hundred and thirty students from Universities of Roehampton and Royal Holloway responded to an online survey via Facebook (delivered on Qualtrics; https://www.qualtrics.com) and were screened using the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003). Fifty-six participants were selected based on the upper and lower quartiles of the sample distribution of the first 100 respondents to establish high CT score (High CT; >40.5, n = 29) and low CT score (Low CT; <29.5, n = 27) groups. Sensitivity analysis shows that the sample size would allow the detection of a medium effect based on 80% power and an alpha = 0.05.
Exclusion criteria, assessed via a self-report pre-screening survey, included presence of contraindications for magnetic resonance imaging (MRI) scanning (i.e. presence of metal, etc), current use of prescribed medication for neuropsychiatric disorders, or history of or presence of psychiatric and neurological disorders and current use of illicit substances misuses. Absence of psychiatric or neurological diagnosis was assessed with two questions in the screening survey: ‘Have you ever been diagnosed with a psychiatric condition (e.g. Attention Deficit Hyperactivity Disorder [ADHD], depression, anxiety, mood disorders)?’ and ‘Have you ever been diagnosed with a neurological disorder or disease (e.g. epilepsy, stroke, head injury, seizures, brain tumours, brain surgery, Parkinson’s disease)?’ Participants in the Low and High-CT groups were matched for age, gender, estimated IQ, tobacco, cannabis and alcohol use. Participants received £20 for participation. All participants provided informed consent. The research protocol was approved by the Ethical Committee at the University of Roehampton.
Clinical, IQ and demographic assessment
All participants completed a demographics form (developed in-house) to determine age, sex, level of education, intellectual functioning (assessed via the Wide Range Achievement Test, Reading Level 2; WRAT-R) (Jastak & Wilkinson, 1984), handedness (assessed via the Annett Hand Preference Questionnaire (Annett, 1970), alcohol consumption (units per day), tobacco consumption (cigarettes per day) and cannabis use (assessed via the Cannabis Experience Questionnaire; CEQ) (Barkus & Lewis, 2008). These measures were used to ensure that the High and Low CT groups were matched for these demographic and environmental/lifestyle factors.
The CTQ (Bernstein et al., 2003), a 28-item questionnaire, designed to quantify self-reported CT history in the home, is divided into five clinical subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect. Each item is rated on a 5-point Likert scale (from 1 = never true to 5 = very often true), with higher scores reflecting trauma history. The CTQ demonstrates good test–retest reliability (coefficients ranging from 0.79 to 0.86) and internal consistency (coefficients ranging from 0.66 to 0.92) (Bernstein et al., 2003).
The Depression and Anxiety Stress Scale (DASS) (Lovibond & Lovibond, 1995), a 42-item questionnaire, is designed to quantify the current (state) levels of depression, anxiety, and stress. Each item is rated on a 4-point Likert scale based on symptom severity/frequency. On the depression subscale, a score of 0–9 indicates no depression, 10–13 mild depression, 14–20 moderate depression, 21–27 severe depression, and 28 + extremely severe depression. On the anxiety subscale, a score of 0–7 indicates no anxiety, 8–9 mild anxiety, 10–14 moderate anxiety, 15–19 severe anxiety, and 20 + extremely severe anxiety. On the stress subscale, a score of 0–14 indicates no stress, 15–18 mild stress, 19–25 moderate stress, 26–33 severe stress, and 34 + extremely severe stress.
We also used the Connor–Davidson Resilience Scale (CD-RISC-25) (Connor & Davidson, 2003), a 25-item questionnaire, to measure resilience. Each item is rated on a 5-point scale (0–4), with higher scores reflecting greater resilience.
MRI acquisition
All MRI scans were acquired on a 3T Siemens Magnetom TIM Trio scanner using a 32-channel head coil at the Combined Universities Brain Imaging Centre (http://www.cubic.rhul.ac.uk/). Structural T1-weighted magnetization-prepared rapid acquisition gradient echo images were acquired with a spatial resolution of 1 mm × 1 mm × 1 mm, in a plane resolution of 256 × 256 × 176 continuous slices and scanning time of approximately 5 min.
1H-MRS data acquisition and analysis
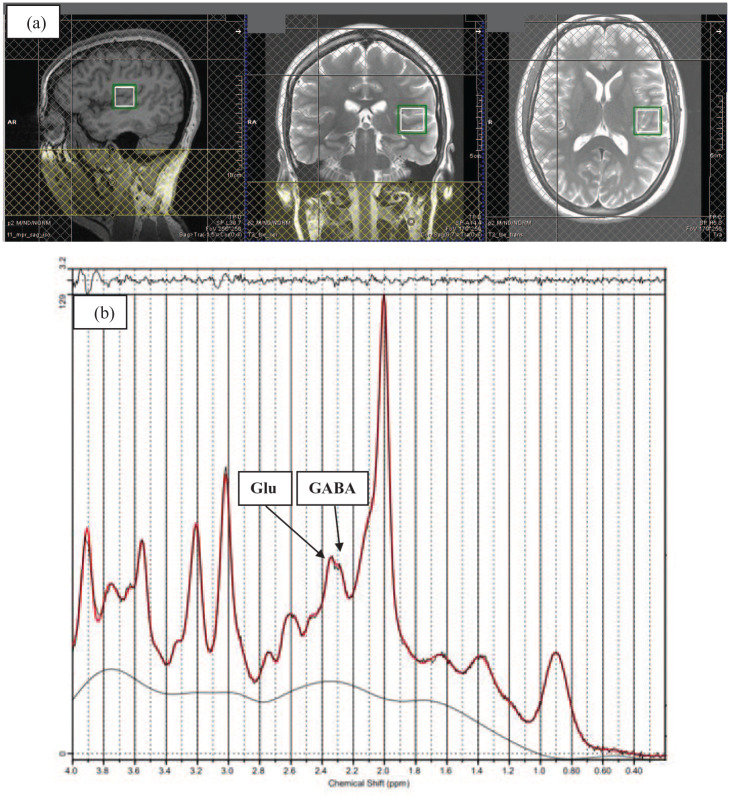
1H-MRS in vivo spectra were acquired from a 20 × 20 × 20 mm voxel located in the left STG during rest (see Figure 1). The voxel was positioned manually in the left STG by reference to an axial T1-weighted gradient echo image. Spectra were acquired using SPin ECho full Intensity-Acquired Localized spectroscopy (SPECIAL) (Mlynárik et al., 2006). The 1H-MRS sequence was acquired with water suppression (TR 3000 ms, TE 8.5 ms, Phase cycle Auto, 192 averages from the STG voxel) in each participant (Godlewska et al., 2015). Water unsuppressed spectra (16 averages) were also acquired. Six outer volume suppression slabs were applied (one on each side at 5 mm from the edge of the cubic voxel) to suppress signals originating from outside the volume of interest and to minimize motion-related image-selected in vivo spectroscopy subtraction artefacts. Spectra were analysed using LCModel 6.3-1L with the basis set consisting of 19 simulated basis spectra: alanine (Ala), ascorbate (Asc), aspartate (Asp), creatine (Cr), GABA, glucose (Glc), glutamine (Gln), glutamate (Glu), glycine (Gly), glutathione (GSH), glycerophosphocholine (GPC), phosphocholine (PCh), lactate (Lac), myo-inositol (mI), N-acetylaspartate (NAA), N-acetylaspartateglutamate (NAAG), phosphorylethanolamine (PE), scyllo-inositol (Scyllo) and taurine (Tau).
Figure 1.
(a) Example of 1H-MRS voxel placement in the left superior temporal gyrus (STG) (sagittal, coronal, and axial orientations) (b) 1H-MRS spectrum obtained from the voxel in A (black line) and the overlay of the spectral fit (red line) (see the Supplemental Figure S1 for another example).
The basis set was simulated using FID-A (Simpson et al., 2017), for TE = 8.5 ms, magnetic field strength = 3T and assuming ideal RF pulses. We excluded spectra with Cramér- Rao lower bounds (CRLB) > 20% as reported by LCModel. In addition to metabolite levels, line widths and signal-to-noise ratios were estimated by LCModel. All spectra had a line width <8 Hz (estimates of the line widths produced by the LC model software) and a mean signal-to-noise ratio (SNR) > 39.72 which are within the accepted ranges (Godlewska et al., 2015; Hollestein et al., 2021). SNR is defined as is defined the ratio of the maximum in the spectrum-minus baseline over the analysis window to twice the root-mean-square (rms) residuals. Following these quality control checks, we reported results form 51 (25 Low CT and 26 High CT) and 56 (27 Low CT and 29 High CT) participants for STG GABA and STG Glu, respectively.
Water referencing and eddy current correction were used to quantify metabolite levels. When quantified in this way, metabolite levels are influenced by cerebral spinal fluid (CSF), grey (GM) and white (WM) matter volumes of the region in which spectra are obtained within the voxel (Srinivasan et al., 2006) and inter-individual differences in cortical grey matter (Huster et al., 2007). In order to account for these confounds, we used the T1-weighted anatomical images to estimate the GM and WM content of the STG voxel in which the 1H-MRS measures were performed using GABA Analysis Toolkit (Gannet 2.0, https://github.com/markmikkelsen/Gannet) adapted to work with Siemens SPECIAL data. The segmentation was performed using ‘new segment’ in SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). CSF, GM volume and WM volume were then accounted for in the expression of GABA and Glu levels using LCModel (Ernst et al., 1993); corrected metabolite levels will be referred to as Glu Corr and GABA Corr using the formula Glu Corr = (Glu*(43300*GMV + 35880*WMV + 55556*CSF))/(35880* (1-CSF)) and GABA Corr = (GABA*(43300*GMV + 35880*WMV + 55556*CSF))/(35880*(1-CSF)). Relaxation corrections were not applied apart from correcting for tissue water relaxation, assuming T2 = 80 ms, by using LCModel parameter ATTH2O = 0.899.
IBM® SPSS Statistics Version 26 and Jamovi 2.2.5 were used for data analysis. Low CT and High CT groups were compared on demographic and clinical measures, as well as STG metabolite levels, SNR, line width and CRLB by using chi-square or independent sample t-tests. Logistic regression analyses were also conducted to predict the CT group (high vs low) from SFG GABA Corr and Glu Corr levels. Relationships between clinical measures and GABA Corr and Glu Corr metabolite concentrations were analysed using bivariate correlations. A statistical significance threshold of p < 0.05 was applied throughout.
VBM and region-of-interest analysis
Images were analysed using Computational Anatomy Toolbox 12 (CAT12; http://www.neuro.uni-jena.de/cat) implemented in SPM12 (Wellcome Trust Centre for Neuroimaging; www.fil.ion.ac.uk/spm/software/spm12). As per standard protocol (see http://www.neuro.uni-jena.de/cat12/CAT12-Manual.pdf), data were skull-stripped using the adaptive probability region-growing approach, normalized to the standard tissue probability map, and segmented into grey matter, white matter, and CSF. These images were ‘modulated normalized’ images (i.e. voxel values were modulated using the Jacobian determinant), derived from the spatial normalization so that the absolute volume of grey matter could be compared between groups. This type of modulation requires group analyses to correct for individual differences in brain size; total intracranial volume was therefore added as a covariate to all group-level general linear models. The images were then registered to the MNI template using DARTEL registration and smoothed using an 8-mm Gaussian Kernel. Data quality was checked based on the image quality ratings (IQR) generated by CAT12, which factors in both noise (e.g. motion) and spatial resolution. The visual inspection revealed no issues. Only the images where the IQR was above the ‘good’ threshold (i.e. B-; 0.80) were included in the analyses; hence, the reported results for VBM and region-of-interest (ROI) analyses are from 49 participants (22 Low CT and 27 High CT).
In order to examine whole-brain level GM volume differences between High CT and Low CT groups, two-sample t-tests that control for TIV were used to determine brain regions in which GMV differed between Low CT group and High CT group. A threshold of p < 0.05 with family-wise error correction for multiple comparisons was applied to all contrasts.
As CAT12 also enables the estimation of mean tissue volumes for different volume-based atlas maps, we used a ROI labelling approach that parcellates each brain into several anatomical regions according to the neuromorphometric atlas (provided by Neuromorphometrics, Inc. (http://Neuromorphometrics.com)) to estimate the sum of local GM inside the left STG. IBM® SPSS Statistics Version 26 was used for data analysis. Low CT and High CT groups were compared on the left IFG GM volume by an independent sample t-test. Relationships between clinical measures, the left STG GABA Corr and Glu Corr metabolite concentrations, and the left STG GM volume were analysed using partial correlations adjusted for TIV. Logistical regression models were also used to test whether the interaction between the STG GMV and metabolite concentrations predicted CT group membership. A statistical significance threshold of p < 0.05 was applied throughout.
Results
Participant characteristics
Due to the slightly differing group configurations for GABA Corr and Glu Corr concentrations and GMV resulting from quality control checks, results are reported separately. Table 1 provides a full summary of participant characteristics in Low and High CT groups for the analysis of GABA Corr and Glu Corr metabolite concentrations and GMV in the left STG. The Low and High CT groups were matched for sex, age, WRAT-R estimated IQ, years in education, tobacco use, alcohol use, cannabis use and handedness, but by design differed significantly on measures of CT. As expected, the Low and High CT groups also differed significantly on measures of depression, anxiety and stress. There was also a difference for CD-RISC (resilience) scores for the participants in the Glu Corr and GMV analyses. Groups did not differ on GM, WM and CSF tissue volumes in the STG voxel.
Table 1.
Demographic summary, questionnaire measures and tissue maps in the Low CT and High CT groups for GABA Corr, Glu Corr metabolite and GMV analysis.
| Characteristic | GABA Corr | Glu Corr | STG GMV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low CT (n = 25) | High CT (n = 26) | t/χ 2 | p | Low CT (n = 27) | High CT (n = 29) | t/χ 2 | p | Low CT (n = 22) | High CT (n = 27) | t/χ 2 | p | |
| Sex (M/F) | 8/17 | 6/20 | 0.51 | 0.475 | 8/19 | 6/23 | 0.59 | 0.440 | 6/16 | 6/21 | 0.17 | 0.683 |
| Age | 19.92 (1.66) | 20.69 (1.49) | −1.75 | 0.086 | 20.00 (1.61) | 20.79 (1.80) | −1.73 | 0.089 | 20.14 (1.70) | 20.67 (1.52) | −1.15 | 0.127 |
| WRAT-R IQ | 75.72 (5.70) | 75.42 (4.47) | 0.21 | 0.837 | 75.92 (5.56) | 75.21 (4.57) | 0.53 | 0.598 | 75.55 (5.96) | 75.89 (3.93) | −0.24 | 0.405 |
| Education, years | 14.88 (3.27) | 15.96 (1.84) | −1.46 | 0.150 | 15.03 (3.20) | 15.38 (3.28) | −0.39 | 0.695 | 15.59 (2.02) | 15.96 (1.79) | −0.69 | 0.248 |
| CTQ-total | 26.88 (1.42) | 57.54 (12.51) | −12.18 | <0.001 | 26.77 (1.42) | 57.21 (12.22) | −12.85 | <0.001 | 26.73 (1.49) | 56.93 (12.58) | −11.17 | <0.001 |
| Emotional abuse | 5.76 (0.97) | 14.38 (3.89) | −10.77 | <0.001 | 5.70 (0.95) | 14.31 (3.82) | −11.38 | <0.001 | 5.55 (0.86) | 14.19 (3.89) | −10.19 | <0.001 |
| Physical abuse | 5.00 (0.00) | 9.96 (5.02) | −4.94 | <0.001 | 5.00 (0.00) | 9.76 (4.78) | −5.17 | <0.001 | 5.00 (0.00) | 10.00 (4.85) | −4.82 | <0.001 |
| Sexual abuse | 5.00 (0.00) | 7.65 (4.63) | −2.86 | 0.006 | 5.00 (0.00) | 7.66 (4.49) | −3.07 | 0.003 | 5.00 (0.00) | 7.48 (4.48) | −2.60 | 0.006 |
| Emotional neglect | 5.96 (1.40) | 14.77 (3.84) | −10.80 | <0.001 | 5.92 (1.35) | 14.79 (3.83) | −11.38 | <0.001 | 6.00 (1.45) | 14.74 (3.77) | −10.26 | <0.001 |
| Physical neglect | 5.16 (0.37) | 10.77 (3.65) | −7.65 | <0.001 | 5.14 (0.36) | 10.69 (3.55) | −8.07 | <0.001 | 5.18 (0.39) | 10.52 (3.62) | −6.87 | <0.001 |
| CD-RISC | 69.60 (8.47) | 62.19 (17.28) | 1.93 | 0.059 | 69.59 (8.54) | 61.69 (18.04) | 2.07 | 0.043 | 70.59 (7.83) | 62.07 (17.86) | 2.08 | 0.022 |
| DASS_Depression | 3.04 (3.56) | 11.23 (7.52) | −4.94 | <0.001 | 3.56 (3.91) | 11.90 (9.29) | −4.32 | <0.001 | 3.23 (3.48) | 11.67 (9.19) | −4.07 | <0.001 |
| DASS_Anxiety | 7.76 (5.77) | 13.50 (7.78) | −2.98 | 0.004 | 3.30 (3.22) | 9.38 (7.73) | −3.79 | <0.001 | 2.91 (3.10) | 8.81 (7.20) | −3.58 | <0.001 |
| DASS_Stress | 3.16 (3.30) | 9.04 (7.98) | −3.41 | <0.001 | 7.44 (5.56) | 14.07 (7.90) | −3.58 | <0.001 | 6.59 (5.60) | 13.56 (7.64) | −3.56 | <0.001 |
| Tobacco use* | 1.55 (3.85) | 0.08 (0.28) | 1.87 | 0.069 | 1.40 (3.68) | 0.37 (1.48) | 1.32 | 0.195 | 0.81 (2.12) | 0.37 (1.48) | 0.812 | 0.211 |
| Alcohol use** | 1.73 (2.34) | 1.44 (1.90) | 0.48 | 0.634 | 1.78 (2.29) | 1.50 (1.94) | 0.50 | 0.617 | 1.96 (2.41) | 1.57 (1.98) | 0.621 | 0.269 |
| CEQ | 3.20 (7.08) | 2.19 (3.45) | 0.65 | 0.519 | 3.03 (6.83) | 2.17 (3.34) | 0.61 | 0.545 | 3.18 (7.22) | 2.11 (3.41) | 0.684 | 0.498 |
| Handedness (R/L) | 20/5 | 23/3 | 0.69 | 0.406 | 21/6 | 26/3 | 1.46 | 0.227 | 6/16 | 24/3 | 2.11 | 0.146 |
| GM volume | 0.63 (0.08) | 0.62 (0.08) | 0.50 | 0.620 | 0.63 (0.08) | 0.62 (0.08) | 0.44 | 0.665 | 0.63 (0.08) | 0.62 (0.08) | 0.241 | 0.405 |
| WM volume | 0.20 (0.14) | 0.25 (0.14) | −1.21 | 0.233 | 0.21 (0.14) | 0.25 (0.14) | −1.06 | 0.293 | 0.23 (0.14) | 0.25 (0.14) | −0.544 | 0.294 |
| CSF volume | 0.17 (0.11) | 0.13 (0.09) | 1.27 | 0.211 | 0.16 (0.11) | 0.13 (0.09) | 1.16 | 0.253 | 0.14 (0.10) | 0.13 (0.09) | 0.576 | 0.284 |
CD-RISC: The Connor–Davidson Resilience Scale; CEQ: Cannabis Experience Questionnaire; CSF: cerebro-spinal fluid; CTQ: Childhood Trauma Questionnaire; DASS: Depression, Anxiety, Stress Scale; F: female; GABA: gamma-aminobutyric acid; GM: grey matter; GMV: grey matter volumes; L: left; M: male; R: right; S: state; STAI: State Trait Anxiety Inventory; STG: superior temporal gyrus; T: trait; WM: white matter; WRAT-R: Wide Range Achievement Test-Revised.
cigarettes/d. ** units/d.
GABA Corr and Glu Corr metabolite concentrations
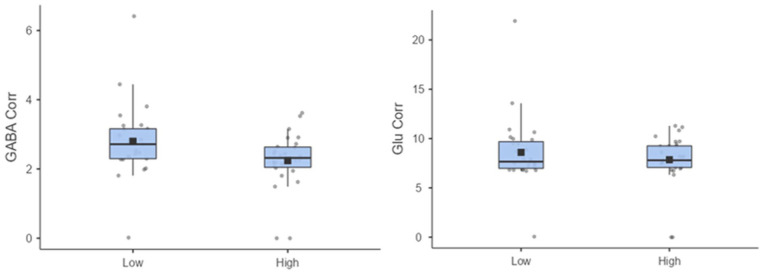
STG GABA Corr and Glu Corr metabolite levels and spectra quality control data for Low and High CT groups are reported in Table 2. All other metabolite levels (NAA, Cr, mI, Glx) are reported in Supplemental Table S1. No significant differences between groups were detected for SNR, line width, or CRLB. The difference between Low and High CT groups for STG Glu Corr was non-significant (p > 0.1). However, the High CT group (M = 2.24, SD = 0.83 institutional units) had significantly lower STG GABA Corr metabolite concentrations compared to the Low CT group (M = 2.79, SD = 1.10 institutional units), (t(49) = 2.03. p = 0.048; Figure 2). After controlling for depression, anxiety, stress and resilience, logistic regression analysis revealed that STG GABA Corr was a significant fit to the model, χ2(5) = 28.66, p < 0.001, Cox and Snell’s R2 = 0.43, Nagelkerke’s R2 = 0.57 and a significant predictor of CTQ group membership (b = −1.08, SE = 0.05, z = 3.08, p = 0.048, CI = [0.12, 0.99]). However, STG Glu Corr was not a significant predictor of CTQ group membership (p > 0.05).
Table 2.
Means, standard deviations and statistical analyses for 1H-MRS quality control measures and STG GABA Corr and Glu Corr levels by Low and High CTQ groups.
| Low CT | High CT | t | p | |
|---|---|---|---|---|
| GABA Corr (IU) | 2.79 (1.10) | 2.24 (0.83) | 2.03 | 0.048 |
| SNR | 44.60 (11.64) | 42.95 (6.86) | 0.59 | 0.559 |
| Line width (Hz) | 5.02 (1.85) | 4.62 (1.16) | 0.90 | 0.372 |
| GABA CRLB (%) | 13.52 (2.72) | 15.07 (2.92) | −1.96 | 0.055 |
| Glu Corr (IU) | 8.60 (3.52) | 7.85 (2.56) | 0.90 | 0.368 |
| SNR | 44.73 (11.54) | 39.72 (11.30) | 1.63 | 0.110 |
| Line width (Hz) | 5.06 (1.83) | 5.72 (3.16) | −0.94 | 0.350 |
| Glu CRLB (%) | 5.25 (2.65) | 5.06 (1.16) | 0.35 | 0.726 |
Corr: corrected; CRLB: Cramér-Rao lower bounds; CT: childhood trauma; GABA: gamma-aminobutyric acid; GLU: Glutamate; 1H-MRS: underwent magnetic resonance spectroscopy; Hz: hertz; IU: institutional units; SNR: signal-to-noise ratio; STG: superior temporal gyrus.
Figure 2.
(Left) Superior temporal gyrus (STG) gamma-aminobutyric acid (GABA) Corr (Right) STG Glu Corr levels by CTQ groups in institutional units. All datapoints are within the expected quality control parameters.
Associations between GABA Corr and Glu Corr metabolite concentrations and clinical measures
Correlations between metabolite concentrations and clinical measures in the High CT group were non-significant (all ps > 0.05). Results for both groups are reported in Supplemental Table S2.
VBM analysis
Following whole-brain VBM analysis, for both contrasts (Low CT > High CT and High CT > Low CT), no GM volume differences were found. The left STG ROI analysis showed that the difference between Low and High CT groups for left STG GM volume was also non-significant (t(54) = 0.392, p = 0.697).
Associations between the left STG GM volume and GABA Corr and Glu Corr concentrations
After controlling for TIV, partial correlations revealed positive associations between the left STG GM volume and (1) GABA Corr (r(17) = 0.502, p = 0.029), and (2) Glu Corr (r(22) = 0.478, p = 0.028) metabolite concentrations in the low CT group, but not in the high CT group (all ps > 0.05).
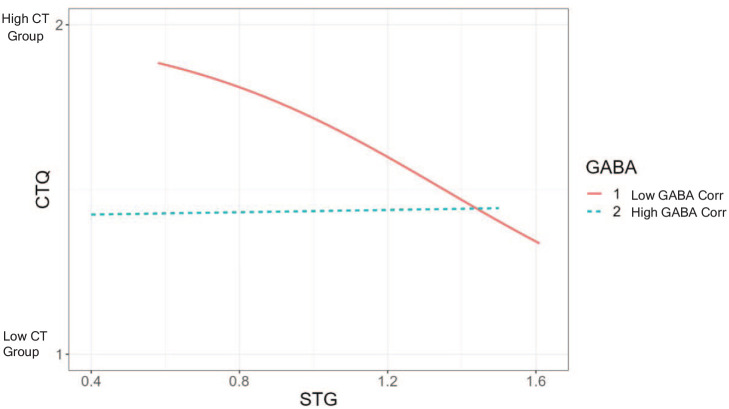
TIV-controlled logistic regression showed that the interaction between GABA Corr levels (categorized as low and high GABA Corr by using a median split) and the left STG GM volume was a significant fit of the model, χ2(2) = 7.16, p < 0.03, Cox and Snell’s R2 = 0.15, Nagelkerke’s R2 = 0.20 and a significant predictor of CTQ group membership (b = −0.95, SE = 0.43, z = 4.69, p < 0.03). Figure 3 shows that participants with low left STG GABA Corr levels and low left STG volumes were significantly more likely to be in the high CTQ group (95%CI 0.17–0.92). The same model using Glu Corr revealed a non-significant result (p > 0.05).
Figure 3.
Logistic regression showing an interaction between the left superior temporal gyrus (STG) GM volume and gamma-aminobutyric acid (GABA) Corr levels.
Associations between the left STG GM volume and clinical measures
By using the estimates of the sum of local grey matter inside the left STG ROI, partial correlations (TIV-controlled) revealed a positive association between the CD-RISC scores and the left STG GM volume in the low CT group (r(22) = 0.484, p = 0.026), but not in the high CT group (p > 0.1). In the low CT group, the left STG GM volume was also found to be negatively associated with DASS-Depression scores (r(19) = −0.451, p = 0.040). All other associations between the left STG volume and clinical measures were non-significant (all ps > 0.05). Results for both groups are reported in Supplemental Table S3.
Discussion
To our knowledge, this is the first study that has investigated the left STG GABA and glutamate, as well as the whole brain and the left STG GM volume differences in young adults in high and low levels of CT. Partially in line with our prediction, we found that individuals in the high CT group had reduced levels of the left STG GABA, but not glutamate, compared to individuals in the low CT group. Furthermore, having lower levels of GABA predicted high CT group membership. Whilst we did not observe any differences between High and Low CT groups in terms of left STG GMV, reduced levels of left STG GABA and lower left STG GMV interacted to predict high CT group membership. Our exploratory analysis also showed that the left STG GMV was positively associated with GABA and glutamate levels, resilience (CD-RISC scores) and negatively associated with depression (DASS-Depression scores) in the low CTgroup, but not in the high CT group.
Our observation of reduced GABA (but not glutamate) levels in the high (vs low) CT group is consistent with experimental work in animals showing the impact of early life adversity on GABA concentrations (Gomes et al., 2016). As no previous studies have linked measures of CT and GABAergic function in healthy humans, we also extended the current clinical evidence that has shown associations between CT and frontal glutamatergic alterations (Duncan et al., 2015; Ousdal et al., 2018, 2019; Sonmez et al., 2021). Our findings of reduced STG GABA metabolite concentration in adults that have experienced CT are also broadly in line with a study in patients with PTSD and trauma exposure (without PTSD) that showed reduced frontal (Sheth et al., 2019) and temporal GABA concentrations (Meyerhoff et al., 2014). Additionally, not only chronic but also acute forms of stress have been shown to decrease prefrontal GABA concentrations by 18% in humans (Hasler et al., 2010). Together, these findings may reflect a selective loss of parvalbumin-containing interneurons (that contain and release GABA) (Lodge et al., 2009) and/or a decrease of mRNA for GAD67 (an enzyme that synthesizes GABA) (Deslauriers et al., 2013; Giovanoli et al., 2013; Stone et al., 2001) in the left STG of young adults that have experienced CT. It might also be speculated that the reduction of the left STG GABA in adults that have experienced CT would reduce GABA’s inhibitory influence on neural circuits involved in responding to stress and/or threat. Converging evidence showing hyperactivity in the limbic structures in individuals who were exposed to early stress and/or childhood maltreatment (Teicher et al., 2003) supports this view.
In terms of our null glutamate finding, it may be the case that CT may selectively alter frontal (but not temporal) glutamatergic function (as evidenced in Duncan et al., 2015; Ousdal et al., 2018, 2019; Sonmez et al., 2021) and temporal GABAergic (as evidenced in the current study) mechanisms separately in healthy individuals. In fact, based on animal studies, it has been shown that stress selectively attenuates (1) excitatory tone (i.e. glutamatergic activity) in the frontal area but not in the amygdala or in the temporal lobe (Knox et al., 2010) and (2) inhibitory tone (i.e. GABAergic activity) in the temporal areas (De Groote & Linthorst, 2007). Moreover, these findings suggest that cortical glutamate levels might be perturbed in high (vs low) CT groups only under specific circumstances. For instance, Duncan et al. (2015) showed that the CTQ scores and frontal glutamate levels correlated with neural BOLD responses to the anticipation of aversive stimuli in areas covering the prefrontal-insular-motor cortex network, suggesting that left STG glutamate might be selectively altered only in the context of affective functioning.
It is important to note that, MEshcher-GArwood Point-RESolved Spectrosocpy (MEGA-PRESS) (Mescher et al., 1998) is the most widely used MRS acquisition protocol, with reproducible within- and between-session GABA measurement at 3T (Baeshen et al., 2020; Brix et al., 2017). However, in the current study and previous studies (Faulkner et al., 2021; Kozhuharova et al., 2021; Godlewska et al., 2015; Morgenroth et al., 2019), SPECIAL was utilized with promising results. Additionally, it has been shown that both GABA (Near et al., 2013) and other metabolite levels (e.g. GSH) (Wijtenburg et al., 2019) were comparable between SPECIAL and more conventional spectral editing techniques – albeit by using larger voxels; hence, we encourage researchers to replicate our findings by using other sequences.
Unlike previous studies, although we did not observe temporal GMV differences between High and Low CT groups (Paquola et al., 2016; Teicher et al., 2018), we found that the left STG GMV was (1) positively associated with GABA and glutamate concentrations as well as resilience, and (2) negatively associated with depression only in the low CT group. Furthermore, we found out that, lower left STG GMV and lower levels of GABA metabolite concentrations interacted to predict high CT group membership. Whilst this finding is difficult to interpret, it is possible that lower left STG GMV and GABA levels are linked to decreased resilience and increased levels of negative affect, such as depression. This may play a role in the risk or the development of psychiatric (or psychiatric-like) symptoms following early traumatic experiences and vulnerability to psychiatric conditions in later life (Nelson et al., 2020).
There are several notable limitations in the current research. First of all, based on the generic power analyses, although the sample size was adequate to detect medium-to-large effects, our results would benefit from replication in a larger sample, which could be achieved by combining 1H-MRS and morphometric data from multiple centres. Secondly, as the CTQ relies upon autobiographical recall that may be biased by current affective states (Vrijsen et al., 2017), and our definition of High and Low CT groups was also arbitrary (i.e. based on the upper and lower quartiles of the 100 first respondents), though not unusual (e.g. Kim et al., 2018), our results must be interpreted with caution. Thirdly, classification of participants based on the total CTQ score (as in the current study) may hinder the potential impact that trauma subtype (sexual abuse, physical abuse, emotional abuse, sexual neglect and physical neglect), severity and duration (single vs prolonged trauma) may have on brain volume and chemistry. Fourthly, given the dimensions and orientation of our 1H-MRS voxel, other temporal lobe structures might have also been included in the voxel, which may have confounded our results. Fifthly, although the CRLB GABA values in the current study are consistent with previous studies (Kozhuharova et al., 2021; Weis et al., 2021) and are within standard limits (Oz et al., 2020), as GABA is a small signal and given the tendency for the high CT group to have higher CRLB for GABA, the possibility of differences in spectral quality driving the results should not be underestimated. Sixthly, by using conventional 1H-MRS, one cannot simply determine whether differences in neurometabolite levels are associated with neurotransmission or metabolism; hence, future research should utilise more sophisticated MRS protocols (Jelen et al., 2018) to address this issue. Seventhly, as some participants were excluded from the volumetric analysis based on image quality, we cannot exclude the possibility that image quality might have affected the tissue segmentation within the MRS voxel. Finally, due to the cross-sectional nature of our study, we could not determine cause and effect relationships; therefore, further longitudinal studies are warranted in order to allow stronger causal inferences to examine the effects of CT on brain volume and chemistry.
In conclusion, our findings suggest that early traumatic experiences are associated with lower left STG GABA neurotransmission/metabolism. Furthermore, although we did not observe a direct group effect in left STG GMV, traumatic stress may influence the left STG GMV through excitotoxicity due to GABA alterations. Further longitudinal studies are warranted to identify neurochemical and neurostructural (and their interaction) correlates of CT throughout development and in populations with other psychiatric disorders, with an aim towards contributing to pharmacological treatments of stress-related mental illness.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811231168243 for Signatures of exposure to childhood trauma in young adults in the structure and neurochemistry of the superior temporal gyrus by Piril Hepsomali, Sandra Machon, Holly Barker, David J Lythgoe, Kenneth Hugdahl, Maria Gudbrandsen and Paul Allen in Journal of Psychopharmacology
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PA was supported by grants from the British Academy and Rosetrees Trust.
ORCID iDs: Piril Hepsomali  https://orcid.org/0000-0001-5812-1081
https://orcid.org/0000-0001-5812-1081
Paul Allen  https://orcid.org/0000-0001-8510-878X
https://orcid.org/0000-0001-8510-878X
Supplemental material: Supplemental material for this article is available online.
References
- Aas M, Dazzan P, Fisher HL, et al. (2011) Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophr Res 129: 12–19. [DOI] [PubMed] [Google Scholar]
- Allen P, Sommer IE, Jardri R, et al. (2019) Extrinsic and default mode networks in psychiatric conditions: Relationship to excitatory-inhibitory transmitter balance and early trauma. Neurosci Biobehav Rev 99: 90–100. [DOI] [PubMed] [Google Scholar]
- Annett M. (1970) A classification of hand preference by association analysis. Br J Psychol 61: 303–321. [DOI] [PubMed] [Google Scholar]
- Arnone D, Job D, Selvaraj S, et al. (2016) Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp 37: 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Fisher HL, et al. (2011) Childhood trauma and children’s emerging psychotic symptoms: A genetically sensitive longitudinal cohort study. Am J Psychiatry 168: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeshen A, Wyss PO, Henning A, et al. (2020) Test–retest reliability of the brain metabolites GABA and Glx with JPRESS, PRESS, and MEGA-PRESS MRS sequences in vivo at 3T. J Magn Reson Imaging 51: 1181–1191. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Van Hasselt FN, Champagne DL, et al. (2009) Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Memory 92: 292–300. [DOI] [PubMed] [Google Scholar]
- Barkus E, Lewis S. (2008) Schizotypy and psychosis-like experiences from recreational cannabis in a non-clinical sample. Psychol Med 38: 1267–1276. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, et al. (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27: 169–190. [DOI] [PubMed] [Google Scholar]
- Bonapersona V, Kentrop J, Van Lissa CJ, et al. (2019) The behavioral phenotype of early life adversity: A 3-level meta-analysis of rodent studies. Neurosci Biobehav Rev 102: 299–307. [DOI] [PubMed] [Google Scholar]
- Brix MK, Ersland L, Hugdahl K, et al. (2017) Within- and between-session reproducibility of GABA measurements with MR spectroscopy. J Magn Reson Imaging 46: 421–430. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR. (2003) Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 18: 76–82. [DOI] [PubMed] [Google Scholar]
- Danielewicz J, Hess G. (2014) Early life stress alters synaptic modification range in the rat lateral amygdala. Behav Brain Res 265: 32–37. [DOI] [PubMed] [Google Scholar]
- De Groote L, Linthorst ACE. (2007) Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience 148: 794–805. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Larouche A, Sarret P, et al. 2013. Combination of prenatal immune challenge and restraint stress affects prepulse inhibition and dopaminergic/GABAergic markers. Prog Neuropsychopharmacol Biol Psychiatry 45: 156–164. [DOI] [PubMed] [Google Scholar]
- Dong XX, Wang Y, Qin ZH. (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Hayes DJ, Wiebking C, et al. (2015) Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: A preliminary multimodal rsfMRI-fMRI-MRS-dMRI study. Hum Brain Mapp 36: 4622–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. (1993) Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson B 102: 1–8. [Google Scholar]
- Faulkner P, Paioni SL, Kozhuharova P, et al. (2021) Relationship between depression, prefrontal creatine and grey matter volume. J Psychopharmacol 35: 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, et al. (2013) Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339: 1095–1099. [DOI] [PubMed] [Google Scholar]
- Godlewska BR, Near J, Cowen PJ. (2015) Neurochemistry of major depression: A study using magnetic resonance spectroscopy. Psychopharmacology 232: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Rincón-Cortés M, Grace AA. (2016) Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev 70: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, et al. (2010) Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 67: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Van der Veen JW, Grillon C, et al. (2010) Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am J Psychiatry 167: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmervik H, Craven AR, Johnsen E, et al. (2022) Negative valence of hallucinatory voices as predictor of cortical glutamatergic metabolite levels in schizophrenia patients. Brain Behav 12: e2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollestein V, Buitelaar JK, Brandeis D, et al. (2021) Developmental changes in fronto-striatal glutamate and their association with functioning during inhibitory control in autism spectrum disorder and obsessive compulsive disorder. NeuroImage: Clin 30: 102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, et al. (2005) Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. Am J Psychiatry 162: 2233–2245. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Craven AR, Nygård M, et al. (2015) Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: A 1H MRS study. Schizophr Res 161: 252–260. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, et al. (2007) Morphologic asymmetry of the human anterior cingulate cortex. NeuroImage 34: 888–895. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Bak M, et al. (2004) Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand 109: 38–45. [DOI] [PubMed] [Google Scholar]
- Jastak J, Wilkinson GS. (1984) Wide range achievement test: Revised edition. Wilmington, DE: Jastak Assoc. [Google Scholar]
- Jelen LA, King S, Mullins PG, et al. (2018) Beyond static measures: A review of functional magnetic resonance spectroscopy and its potential to investigate dynamic glutamatergic abnormalities in schizophrenia. J Psychopharmacol 32: 497–508. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Collin G, Guimond S, et al. (2020) Neuroimaging in schizophrenia. Neuroimag Clin N Am 30: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, et al. (2010) Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry 197: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim JS, Jin MJ, et al. (2018) Dysfunctional frontal lobe activity during inhibitory tasks in individuals with childhood trauma: An event-related potential study. NeuroImage: Clin 17: 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, et al. (2010) Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci Lett 480: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhuharova P, Diaconescu AO, Allen P. (2021) Reduced cortical GABA and glutamate in high schizotypy. Psychopharmacology (Berl) 238: 2459–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim HJ, Kim JG, et al. (2007) Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res 58: 32–39. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. (2009) A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci Off J Soc Neurosci 29: 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2007) Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27: 11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2011) Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 32: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. (1995) Manual for the Depression Anxiety Stress Scales, 2nd edn. Sydney: Psychology Foundation. [Google Scholar]
- Madonna D, Delvecchio G, Soares JC, et al. (2019) Structural and functional neuroimaging studies in generalized anxiety disorder: A systematic review. Braz J Psychiatry 41: 336–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang Rui R, et al. (2013) Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci 110: 18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, et al. 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11: 266–272. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Mon A, Metzler T, et al. (2014) Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 37: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynárik V, Gambarota G, Frenkel H, et al. (2006) Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med 56: 965–970. [DOI] [PubMed] [Google Scholar]
- Monroy E, Hernández-Torres E, Flores G. (2010) Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat 40: 93–101. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, et al. (2006) A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: Implications for the neuropathology of schizophrenia. Biol Psychiatry 60: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenroth E, Orlov N, Lythgoe DJ, et al. (2019) Altered relationship between prefrontal glutamate and activation during cognitive control in people with high trait anxiety. Cortex 117: 53–63. [DOI] [PubMed] [Google Scholar]
- Near J, Andersson J, Maron E, et al. (2013) Unedited in vivo detection and quantification of γ-aminobutyric acid in the occipital cortex using short-TE MRS at 3 T. NMR Biomed 26: 1353–1362. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bhutta ZA, Burke Harris N, et al. (2020) Adversity in childhood is linked to mental and physical health throughout life. BMJ 371: m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousdal OT, Huys QJ, Milde AM, et al. (2018) The impact of traumatic stress on Pavlovian biases. Psychol Med 48: 327–336. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Milde AM, Craven AR, et al. (2019) Prefrontal glutamate levels predict altered amygdala-prefrontal connectivity in traumatized youths. Psychol Med 49: 1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Deelchand DK, Wijnen JP, et al. (2020) Advanced single voxel 1 H magnetic resonance spectroscopy techniques in humans: Experts’ consensus recommendations. NMR Biomed 34: e4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola C, Bennett MR, Lagopoulos J. (2016) Understanding heterogeneity in grey matter research of adults with childhood maltreatment – A meta-analysis and review. Neurosci Biobehav Rev 69: 299–312. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9: 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S, Locatelli C, Falini A, et al. (2016) Adverse childhood experiences associate to reduced glutamate levels in the hippocampus of patients affected by mood disorders. Progr Neuro-Psychopharmacol Biol Psychiatry 71: 117–122. [DOI] [PubMed] [Google Scholar]
- Read J, Van Os J, Morrison AP, et al. (2005) Childhood trauma, psychosis and schizophrenia: A literature review with theoretical and clinical implications. Acta Psychiatr Scand 112: 330–350. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, et al. (2003) Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Hormones Behav 43: 561–567. [DOI] [PubMed] [Google Scholar]
- Scheepens DS, Van Waarde JA, Lok A, et al. (2020) The link between structural and functional brain abnormalities in depression: A systematic review of multimodal neuroimaging studies [systematic review]. Front Psychiatry 11: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, et al. (2013) Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth C, Prescot AP, Legarreta M, et al. (2019) Reduced gamma-amino butyric acid (GABA) and glutamine in the anterior cingulate cortex (ACC) of veterans exposed to trauma. J Affect Disord 248: 166–174. [DOI] [PubMed] [Google Scholar]
- Simpson R, Devenyi GA, Jezzard P, et al. (2017) Advanced processing and simulation of MRS data using the FID appliance (FID-A) – An open source, MATLAB-based toolkit. Magn Reson Med 77: 23–33. [DOI] [PubMed] [Google Scholar]
- Smith KE, Pollak SD. (2020) Early life stress and development: Potential mechanisms for adverse outcomes. J Neurodev Disord 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonmez AI, Lewis CP, Port JD, et al. (2021) A pilot spectroscopy study of adversity in adolescents. Biomark Neuropsychiatry 5: 100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Cunningham C, Chen A, et al. (2006) TE-averaged two-dimensional proton spectroscopic imaging of glutamate at 3 T. NeuroImage 30: 1171–1178. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Walsh JP, Sebro R, et al. (2001) Effects of pre- and postnatal corticosterone exposure on the rat hippocampal GABA system. Hippocampus 11: 492–507. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, et al. (2003) The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 27: 33–44. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, et al. (2018) Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage 169: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge J, Williamson KE, Aoyama N, et al. (2007) Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry 191: 325–334. [DOI] [PubMed] [Google Scholar]
- Trzesniak C, Araújo D, Crippa JAS. (2008) Magnetic resonance spectroscopy in anxiety disorders. Acta Neuropsychiatr 20: 56–71. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, et al. (2012) Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 38: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman TN, Krishnan RR, Steffens DC, et al. (2009) Biochemical abnormalities of the medial temporal lobe and medial prefrontal cortex in late-life depression. Psychiatry Res Neuroimag 172: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen JN, Van Amen CT, Koekkoek B, et al. (2017) Childhood trauma and negative memory bias as shared risk factors for psychopathology and comorbidity in a naturalistic psychiatric patient sample. Brain Behav 7: e00693–e00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J, Persson J, Frick A, et al. (2021) GABA quantification in human anterior cingulate cortex. PLoS One 16: e0240641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield CL., Dube SR, Felitti VJ, et al. (2005) Adverse childhood experiences and hallucinations. Child Abuse Negl 29: 797–810. [DOI] [PubMed] [Google Scholar]
- Wijtenburg SA, Near J, Korenic SA, et al. (2019) Comparing the reproducibility of commonly used magnetic resonance spectroscopy techniques to quantify cerebral glutathione. J Magn Reson Imaging 49: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. (2014) Glutamate as a neurotransmitter in the healthy brain. J Neural Trans (Vienna, Austria: 1996) 121: 799–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811231168243 for Signatures of exposure to childhood trauma in young adults in the structure and neurochemistry of the superior temporal gyrus by Piril Hepsomali, Sandra Machon, Holly Barker, David J Lythgoe, Kenneth Hugdahl, Maria Gudbrandsen and Paul Allen in Journal of Psychopharmacology