Abstract
Background: Programmed cell death protein-1 inhibitors combined with lenvatinib have become a popular treatment option for patients with unresectable hepatocellular carcinoma. Transarterial chemoembolization combined with programmed cell death protein-1 inhibitors and lenvatinib has also shown preliminary efficacy in the unresectable hepatocellular carcinoma. We conducted this observational, retrospective, cohort study to compare the clinical outcomes and safety of transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib versus programmed cell death protein-1 inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Methods: Between November 2019 and November 2021, patients who were diagnosed with unresectable hepatocellular carcinoma and received transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib or programmed cell death protein-1 inhibitors plus lenvatinib treatment were reviewed for eligibility. The primary endpoints included objective response rate, overall survival, and progression-free survival. The secondary endpoint was the frequency of key adverse events. Results: In total, 105 patients were eligible for the present study, and they were divided into the transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib group (n = 46) and the programmed cell death protein-1 inhibitors plus lenvatinib group (n = 59). The patient cohort after a one-to-one propensity score matching (n = 86) was also analyzed. The transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib group had a higher objective response rate both in the patient cohort before propensity score matching (54.3% vs 25.4%, P = .002) and after propensity score matching (55.8% vs 30.2%, P = .017). The patients in the transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib group had prolonged overall survival (median, 20.5 vs 12.6 months, P = .015) and progression-free survival (median, 10.2 vs 7.4 months, P = .035). For patient cohort- propensity score matching, the overall survival (20.5 vs 12.8 months, P = .013) and progression-free survival (12.1 vs 7.8 months, P = .030) were also significantly better in the transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib group than in the programmed cell death protein-1 inhibitors plus lenvatinib group. There were no significant differences between the 2 groups concerning adverse reactions caused by immunotherapy and lenvatinib. The adverse reactions caused by transarterial chemoembolization were transient and were quickly reversed. Conclusions: Compared to programmed cell death protein-1 inhibitors plus lenvatinib, transarterial chemoembolization combined with programmed cell death protein-1 inhibitors plus lenvatinib may provide better treatment response and survival benefits for patients with unresectable hepatocellular carcinoma, and the adverse events were manageable.
Keywords: transarterial chemoembolization, lenvatinib, PD-1 inhibitor, combined therapy, hepatocellular carcinoma
Introduction
Liver cancer remains a global health challenge, with an estimated incidence of 1.4 million cases by the year 2040.1,2 Hepatocellular carcinoma (HCC), the most prevalent type of primary liver cancer, ranks sixth in terms of incidence among malignancies and is the third leading cause of cancer-related death globally. 3
Approximately 70% of patients with HCC are unable to undergo radical resection due to late-stage diagnosis and liver dysfunction, which results in poor survival.4,5 According to the new Barcelona Clinic Liver Cancer (BCLC) staging system, patients in the BCLC-B stage with extensive bilobar liver involvement and BCLC-C stage are recommended to receive systemic therapy.6 Lenvatinib, a novel multitargeted tyrosine kinase inhibitor (TKI) that targets VEGFR 1-3, fibroblast growth factor receptor 1-4, PDGFR, RET, and KIT,7 gained approval in 2018 for the first-line treatment of unresectable HCC (uHCC) based on the phase III REFLECT study. 8 Recently, immune checkpoint inhibitors, including programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) inhibitors, have exhibited a promising clinical benefit to uHCC patients.9,10 However, the efficacy of monotherapy with either TKIs or PD-1 inhibitors is modest, combination treatment strategies are critical to improving treatment efficacy for uHCC. In an open-label multicenter study, lenvatinib plus pembrolizumab showed a median overall survival (OS) of 22 months and a median progression-free survival (PFS) of 8.6 months in patients with uHCC. 11 PD-1 inhibitors combined with lenvatinib show excellent antitumor effects and have become a popular treatment option for uHCC.
In addition to pharmacological combinations, locoregional therapies can be used as adjunct treatments for uHCC. Transarterial chemoembolization (TACE), the most common nonsurgical treatment for HCC, combined with PD-1 inhibitors and lenvatinib has shown preliminary efficacy in the uHCC.12,13 However, all of these studies are one-armed, and a comparison of the efficacy and safety of PD-1 inhibitors plus lenvatinib versus those of TACE combined with PD-1 inhibitors plus lenvatinib in the treatment of uHCC has not previously been conducted. Therefore, this retrospective study was designed to compare the clinical outcomes and safety of patients with uHCC who received TACE combined with PD-1 inhibitors plus lenvatinib (TPL) versus those who received PD-1 inhibitors plus lenvatinib (PL) to provide a reference for the treatment of uHCC.
Materials and Methods
Study Design and Patients Selection
The study was designed as an observational, retrospective, cohort study. The reporting of this study conforms to STROBE guidelines. 14 Between November 2019 and November 2021, 152 consecutive patients with uHCC who received TPL or PL at the Fourth Hospital of Hebei Medical University with accessible baseline data were included in a retrospective analysis. We have de-identified all patient details. The study was reviewed and approved by the Institutional Review Board of the Fourth Hospital of Hebei Medical University. Patients were included based on the following criteria: (1) age ≥18 years; (2) patients were diagnosed with uHCC through imaging or pathology according to the AASLD practice guidelines 15 ; (3) eligible patients had not previously received systemic treatment for HCC, and patients who had progressed after surgery, TACE, or radiofrequency ablation were also enrolled. No other cancer-related therapies were involved during TPL or PL; (4) patients in BCLC stage B or C were included, and the BCLC B patients must have extensive bilobar liver involvement, which cannot be entirely cured by TACE; (5) Child-Pugh was classified as A or B; (6) Eastern Cooperative Oncology Group (ECOG) performance status score ≤ 1; (7) at least one measurable target nodule according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) 16 ; (8) no other malignant tumors were diagnosed; (9) acceptable heart, kidney, and bone marrow function; and (10) complete medical and follow-up data were available. All laboratory serum test data were collected within 3 days before initiating treatment. Abdominal and chest imaging evaluation, including enhanced computed tomography (CT) or magnetic resonance imaging (MRI), was performed within a week of treatment initiation.
Treatment Procedure
Lenvatinib (Eisai Co., Ltd) was taken orally (8-12 mg according to body weight). Standard doses of the PD-1 inhibitors, pembrolizumab 200 mg, camrelizumab 200 mg, sintilimab 200 mg, were given intravenously every 3 weeks. The first use of PD-1 inhibitors was within 7 days of initiation of lenvatinib. For the TPL group, the patients received TACE treatment prior to combination drug treatment. A 5F-Yashiro vascular sheath was inserted following percutaneous femoral artery puncture using the Seldinger technique. 17 The tip of the catheter was inserted into tumor-feeding arteries according to tumor size, location, and arterial supply. Based on the angiography results, chemotherapy drugs, such as epirubicin, raltitrexed, and oxaliplatin, were infused into the target arteries. Then, embolization was conducted using lipiodol, or varying diameter microspheres, and the trunk was embolized using an absorbable gelatin sponge until the bleeding stopped. TACE was repeated “on demand” upon the presence of active lesions by follow-up CT or MRI in patients with adequate liver function and good performance status. Treatment was discontinued if patients experienced intolerable adverse effects, disease progression, or required changes in the treatment plan.
According to the principle of intent-to-treat, patients were informed of any potential side effects and the cost of long-term treatment before treatment. The treatment protocols were reviewed by the attending physician and the final decision on receiving which therapeutic regime was made by the patients.
Outcomes and Assessments
The primary endpoints were objective response rate (ORR), OS, and PFS. In the present study, objective tumor response was evaluated using CT or MRI scans repeated every 2 or 3 cycles of combined treatment of PD-1 inhibitors and lenvatinib according to mRECIST. Overall survival was defined from the date of initiation of combination therapy to the date of death from any cause. PFS was defined from the date of the initiation of combination therapy to the date of progression or the date of death from any cause, whichever occurred first. The secondary endpoint was the frequency of key adverse events (AEs), which were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v5.0). The severity of AEs was evaluated based on clinical manifestation, imaging and laboratory test, activities of daily living, as well as whether and what kind of treatment is needed, all of which were obtained by telephone follow-up and reviewing medical records.
Statistical Analysis
Continuous data in the baseline characteristics were compared using t tests or Mann-Whitney U tests and were described using the mean ± standard error (SE) or the median and range. Categorical variables were compared using χ2 test or Fisher exact test. The cumulative survival rates after initiation of combination therapy were estimated using the Kaplan-Meier method, followed by comparison using the log-rank test. Any survival-related variable with P < .10 in the univariate analysis was included in a multivariable Cox proportional hazards model. Hazard risk was calculated using a Cox proportional hazards model. A 2-tailed P < .05 was considered statistically significant. All statistical analyses were conducted using SPSS 25.0 (IBM Corp) or R language (v 4.1.3).
Due to the retrospective nature of present study, we conducted a propensity score matching (PSM) analysis to reduce the influence of selection bias and potential confounding factors between arms, and the data after PSM formed the patient cohort-PSM (after PSM). All parameters included in PSM (age, gender, ECOG, etiology, ALT, AST, AFP, liver cirrhosis, Child-Pugh class, BCLC stage, size of largest nodule, tumor number, macrovascular invasion, extrahepatic metastasis, previous therapy, and PD-1 Inhibitors categories). PSM analysis was performed using a one-to-one nearest-neighbor method without replacement using caliper widths of 0.2. A subgroup analysis comparing OS between the 2 groups was conducted, including age, gender, tumor size, tumor number, macrovascular invasion, extrahepatic metastasis, BCLC stage, AFP, hepatitis, cirrhosis, and Child-Pugh grade. To separately evaluate the predictive value of each parameter for predicting the survival benefit, each parameter's interaction term with treatment was tested. 18
Results
Patient Identification and Characteristics
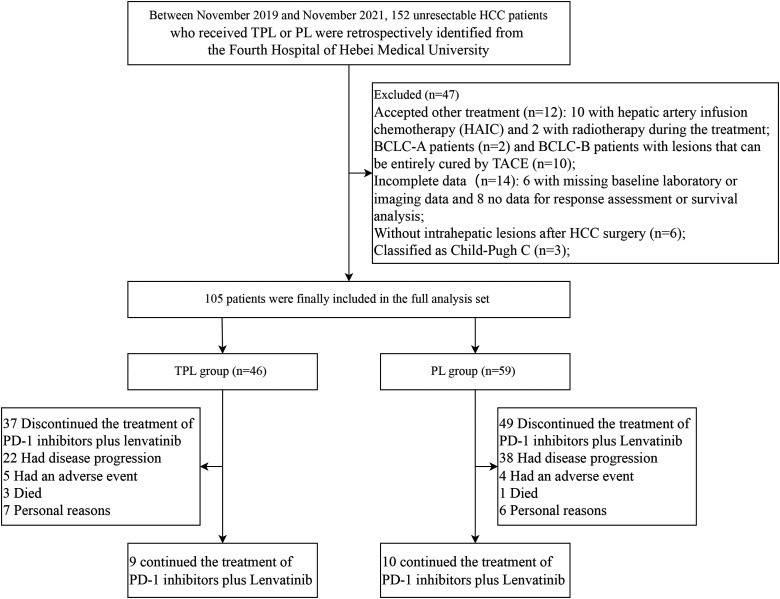
From November 2019 to November 2021, 152 patients with HCC who received TPL or PL were screened. Twelve patients received other treatments, including 10 who underwent hepatic artery infusion chemotherapy and 2 who underwent radiotherapy during treatment. Ten BCLC-B patients with lesions that can be entirely cured by TACE. Six patients had missing baseline laboratory or imaging data, and 8 patients had no data for response assessment or survival analysis. Six patients presented without intrahepatic lesions because of extrahepatic metastases after HCC surgery, 3 patients were classified as Child-Pugh C, and 2 patients were classified as BCLC stage A. A total of 105 patients who met the inclusion criteria were included in the study, and the patients were divided into the TPL group (n = 46) and the PL group (n = 59) (Figure 1).
Figure 1.
Flow diagram summarizing the disposition process of patients.
The clinical characteristics and treatments of 2 groups before and after PSM are summarized in Table 1. No significant difference was observed in the baseline characteristics of the patient cohorts before or after PSM. The mean of the largest nodule size in the TPL group and PL group was 7.73 ± 3.33 cm and 8.53 ± 4.55 cm, respectively. The median of the largest nodule size in the TPL group and PL group was 6.70 cm (interquartile range [IQR] 5.15, 9.95) and 7.20 cm (IQR 5.20, 12.00), and the P value was .518. In the patient cohort after PSM, the mean of the largest nodule size in the TPL group and PL group was 7.9 ± 3.4 cm and 8.3 ± 4.1 cm, respectively. The median of the largest nodule size in the TPL group and PL group was 7.0 cm (IQR 5.2, 10.4) and 6.8 cm (IQR 5.2, 11.9), and the P value was .809. In the TPL group, the median number of PD-1 inhibitor plus lenvatinib cycles was 8.5 (range 1-30), and the median number of TACE was 1 (range 1-4). In the PL group, the cycles of PD-1 inhibitors plus lenvatinib ranged from 1 to 28, with a median number of 7. The median duration of lenvatinib administration was 8.7 (1.7-29.1) months for TPL group, and 6.3 (0.7-27.2) months for PL group (P = .150). Thirty-seven patients discontinued the treatment of PD-1 inhibitors plus lenvatinib in the TPL group, 22 due to disease progression, 5 due to AE, 3 died, and 7 due to personal reasons; In the PL group, 49 patients stopped the treatment of PD-1 inhibitors plus lenvatinib for 1 of 4 reasons: disease progression in 38 cases, adverse effects in 4, died in 1, and personal reasons in 6 (Figure 1).
Table 1.
Patient Baseline Clinical Characteristics of 2 Groups Before and After PSM.
| Characteristic | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| TPL (n = 46) | PL (n = 59) | P | TPL(n = 43) | PL (n = 43) | P | |
| Age | 55.54 ± 11.92 | 58.56 ± 9.82 | .158 a | 57.07 ± 10.53 | 58.00 ± 10.52 | .683 a |
| Gender | .678 b | .747 b | ||||
| Male | 41 (89.1%) | 51 (86.4%) | 38 (88.4%) | 37 (86.0%) | ||
| Female | 5 (10.9%) | 8 (13.6%) | 5 (11.6%) | 6 (14.0%) | ||
| ECOG | .745 b | .651 b | ||||
| 0 | 17 (37.0%) | 20 (33.9%) | 16 (37.2%) | 14 (32.6%) | ||
| 1 | 29 (63.0%) | 39 (66.1%) | 27 (62.8%) | 29 (67.4%) | ||
| Etiology | .838 b | .333 b | ||||
| HBV/HCV | 42 (91.3%) | 52 (88.1%) | 39 (90.7%) | 36 (83.7%) | ||
| None | 4 (8.7%) | 7 (11.9%) | 4 (9.3%) | 7 (16.3%) | ||
| ALT (U/L) | 43.4 (29.4, 54.8) | 34.1 (24.8, 53.1) | .246 c | 43.1 (28.3,56.0) | 34.9 (24.6,53.1) | .278 c |
| AST (U/L) | 46.9 (33.8, 83.2) | 53.4 (37.0, 98.8) | .329 c | 47.7 (36.6,83.7) | 51.3 (37.0,79.7) | .990 c |
| AFP (ng/mL) | .172 b | .387 b | ||||
| ≥400 | 28 (60.9%) | 28 (47.5%) | 25 (58.1%) | 21 (48.8%) | ||
| <400 | 18(39.1%) | 31 (52.5%) | 18 (41.9%) | 22 (51.2%) | ||
| Liver cirrhosis | .440 b | .366 b | ||||
| Absent | 6 (13.0%) | 11 (18.6%) | 5 (11.6%) | 8 (18.6%) | ||
| Present | 40 (87.0%) | 48 (81.4%) | 38 (88.4%) | 35 (81.4%) | ||
| Child-Pugh class | .429 b | .596 b | ||||
| A | 38 (82.6%) | 45 (76.3%) | 35 (81.4%) | 33 (76.7%) | ||
| B | 8 (17.4%) | 14 (23.7%) | 8 (18.6%) | 10 (23.3%) | ||
| BCLC stage | .768 b | .776 b | ||||
| B | 8 (17.4%) | 9 (15.3%) | 8 (18.6%) | 7 (16.3%) | ||
| C | 38 (82.6%) | 50 (84.7%) | 35 (81.4%) | 36 (83.7%) | ||
| Tumor size | .759 b | .829 b | ||||
| ≥7 cm | 22 (47.8%) | 30 (50.8%) | 22 (51.2%) | 21 (48.8%) | ||
| <7 cm | 24 (52.2%) | 29 (49.2%) | 21 (48.8%) | 22 (51.2%) | ||
| Tumor number | .580 b | 1.000 b | ||||
| Solitary | 18 (39.1%) | 20 (33.9%) | 17 (39.5%) | 17 (39.5%) | ||
| Multiple | 28 (60.9%) | 39 (66.1%) | 26 (60.5%) | 26 (60.5%) | ||
| Macrovascular invasion | .685 b | .828 b | ||||
| Present | 20 (43.5%) | 28 (47.5%) | 19 (44.2%) | 18 (41.9%) | ||
| Absent | 26 (56.5%) | 31 (52.5%) | 24 (55.8%) | 25 (58.1%) | ||
| Extrahepatic metastasis | .577 b | .516 b | ||||
| Present | 24 (52.2%) | 34 (57.6%) | 22 (51.2%) | 25 (58.1%) | ||
| Absent | 22 (47.8%) | 25 (42.4%) | 21 (48.8%) | 18 (41.9%) | ||
| Previous therapy | ||||||
| Surgical resection | 11(23.9%) | 9 (15.3%) | .262 b | 9 (20.9%) | 5 (11.6%) | .243 b |
| TACE | 11 (23.9%) | 15 (25.4%) | .859 b | 11 (25.6%) | 9 (20.9%) | .610 b |
| RFA | 2 (4.3%) | 7 (11.9%) | .311 b | 2 (4.7%) | 5 (11.6%) | .430 b |
| PD-1 inhibitors categories | .184 b | .121 b | ||||
| Pembrolizumab | 2 (4.3%) | 7 (11.9%) | 1 (2.3%) | 5 (11.6%) | ||
| Camrelizumab | 19 (41.3%) | 29 (49.1%) | 17 (39.6%) | 21 (48.8%) | ||
| Sintilimab | 25 (54.4%) | 23 (39.0%) | 25 (58.1%) | 17 (39.6%) | ||
Abbreviations: TACE, transarterial chemoembolization; PSM, propensity score matching; BCLC, Barcelona Clinic Liver Cancer; RFA, radiofrequency ablation.
Independent samples t test.
Chi-square test or Fisher exact test.
Mann-Whitney U test.
Survival
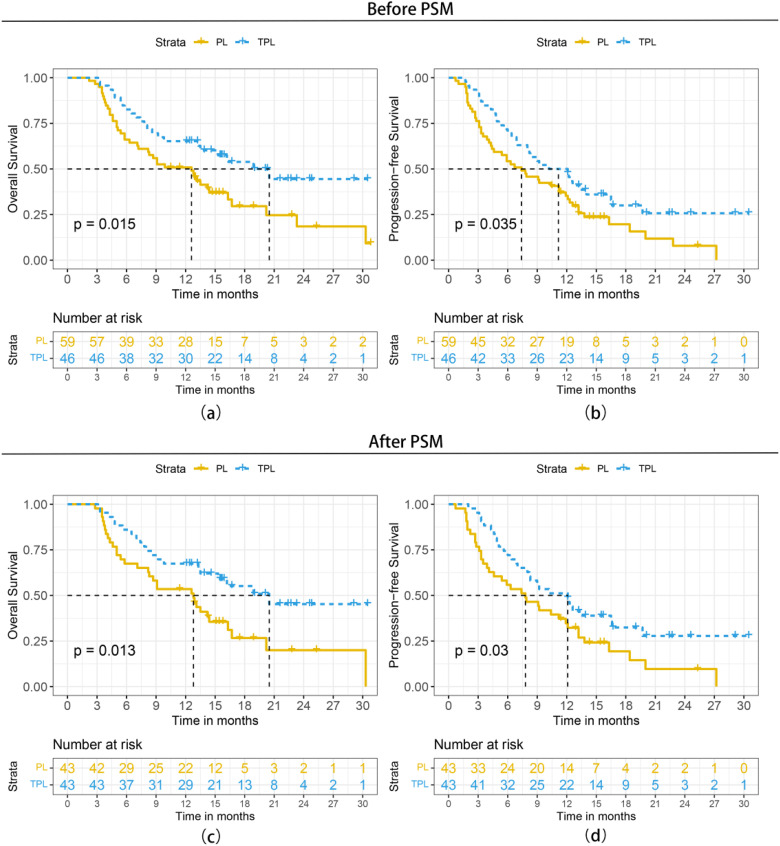
The final follow-up was on October 25, 2022, and the median follow-up time was 17.5 months (95% CI: 14.3-20.7 months). A total of 41 (69.5%) patients in PL group and 22 (47.8%) in TPL group had died. Patients in the TPL group had significantly better survival outcomes than those in the PL group. The 3-, 6-, and 12-month OS was 97.8%, 80.4%, and 62.7%, respectively, in the TPL group, and 94.9%, 64.4%, and 49.0%, respectively, in the PL group. The median OS was 20.5 months (95% CI: 13.5-27.5 months) in the TPL group and 12.6 months (95% CI: 8.1-17.1months) in the PL group (P = .015, hazard rate [HR] = 0.531, 95% CI: 0.315-0.894). The 3-, 6-, and 12-month PFS was 89.1%, 69.6%, and 47.8%, respectively, in the TPL group, and 72.9%, 52.5%, and 33.4%, respectively, in the PL group. The median PFS was 10.2 months (5.8-14.6 months) in the TPL group and 7.4 months (95% CI: 3.9-10.9 months) in the PL group (P = .035, HR = 0.621, 95% CI: 0.397-0.972). The survival curves are shown in Figure 2A and B. For patient cohort-PSM, the OS (20.5 months vs 12.8 months, P = .013, HR = 0.494, 95% CI: 0.281-0.871) and PFS (12.1 months vs 7.8 months, P = .030, HR = 0.585, 95% CI: 0.358-0.956) were also statistically significantly better in the TPL group than in the PL group (Figure 2C and D).
Figure 2.
Kaplan-Meier curves of survival outcomes of patients in the 2 groups before and after propensity score matching (PSM). (A) Overall survival before PSM, (B) progression-free survival before PSM, (c) overall survival after PSM, and (D) progression-free survival after PSM.
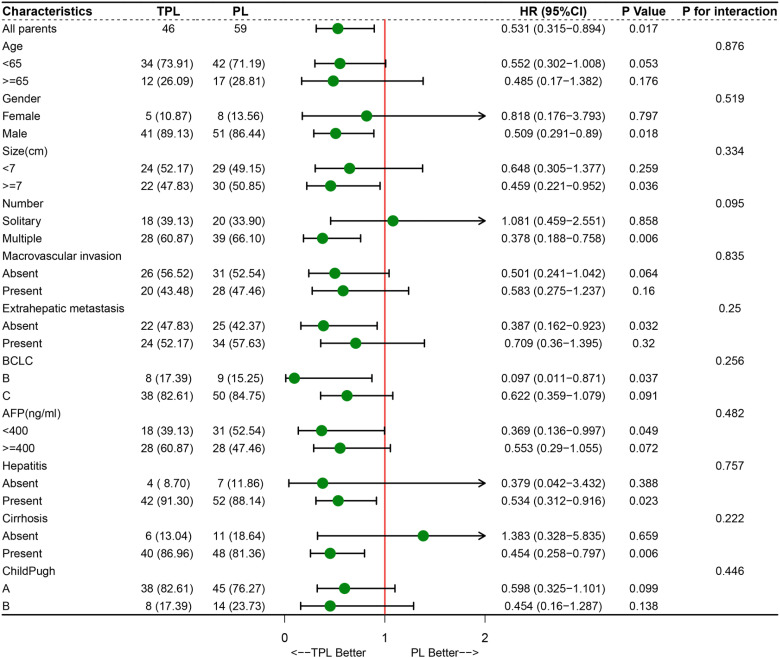
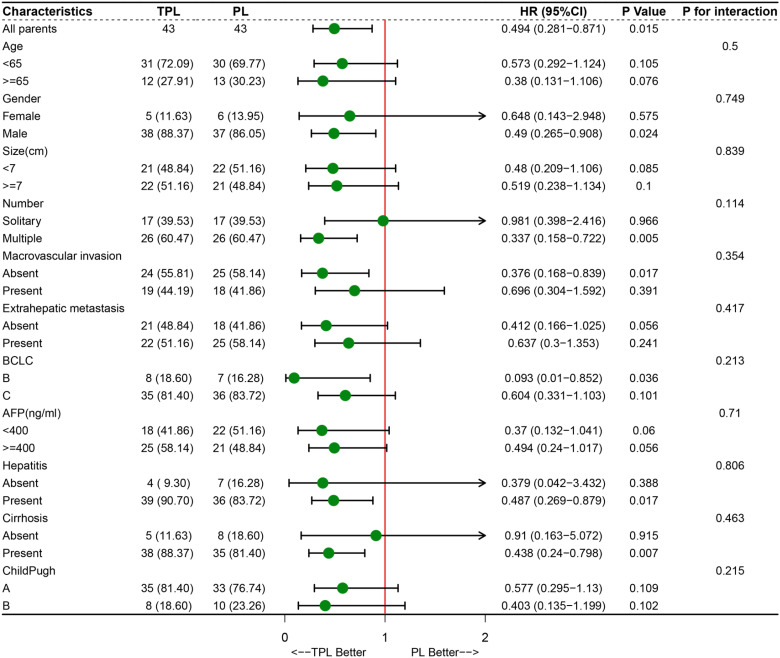
Furthermore, we conducted a subgroup analysis to determine which patients were candidates for combined local and systemic therapy. The results showed that male patients (P = .018, HR = 0.509, 95% CI: 0.291-0.890), patients with the largest tumor size ≥7 cm (P = .036, HR = 0.459, 95% CI: 0.221-0.952), patients with multinodular (P = .006, HR = 0.378, 95% CI: 0.188-0.758), patients without extrahepatic metastasis (P = .032, HR = 0.387, 95% CI: 0.162-0.923), patients in BCLC B classification (P = .037, HR = 0.097, 95% CI: 0.011-0.871), patients with AFP <400 U/mL (P = .049, HR = 0.369, 95% CI: 0.136-0.997), patients with hepatitis (P = .023, HR = 0.534, 95% CI: 0.312-0.916), and patients with cirrhosis (P = .006, HR = 0.454, 95% CI: 0.258-0.797), were more likely to benefit from TPL treatment (Figure 3). The subgroup analysis of patient cohort after PSM showed a similar trend with the patient cohort before PSM (Figure 4). In addition, all of the parameters had no interaction with treatment in the 2 groups whether or not it is performed PSM (Figures 3 and 4).
Figure 3.
Forest plot for overall survival of the matched cohorts of patients before propensity score matching (PSM).
Figure 4.
Forest plot for overall survival of the matched cohorts of patients after propensity score matching (PSM).
Tumor Response
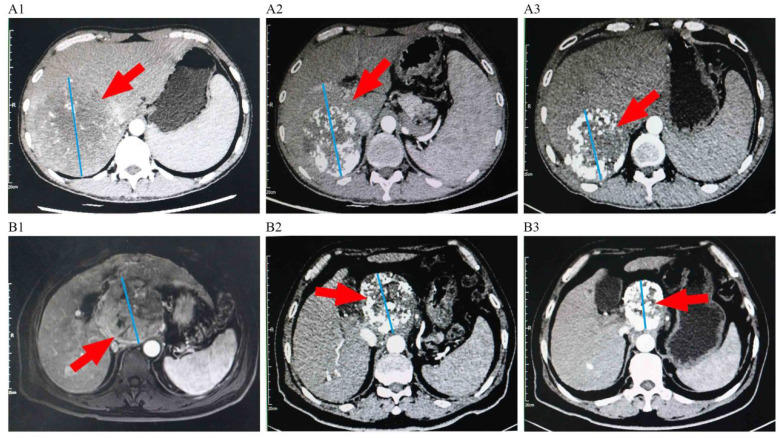
Treatment response is summarized in Table 2. Based on mRECIST, the ORR was higher in the TPL group (54.3%) than that in the PL group (25.4%) (P = .002). Compared with the PL group, the TPL group had a higher DCR (82.6 vs 64.4%; P = .038). In the patient cohort after PSM, the ORR (55.8% vs 30.2%, P = .017) and DCR (86.0 vs 65.1%, P = .024) in the TPL group were also higher than those in the PL group (Table 2). Representative images from 2 patients treated with TACE combined with PD-1 inhibitors plus lenvatinib therapy before and after treatment are shown in Figure 5.
Table 2.
Therapeutic Efficacy of 2 Groups Before and After PSM.
| variable | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| TPL (n = 46) | PL (n = 59) | P a | TPL (n = 43) | PL (n = 43) | P a | ||
| CR | 0 | 0 | 0 | 0 | |||
| PR | 25 | 15 | 24 | 13 | |||
| SD | 13 | 23 | 13 | 15 | |||
| PD | 8 | 21 | 6 | 15 | |||
| ORR | 54.3% | 25.4% | .002 | 55.8% | 30.2% | .017 | |
| DCR | 82.6% | 64.4% | .038 | 86.0% | 65.1% | .024 | |
Abbreviations: ORR, objective response rate; PSM, propensity score matching.
Chi-square test.
Figure 5.
Representative images of 2 patients before and after the treatment of transarterial chemoembolization (TACE) combined with PD-1 inhibitors plus Lenvatinib. A1: Imaging manifestations of a patient before the treatment, showing a massive tumor in the right lobe of the liver. A2: Imaging manifestations of the patient after 2 TACE sessions and 4 cycles of camrelizumab plus lenvatinib, showing the tumor with partial inactivation and shrinkage. A3: Imaging manifestations of the patient after 3TACE sessions and 8 cycles of camrelizumab plus lenvatinib, showing the tumor with evident inactivation and shrinkage; B1: Imaging manifestations of another patient before the treatment, showing a massive tumor in the hepatic hilar region. B2: Imaging manifestations of the patient after 2 TACE sessions and 6 cycles of sintilimab plus lenvatinib, showing the tumor with partial inactivation and shrinkage.B3: Imaging manifestations of the patient after 3 TACE sessions and 10 cycles of sintilimab plus lenvatinib, showing the tumor was generally inactivated and obviously shrinked.
Prognostic Factor Analysis
In the multivariable analysis, PL was identified as an independent risk factor for OS (P = .004, HR = 2.202; 95% CI: 1.290-3.760) and PFS (P = .031, HR = 1.638; 95% CI: 1.045-2.566). In addition, multivariate analysis showed that patients classified as Child-Pugh B (P = .009, HR = 2.131, 95% CI: 1.208-3.760), patients with AFP ≥400 ng/mL (P = .017, HR = 1.882; 95% CI:1.120-3.161) were at risk for poor OS, and classification as Child-Pugh B (HR = 1.982, 95% CI: 1.188-3.308, P = .009) was a risk factor for worse PFS (Table 3). In the patient cohort after PSM, PL was also identified as an independent risk factor for OS (P = .003, HR = 2.450; 95% CI: 1.365-4.398) and PFS (P = .016, HR = 1.841; 95% CI: 1.119-3.029) (Table 4).
Table 3.
Univariate and Multivariate Analysis of Risk Factors for Overall Survival and Progression-Free Survival Before PSM.
| Characteristics | Overall survival | Progression-free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI for HR | P | HR | 95% CI for HR | P | HR | 95% CI for HR | P | HR | 95% CI for HR | P | |
| Age, years (<65 vs ≥65) | 0.953 | 0.545-1.665 | .865 | 0.794 | 0.478-1.316 | .371 | ||||||
| Gender (Female vs Male) | 0.982 | 0.465-2.071 | .962 | 0.975 | 0.500-1.902 | .941 | ||||||
| Tumor size, cm (<7 vs ≥7) | 1.594 | 0.968-2.622 | .067 | 1.406 | 0.905-2.182 | .129 | ||||||
| Tumor number, (Single vs Multiple) | 1.101 | 0.652-1.860 | .718 | 0.975 | 0.620-1.532 | .912 | ||||||
| Macrovascular invasion, (No vs Yes) | 1.302 | 0.793-2.140 | .297 | 1.038 | 0.668-1.612 | .869 | ||||||
| Extrahepatic metastasis, (No vs Yes) | 1.233 | 0.743-2.045 | .418 | 1.421 | 0.907-2.227 | .125 | ||||||
| BCLC stage (Stage B vs C) | 1.696 | 0.806-3.567 | .164 | 1.773 | 0.913-3.445 | .091 | ||||||
| Child-Pugh class (Class A vs B) | 2.304 | 1.316-4.036 | .003 | 2.131 | 1.208-3.760 | .009 | 1.944 | 1.166-3.243 | .011 | 1.982 | 1.188-3.308 | .009 |
| ALT, U/L | 1.002 | 0.997-1.007 | .393 | 1.001 | 0.996-1.005 | .725 | ||||||
| AST, U/L | 1.005 | 1.001-1.009 | .018 | 1.003 | 0.999-1.007 | .113 | ||||||
| AFP, ng/mL (<400 vs ≥400) | 1.805 | 1.084-3.007 | .023 | 1.882 | 1.120-3.161 | .017 | 1.433 | 0.922-2.227 | .110 | |||
| Hepatitis B/C, (No vs Yes) | 1.564 | 0.625-3.912 | .339 | 1.485 | 0.682-3.230 | .319 | ||||||
| Liver cirrhosis, (No vs Yes) | 1.213 | 0.597-2.462 | .594 | 1.363 | 0.719-2.581 | .342 | ||||||
| Treatment (PL versus TPL) | 1.884 | 1.118-3.172 | .017 | 2.202 | 1.290-3.760 | .004 | 1.610 | 1.029-2.521 | .037 | 1.638 | 1.045-2.566 | .031 |
Abbreviations: TPL, transarterial chemoembolization combined with PD-1 inhibitors plus lenvatinib; PL, PD-1 inhibitors plus lenvatinib; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; HR, hazard rate; CI, confidence interval; PSM, propensity score matching; BCLC, Barcelona Clinic Liver Cancer.
Table 4.
Univariate and Multivariate Analysis of Risk Factors for Overall Survival and Progression-Free Survival After PSM.
| Characteristics | Overall survival | Progression-free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR | 95% CI for HR | P | HR | 95% CI for HR | P | HR | 95% CI for HR | P | HR | 95% CI for HR | P | |
| Age, years (<65 vs ≥65) | 1.265 | 0.695-2.302 | .441 | 0.980 | 0.568-1.689 | .941 | ||||||
| Gender (Female vs Male) | 0.759 | 0.355-1.621 | .476 | 0.709 | 0.358-1.402 | .323 | ||||||
| Tumor size, cm (<7 vs ≥7) | 1.381 | 0.795-2.398 | .251 | 1.306 | 0.800-2.130 | .286 | ||||||
| Tumor number, (Single vs Multiple) | 1.074 | 0.608-1.897 | .805 | 0.917 | 0.559-1.504 | .731 | ||||||
| Macrovascular invasion, (No vs Yes) | 1.264 | 0.727-2.197 | .406 | 0.928 | 0.564-1.527 | .769 | ||||||
| Extrahepatic metastasis, (No vs Yes) | 1.219 | 0.698-2.130 | .487 | 1.453 | 0.880-2.399 | .144 | ||||||
| BCLC stage (Stage B vs C) | 1.677 | 0.754-3.731 | .205 | 1.644 | 0.811-3.333 | .168 | ||||||
| Child-Pugh class (Class A vs B) | 2.667 | 1.440-4.940 | .002 | 2.821 | 1.515-5.252 | .001 | 2.183 | 1.232-3.868 | .007 | 2.378 | 1.334-4.239 | .003 |
| ALT, U/L | 1.000 | 0.993-1.006 | .920 | 0.999 | 0.993-1.005 | .663 | ||||||
| AST, U/L | 1.003 | 0.998-1.008 | .258 | 1.001 | 0.996-1.006 | .733 | ||||||
| AFP, ng/mL (<400 vs ≥400) | 1.982 | 1.121-3.504 | .019 | 2.224 | 1.248-3.963 | .007 | 1.479 | 0.903-2.422 | .120 | |||
| Hepatitis B/C, (No vs Yes) | 1.509 | 0.597-3.813 | .385 | 1.395 | 0.635-3.062 | .407 | ||||||
| Liver cirrhosis, (No vs Yes) | 1.351 | 0.574-3.181 | .491 | 1.435 | 0.683-3.013 | .341 | ||||||
| Treatment (PL versus TPL) | 2.023 | 1.148-3.563 | .015 | 2.450 | 1.365-4.398 | .003 | 1.710 | 1.046-2.796 | .033 | 1.841 | 1.119-3.029 | .016 |
Abbreviations: TPL, transarterial chemoembolization combined with PD-1 inhibitors plus lenvatinib; PL, PD-1 inhibitors plus lenvatinib; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein; HR, hazard rate; CI, confidence interval; PSM, propensity score matching; BCLC, Barcelona Clinic Liver Cancer.
Safety
We assessed AEs for each of the 105 patients included in our study. Adverse events are summarized in Table 5. Thirty-nine patients (84.8%) in the TPL group and 42 patients (71.2%) in the PL group experienced at least one AE. The vast majority of AEs were characterized as mild and manageable, and the most common grade ≥3 AEs were hypertension and elevated ALT and AST. More patients in the TPL group experienced fever (P = .002), abdominal pain (P = .000), elevated ALT (P = .020), and elevated AST (P = .001). Among grade ≥3 AEs, more patients in the TPL experienced elevated AST (P = .033). No toxicity-associated deaths occurred in our sample. In the patient cohort after PSM, more any grade AEs including fever (P = .011), abdominal pain (P = .002), elevated ALT (P = .013) and elevated AST (P = .000) occurred in the TPL group. There was no difference in ≥3 AE between the 2 group in the patient cohort-PSM (Table S1).
Table 5.
Treatment-Related Adverse Events in the 2 Groups (Before PSM).
| Adverse events | Any grade | Grade ≥ 3 | ||||
|---|---|---|---|---|---|---|
| TPL (n = 46) | PL (n = 59) | P | TPL (n = 46) | PL (n = 59) | P | |
| Rash | 6 (13.0%) | 6 (10.2%) | .646 a | 1 (2.2%) | 1 (1.7%) | 1.000 b |
| Pruritus | 1 (2.2%) | 3 (5.1%) | .795 a | 0 (0%) | 0 (0%) | - |
| Hand-foot skin reaction | 4 (8.7%) | 6 (10.2%) | 1.000 a | 0 (0%) | 0 (0%) | - |
| Fever | 16 (34.8%) | 6 (10.2%) | .002 a | 0 (0%) | 0 (0%) | - |
| Abdominal Pain | 19 (41.3%) | 6 (10.2%) | .000 a | 0 (0%) | 0 (0%) | - |
| Diarrhea | 8 (17.4%) | 10 (16.9%) | .952 a | 2 (4.3%) | 1 (1.7%) | .826 a |
| Fatigue | 9 (19.6%) | 12 (20.3%) | .922 a | 0 (0%) | 0 (0%) | - |
| Nausea | 6 (13.0%) | 4 (6.8%) | .453 a | 0 (0%) | 0 (0%) | - |
| Stomatitis | 2 (4.3%) | 4 (6.8%) | .913 a | 0 (0%) | 0 (0%) | - |
| Hypertension | 12 (26.1%) | 16 (27.1%) | .906 a | 5 (10.9%) | 7 (11.9%) | .874 a |
| Hypothyroidism | 3 (6.5%) | 2 (3.4%) | .775 a | 0 (0%) | 0 (0%) | - |
| Thrombocytopenia | 3 (6.5%) | 2 (3.4%) | .775 a | 1 (2.2%) | 1 (1.7%) | 1.000 b |
| ALT elevated | 17 (36.9%) | 10 (16.9%) | .020 a | 4 (8.7%) | 0 (0%) | .073 a |
| AST elevated | 20 (43.5%) | 8 (13.6%) | .001 a | 5 (10.9%) | 0 (0%) | .033 a |
| Syndrome of inappropriate secretion of antidiuretic hormone | 1 (2.2%) | 0 (0%) | .438 b | 1 (2.2%) | 0 (0%) | .438 b |
| Pneumonia | 1 (2.2%) | 1 (1.7%) | 1.000 b | 1 (2.2%) | 0 (0%) | .438 b |
Abbreviation: PSM, propensity score matching.
Chi-square test.
Fisher exact test.
-, No statistical analysis.
Discussion
The combination of PD-1 inhibitors and lenvatinib has been shown to increase survival in patients with uHCC.19–21 This combination therapy strategy may enhance dendritic cell and cytotoxic T lymphocyte activity and inhibit tumor-associated macrophage, regulatory T-cell, and myeloid-derived suppressor cell regulation of the immune microenvironment, thereby creating an inflammatory microenvironment associated with relatively effective and long-lasting responses to checkpoint inhibitors. 22 Some studies have evaluated the efficacy of TACE in combination with PD-1 inhibitors plus lenvatinib and found that this therapeutic strategy significantly improved survival.12,13 However, no previous studies have compared the treatment of uHCC with TACE combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib. Therefore, we compared the 2 treatment regimens.
Our results showed that the ORR in the TPL group was 54.3%, and the mPFS and mOS were 10.2 months and 20.5 months, respectively. Li et al investigated 114 uHCC patients who received LePD1-TACE triple therapy, the result showed the ORR was 69.3%. 12 In addition, another research on the efficacy of lenvatinib-TACE in uHCC patients showed the ORR was 68.3%. 24 The ORR in our study was significantly lower than in previous studies. One possible reason for the difference is that the patients in our study had worse liver function, and more patients were classified as Child-Pugh B (17.4% vs 2.6%). Another reason is that the patients enrolled in this study had more advanced disease stages with higher proportions of BCLC stage C (82.6% vs 60.5%) and extrahepatic metastases (52.2% vs 20.2%) compared to the patients enrolled in the previous study. 12 Meanwhile, differences in the PDL1, TMB, and MSI statuses of the patients in the 2 studies are unknown, which may also have affected efficacy. In addition, compared with other studies in the literature,12,13,23 the mOS and mPFS of TACE combined with PD-1 inhibitors plus lenvatinib were 16.9 to 23.6m, and 7.3 to 13.3m, respectively. However, the mOS and mPFS in our study were 20.5 months and 10.2 months, which were in the upper range. The different results between these studies and our data should be viewed with caution. In the PL group, the ORR was 25.4%, and the mOS was 12.6 months, which were lower than those observed in the IMbrave150 25 and Keynote-524 11 trials. These differences may have resulted from the following factors: (1) The patients in our study were relatively late-staged and had worse tumor biological behaviors, with 84.7% of patients classified as BCLC C stage, 47.5% of patients exhibited macrovascular invasion, and 57.6% of patients with extrahepatic metastases; (2) Compared to 2 global clinical trials, the patients who received PD-1 inhibitors plus lenvatinib in our study had a worse liver function score, and 23.7% patients were in the Child-Pugh B class; (3) Patients in our study had worse ECOG scores, and over 60% of patients in the 2 global clinical trials had ECOG scores of 0. In contrast, the proportion of patients with ECOG scores of 0 in our study was only 33.9%.
Our study showed that patients with uHCC who received TPL had higher ORR and longer survival than those who received PL. From 2004 to 2018, the scenario of HCC management was continuously and rapidly evolving, and the locoregional treatments showed an improved outcome. 26 In the latest 5 years, systemic treatment exerts a remarkable therapeutic effect with the rapid development of molecular-targeted and immuno-oncology agents in HCC. In addition, consistent with our findings, mounting evidence has indicated the fascinating combination of strategies involving targeted therapies, immunotherapies, and TACE markedly improves the clinical outcome of patients, which may change the current landscape of HCC management in the future. The excellent efficacy of TPL may have resulted from the following factors: (1) Prior studies27,28 have shown the anticancer activity of TACE in intrahepatic tumors, which was attributed to local anticancer effects; (2) TACE can cause immunogenic cell death (ICD), resulting in the release of various tumor antigens, and subsequent activation of the immune response. 29 In addition, chemotherapy such as doxorubicin and oxaliplatin in combination with TACE can induce apoptosis, leading to ICD and immune activation30,31; (3) TACE can cause changes in the immune microenvironment that makes it more responsive to immunotherapy. Pinato et al 32 found that TACE was associated with a lower density of immune-exhausted effector cytotoxic and T-regs, with significant upregulation of pro-inflammatory pathways. This demonstrates the pleiotropic effects of TACE in modulating the tumor microenvironment and strengthens the rationale for developing immunotherapy alongside TACE. (4) Recently, a biopsy-matched study showed increased expression of hypoxia markers such as VEGF in the subgroup of patients treated with TACE when compared to controls (P = .046), which suggested that the hypoxic environment induced by TACE could stimulate tumor growth. 33 Lenvatinib can inhibit the kinase activities of VEGF receptors (VEGFR-12,3) and mitigate the upregulation of proangiogenic factors following TACE. 24 The combination of lenvatinib with TACE may block pathogenic angiogenesis and tumor growth.34,35
Our subgroup analysis showed that patients classified as BCLC stage B, without extrahepatic metastasis were more likely to benefit from TPL treatment, which further confirmed that TACE may be a locoregional approach to control intrahepatic lesions, but that it may not effectively manage extrahepatic metastases. In our study, patients with multinodular were more likely to benefit from triple therapy. Similarly, a previous retrospective study reported that LePD1-TACE significantly improved survival over lenvatinib-TACE in advanced HCC patients with tumor number > 3. 23 However, in a real-world study in China on the efficacy and safety of lenvatinib combined with PD-1 inhibitors plus TACE for uHCC, tumor number < 3 was identified as the independent predictor of OS. 12 Therefore, the relationship between the number of intrahepatic tumors and the efficacy of the combined treatment needs to be further investigated. For patients with the largest tumor size ≥7 cm, the TPL treatment has a higher OS than PL, which may be explained as TACE can more promptly and effectively combat tumors than systemic therapy alone. Although our results show that AFP <400 ng/mL was more likely to benefit from TPL (P = .049), the P value in the AFP ≥400 ng/mL group was .072, which is very close to .05; therefore, the result needs to be further studied. Our subgroup analysis also demonstrated that male patients, patients with hepatitis and cirrhosis showed better survival in response to TPL treatment compared with PL treatment. The associations between outcomes and gender, hepatitis, and cirrhosis had large standard deviations, which indicated that larger sample sizes are needed to further characterize these associations. Furthermore, subgroup analysis for OS revealed that none of the factors interacted with treatment, indicating the need for additional research to identify the patients who are more likely to benefit from TPL treatment.
More patients in the TPL group suffered from fever, abdominal pain, and hepatic impairment than in the PL group. These AEs were mainly caused by TACE treatment. However, these adverse reactions were transient and were quickly reversed. There were no significant differences between the 2 groups concerning adverse reactions caused by immunotherapy and lenvatinib. In general, there were no unexpected safety-related complications caused by TPL treatment, which indicated that this treatment strategy was safe and the associated adverse effects were manageable.
There were several limitations in our study. First, this was a retrospective, single-center study. Future studies should be multicentered. Second, the sample sizes were relatively small, which may have led to large confidence intervals and an inability to detect clinically relevant differences between the treatment regimens. Larger sample sizes are needed for stratified analyses. Third, the PD-1 inhibitors were varied, which affected the consistency of treatment regimens. However, the kinds of PD-1 inhibitors in the 2 groups were well balanced; therefore, the results should be genuine. Finally, this study did not include pathological data, including PDL1 expression, MSI, or TMB. Findings from this study should be further verified by incorporating pathological indicators.
Conclusions
Based on our results, TPL was associated with significantly better treatment responses and survival benefits compared to PL, and the AEs were manageable. Therefore, TPL may be a potential novel treatment option for uHCC.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338231166765 for Transarterial Chemoembolization Combined With PD-1 Inhibitors Plus Lenvatinib Showed Improved Efficacy for Treatment of Unresectable Hepatocellular Carcinoma Compared With PD-1 Inhibitors Plus Lenvatinib by Jinfeng Wang, Man Zhao, Guangjie Han, Xin Han, Jianfei Shi, Lili Mi, Ning Li, Xiaolei Yin, Xiaoling Duan, Jiaojiao Hou and Fei Yin in Technology in Cancer Research & Treatment
Acknowledgments
The authors would like to thank all colleagues involved in the study for their contributions.
Abbreviations
- AEs
adverse events
- BCLC
Barcelona Clinic Liver Cancer
- CT
computed tomography
- DCR
disease control rates
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- ORR
objective response rates
- OS
overall survival
- PD
progressive disease
- PD1
programmed death 1
- PD-L1
programmed death ligand 1
- PL
PD-1 inhibitors plus lenvatinib
- PFS
progression-free survival
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- RFA
radiofrequency ablation
- SD
stable disease
- TACE
Transarterial chemoembolization
- TPL
transarterial chemoembolization combined with PD-1 inhibitors plus lenvatinib
- uHCC
unresectable hepatocellular carcinoma.
Footnotes
Authors’ Note: This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (approval number: 2022KS044). Informed consent was waived by the committee because of the retrospective nature of this study. We have de-identified all patient details to ensure the confidentiality of patient information. The study was conducted in accordance with the Declaration of Helsinki, and approved by the institutional review board of the Fourth Hospital of Hebei Medical University (No.2022KS044, approval time: September 22, 2022). Patient consent was waived due to a retrospective study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jinfeng Wang https://orcid.org/0000-0001-6074-9118
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 2.Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598-1606. doi: 10.1016/j.jhep.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primer. 2016;2(Apr):16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1(3-4):144-158. doi: 10.1159/000343828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681-693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo M. Lenvatinib in advanced hepatocellular carcinoma. Liver Cancer. 2017;6(4):253-263. doi: 10.1159/000479573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet Lond Engl. 2018;391(10126):1163-1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 9.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960-2970. doi: 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Fu Z, Chen X, et al. Efficacy and safety of lenvatinib combined with PD-1 inhibitors plus TACE for unresectable hepatocellular carcinoma patients in China real-world. Front Oncol. 2022;12(Jul):950266. doi: 10.3389/fonc.2022.950266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11(Dec):783480. doi: 10.3389/fonc.2021.783480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800-804. doi: 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835-853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):052-060. doi: 10.1055/s-0030-1247132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seldinger SI. Catheter replacement of the needle in percutaneous arteriography: a new technique. Acta Radiol. 1953;39(5):368-376. doi: 10.3109/00016925309136722 [DOI] [PubMed] [Google Scholar]
- 18.He M, Li Q, Shen J, et al. Predictive factors for the benefit of triple-drug transarterial chemoembolization for patients with unresectable hepatocellular carcinoma. Cancer Med. 2019;8(9):4200-4213. doi: 10.1002/cam4.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu S, Liu C, Dong Y, Shao J, Liu B, Shen J. A retrospective study of lenvatinib monotherapy or combined with programmed cell death protein 1 antibody in the treatment of patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma in China. Front Oncol. 2021;11(Dec):788635. doi: 10.3389/fonc.2021.788635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, Zhang Q, Mei J, Yang Z, Chen M, Liang T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer. 2022;22(1):293. doi: 10.1186/s12885-022-09405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K, Wei W, Liu L, et al. Lenvatinib with or without immune checkpoint inhibitors for patients with unresectable hepatocellular carcinoma in real-world clinical practice. Cancer Immunol Immunother. 2022;71(5):1063-1074. doi: 10.1007/s00262-021-03060-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primer. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 23.Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13(Mar):848387. doi: 10.3389/fimmu.2022.848387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663-675. doi: 10.1007/s12072-021-10184-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 26.Garuti F, Neri A, Avanzato F, et al. The changing scenario of hepatocellular carcinoma in Italy: an update. Liver Int. 2021;41(3):585-597. doi: 10.1111/liv.14735 [DOI] [PubMed] [Google Scholar]
- 27.Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2020;72(1):198-212. doi: 10.1002/hep.31022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2261-2273. doi: 10.1002/hep.26256 [DOI] [PubMed] [Google Scholar]
- 29.Kohles N, Nagel D, Jüngst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumor Biol. 2012;33(6):2401-2409. doi: 10.1007/s13277-012-0504-2 [DOI] [PubMed] [Google Scholar]
- 30.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690-714. doi: 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol. 2020;43(6):1203-1214. doi: 10.1007/s13402-020-00552-2 [DOI] [PubMed] [Google Scholar]
- 32.Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9(9):e003311. doi: 10.1136/jitc-2021-003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nahm JH, Rhee H, Kim H, et al. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: a biopsy and resection matched study. Oncotarget. 2017;8(59):99359-99371. doi: 10.18632/oncotarget.22078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimose S, Kawaguchi T, Tanaka M, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett. 2020;20(3):2257-2265. doi: 10.3892/ol.2020.11758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamura Y, Kobayashi M, Shindoh J, et al. Lenvatinib-tansarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020;9(6):756-770. doi: 10.1159/000510299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338231166765 for Transarterial Chemoembolization Combined With PD-1 Inhibitors Plus Lenvatinib Showed Improved Efficacy for Treatment of Unresectable Hepatocellular Carcinoma Compared With PD-1 Inhibitors Plus Lenvatinib by Jinfeng Wang, Man Zhao, Guangjie Han, Xin Han, Jianfei Shi, Lili Mi, Ning Li, Xiaolei Yin, Xiaoling Duan, Jiaojiao Hou and Fei Yin in Technology in Cancer Research & Treatment