Abstract
Objectives
The aim of this study was to investigate central sensitization and associated factors in knee osteoarthritis (OA) patients and compare them with rheumatoid arthritis (RA) patients and healthy controls.
Patients and methods
This cross-sectional study was conducted with 125 participants (7 males, 118 females; mean age: 57.2±8.2 years; range, 45 to 75 years) between January 2017 and December 2018. Sixty-two patients with symptomatic knee OA, 32 RA patients with knee pain, and 31 healthy controls constituted the participants. Central sensitization was investigated with the Central Sensitization Inventory (CSI) and pressure pain threshold (PPT) measurements. Pain, functional status, and psychosocial features were assessed with self-reported questionnaires.
Results
The OA and RA groups had significantly lower PPT values at local, peripheral, and remote regions compared to the healthy controls. Pressure hyperalgesia was shown at the knee with a 43.5% prevalence, 27.4% at the leg, and 8.1% at the forearm of OA patients. Pressure hyperalgesia was present at the knee, leg, and forearm in 37.5%, 25%, and 9.4% of RA patients, respectively. Pressure pain threshold values, CSI scores, frequency of pressure hyperalgesia, and frequency of central sensitization according to the CSI were not statistically different between the OA and RA groups. Psychosocial features and structural damage were not correlated with PPT values in the OA group.
Conclusion
The severity of chronic pain and functional status may be the clinical clues to recognizing patients with central sensitization since local joint damage does not play a direct role in the etiopathogenesis of central sensitization in OA patients and severe pain persisting in the chronic process is associated with central sensitization regardless of the pathogenesis.
Keywords: Central sensitizisation, knee osteoarthritis, pressure pain threshold, rheumatoid arthritis.
Introduction
Osteoarthritis (OA), which is the most common joint disease, is one of the leading causes of pain and disability, particularly in the elderly.[1] The pathophysiology of pain in OA is not fully understood. Synovial inflammation, raised intraosseous pressure, subchondral ischemia, and mechanical stresses on ligaments are known local causes of pain in OA.[2] Some of these changes observed on plain radiographs are joint space narrowing, osteophytes, and subchondral sclerosis. The correlation between symptoms and these radiographic findings is known to be weak.[1,3] In some patients, standard medical treatments are not effective in controlling joint pain, and pain may persist even after joint replacement.[4] These findings suggest that sensitization mechanisms may contribute to the pathogenesis of pain in OA.
Woolf[5] defines central sensitization as the “amplification of neural signaling within the central nervous system that elicits pain hypersensitivity.” Local damage in osteoarthritic joints and increased chemical mediators may lower the threshold for local nerve excitation, which is named peripheral sensitization leading to primary hyperalgesia.[5] Fingleton et al.[6] revealed the role of central sensitization in osteoarthritic knee pain. Central sensitization leading to pain hypersensitivity may cause pain at remote sites away from the arthritic knee as widespread hyperalgesia.[6] However, it is unclear whether central sensitization is pathophysiologically related to the OA process or a feature of chronic pain, arising from the genetic or psychosocial history of patients.[7] In addition to OA patients, a healthy control group and rheumatoid arthritis (RA) patients with a different cause of chronic knee pain were included in our study to understand the mechanisms of central sensitization. Rheumatoid arthritis is a systemic inflammatory disease and inflammation plays a major role in pain pathogenesis. It is also known that disease activity scores are not always correlated with symptoms in early RA patients.[8] Central mechanisms are now also thought to contribute to pain expression in RA.[9]
Chronic joint pain, which is the most common and disabling symptom of both OA and RA, also affects the sleep, mood, and life quality of these patients. Identification of pain mechanisms involved in these chronic joint pain patients (nociceptive pain and pain associated with sensitization mechanisms) is critical to ensure an individualized and appropriate treatment approach for these patients. Hence, the aim of this study was to investigate central sensitization and possible associated clinical features in a sample of chronic symptomatic knee OA patients and to compare them with RA patients and healthy controls.
Patients and Methods
The cross-sectional study was conducted with 125 participants (7 males, 118 females; mean age: 57.2±8.2 years; range, 45 to 75 years) at the Ankara University Faculty of Medicine, Department of Physical Medicine and Rehabilitation between January 2017 and December 2018. Sixtytwo patients with symptomatic knee OA based on the American College of Rheumatology (ACR) clinical criteria, 32 patients with RA based on the 2010 ACR/European Alliance of Associations for Rheumatology (EULAR) criteria, and 31 healthy participants who had no history of knee pain in the last six months constituted the participants.[10,11] Patients with OA and RA described knee pain for at least six months. Individuals who had uncontrolled systemic disease, active RA, peripheral neuropathy, fibromyalgia syndrome, cognitive impairment, pain in the forearm, history of total knee arthroplasty, and those who used nonsteroidal anti-inflammatory drugs, gabapentinoids, antidepressants, or anxiolytics in the last 24 h were excluded.
Demographic and clinical characteristics were assessed in all participants by medical history, clinical examination, and questionnaires. Pain intensity in the last week was assessed with the Visual Analog Scale (VAS), and pain and functional status were evaluated with the validated Turkish version of the Graded Chronic Pain Scale (GCPS).[12,13] Central sensitization was assessed with pressure pain threshold (PPT) measurements and the validated Turkish version of the Central Sensitization Inventory (CSI).[14] A hand-held digital pressure algometer (JTECH Medical, Midvale, UT, USA) was used to measure PPT. The probe was placed perpendicular to the skin, and the pressure was increased progressively until the participant defined the pressure as pain.[15] For each site, an initial practice was followed by two recorded trials, with the mean values used for analysis. The PPT was assessed at the painful knee joint (if both knees were painful, the more painful one was chosen), ipsilateral leg, and contralateral forearm. As the most affected compartment in knee OA is the medial tibiofemoral compartment, knee measurements were performed over the medial joint line. Leg measurements were performed on the tibialis anterior muscle, 5 cm distal to the tibial tuberosity, to assess the peripheral distribution of pain. Forearm measurements were performed over 5 cm distal to the medial epicondyle at the volar side to assess pain sensitization in the painless remote region.
The CSI has two parts: A and B. Central Sensitization Inventory-A is composed of 25 items of symptoms that are common to central sensitization syndromes. Total score ranges from 0 to 100, and higher scores are associated with a higher degree of symptomatology.[16] Neblett et al.[17] determined that a CSI score of 40 out of 100 best distinguished central sensitization patients with 81% sensitivity and 75% specificity.
Various psychosocial features associated with chronic pain were assessed with some self-reported questionnaires. Beck Depression Inventory (BDI) consists of 21 questions that evaluate depressive symptoms in the past week. Each question is scored between 0 and 3, and higher scores indicate more severe symptoms.[18] Insomnia Severity Index (ISI) is a seven-item questionnaire assessing the nature, severity, and impact of insomnia, and a 5-point Likert score is used for rating.[19] Pain Catastrophization Scale (PCS) consists of 13 items that describe the feelings and thoughts about having pain; participants are asked to reflect on past painful experiences and to indicate the degree of each item on a 5-point scale (0= not at all, 4=all the time).[20] The validated Turkish versions of these questionnaires were used.[21-23] Knee radiographs of OA patients were graded by a physical medicine and rehabilitation specialist using the Kellgren-Lawrence (KL) scale (0=normal, 4=severe).[24]
Statistical analysis
The minimum sample size was calculated in R 3.0.1 software (The R Foundation, Vienna, Austria) with 80% power and 5% type 1 error as 22 individuals for each group following the study of Moss et al.[25] The PPT was used as primary outcome measure to calculate sample size.
Data were analyzed using the SPSS version 11.5 software (SPSS Inc., Chicago, IL, USA). Since n<50 for the RA and control groups, the Shapiro-Wilk test was used in addition to the Kolmogorov-Smirnov test for the assumption of normality. Group comparisons were analyzed using the analysis of variance for normally distributed variables, the Kruskal-Wallis test for nonnormally distributed continuous variables, and Fisher exact test for categorical variables. Pairwise comparisons were made by Tukey’s honestly significant difference test for one-way analysis of variance and the Dunn-Bonferroni test for the Kruskal-Wallis test. Correlations of the pain-related variables and PPT were analyzed with Spearman’s correlation analysis due to the nonnormal distribution of PPT results.
Z-transformation was used to compare individual patients’ PPT results with normative data obtained from the healthy controls. For Z-scores to be calculated, PPT results were logarithmically transformed to meet the assumptions of normality, as suggested by the German Research Network on Neuropathic Pain.[26] The Z-scores were then separately calculated for the individual participant and measurement site. The equation used to calculate the Z-scores was Z-score= (Xindividual-Meancontrols)/Standard deviationcontrol.
Results
The demographic data of the groups are presented in Table 1. No statistically significant difference was found in the comparison of the median ages of OA and RA patients (59.5 years vs. 57.5 years, p=0.768), but the control group (49 years) was significantly younger than the patient groups (p<0.001). The sex distribution between the groups was not statistically different. The mean BMI values were not statistically different between OA and RA groups (p=0.285), but this value in the control group was significantly lower (p<0.001).
Table 1. Comparison of demographic and clinical characteristics of groups.
| OA group (n=62) | RA group (n=32) | Control group (n=31) | ||||||||||||||
| n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | p | |
| Age (year) | 59.5 | 47-75 | 57.5 | 45-72 | 49 | 45-61 | <0.001 | |||||||||
| Sex | ||||||||||||||||
| Female | 59 | 95.2 | 30 | 93.8 | 29 | 93.5 | 1.000 | |||||||||
| Education | <0.001 | |||||||||||||||
| Illiterate | 12 | 19.4 | 1 | 3.1 | 0 | 0 | ||||||||||
| Primary education | 35 | 56.5 | 21 | 65.6 | 5 | 16.1 | ||||||||||
| High school-college | 15 | 24.1 | 10 | 31.3 | 26 | 83.9 | ||||||||||
| CS related disease* | 8 | 12.9 | 3 | 9.4 | 0 | 0.124 | ||||||||||
| Depression | 5 | 1 | ||||||||||||||
| Anxiety | 1 | |||||||||||||||
| Migraine/tension type headache | 1 | 2 | ||||||||||||||
| Irritable bowel syndrome | 1 | |||||||||||||||
| BMI (kg/m2) | 32.6±5.1 | 30.9±5.7 | 26.4±4.3 | <0.001 | ||||||||||||
| Knee pain duration | 5 | 0.5-30 | 5 | 0.5-33 | 0.653 | |||||||||||
| CS: Central sensitization; BMI: Body mass index; * Except for fibromyalgia syndrome. | ||||||||||||||||
The severity of OA was graded according to the KL system as listed: seven (11.3%) patients in Grade 1, 24 (38.7%) patients in Grade 2, 18 (29%) patients in Grade 3, and 13 (21%) patients in Grade 4. The duration of RA ranged from 0.58 to 33 years, with a mean of 15.5±9.9 years. The mean Disease Activity Score 28 was 3.01±0.67 showing low disease activity. The duration of pain in the patient groups was not statistically different (p=0.653).
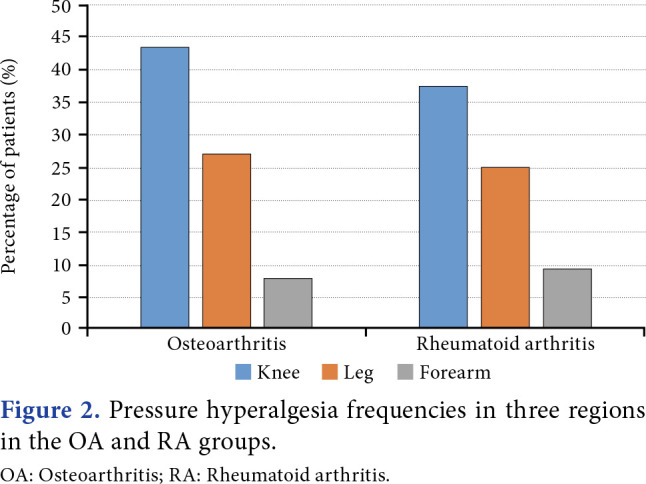
The comparison of pain-related characteristics and scores of assessed parameters are shown in Table 2. While VAS, PCS, BDI, and ISI scores were not statistically different between OA and RA groups, GCPS was significantly higher in the OA group. Osteoarthritis and RA groups had significantly lower median PPTs than the control group at the knee (p<0.001 for both), indicating local pressure hyperalgesia, and at the leg (p<0.001 for both), indicating spreading sensitization. Osteoarthritis patients had significantly lower median PPTs than the control group at the forearm (p=0.033), indicating distant pressure hyperalgesia. Rheumatoid arthritis patients had numerically lower median PPTs than the control group at the forearm, but this difference was statistically insignificant (p=0.217). There was no statistically different between the median PPTs of OA and RA patients in all three regions (p=1.000 for all three regions, Figure 1). The PPT values of all participants were separately compared for three regions with the normal value obtained by using the data of the control group using z transformation to investigate the presence of central sensitization. In the OA group, 43.5% of patients reported pressure hyperalgesia at the knee, 27.4% at the leg, and 8.1% at the forearm. In the RA group, 37.5% of patients reported pressure hyperalgesia at the knee, 25% at the leg, and 9.4% at the forearm (Figure 2). There was no significant difference between the OA and RA groups’ percentages of pressure hyperalgesia (p=0.661 for the knee, p=1.000 for the tibialis anterior muscle and forearm). None of the participants in the control group reported pressure hyperalgesia in any region. The PPTs for three regions were separately examined in the OA group. There was no significant correlation between PPTs at any region and age, BMI, duration of knee pain, VAS, BDI, PCS, ISI, and CSI scores. There was a weak negative correlation between the GCPS pain score and PPTs at the leg and forearm (r=-0.270, p=0.036 and r=-0.276, p=0.030, respectively). There was a weak negative correlation between the total GCPS score and PPT at the knee (r=-0.251, p=0.049). The PPT at the knee showed a strong positive correlation with PPT at the leg and forearm (r=0.700, p<0.001 and r=0.633, p<0.001, respectively). There was a strong positive correlation between PPTs at the forearm and the leg (r=0.727, p<0.001). The KL grade showed no significant correlation with PPT values, VAS scores, or CSI scores.
Table 2. Comparison of pain-related characteristics of groups.
| OA group | RA group | Control group | |||||||||||||||||
| n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | n | % | Mean±SD | Median | Min-Max | p | p1 | p2 | p3 | |
| VAS score (0-100) | 70 | 36-100 | 55 | 10-100 | 7 | 0-65 | <0.001 | <0.001 | <0.001 | 0.157 | |||||||||
| PCS score (0-52) | 16 | 1-40 | 16.5 | 1-69 | 10 | 0-42 | 0.055 | ||||||||||||
| BDI score (0-63) | 14 | 0-49 | 19 | 1-46 | 5 | 0-23 | <0.001 | <0.001 | <0.001 | 0.125 | |||||||||
| ISI score (0-28) | 8.5 | 0-19 | 5.5 | 0-22 | 3 | 0-14 | 0.008 | 0.006 | 0.318 | 0.632 | |||||||||
| GCPS pain score* (0-100) | 76.6±13.4 | 50-100 | 70±15 | 40-100 | - | - | 0.031 | ||||||||||||
| GCPS disability score (0-6) | 4 | 0-6 | 4 | 1-6 | - | - | 0.442 | ||||||||||||
| CSI score* (0-100) | 39.3±15.1 | 39.2±12.6 | 25.2±12.8 | <0.001 | <0.001 | <0.001 | 1.000 | ||||||||||||
| CSI >40 | 33 | 53.2 | 19 | 59.4 | 4 | 12.9 | <0.001 | <0.001 | <0.001 | 0.570 | |||||||||
| PPT at knee (kPa) | 96 | 15-277 | 98 | 34-295 | 239 | 96-631 | <0.001 | <0.001 | <0.001 | 1.000 | |||||||||
| PPT at leg (kPa) | 147 | 15-495 | 138 | 26-526 | 255 | 96-840 | <0.001 | <0.001 | <0.001 | 1.000 | |||||||||
| PPT at forearm (kPa) | 165.5 | 21-463 | 178 | 26-505 | 219 | 79-642 | 0.036 | 0.033 | 0.217 | 1.000 | |||||||||
| SD: Standard deviation; VAS: Visual analogue scale; PCS: Pain catastrophization scale; BDI: Beck depression inventory; ISI: Insomnia severity index; GCPS: Graded Chronic Pain Scale; CSI: Central sensitization inventory; PPT: pressure pain treshold; p: Comparison of all groups; p1: OA vs. control groups; p2: RA vs. control groups; p3: OA vs. RA groups. | |||||||||||||||||||
Figure 1. Comparison of (a) knee, (b) leg, and (c) forearm PPTs. PPT: Pressure pain threshold; OA: Osteoarthritis; RA: Rheumatoid arthritis; * Shows statistical significance between groups.
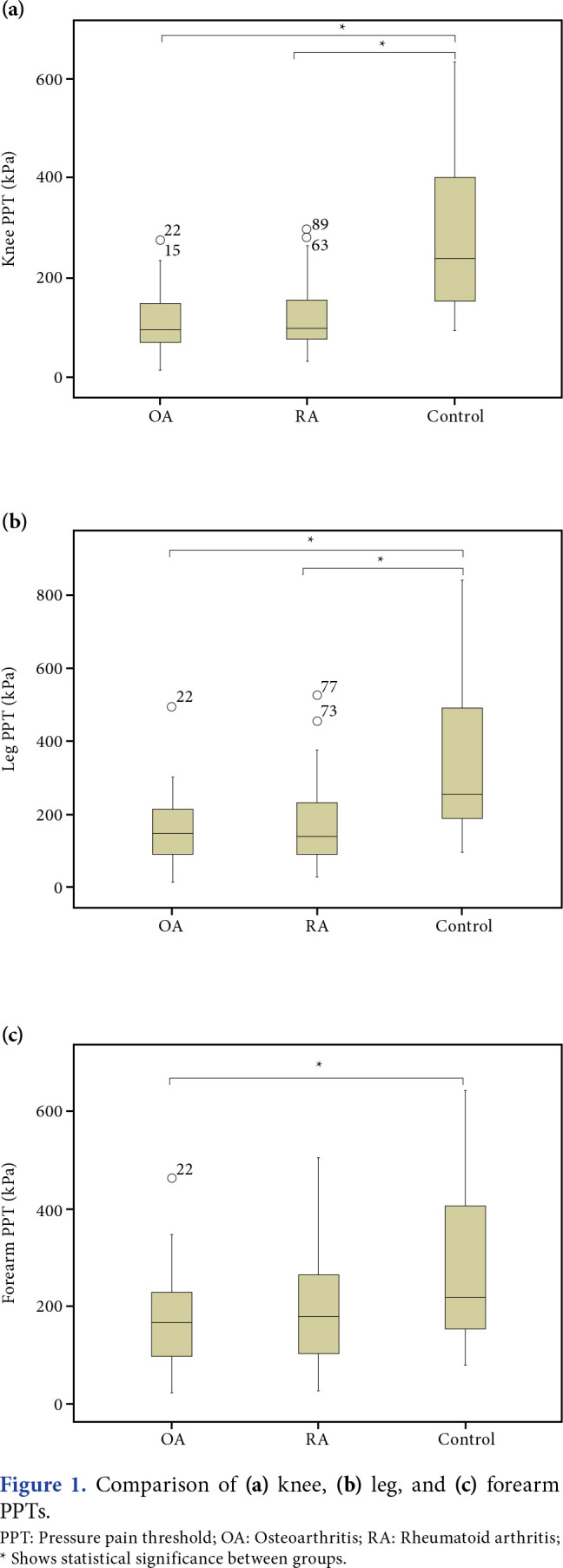
Figure 2. Pressure hyperalgesia frequencies in three regions in the OA and RA groups. OA: Osteoarthritis; RA: Rheumatoid arthritis.
Central Sensitization Inventory scores of the OA and RA groups were significantly higher than the control group (p<0.001 for both); however, there was no statistical difference between them (p=1.000). The frequency of those with central sensitization according to the CSI (score ≥40) was 53.2% in the OA group, 59.4% in the RA group, and 12.9% in the control group. While the frequency of central sensitization in the OA and RA groups was statistically significantly higher than in the control group (p<0.001), there was no significant difference between the OA and RA groups (p=0.570).
Discussion
In this study, the presence and possible mechanisms of central sensitization were examined by comparing OA and RA patients with healthy controls. The pressure hyperalgesia at local, peripheral, and painless remote regions in both OA and RA patients with chronic knee pain suggests central sensitization. Pain sensitization in patients with OA was also supported by high CSI and central sensitization frequency. Some studies that have assessed PPT in knee OA patients have found pressure hyperalgesia in the painful knee and other pain-free body regions.[14,27,28] There was no relationship between CSI and PPT in the OA group in this study. Gervais-Hupé et al.[29] reported a weak negative correlation of CSI with local and distant PPT in 133 knee OA patients. They also reported that CSI is more significantly associated with psychological factors, such as somatization and anxiodepressive symptoms, in patients with knee OA.
Prolonged or repetitive painful stimulation in the periphery plays a role in the development of central sensitization.[30] Therefore, a relationship between pain duration and PPT can be expected; however, there are controversial reports in the literature. In our study, there was no relationship between pain duration and PPT in knee OA patients consistent with the results of Skou et al.’s[31] study. Nonetheless, Arendt-Nielsen et al.[32] suggested an association between pain sensitization and longer duration of symptoms. Pain sensitization has been shown to be associated with pain severity.[5,32] There was no relationship between the VAS score and PPT at any region in our OA group. However, GCPS had a weak negative correlation with PPT at the leg and forearm, which shows that severe pain persisting in the chronic process may be associated with central sensitization. The GCPS evaluates the severity of pain over the past six months.[12] This feature of GCPS may have provided a better correlation with pain sensitivity in these chronic joint pain patients. There was also a significant but weak negative correlation between GCPS total score and PPT at the knee; patients with local pressure hyperalgesia may tend to express more pain and disability.
Psychosocial factors such as depression, pain catastrophizing, and sleep disturbances are known to be associated with pain experience in knee OA patients.[33] In our study, while both BDI and ISI scores were higher in the OA group than in the control group, this significance was found only in BDI in the RA group. Finan et al.[34] reported that anxiety, depression, and pain disaster symptoms increased in patients with high pain/low knee OA grade compared to low pain groups; however, group differences in quantitative sensory testing (QST) measurements were independent of psychosocial functionality. In another study exploring the association of sleep efficiency and catastrophic thinking to central sensitization in knee OA patients, it has been suggested that those with low sleep efficiency and higher catastrophizing scores reported increased levels of central sensitization.[35] In this study, PPT scores were not correlated with BDI, PCS, and ISI scores. This suggests that pain sensitization in OA patients cannot be explained by psychosocial factors alone.
Structural damage is thought to be a source of pain, and sustained severe nociceptive input from this may lead to pain sensitivity in OA patients.[27] We did not find a relationship between the KL grade and PPT and CSI consistent with the literature.[14] Therefore, central sensitization appears to be independent of the severity of structural damage. The fact that the CSI value is ≥40 in 59.4% of RA patients, which is a prototype of systemic inflammatory joint diseases, causes the relationship between this structural damage and central sensitization to be questioned. Zhang and Lee[9] also reported peripheral sensitization, as well as features suggestive of neuropathic pain and changes in central pain mechanisms in RA patients. The PPT, CSI, pressure hyperalgesia frequency, and central sensitization frequency according to the CSI were found to be similar between the RA and OA groups. To our knowledge, there are few studies comparing pain thresholds and pain sensitization in OA and RA patient groups. In the first study published in 1989, pain thresholds in the OA group were reported to be higher compared to the RA and control groups.[36] The second study, comparing mechanical sensations and pain thresholds in OA and RA patients with normal control subjects, demonstrated that OA and RA patients exhibit higher mechanical sensation thresholds and lower cutaneous mechanical pain thresholds (allodynia) compared to normal control subjects, further suggesting that there may be similar central mechanisms involved in OA and RA patients.[37] The third study, comparing OA and RA patients with healthy controls by using the pain-DETECT questionnaire and QST, revealed that both OA and RA patients have evidence of neuropathic features and exhibit peripheral and central sensitization.[38]
The prevalence of hyperalgesia in patients with OA and RA was 43.5% and 37.5% in the knee, 27.4% and 25% in the leg, and 8.1% and 9.4% in the forearm, respectively. Wylde et al.[39] reported that primary and secondary pressure hyperalgesia was not a common occurrence in knee OA patients; localized pressure pain sensitization was found in 32% and distant pressure pain sensitization in 20% of knee OA patients. A recent study exploring the role of CS pain in various rheumatic diseases found central sensitization according to the CSI in 41% of RA and 62% of OA patients.[40]
There are several limitations to this study. As our study design was cross-sectional, it does not allow the determination of the direction of associations between pain sensitization measures and clinical variables. Although age was not found to be correlated with PPT in the current study, the fact that the control group was younger than the chronic pain groups might have influenced other variables. The number of patients to be included in the study was calculated to investigate whether there was a difference between the OA and the control group in terms of PPT; however, the relatively small sample size might have been insufficient to evaluate other outcomes. The low number of male patients does not allow the study results to be generalized to the entire knee OA population.
In conclusion, central sensitization is encountered in some patients with OA and RA, which cause chronic knee pain by different mechanisms. The absence of a positive relationship between the severity of structural damage and central sensitization in OA patients and the lack of difference in central sensitization prevalence between RA and OA patients support the idea that chronic pain may be a more vital factor in the pathophysiology of central sensitization. Future studies are needed to determine the clinical features and diagnostic criteria of patients with central sensitization, whose treatment strategies and prognosis may differ from the general knee OA and RA patient population.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Ankara University Faculty of Medicine Ethics Committee (date: 12.12.2016, no: 19-964-16). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Contributed all parts of the study: H.Ö.K.; Contributed all parts of the study except for control/ supervision and data collection: A.G.; Contributed all parts of the study except for data collection and analysis: S.T.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.
References
- 1.O'Neill TW, Felson DT. Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep. 2018;16:611–616. doi: 10.1007/s11914-018-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidd BL. Osteoarthritis and joint pain. Pain. 2006;123:6–9. doi: 10.1016/j.pain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116–116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: Prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152:566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. S2-S15Pain. 2011;152(3 Suppl) doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:1043–1056. doi: 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 7.Walsh DA, McWilliams DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:581–592. doi: 10.1038/nrrheum.2014.64. [DOI] [PubMed] [Google Scholar]
- 8.McWilliams DF, Rahman S, James RJE, Ferguson E, Kiely PDW, Young A, et al. Disease activity flares and pain flares in an early rheumatoid arthritis inception cohort; characteristics, antecedents and sequelae. BMC Rheumatol. 2019;3:49–49. doi: 10.1186/s41927-019-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang A, Lee YC. Mechanisms for joint pain in rheumatoid arthritis (RA): From cytokines to central sensitization. Curr Osteoporos Rep. 2018;16:603–610. doi: 10.1007/s11914-018-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 12.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 13.Ozdemir-Karatas M, Peker K, Balık A, Uysal O, Tuncer EB. Identifying potential predictors of pain-related disability in Turkish patients with chronic temporomandibular disorder pain. J Headache Pain. 2013;14:17–17. doi: 10.1186/1129-2377-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Düzce E. Santral Sensitizasyon Ölçeğinin Türkçe Geçerlilik ve Güvenilirliği. [Uzmanlık Tezi] Edirne: Trakya Universitesi Tıp Fakültesi Fizik Tedavi ve Rehabilitasyon Anabilim Dalı; 2017. [Google Scholar]
- 15.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12:276–285. doi: 10.1111/j.1533-2500.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, et al. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 2013;14:438–445. doi: 10.1016/j.jpain.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 21.Aktürk Z, Dağdeviren N, Türe M, Tuğlu C. The reliability and validity analysis of the Turkish version of beck depression inventory for primary care. Türk Aile Hek Derg. 2005;9:117–122. [Google Scholar]
- 22.Boysan M, Güleç M, Beşiroğlu L, Kalafat T. Psychometric properties of the Insomnia Severity Index in Turkish sample. Anadolu Psikiyatri Dergisi. 2010;11:248–252. [Google Scholar]
- 23.Süren M, Okan I, Gökbakan AM, Kaya Z, Erkorkmaz U, Arici S, et al. Factors associated with the pain catastrophizing scale and validation in a sample of the Turkish population. Turk J Med Sci. 2014;44:104–108. doi: 10.3906/sag-1206-67. [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss P, Knight E, Wright A. Subjects with knee osteoarthritis exhibit widespread hyperalgesia to pressure and cold. e0147526PLoS One. 2016;11 doi: 10.1371/journal.pone.0147526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis Rheum. 2008;59:1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 28.De Oliveira Silva D, Rathleff MS, Petersen K, Azevedo FM, Barton CJ. Manifestations of pain sensitization across different painful knee disorders: A systematic review including meta-analysis and metaregression. Pain Med. 2019;20:335–358. doi: 10.1093/pm/pny177. [DOI] [PubMed] [Google Scholar]
- 29.Gervais-Hupé J, Pollice J, Sadi J, Carlesso LC. Validity of the central sensitization inventory with measures of sensitization in people with knee osteoarthritis. Clin Rheumatol. 2018;37:3125–3132. doi: 10.1007/s10067-018-4279-8. [DOI] [PubMed] [Google Scholar]
- 30.Woolf CJ. Central sensitization: Uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- 31.Skou ST, Graven-Nielsen T, Rasmussen S, Simonsen OH, Laursen MB, Arendt-Nielsen L. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. Pain. 2013;154:1588–1594. doi: 10.1016/j.pain.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Arendt-Nielsen L, Skou ST, Nielsen TA, Petersen KK. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep. 2015;13:225–234. doi: 10.1007/s11914-015-0276-x. [DOI] [PubMed] [Google Scholar]
- 33.Somers TJ, Keefe FJ, Godiwala N, Hoyler GH. Psychosocial factors and the pain experience of osteoarthritis patients: New findings and new directions. Curr Opin Rheumatol. 2009;21:501–506. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- 34.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: Findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res (Hoboken) 2015;67:1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol. 1989;8:467–474. doi: 10.1007/BF02032098. [DOI] [PubMed] [Google Scholar]
- 37.Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain. 2003;4:203–211. doi: 10.1016/s1526-5900(03)00557-1. [DOI] [PubMed] [Google Scholar]
- 38.Chua J, Ishihara S, Riad M, Castrejon I, Miller R, Malfaid A-M, et al. Peripheral and central sensitization and neuropathic pain are present in both osteoarthritis and rheumatoid arthritis. S414Abstracts/ Osteoarthritis and Cartilage. 2019;27 [Google Scholar]
- 39.Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology (Oxford) 2012;51:535–543. doi: 10.1093/rheumatology/ker343. [DOI] [PubMed] [Google Scholar]
- 40.Guler MA, Celik OF, Ayhan FF. The important role of central sensitization in chronic musculoskeletal pain seen in different rheumatic diseases. Clin Rheumatol. 2020;39:269–274. doi: 10.1007/s10067-019-04749-1. [DOI] [PubMed] [Google Scholar]


