Abstract
Background:
The aim of the present study was to evaluate the effect of ipratropium bromide with violet flower extract, ipratropium bromide with budesonide, and ipratropium bromide alone on the cuff-leak of the endotracheal tube and changes in hemodynamic parameters in intubated patients admitted to the intensive care unit.
Materials and Methods:
The present randomized clinical trial study was performed on 195 intubated patients in three groups of 65 patients. The first group received nebulized ipratropium bromide with budesonide (I + B group), the second group in addition to ipratropium bromide, received one tablespoon of the violet flower extract syrup every 8 hours (I + V group), and the third group received nebulized ipratropium bromide alone (I group). Hemodynamic parameters and the cuff-leak ratio (CLR) of patients were evaluated up to 72 hours after intubation.
Results:
The results of the present study revealed that 12 hours after intubation, the mean of CLR was significantly lower in group I (with a mean of 0.14 ± 0.02) as compared with that of the I + V and I + B groups (with the means of 0.16 ± 0.05 and 0.23 ± 0.05, respectively) (P < 0.001). In addition, 24 hours after intubation, the mean of CLR in group I + V was higher than that of I + B and I groups (P < 0.05).
Conclusion:
According to the results of this study, the use of violet extract syrup in patients under intubation can significantly improve patients' ratio of cuff-leak and SpO2. It seems that the use of violet extract syrup is effective to prevent unwanted complications during intubation and to facilitate patients' breathing.
Keywords: Budesonide, cuff-leak ratio, expiratory flow volume, intubation, ipratropium, violet plant
INTRODUCTION
Endotracheal intubation is a significant action that is taken in the operating room and intensive care unit (ICU) to facilitate patient breathing; however, if intubation is prolonged, it can lead to complications such as upper airway injuries presented as wounds, necrosis, and tracheal stenosis due to the excessive cuff pressure on the tracheal wall, airway obstruction, infection due to the improper size of the tracheal tube, and prolonged intubation (approximately more than 36 hours). Moreover, complications such as the subglottic edema including the nasopharynx, oropharynx, and larynx or the lower parts such as vocal cords may occur as well.[1,2]
Drugs such as salbutamol, atropine, epinephrine, ipratropium bromide, and lidocaine can be used through the endotracheal tube. Ipratropium bromide is a bronchodilator. The inhaled form of this drug belongs to a group of drugs called anticholinergic drugs, which have antimuscarinic and antispasmodic properties. These drugs are often used to treat similar diseases and relax the airway smooth muscles, which prevents shortness of breath and increases airflow to the lungs.[3]
In addition, one of the drugs routinely used to reduce edema in these patients is pulmicort, which is the brand name for budesonide and is an inhaled corticosteroid that reduces upper airway edema.[4] So far, many studies have indicated that the use of corticosteroid can be effective in reducing or preventing airway obstruction after extubation.[5,6] In contrast, some other studies have found corticosteroids to be ineffective in preventing re-intubation and have not recommended its use.[7] In addition, budesonide has been demonstrated to be a steroid used to treat asthma and allergic rhinitis, and prevent and treat nasal polyposis.[8,9] However, this drug may be contraindicated in some patients and have some complications such as the risk of respiratory infection, headache, cough, and bronchitis.[10]
Another option in this regard is the fragrant violet, also recognized as Viola odorata, which is a plant belonging to the Violaceae family, and grows in most parts of Iran and mostly in mountainous and humid areas. The Violaceae has long been of great significance in herbal medicine. Viola odorata is a rich source of vitamin C, has long been used to treat jaundice, and has anti-edema, antifever, antibacterial, and protective effects on the liver. Its syrup inhibits cough in children with asthma. Its intranasal spray has a soothing effect on the brain and is effective in treating insomnia. Moreover, it has also been prescribed in the Chinese medical system to inhibit the proliferation of cancer cells.[10]
So far, various studies have been conducted to address the effects of violet flower. Moreover, the antibacterial effects of its extract on the treatment of sinusitis, its inhibitory effect on the cancer cell proliferation, and its managerial and improving effects on the respiratory problems in children and adults have been addressed in the literature.[10,11,12,13]
Moreover, it has also been indicated that the aqueous extract of violet, as compared with hydrocortisone, has a significant effect on the improvement of formalin-induced lung damage in rats.[14] In many other studies, the anti-edema effects of the violet plant dissolved in polysaccharide solution have been confirmed.[14,15,16] These water-soluble polysaccharides containing galactose, glucose, and galacturonic acid reduce the stages of edema and alter capillary permeability.[15]
Therefore, it seems that violet flower extract has significant anti-edema effects. In addition, its concomitant use with ipratropium bromide can be effective in the better treatment, control, and suppression of respiratory edema in patients. Moreover, considering the common use of intubation in ICUs and its complications, the most common of which is upper respiratory edema in patients, the present study aimed at comparing the effect of ipratropium bromide with violet flower extract, ipratropium bromide with budesonide, and ipratropium bromide alone on the cuff-leak of the endotracheal tube, and changes in hemodynamic parameters in intubated patients admitted to ICU.
MATERIALS AND METHODS
The present study was a double-blind clinical trial. The study population consisted of all intubated patients admitted to the ICU and to the Level 3 medical center of Al-Zahra Hospital from January 2020 to May 2021. The sample size of 195 patients (65 patients in each group) was considered from the mentioned population according to the sample size formula for between-group comparisons, at a 95% confidence interval, a test power of 80%, the percentage of the respiratory improvement in the two groups with and without the use of violet plant in previous studies to be equal to 0.60% and 0.36%,[13] respectively, and the error level of 0.24 (P1-P2).
The inclusion criteria included patients that were intubated, aged 18 to 65 years, required intubation for more than 48 hours, had no history of treatment with corticosteroids or NSAIDs prior to participation in the study, had no history of respiratory diseases including asthma or chronic obstructive pulmonary disease (COPD), and satisfied to participate in the study. In addition, if patients had a history of allergy to budesonide or the drugs used in the current study, they were not included in the study. Moreover, in the event of death within the first 24 hours, more than one week of extubation, or unwillingness to continue their cooperation with the study, they were excluded from the study and replaced with another sample.
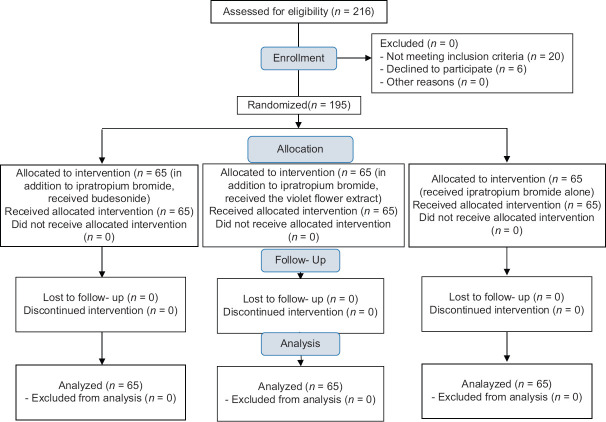
After obtaining the code of ethics from the Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.139), the clinical trial code (IRCT20120716010297N8), and informed written consent from eligible patients' companion, 195 patients were included in the study using the convenience sampling. Then, the patients were divided into three groups of 65 using the random allocation software [Figure 1].
Figure 1.
CONSORT flowchart of patients
The patients' demographic information including age, sex, body mass index (BMI), cause of intubation, intubation times, and hemodynamic parameters including mean arterial pressure (MAP), heart rate (HR), and oxygen saturation percentage (SpO2) were recorded before the intervention.
In order to observe the blindness condition in the present study, three drugs, namely, ipratropium bromide alone, ipratropium bromide with budesonide, and ipratropium bromide with violet flower extract were prepared by the pharmacist, put in the coded packages, and delivered daily to the anesthesiologist, who prescribed them without being informed of the type of any of the drugs. In addition, the person recording the clinical and basic information of the patients as well as the statistical analyst was not aware of the type of intervention.
The following medications were provided for the three groups for 72 hours as follows: The first group, in addition to ipratropium bromide, received budesonide (at the dose of 0.25 mg reached to 4 cc with the oxygen flow rate of 6 liters per minute) via a nebulizer every 12 hours for 20 minutes (I + B group); the second group, in addition to ipratropium bromide, received one tablespoon of the violet flower extract syrup every 8 hours (I + V group); the third group, as the control group, received ipratropium bromide alone via a nebulizer every 12 hours for 20 minutes (I group).
It should be noted that in all three groups, the tracheal tubes were of the same size and the same material, that is, poly vinyl chloride (PVC). In addition, the endotracheal tube cuff pressure was checked using a special manometer and was adjusted to be equal to 25 mmHg.
During the study, patients underwent pulse oximetry and cardiac monitoring, and their hemodynamic parameters such as SpO2, MAP, HR, and cuff-leak ratio (CLR) were recorded 12, 24, 36, 48, and 72 hours after intubation.
In order to calculate the CLR of patients, the device mode was first set to volume-assisted control, tidal volume (TV) as 8 cc/kg, and PEEP as 0. Then, the cuff of the endotracheal tube was emptied, and the expiratory TV was checked in six respiratory cycles. Then, the mean of the three samples with the lowest TV was calculated. Moreover, the CLR of the patient was calculated as follows[10]:

Finally, the collected information was entered into SPSS software (version 25). Data were presented as mean ± standard deviation or frequency (percentage). At the level of inferential statistics, according to the result of Kolmogorov–Smirnov test indicating the normal distribution of data, tests such as one-way analysis of variance, repeated measures analysis of variance, independent samples t test, and Chi-squared test were used. A significance level of less than 0.05 was considered in all analyses.
RESULTS
In the present study, the I + V group consisted of 40 males (61.5%) and 25 females (38.5%) with the mean age of 45.52 ± 14.67 years; I + B group included 38 males (58.5%) and 27 females (41.5%) with the mean age of 40.38 ± 13.64 years; and group I comprised of 31 males (47.7%) and 34 females (52.3%) with the mean age of 44.45 ± 15.21 years (P > 0.05) [Table 1].
Table 1.
Basic and clinical characteristics of patients in the three groups
| Variables | I + V group | I + B group | I group | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 40 (61.5%) | 38 (58.5%) | 31 (47.7%) | 0.248 |
| Female | 25 (38.5%) | 27 (41.5%) | 34 (52.3%) | |
| Age (years) | 45.52±14.67 | 40.38±13.64 | 44.45±15.21 | 0.106 |
| BMI (kg/m2) | 25.11±3.43 | 25.07±3.24 | 24.81±3.63 | 0.450 |
| Cause of Intubation | ||||
| Trauma | 21 (31.8%) | 30 (46.2%) | 38 (58.5%) | 0.075 |
| Loss of Consciousness | 21 (31.8%) | 17 (26.2%) | 17 (26.2%) | |
| Surgery | 17 (25.8%) | 13 (20.0%) | 6 (9.2%) | |
| Poisoning | 7 (10.6%) | 5 (7.7%) | 4 (6.2%) | |
| Intubation time (days) | 7.63±6.11 | 9.03±6.64 | 8.69±7.06 | 0.455 |
Data is presented as n (%) or Mean±SD. I + V group: Received ipratropium bromide and budesonide via a nebulizer. I + B group: In addition to ipratropium bromide, received the violet flower extract syrup. I group: Received ipratropium bromide alone via a nebulizer
In addition, the evaluation of hemodynamic parameters revealed that HR and MAP of patients before the intervention to 48 hours after intubation were not significantly different between the three groups (P > 0.05). However, the SpO2 of patients 12, 24, 36, and 72 hours after intubation in the I + V group with the mean of 96.03 ± 1.63, 95.89 ± 1.81, and 96.30 ± 2.22 was significantly higher than that of the I + B group with the mean of 94.60 ± 2.03, 94.66 ± 2.35, and 95.48 ± 2.50 and the I group with the mean of 95.12 ± 2.28, 94.89 ± 2.04, and 95.38 ± 1.83, respectively [Table 2].
Table 2.
Determination and comparison of hemodynamic parameters of patients in the three groups
| Variables | Groups | 0 h | 12 h | 24 h | 36 h | 48 h | 72 h | P□ |
|---|---|---|---|---|---|---|---|---|
| HR | I + V group | 89.12±14.81 | 88.85±15.25 | 85.59±14.38 | 83.56±13.30 | 82.26±8.35 | 84.26±8.35 | <0.001 |
| I + B group | 89.15±10.65 | 85.60±9.16 | 84.35±11.16 | 80.18±8.42 | 80.45±5.28 | 83.45±8.28 | <0.001 | |
| I group | 89.29±12.21 | 86.63±6.48 | 84.32±10.89 | 82.38±12.66 | 81.54±5.36 | 84.54±5.37 | <0.001 | |
| P* | 0.950 | 0.592 | 0.989 | 0.0.284 | 0.339 | 0.329 | ||
| P** | 0.939 | 0.248 | 0.555 | 0.565 | 0.257 | 0.265 | ||
| P*** | 0.988 | 0.988 | 0.564 | 0.100 | 0.112 | 0.100 | ||
| SpO2 | I + V group | 95.74±2.18 | 96.03±1.63 | 95.89±1.81 | 96.30±2.22 | 96.88±2.12 | 96.58±2.11 | <0.001 |
| I + B group | 95.15±1.77 | 94.60±2.03 | 94.66±2.35 | 95.48±2.50 | 95.92±2.65 | 95.62±2.65 | <0.001 | |
| I group | 95.34±1.50 | 95.12±2.28 | 94.89±2.04 | 95.38±1.83 | 96.38±1.67 | 96.08±1.67 | <0.001 | |
| P* | 0.568 | 0.137 | 0.529 | 0.811 | 0.230 | 0.213 | ||
| P** | 0.211 | 0.010 | 0.007 | 0.018 | 0.197 | 0.211 | ||
| P*** | 0.069 | <0.001 | 0.001 | 0.033 | 0.382 | 0.368 | ||
| MAP | I + V group | 86.27±12.20 | 86.05±11.84 | 84.06±8.95 | 83.32±9.95 | 83.26±9.49 | 85.26±9.48 | 0.40 |
| I + B group | 89.45±10.74 | 86.78±12.60 | 83.89±7.40 | 82.18±8.97 | 81.22±9.72 | 83.22±9.72 | <0.001 | |
| I group | 87.57±10.35 | 88.92±7.98 | 86.06±9.48 | 83.17±8.62 | 83.28±9.02 | 85.28±9.06 | <0.001 | |
| P* | 0.338 | 0.269 | 0.155 | 0.543 | 0.214 | 0.215 | ||
| P** | 0.506 | 0.136 | 0.187 | 0.926 | 0.991 | 0.988 | ||
| P*** | 0.104 | 0.912 | 0.912 | 0.482 | 0.217 | 0.203 |
I + V group: Received ipratropium bromide and budesonide via a nebulizer. I + B group: In addition to ipratropium bromide, received the violet flower extract syrup. I group: Received ipratropium bromide alone via a nebulizer. HR: Heart Rate, SpO2: oxygen saturation, MAP: Mean Arterial Pressure. □: In the inter-group comparison: Level of significance for comparing each group in the follow-up. In the intra-group comparison, three comparisons have been made as follows: *Level of significance for comparing I group vs I + B group, **Level of significance for comparing I group vs I + V group, ***Level of significance for comparing I + B group vs I + V group
Finally, 12 hours after intubation, the mean CLR in the I + V and I + B groups with the means of 0.16 ± 0.05 and 0.23 ± 0.05, respectively, was higher than that of the I group with the mean of 0.14 ± 0.02 (P < 0.001). However, there was no significant difference between the I + V and I + B groups in this regard (P = 0.921). Moreover, 24, 36, 48, and 72 hours after intubation, CLR of I + V group with the mean of 0.28 ± 0.06, 0.35 ± 0.06, 0.40 ± 0.07, and 0.50 ± 0.06, respectively was higher than that of the I + B group with the mean of 0.024 ± 0.06, 0.28 ± 0.06, 0.32 ± 0.06, and 0.42 ± 0.06, respectively, and I group with the mean of 0.16 ± 0.02, 0.18 ± 0.02, 0.21 ± 0.02, and 0.31 ± 0.02, respectively (P < 0.05) [Table 3].
Table 3.
Determination and comparison of the mean of CLR for three groups
| Variables | Groups | 0 h | 12 h | 24 h | 36 h | 48 h | 72 h | P□ |
|---|---|---|---|---|---|---|---|---|
| CLR | I + V group | 0.16±0.05 | 0.23±0.10 | 0.28±0.06 | 0.35±0.06 | 0.40±0.07 | 0.50±0.06 | <0.001 |
| I + B group | 0.16±0.10 | 0.23±0.05 | 0.24±0.06 | 0.28±0.06 | 0.32±0.06 | 0.42±0.06 | <0.001 | |
| I group | 0.14±0.03 | 0.14±0.02 | 0.16±0.02 | 0.18±0.02 | 0.21±0.02 | 0.31±0.02 | <0.001 | |
| P* | 0.051 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| P** | 0.154 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| P*** | 0.742 | 0.921 | <0.001 | <0.001 | <0.001 | <0.001 |
CLR: Cuff-Leak Ratio. □: In the inter-group comparison: Level of significance for comparing each group in the follow-up. In the intra-group comparison, three comparisons have been made as follows: *Level of significance for comparing I group vs I + B group, **Level of significance for comparing I group vs I + V group, ***Level of significance for comparing I + B group vs I + V group
DISCUSSION
Considering the lack of gag reflex in intubated patients and the presence of a foreign body in the upper respiratory tract, these patients will have upper airway edema. In this regard, budesonide is used as an inhaled corticosteroid to reduce the upper airway edema. However, this drug is associated with some complications such as the increased risk of respiratory infection, headache, and cough.[6,10] In contrast, violet flower extract, one of the plants used in herbal medicine, has anti-edema effects and has long been used for a wide range of lung diseases.[11,12,13] Therefore, the present study evaluated the effect of ipratropium bromide with budesonide, ipratropium bromide with violet flower extract, and ipratropium bromide alone on the cuff-leak of the endotracheal tube, and changes in hemodynamic parameters in intubated patients. The results of this study revealed that nebulized ipratropium bromide with violet flower extract syrup, as compared to ipratropium bromide with budesonide and ipratropium bromide alone, was significantly effective in increasing patients' SpO2. In fact, the administration of violet flower extract has played a significant role in improving the patients' respiratory status along with the stability of patients' other hemodynamic parameters.
In this regard, the effect of corticosteroids on reducing or preventing airway obstruction after extubation has been reported in many pertinent studies[5,6] although some studies have not considered this type of intervention to be effective and have not recommended it.[7]
In addition, according to some other studies, the steroidal budesonide can be effective in improving the symptoms of allergic rhinitis and asthma, and preventing nasal polyps.[8,9]
Although no study has been performed to address the effects of violet flower extract, the anti-inflammatory effects of violet have been confirmed in many previous studies because the water-soluble polysaccharides extracted from this plant show anti-inflammatory activity, which has been characterized by the suppression of the secretion and proliferation of inflammatory stages and changes in capillary permeability.[14,15]
Moreover, the results of the present study revealed that from 12 hours after intubation, the mean of CLR was significantly higher in the two groups receiving nebulized ipratropium bromide with violet flower extract syrup (I + V group) and ipratropium bromide with budesonide (I + B group) as compared with the ipratropium alone group (I group). In addition, the most crucial point is that this difference was also significant between the two I + V and I + B groups 24 hours after intubation such that the mean of CLR in I + V group was significantly higher than that of the I + B group.
So far, various studies have been conducted on the effects of violets. For instance, the antibacterial effect of violet flower extract on the treatment of sinusitis[16] and the inhibitory effect of violet flower extract on cancer cell proliferation in breast cancer[11] have been confirmed in previous studies.
Mahboubi et al.[12] in their review study on the role of violet flower as a traditional medicinal plant in the management of respiratory problems indicated that this plant can be effective in treating cough, headache, migraine, insomnia, sore throat, and epilepsy in children and adults. In addition, the syrup of this plant has been recognized as a safe treatment for managing respiratory diseases in children. In addition, its effect on pain, fever, infection, and edema is also known to be acceptable.
The results of the study conducted by Koochek et al.[14] also showed that the aqueous extract of violet, as compared with hydrocortisone, had a comparable preventive effect on the formalin-induced lung damage in rats. Moreover, the violet extract significantly reduces the degree of damage, rupture, and epithelial lesions.
Another clinical trial reported that violet flower syrup can quickly improve the severity and frequency of children's coughs.[13] In addition, the results of an in-vivo study by Ghorbani et al.[17] indicated the CNS-depressant and hypnotic effects of another genus of Violaceae. The mentioned study reported increased sleep duration without any neuronal toxicity as the result of Viola tricolor extracts. The hypnotic effect of V. tricolor in this study was attributed to the intermediate-to-low polar agents including some sterols, alkanes, flavonoids, and some terpenoids. Moreover, the sedative effects of V. tricolor on the brain have been reported in literature.[17] Another study has reported sesquiterpene and monoterpenes as the central components in the essential oil of V. odorata flowers that could justify its hypnotic effects.[18]
Comparison of the results of other studies with those of the present study is not feasible as none of the pertinent studies have evaluated the effect of violet flower on CLR and hemodynamic parameters of intubated patients. However, considering the results of previous studies on the significant effects of this plant on drowsiness and sedation of the brain as well as its positive effects on respiratory problems of patients, it seems that the use of this plant in intubated patients that require sedation, stability of hemodynamic parameters, and good respiratory volume can be useful and effective. Therefore, the novelty of this issue and its examination in three groups can be one of the strengths of the present study. However, the non-evaluation of patients' other hemodynamic parameters as well as the non-evaluation of complications caused by intubation is one of the limitations of this study. Therefore, it is recommended that future studies take into account other respiratory parameters as well as the incidence of complications so that the obtained results can be generalized to the community with more confidence.
CONCLUSION
According to the results of the present study, there was no significant difference between the patients' blood pressure and heart rate in any of the three groups, and the patients were in a stable condition. However, the mean of SpO2 in the group receiving violet flower extract syrup with nebulized ipratropium bromide was higher than that of the other two groups receiving nebulized ipratropium bromide with budesonide or nebulized ipratropium bromide alone. Moreover, the effect of this plant on increasing the mean of CLR in the group receiving violet flower extract syrup with nebulized ipratropium bromide, as compared to the other two groups, was significantly evident 24 hours after intubation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kuriyama A, Umakoshi N, Sun R. Prophylactic corticosteroids for prevention of postextubation stridor and reintubation in adults: A systematic review and meta-analysis. Chest. 2017;151:1002–10. doi: 10.1016/j.chest.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Parajuli B, Baranwal AK, Kumar-M P, Jayashree M, Takia L. Twenty-four-hour pretreatment with low dose (0.25 mg/kg/dose) versus high dose (0.5 mg/kg/dose) dexamethasone in reducing the risk of postextubation airway obstruction in children: A randomized open-label noninferiority trial. Pediatr Pulmonol. 2021;56:2292–301. doi: 10.1002/ppul.25388. [DOI] [PubMed] [Google Scholar]
- 3.Longhini F, Navalesi P. Inhalation therapy in ventilated patients. ERS Practical Handbook of Invasive Mechanical Ventilation. European Respiratory Society. 2019:201. [Google Scholar]
- 4.Kothe TB, Kemp MW, Schmidt A, Royse E, Salomone F, Clarke MW, et al. Surfactant plus budesonide decreases lung and systemic inflammation in mechanically ventilated preterm sheep. Am J Physiol Lung Cell Mol Physiol. 2019;316:L888–93. doi: 10.1152/ajplung.00477.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuriyama A, Jackson JL, Kamei J. Performance of the cuff leak test in adults in predicting post-extubation airway complications: A systematic review and meta-analysis. Crit Care. 2020;24:640. doi: 10.1186/s13054-020-03358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnell D, Planquette B, Berger A, Merceron S, Mayaux J, Strasbach L, et al. Cuff leak test for the diagnosis of post-extubation stridor: A multicenter evaluation study. J Intensive Care Med. 2019;34:391–6. doi: 10.1177/0885066617700095. [DOI] [PubMed] [Google Scholar]
- 7.Young D, Watkinson P. Preventing postextubation airway complications in adults. BMJ. 2008;337:a1565. doi: 10.1136/bmj.a1565. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen ES, Smulders CA, Kappen TH, Calis JC, van Woensel J, Raymakers-Janssen PA, et al. Post-extubation stridor in respiratory syncytial virus bronchiolitis: Is there a role for prophylactic dexamethasone? PLoS One. 2017;12:e0172096. doi: 10.1371/journal.pone.0172096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha A, Jayashree M, Singhi S. Aerosolized L-epinephrine vs budesonide for post extubation stridor: A randomized controlled trial. Indian Pediatr. 2010;47:317–22. doi: 10.1007/s13312-010-0060-z. [DOI] [PubMed] [Google Scholar]
- 10.Rafieian-Kopaei M, Baradaran A, Rafieian M. Plants antioxidants: From laboratory to clinic. J Nephropathol. 2013;2:152–3. doi: 10.12860/JNP.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooshyar N, Sedighi M, Hooshmand M, Valizadeh R, Ebrahimi S, Khosravifarsani MR, et al. Mechanistic impact of medicinal plants affecting cisplatin-induced nephrotoxicity; an overview. Immunopathol Persa. 2019;5:e07. [Google Scholar]
- 12.Mahboubi M, Kashani LM. A Narrative study about the role of Viola odorata as traditional medicinal plant in management of respiratory problems. Adv Integr Med. 2018;5:112–8. [Google Scholar]
- 13.Asgharpour M, Tolouian A, Bhaskar LVKS, Tolouian R, Massoudi N. Herbal antioxidants and renal ischemic-reperfusion injury; an updated review. J Nephropharmacol. 2021;10:e03. [Google Scholar]
- 14.Koochek MH, Pipelzadeh MH, Mardani H. The effectiveness of Viola odorata in the prevention and treatment of formalin-induced lung damage in the rat. J Herbs Spices Med Plants. 2003;10:95–103. [Google Scholar]
- 15.Drozdova IL, Bubenchikov RA. Composition and antiinflammatory activity of polysaccharide complexes extracted from sweet violet and low mallow. Pharm Chem J. 2017;39:197–200. [Google Scholar]
- 16.Asheesh K, Suresh C, Meenakshi P. A brief knowledge of banafsha (Viola odorata linn.) and other Viola species. Int J Ayurveda Res. 2017;5:1–10. [Google Scholar]
- 17.Ghorbani A, Youssofabad NJ, Rakhsh H. Effect of Viola tricolor on pentobarbital-induced sleep in mice. Afr J Pharm Pharmacol. 2012;6:2600–6. [Google Scholar]
- 18.Hammami I, Kamoun N, Rebai A. Biocontrol of Botrytis cinerea with essential oil and methanol extract of Viola odorata L.flowers. Arch Appl Sci Res. 2011;3:44–51. [Google Scholar]