Abstract
Objective
To measure the impact of the covid-19 pandemic on admissions to hospital and interventions for acute ischemic stroke and acute myocardial infarction.
Design
A retrospective analysis.
Setting
746 qualifying hospitals in the USA from the Premier Healthcare Database.
Participants
Patients aged 18 years and older who were admitted to hospital with a primary diagnosis of acute ischemic stroke or acute myocardial infarction between 1 March 2019 and 28 February 2021.
Main outcome measures
Relative changes in volumes were assessed for acute ischemic stroke and acute myocardial infarction hospital admissions as well as intravenous thrombolysis, mechanical thrombectomy, and percutaneous coronary intervention (overall and for acute myocardial infarction only) across the first year of the pandemic versus the prior year. Mortality in hospital and length of stay in hospital were also compared across the first year of the pandemic versus the corresponding period the year prior. These metrics were explored across the different pandemic waves.
Results
Among 746 qualifying hospitals, admissions to hospital were significantly reduced after the covid-19 pandemic compared with before the pandemic for acute ischemic stroke (−13.59% (95% confidence interval−13.77% to −13.41%) and acute myocardial infarction (−17.20% (−17.39% to −17.01%)), as well as intravenous thrombolysis (−9.47% (−9.99% to −9.02%)), any percutaneous coronary intervention (−17.89% (−18.06% to −17.71%)), and percutaneous coronary intervention for acute myocardial infarction (−14.36% (−14.59% to −14.12%)). During the first year of the pandemic versus the previous year, the odds of mortality in hospital for acute ischemic stroke were 9.00% higher (3.51% v 3.16%; ratio of the means 1.09 (95% confidence interval (1.03 to 1.15); P=0.0013) and for acute myocardial infarction were 18.00% higher (4.81% v 4.29%; ratio of the means 1.18 (1.13 to 1.23); P<0.0001).
Conclusions
We observed substantial decreases in admissions to hospital with acute ischemic stroke and acute myocardial infarction, but an increase in mortality in hospital throughout the first year of the pandemic. Public health interventions are needed to prevent these reductions in future pandemics.
Keywords: COVID-19, Stroke, Myocardial infarction
WHAT IS ALREADY KNOWN ON THIS TOPIC
Covid-19 is related to thromboembolic complications including cryptogenic strokes in the young population and worse outcomes in acute myocardial infarction
By contrast, reports have described a decrease in admissions to hospital and acute reperfusion treatment rates
WHAT THIS STUDY ADDS
An important decline was reported in the numbers of admissions and in volumes for intravenous thrombolysis and percutaneous coronary intervention
However, adjusted rates of mortality in hospital were increased for intravenous thrombolysis and percutaneous coronary intervention
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
The general public should be engaged and informed on the consequences of untreated acute conditions, and public health interventions are needed to prevent these reductions in future pandemics
Introduction
Since emerging in Wuhan, China in December 2019, the virus SARS-CoV-2 caused a global pandemic of unprecedented impact.1 In the United States of America (US) alone, more than 42.7 million people have been diagnosed with the resultant disease covid-19 and more than 680 000 people have died.2 SARS-CoV-2 is more than a respiratory virus and can clearly affect multiple organs, including the endothelium by promoting a clotting diathesis that can result in life threatening thrombotic complications.3 Several studies have described high rates of cryptogenic strokes among younger patients (18-50 years) with covid-19.3–6 Similarly, patients with concurrent acute myocardial infarction (AMI) and covid-19 might have a higher thrombus burden and worse outcomes.7 By contrast, reductions in the rates of hospital admission and acute reperfusion treatment for acute ischemic stroke (AIS)5 8–12 and acute coronary syndromes13–19 have been commonly reported during the pandemic. As such, the indirect consequences of the outbreak on public behavior and on the organization of the systems of care seemingly represent a greater healthcare problem than the potential direct effect of AIS and AMI caused by the SARS-CoV-2 infection.
Using a nationwide hospital database, we aim to quantify the changes in the number of hospital admissions and interventions provided for AIS and AMI in the US in relation to the first year of the covid-19 pandemic. We also explore temporal trends across the pandemic and potential differences in baseline characteristics and outcomes for these conditions across the prepandemic and pandemic periods. We made five hypotheses: (1) a reduction in AIS and AMI admissions as well as use of intravenous thrombolysis, mechanical thrombectomy, and percutaneous coronary intervention would occur; (2) the magnitude of these changes in AIS and AMI would temporarily correlate with the mortality burden from covid-19; (3) the proportion of patients receiving reperfusion therapies would remain the same or increase, despite absolute reductions in volume, as a reflection of a greater decline in the hospital admissions for milder syndromes; (4) rates of mortality in hospital would increase during the pandemic; and (5) the effects of covid-19 on hospital admissions and interventions would decrease over time as systems would be more strategically ready than the first few months that they faced the pandemic.
Methods
Data source
The Premier Healthcare Database contains detailed information about patient demographics, diagnosis, and procedure coding, hospital costs, and patient billing data from more than 1000 hospitals, representing approximately 20% of all acute inpatient care in the US. The Premier Healthcare Database collects data from voluntary participating hospitals to improve patient safety. A subset of these hospitals agreed to provide more real-time data to facilitate analyses during covid-19. For the purposes of the study, hospitals were required to contribute inpatient data on a continuous basis to Premier Healthcare Database from 1 March 2019 to 28 February 2021.
Study population
To identify the study population, we used diagnoses, procedures, and payment codes from the International Classification of Diseases system, using the 10th Revision clinical modification and procedure coding system, as well as current procedural terminology and diagnosis related group codes.
Patients aged 18 years and older who were admitted to hospital with a primary diagnosis of AIS or AMI between 1 March 2019 and 28 February 2021 were identified across qualifying hospitals. We defined the prepandemic and pandemic cut-off of 1 March 2020, the day that New York City, the initial US epicenter of the pandemic, reported its first covid-19 hospital admission. Furthermore, we classified the pandemic period into three waves: first wave from 1 March 2020-31 May 2020; second wave from 1 June 2020-31 August 2020; and third wave from 1 September 2020-28 February 2021. The intervals were based on a graph from Centres for Disease Control for deaths.20 For binary comparisons of baseline characteristics and outcomes, the precovid-19 sample included patients admitted between 1 March 2019-29 February 2020 (and considered them in three periods corresponding with the three pandemic waves in 2020-21), to account for potential seasonal variations in the occurrence of AIS and AMI.21 22
Study outcomes
The primary study analysis assessed the relative changes in proportions of AIS and AMI hospital admissions as well as use of intravenous thrombolysis, mechanical thrombectomy, and percutaneous coronary intervention (both overall and for AMI only) across the first year of the pandemic versus the prior year. AIS and AMI volume was based on inpatient admissions with a primary diagnosis of these two conditions; mechanical thrombectomy and intravenous thrombolysis were based on inpatient admissions associated with primary or secondary diagnosis of acute ischemic stroke; percutaneous coronary intervention volume was based on inpatient admissions associated with these procedures irrespective of diagnosis; and percutaneous coronary intervention with acute myocardial infarction was based on inpatient admissions associated with acute myocardial infarction primary diagnosis.
Outcomes at patient level, of mortality and length of stay, were assessed and compared during the periods of covid and before covid. Use of intravenous thrombolysis, mechanical thrombectomy, and percutaneous coronary intervention was then examined, to understand variation in treatment use during the covid and precovid era. Patient demographic and hospital provider characteristics were evaluated. We performed secondary analyses considering the comparisons across each of the three waves of the pandemic versus their respective equivalent periods the year prior. Additionally, use of mechanical thrombectomy has steadily grown over the past several years,23 24 therefore, we also compared the three months of the first covid-19 wave (March 2020-May 2020) with the three months immediately preceding the pandemic (December 2019-February 2020).
Statistical analysis
Descriptive analyses were used to characterize patient demographics and hospital characteristics. The 95% confidence intervals for percentage change were calculated using the Wilson procedure without correction for continuity. Assessing between the precovid-19 and covid-19 periods, bivariate tests (χ2 or t tests) compared patient and hospital characteristics among patients who were admitted to hospital with AIS or AMI. Multivariable regression using generalized estimating equation with applicable links (logit link for mortality; log link for length of stay) and distribution functions (binomial distribution for mortality; negative binomial distribution for length of stay) were used to examine differences in study outcomes between the study periods. The generalized estimating equation models included adjustment for potential clustering of outcomes within hospitals as we controlled for clustering by accounting for provider id (hospital identifier) in our regression (generalized estimating equation) model, and included all study covariates. Study covariates included age; gender; race; marital status; payor; elixhauser score; presence of congestive heart failure, hypertension, diabetes, obesity, atrial fibrillation, transient ischemic attack, coronary artery disease, or dyslipidemia; smoking; number of beds; region; urban-rural status; hospital teaching status; covid-19 period; also, tissue plasminogen activator and thrombectomy (added for AIS); and percutaneous coronary intervention for AMI models. All analyses were done by use of SAS Enterprise Guide for Windows (SAS Institute Inc, Cary, NC, USA).
Patient and public involvement
Due to study design constraints, neither patients nor members of the public were involved in the conduct of this study. The patients’ data are de-identified so we will not be able to directly disseminate the results to individual participants, but we will be able to share it with sites. Our findings will be also shared on social media and relevant websites via graphical and simplified summaries.
Results
Primary analysis
Acute ischemic stroke
Of the 746 hospitals, 721 (97%) hospitals had at least one inpatient admission with a primary diagnosis of AIS during the study period (March 2019-February 2021) (figure 1). Overall, 519 (69.57%) had fewer than 300 beds and 537 (72%) were non-teaching hospitals (table 1). As compared with their prepandemic year counterparts, patients admitted to hospital with AIS in the first year of the covid-19 pandemic were significantly different in distribution for age, race, marital status, and health insurance payors. Additionally, patients during the pandemic period had a significantly higher burden of some comorbidities (table 2). Slightly more AIS admissions were to smaller hospitals (0-299 beds) during the pandemic. Additionally, the geographical redistribution modestly changed with a slight increase in AIS hospital admissions in the Midwest and a mild reduction in the Northeast.
Figure 1.
Flow diagram of hospitals contributing inpatient data continuously from March 2019 to February 2021
Table 1.
Characteristics of hospitals contributing inpatient data continuously from March 2019 to February 2021
| Characteristics | Continuous inpatient data | AIS episodes | MT episodes | IVT episodes | AMI episodes | PCI episodes | PCI with AMI episodes |
| No of hospitals | 746 (100) | 721 (100) | 328 (100) | 549 (100) | 702 (100) | 470 (100) | 444 (100) |
| No of beds: | |||||||
| 0-299 | 519 (69.57) | 496 (68.79) | 130 (39.63) | 323 (58.83) | 477 (67.95) | 249 (52.98) | 226 (50.90) |
| 300-500 | 142 (19.03) | 140 (19.42) | 115 (35.06) | 141 (25.68) | 140 (19.94) | 137 (29.15) | 134 (30.18) |
| ≥500 | 85 (11.39) | 85 (11.79) | 83 (25.30) | 85 (15.48) | 85 (12.11) | 84 (17.87) | 84 (18.92) |
| Region: | |||||||
| Midwest | 214 (28.69) | 208 (28.85) | 89 (27.13) | 143 (26.05) | 207 (29.49) | 130 (27.66) | 126 (28.38) |
| Northeast | 87 (11.66) | 85 (11.79) | 44 (13.41) | 74 (13.48) | 85 (12.11) | 50 (10.64) | 44 (9.91) |
| South | 328 (43.97) | 313 (43.41) | 144 (43.90) | 239 (43.53) | 300 (42.74) | 212 (45.11) | 201 (45.27) |
| West | 117 (15.68) | 115 (15.95) | 51 (15.55) | 93 (16.94) | 110 (15.67) | 78 (16.60) | 73 (16.44) |
| Urban-rural status: | |||||||
| Rural | 239 (32.04) | 232 (32.18) | 35 (10.67) | 110 (20.04) | 217 (30.91) | 68 (14.47) | 65 (14.64) |
| Urban | 507 (67.96) | 489 (67.82) | 293 (89.33) | 439 (79.96) | 485 (69.09) | 402 (85.53) | 379 (85.36) |
| Teaching | 209 (28.02) | 202 (28.02) | 151 (46.04) | 190 (34.61) | 199 (28.35) | 183 (38.94) | 171 (38.51) |
AIS=acute ischemic stroke; AMI=acute myocardial infarction; IVT=intravenous thrombectomy; MT=mechanical thrombectomy; PCI=percutaneous coronary intervention.
Table 2.
Patient and provider characteristics for patients with acute ischemic stroke (AIS) in the covid-19 (March 2020-February 2021) versus precovid-19 study period (March 2019-February 2020)
| Characteristics | Precovid-19 era (March 2019-February 2020) (n=125 116) |
Covid-19 era (March 2020-February 2021) (n=105 081) |
P value |
| Age, years: | |||
| Mean, SD | 70.42 (13.10) | 70.14 (13.12) | 0.6144 |
| 18-49 | 8489 (6.78) | 7551 (7.19) | <0.0001 |
| 50-59 | 16 536 (13.22) | 13 754 (13.09) | |
| 60-69 | 30 013 (23.99) | 25 614 (24.38) | |
| ≥70 | 70 078 (56.01) | 58 162 (55.35) | |
| Sex: | |||
| Male | 64 603 (51.63) | 54 558 (51.92) | 0.1720 |
| Female | 60 513 (48.37) | 50 523 (48.08) | |
| Race: | |||
| White | 95 247 (76.13) | 80 425 (76.54) | <0.0001 |
| Black | 18 726 (14.97) | 16 181 (15.40) | |
| Asian | 2485 (1.99) | 2000 (1.90) | |
| Other | 8658 (6.92) | 6475 (6.16) | |
| Marital status: | |||
| Married | 53 666 (42.89) | 44 538 (42.38) | 0.0130 |
| Single | 60 492 (48.35) | 51 455 (48.97) | |
| Other | 10 958 (8.76) | 9088 (8.65) | |
| Payor: | |||
| Commercial | 19 711 (15.75) | 16 891 (16.07) | <0.0001 |
| Medicaid | 10 432 (8.34) | 9312 (8.86) | |
| Medicare | 87 343 (69.81) | 72 038 (68.55) | |
| Other | 7630 (6.10) | 6840 (6.51) | |
| Elixhauser Score: | |||
| 0 | 2146 (1.72) | 1479 (1.41) | <0.0001 |
| 1 | 8896 (7.11) | 6667 (6.34) | |
| ≥2 | 114 074 (91.17) | 96 935 (92.25) | |
| Congestive heart failure | 24 176 (19.32) | 20 605 (19.61) | 0.0840 |
| Hypertension | 108 123 (86.42) | 91 404 (86.98) | 0.0001 |
| Diabetes | 49 057 (39.21) | 41 473 (39.47) | 0.2060 |
| Obesity | 20 035 (16.01) | 18 603 (17.70) | 0.0001 |
| Atrial fibrillation | 29 959 (23.94) | 25 507 (24.27) | 0.0660 |
| Transient ischemic attack | 2004 (1.60) | 1907 (1.81) | 0.0001 |
| Coronary artery disease | 38 762 (30.98) | 32 010 (30.46) | 0.0070 |
| Dyslipidemia | 82 673 (66.08) | 70 545 (67.13) | 0.0001 |
| Smoking | 26 133 (20.89) | 22 097 (21.03) | 0.4060 |
| No of beds: | |||
| 0-299 | 39 522 (31.59) | 33 893 (32.25) | 0.0010 |
| 300-500 | 38 696 (30.93) | 31 876 (30.33) | |
| >500 | 46 898 (37.48) | 39 312 (37.41) | |
| Region: | |||
| Midwest | 29 372 (23.48) | 25 507 (24.27) | <0.0001 |
| Northeast | 16 467 (13.16) | 13 167 (12.53) | |
| South | 62 014 (49.57) | 51 937 (49.43) | |
| West | 17 263 (13.80) | 14 470 (13.77) | |
| Urban-rural status: | |||
| Rural | 15 180 (12.13) | 12 526 (11.92) | 0.1190 |
| Urban | 109 936 (87.87) | 92 555 (88.08) | |
| Teaching | 64 723 (51.73) | 54 236 (51.61) | 0.5760 |
Data are numerator (percentage). IQR=interquartile range; SD=standard deviation.
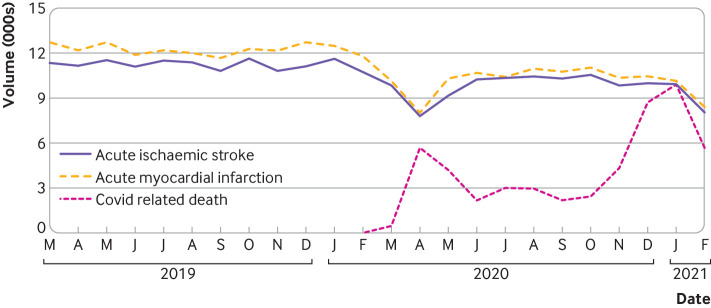
We depict trends in hospital admissions with AIS (figure 2), and intravenous thrombolysis and mechanical thrombectomy volumes (figure 3), in relation to the national covid-19 mortality across the entire study period. In the 12 months preceding the pandemic, 134 768 people were admitted to hospital with AIS compared with 116 452 during the pandemic year, representing a 13.59% reduction (95% confidence interval −13.77% to −13.41%) (table 3). Additionally, use of intravenous thrombolysis was reduced by 9.47% (−9.99% to −9.02%; from 16 202 to 14 668) in the 549 hospitals providing this intervention. During the study, 328 hospitals provided mechanical thrombectomy, with 9343 procedures prior to the pandemic versus 9711 over the first year of the pandemic. Although this corresponds to an increase of 3.94% (3.55% to 4.36%) in relation to the prior year, this increase has to be considered in the context that use of mechanical thrombectomy has increased steadily over the years. Mechanical thrombectomy volumes grew by 12.65% when comparing the very first month of the pandemic (March 2020, when the mortality rates due to covid-19 were still low) with March 2019 but that was followed by reductions of over 5% for the monthly comparisons of April and May 2020 (when covid-19 mortality spiked) versus 2019. Moreover, use of mechanical thrombectomy was lower by 11.26% (−12.54% to −10.09%) when comparing the first three months of the pandemic (March-May 2020) with the immediately preceding three months (December 2019-February 2020). Notably, comparing pandemic versus prepandemic periods, the proportion of patients admitted to hospital with AIS who received intravenous thrombolysis (from 9.75% to 10.1%, p=0.0049) or mechanical thrombectomy (from 6.33% to 7.59%, P<0.0001) significantly increased.
Figure 2.
Proportion of hospital admissions from acute ischemic stroke and acute myocardial infarction, and covid-19 related deaths from March 2019-February 2021. Deaths due to covid-19 are expressed in numbers ×10-1 (pink line)20
Figure 3.
Volume of intravenous thrombolysis, mechanical thrombectomy, any percutaneous coronary intervention, and percutaneous coronary intervention with acute myocardial infarction shown as number of admissions as well as covid-19 related deaths per month from March 2019-February 2021. Deaths due to covid-19 are expressed in numbers ×10-1 (pink line)20
Table 3.
Volume for acute ischemic stroke (AIS), acute myocardial infarction (AMI), intravenous thrombolysis (IVT), mechanical thrombectomy (MT), and percutaneous coronary intervention (PCI) during the covid-19 period and precovid-19 period
| Outcome | Precovid-19 period | Covid-19 period | Percentage volume change (95% CI) |
| Overall* | |||
| AIS | 134 768 | 116 452 | −13.59 (−13.77 to −13.41) |
| MT | 9343 | 9711 | 3.94 (3.55 to 4.36) |
| IVT | 16 202 | 14 6683 | −9.47 (−9.99 to −9.02) |
| AMI | 146 800 | 121 551 | −17.20 (−17.39 to −17.01) |
| PCI | 184 336 | 151 364 | −17.89 (−18.06 to −17.71) |
| PCI+AMI | 88 935 | 76 168 | −14.36 (−14.59 to −14.12) |
| First wave† | |||
| AIS | 34 053 | 26 779 | −21.36 (−21.80 to −20.92) |
| MT: | |||
| Overall | 2249 | 2262‡ | 0.58 (0.32 to 1.00) |
| March | 743 | 837 | 12.65 (10.39 to 15.30) |
| April | 728 | 687 | −5.63 (−7.63 to −4.12) |
| May | 778 | 738 | −5.14 (−7.00 to −3.47) |
| IVT | 3957 | 3466 | −12.91 (−13.99 to −11.90) |
| AMI | 37 632 | 28 458 | −24.38 (−24.81 to −23.94) |
| PCI | 47 978 | 33 699 | −29.76 (−30.17 to −29.35) |
| PCI+AMI | 22 690 | 18 182 | −19.87 (−20.39 to 19.35) |
| Second wave§ | |||
| AIS | 33 970 | 31 025 | −8.67 (−8.97 to −8.37) |
| MT | 2211 | 2601 | 17.64 (16.08 to 19.30) |
| IVT | 4068 | 3917 | −3.71 (−4.34 to −3.17) |
| AMI | 36 047 | 31 989 | −11.26 (−11.59 to −10.93) |
| PCI | 46 102 | 40 980 | −11.11 (−11.40 to −10.82) |
| PCI+AMI | 22 059 | 20 168 | −8.57 (−8.95 to −8.21) |
| Third wave¶ | |||
| AIS | 66 745 | 58 648 | −12.13 (−12.38 to −11.88) |
| MT | 4883 | 4848 | −0.72 (−1.00 to −0.51) |
| IVT | 8177 | 7305 | −10.66 (−11.35 to −10.01) |
| AMI | 73 121 | 61 104 | −16.43 (−16.70 to −16.17) |
| PCI | 90 256 | 76 685 | −15.04 (−15.27 to −14.80) |
| PCI+AMI | 44 186 | 37 818 | −14.41 (−14.74 to −14.09) |
The average volume of PCI in this table is higher than the average volume of AMI because PCI procedures might be associated with coronary artery disease treatment (besides AMI).
CI=confidence interval. AIS=acute ischemic stroke; AMI=acute myocardial infarction; IVT=intravenous thrombectomy; MT=mechanical thrombectomy; PCI=percutaneous coronary intervention.
*Covid-19 period of March 2020-February 2021 v precovid-19 period of March 2019-February 2020. †MT volume reduced by 26% reduction (95% CI −12.54% to −10.09%) when comparing the first three months of the pandemic (March-May 2020) with the immediately preceding three months (December 2019-February 2020). ‡Covid-19 period of March-May 2020 v precovid-19 period of March-May 2019. §Covid-19 period of June-August 2020 v precovid-19 period of June-August 2019. ¶Covid-19 period of September 2020-February 2021 v precovid-19 period of September 2019-February 2020
Results from multivariable generalized estimating equation models showed that patients with AIS who were admitted during the first year of the covid-19 pandemic had 9% higher odds of mortality in hospital (3.51% v 3.16%; ratio of the means 1.09 (95% confidence interval 1.03 to 1.15); p=0.0013) as compared with those admitted in the year prior to the pandemic. No difference in length of stay was noted across the pandemic and prepandemic periods (adjusted mean 4.27 (standard error 0.04) v 4.24 (0.04); ratio of the means 1.01 (95% confidence interval 0.99 to 1.03); p=0.3121) (online supplemental eTable 1).
bmjmed-2022-000207supp001.pdf (446KB, pdf)
Acute myocardial infarction
A total of 702 hospitals had at least one inpatient admission with a primary diagnosis of AMI during the study period. As seen for hospital admissions from AIS, more than two thirds of these centers had fewer than 300 beds, and 537 (72%) of 746 were non-teaching hospitals (table 1). As compared with people admitted to hospital over the year preceding the pandemic, patients admitted with AMI during the first year of the covid-19 pandemic tended to be younger and more often were male. The distribution of these patients also differed by racial profile, marital status, and health insurance payors, and patients had a significantly higher comorbidity burden than before the pandemic. Slightly more admissions with AMI were to smaller hospitals and non-teaching hospitals during the pandemic than before the pandemic. Additionally, the geographical redistribution in admissions to hospital from AMI in relation to the pandemic was very modest (table 4).
Table 4.
Patient and provider characteristics for patients with acute myocardial infarction in the covid-19 (March 2020-February 2021) versus precovid-19 study period (March 2019-February 2020)
| Characteristics | Precovid-19 period (March 2019-February 2020) (n=138 133) |
Covid-19 period (March 2020-February 2021) (n=111 580) |
| Age, years: | ||
| Mean, SD | 66.63 (13.12) | 66.17 (13.04) |
| 18-49 | 14 553 (10.54) | 12 172 (10.91) |
| 50-59 | 26 644 (19.29) | 22 102 (19.81) |
| 60-69 | 37 339 (27.03) | 31 056 (27.83) |
| ≥70 | 59 597 (43.14) | 46 250 (41.45) |
| Gender: | ||
| Male | 87 259 (63.17) | 71 598 (64.17) |
| Female | 50 874 (36.83) | 39 982 (35.83) |
| Race: | ||
| White | 109 802 (79.49) | 90 108 (80.76) |
| Black | 14 740 (10.67) | 11 866 (10.63) |
| Asian | 2914 (2.11) | 2276 (2.04) |
| Other | 10 677 (7.73) | 7330 (6.57) |
| Marital Status: | ||
| Married | 65 621 (47.51) | 53 081 (47.57) |
| Single | 59 314 (42.94) | 48 327 (43.31) |
| Other | 13 198 (9.55) | 10 172 (9.12) |
| Payor: | ||
| Commercial | 32 532 (23.55) | 27 356 (24.52) |
| Medicaid | 12 817 (9.28) | 11 048 (9.90) |
| Medicare | 80 576 (58.33) | 63 131 (56.58) |
| Other | 12 208 (8.84) | 10 045 (9.00) |
| Elixhauser score: | ||
| 0 | 4615 (3.34) | 3602 (3.23) |
| 1 | 15 372 (11.13) | 11 913 (10.68) |
| ≥2 | 118 146 (85.53) | 96 065 (86.10) |
| Congestive heart failure: | 58 943 (42.67) | 47 645 (42.70) |
| Hypertension | 114 858 (83.15) | 92 434 (82.84) |
| Diabetes | 57 420 (41.57) | 45 391 (40.68) |
| Obesity | 32 109 (23.24) | 28 552 (25.59) |
| Atrial fibrillation | 22 634 (16.39) | 17 781 (15.94) |
| Transient ischemic attack | 312 (0.23) | 258 (0.23) |
| Coronary artery disease | 117 244 (84.88) | 94 567 (84.75) |
| Dyslipidemia | 100 739 (72.93) | 82 526 (73.96) |
| Smoking | 36 603 (26.50) | 30 140 (27.01) |
| No of beds: | ||
| 0-299 | 46 559 (33.71) | 38 813 (34.78) |
| 300-500 | 45 620 (33.03) | 36 249 (32.49) |
| >500 | 45 954 (33.27) | 36 518 (32.73) |
| Region: | ||
| Midwest | 34 822 (25.21) | 28 534 (25.57) |
| Northeast | 18 047 (13.06) | 14 175 (12.70) |
| South | 65 493 (47.41) | 52 906 (47.42) |
| West | 19 771 (14.31) | 15 965 (14.31) |
| Urban-rural status: | ||
| Rural | 18 056 (13.07) | 14 628 (13.11) |
| Urban | 120 077 (86.93) | 96 952 (86.89) |
| Teaching | 69 133 (50.05) | 54 760 (49.08) |
| Percutaneous coronary intervention | 84 046 (60.84) | 70 491 (63.18) |
Data are number (percentage), unless otherwise specified. IQR=interquartile range; SD=standard deviation.
We depict the number of people admitted to hospital with AMI (figure 1) and people who received a percutaneous coronary intervention (figure 2), in relation to the national covid-19 mortality over the study period. In the year prior to the pandemic 146 800 people were admitted to hospital for AMI versus 121 551 over the pandemic year, representing a 17.20% decrease (95% confidence interval −17.39% to −17.01%) (table 2). Similarly, the use of any percutaneous coronary intervention (including AMI and other indications) declined by 17.89% ((−18.06% to −17.71%); from 184 336 to 1 51 364) across 470 hospitals providing this intervention whereas use of percutaneous coronary intervention for AMI declined by 14.36% ((−14.59% to −14.12%); from 88 935 to 76 168) across 444 hospitals. Despite this reduction in the absolute volumes of percutaneous coronary intervention, the proportion of patients who were admitted to hospital who received percutaneous coronary intervention for any indication significantly increased in relation to the number of AMI admissions (60.84% to 63.18%, p=0.0001) in the pandemic versus prepandemic period.
Multivariable generalized estimating equation computations showed that patients with AMI who were admitted to hospital over the first year of the covid-19 pandemic had 18.00% higher odds of mortality in hospital (4.81% v 4.29%; ratio of the means 1.18 (1.13 to 1.23); P<0.0001) and significantly shorter length of stay (adjusted mean (standard error), 3.81 (0.04) v 3.96 (0.04) days; ratio of the means 0.96 (0.95 to 0.97); P<0.0001) in the pandemic versus prepandemic periods (online supplemental eTable 2).
Secondary analyses
As compared with their corresponding periods in the prior year, admission to hospital for AIS significantly reduced across all three pandemic waves. These declines were more pronounced over the first wave (−21.36% (−21.80% to −20.92%)) than the third (−12.13% (−12.38% to −11.88%)) and second (−8.67%; (−8.97% to −8.37%)) waves. Similarly, more prominent reductions in intravenous thrombolysis were noted in the first wave (−12.91%; (13.99% to −11.90%)) and third wave (−10.66%; (−11.35% to −10.01%)) versus the second wave (−3.71%; (−4.34% to −3.17%)). The mechanical thrombectomy volumes over the first (0.58%; (0.32% to 1.00%)) and third (−0.72%; (−1.00% to −0.51%)) wave were nearly unchanged but volume increased by 17.64% (16.08% to 19.30%) over the second wave (table 3). We provide data for characteristics of patients with AIS and of the provider, as well as mortality in hospital and length of stay analyses for each covid-19 wave in the supplement (online supplemental eTables 3–8). mortality from AIS in hospital was highest during the second wave (3.43% v 2.84%; ratio of the means 1.16 (1.06 to 1.27); p=0.0010) and had non-significant increases over the first and third wave.
Similarly, significant reductions in AMI hospital admission volumes were noticed across all three pandemic waves with greater prominence over the first wave (−24.38% (−24.81% to −23.94%)) than the third (−16.43%; (−16.70% to −16.17%)) and second (−11.26%; (−11.59% to −10.93%)) waves. Absolute volumes of percutaneous coronary intervention also decreased throughout all three pandemic waves (table 3). Data for AMI patient and provider characteristics as well as mortality in hospital and length of stay analyses for each wave are provided in the supplement (online supplemental eTables 9–14). Mortality from AMI in hospital showed a significant and gradual increase across all three covid-19 waves in relation to their corresponding periods over the preceding year, reaching its peak at 21% higher odds over the third wave (5.08% v 4.39%; ratio of the means 1.21 (1.14 to 1.28); P<0.0001).
We also provide additional analysis including the timeframe for covid-19 from March 2020 to December 2021 in which we looked at hospitals that provided continuous inpatient and outpatient data for 34 months (online supplemental file 1, online supplemental eTable 15). The results are consistent with trends seen in prior analysis (online supplemental efigures 2 and 3)
Discussion
By use of a large multihospital database, we examined the impact of the first year of the covid-19 pandemic on acute care of AIS and AMI in the US. Our study documents an important reduction in all measured metrics for AIS and AMI care, including the change in numbers of admissions for AIS (−13.59%) and AMI (−17.20%) during the pandemic versus before the pandemic. We also noted significant decreases in volumes for intravenous thrombolysis (−9.47%) as well as any percutaneous coronary intervention (−17.89%) and percutaneous coronary intervention for AMI only (−14.36%). Although the comparative mechanical thrombectomy changes in relation to the prior year were presumably masked by the temporal increases in mechanical thrombectomy volumes observed over the recent years (as suggested by the March 2019 to March 2020 trends in figure 2),23 24 comparison of the first three pandemic months with three months immediately preceding the pandemic showed a 11.26% reduction in mechanical thrombectomy procedures. This mirrors the decline described across the same three month periods in a recent large multicenter global analysis.25 Notably, the extent of the changes in AIS and AMI care had a distinct temporal correlation with spikes in deaths related to covid-19 (figures 1 and 2). Additionally, adjusted rates of mortality in hospital increased for both AIS and AMI while length of stay was shorter for AMI but not AIS.
Our results are in alignment with several previous studies that showed significant reductions in the care for AIS and AMI during the first wave of covid-19 pandemic worldwide.25–31 However, prior studies were largely focused on the first few months of the pandemic and, until now, whether these effects would be limited to the early phases or would continue to occur throughout the pandemic was unclear. In this context, a few recent studies have suggested a potential recovery in the volumes of AIS and AMI care over the subsequent months of the pandemic.26–29 Although our study showed similar trends over these same periods, our findings show that, in the longer term, the collateral effects of the pandemic over the care of AIS and AMI seem to reliably recur with any new upsurges in covid-19, suggesting that systems of care and populational behaviors did not readapt over time.
Among patients being admitted with a diagnosis of AIS or AMI during the covid-19 period, we observed an increase in treatment rates as compared with those for patients admitted before covid-19 emergence. The observed relative increase in the reperfusion treatment rates suggests a shift towards greater clinical severity for patients in hospital during the pandemic. AIS studies have reported a greater reduction in the admissions for transient ischemic attack and mild strokes compared with more severe stroke presentations.3 8 Similarly, acute coronary syndromes studies showed greater reductions in volumes for unstable angina and non-ST-elevation myocardial infarction than for ST-elevation myocardial infarction.13 17 32 Notably, the outbreak appears to have not only impacted AIS and AMI treatment volumes but also their workflow leading to increases in times from symptom onset to first medical contact and door to reperfusion for both conditions.8 10 12 33–35
A particularly important novel finding of our study is the significantly higher mortality in hospital for AIS and AMI over the first year of the pandemic. Although we could not find a convincing explanation for the reported increase in mortality, the aforementioned workflow delays combined with the relative increase in disease severity were presumably main contributors to the increased mortality in hospital. Additionally, despite their overall low frequency, patients presenting with concomitant diagnosis of covid-19 and AIS or AMI are known to have much poorer outcomes.36 37 Such combined presentations might have contributed to observed increases in mortality. The observed length of stay changes in AMI were possibly a reflection of pressures on healthcare systems to improve efficiencies of hospital resources, such as accelerated treatment and discharge, to reduce risks of viral exposure and optimize bed capacity during the pandemic.
Our study is limited by the design of any retrospective analysis, such as the availability of data for relevant clinical outcomes. Although we could assess patient comorbidity status, clinical metrics such as modified Rankin scale scores were not available. We also tried to be comprehensive as much as we could by including hospitals with a minimum criterion of admissions of ‘one’ during the study period. This criterion could have created some noise in the data and would limit hospitals that have some minimum threshold enough to get stable estimates. Additionally, sampling could be a possible limitation that could affect generalizability given that Premier Healthcare Database collects data from voluntary participating hospitals with possible selection bias. Furthermore, some of the absolute differences observed were small and might not be clinically relevant. We only had missing data for race and marital status. The rate of missing data for patients with AIS in precovid-19 period sample was 2.98% and for postcovid-19 period was 3.49%. The rate of missing data for AMI patients in precovid-19 period sample was 2.33% and for post-covid-19 period was 2.90%. Given the small proportion of missing information, we do not expect our results to have been affected by removal of patients with missing data. Moreover, billing and coding errors, especially as hospitals face logistical and administrative stress during the pandemic, could have influenced the accuracy of study results.
Conclusions
This large multihospital database analysis adds to the increasing evidence evaluating the side effects of the covid-19 pandemic in the treatment and outcomes for acute care conditions across US hospitals. Although improved efficiencies in hospital length of stay were observed in AMI during the covid-19 era, inpatient mortality for AIS and AMI was higher and the number of hospital admissions was lower than expected. The persistence of these deleterious effects one year into the pandemic is particularly troublesome. Many factors could be considered including reduced capacity at hospitals, changes in service organisation and how to access care, and delays due to health services and intensive care facilities being overwhelmed. From a public policy perspective, public health interventions (eg, awareness media campaigns and relevant health education) should be used to engage and inform the public about the effects of untreated acute conditions and to prevent these observed decreases in patient admissions to hospital in future pandemics.
Footnotes
Contributors: RGN designed study conception and work, reviewed the literature, interpreted data, and drafted the manuscript. RK, SI, CW, and KE collected data, did the statistical analysis, reviewed the literature, and critically revised the manuscript. Other coauthors critical revised the manuscript. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RGN is identified as guarantor and is responsible for the overall content. Transparency: The lead author (RGN) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Funding: We declare no specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organization for the submitted work; RGN reports consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Hybernia, Imperative Care, Medtronic, Phenox, Philips, Prolong Pharmaceuticals, Stryker Neurovascular, Shanghai Wallaby, Synchron, and stock options for advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz-AI, RapidPulse and Perfuze. RGN is one of the Principal Investigators of the “Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW) trial. Funding for this project is provided by Cerenovus. RGN is an investor in Viz-AI, Perfuze, Cerebrotech, Reist/Q’Apel Medical, Truvic, and Viseon. TNN reports research support from Medtronic, Society of Vascular and Interventional Neurology (SVIN) and Data Safety Monitoring Board participation on the TESLA, ENDOLOW, SELECT-2, PROST, CREST-2, and WE-TRUST trials. KE, SI, CW, and RK are employees of Johnson & Johnson and provided analytical support for this manuscript. DCH reports consulting fees from Stryker, Cerenovus and Vesalio and stock options in VizAI. CMD reports consulting fees from Medtronic, ReCor Medical, Vascular Dynamics and Proctor fees from Edwards Lifesciences. ARAB reports consulting fees from Stryker.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
References
- 1.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ 2020;368:m1036. 10.1136/bmj.m1036 [DOI] [PubMed] [Google Scholar]
- 2.CDC . Covid data tracker [Internet]. Centers for Disease Control and Prevention. 2020. Available: https://covid.cdc.gov/covid-data-tracker
- 3.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med 2020;382:NEJMc2009787. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaghi S, Ishida K, Torres J, et al. SARS-cov-2 and stroke in a new York healthcare system. Stroke 2020;51:2002–11. 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke 2020;51:1991–5. 10.1161/STROKEAHA.120.030329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onteddu SR, Nalleballe K, Sharma R, et al. Underutilization of health care for strokes during the COVID-19 outbreak. Int J Stroke 2020;15:9–10. 10.1177/1747493020934362 [DOI] [PubMed] [Google Scholar]
- 7.Choudry FA, Hamshere SM, Rathod KS, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2020;76:1168–76. 10.1016/j.jacc.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak: decreased activity, and increased care delays. Stroke 2020;51:2012–7. 10.1161/STROKEAHA.120.030373 [DOI] [PubMed] [Google Scholar]
- 9.Montaner J, Barragán-Prieto A, Pérez-Sánchez S, et al. Break in the stroke chain of survival due to COVID-19. Stroke 2020;51:2307–14. 10.1161/STROKEAHA.120.030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baracchini C, Pieroni A, Viaro F, et al. Acute stroke management pathway during coronavirus-19 pandemic. Neurol Sci 2020;41:1003–5. 10.1007/s10072-020-04375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Li H, Kung D, et al. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke 2020;51:1996–2001. 10.1161/STROKEAHA.120.030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schirmer CM, Ringer AJ, Arthur AS, et al. Delayed presentation of acute ischemic strokes during the COVID-19 crisis. J Neurointerv Surg 2020;12:639–42. 10.1136/neurintsurg-2020-016299 [DOI] [PubMed] [Google Scholar]
- 13.Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in england. Lancet 2020;396:381–9. 10.1016/S0140-6736(20)31356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholz KH, Lengenfelder B, Thilo C, et al. Impact of COVID-19 outbreak on regional STEMI care in Germany. Clin Res Cardiol 2020;109:1511–21. 10.1007/s00392-020-01703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, et al. Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. RECICE 2022;4060. 10.24875/RECICE.M20000123 [DOI] [Google Scholar]
- 16.Lantelme P, Couray Targe S, Metral P, et al. Worrying decrease in hospital admissions for myocardial infarction during the COVID-19 pandemic. Arch Cardiovasc Dis 2020;113:443–7. 10.1016/j.acvd.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papafaklis MI, Katsouras CS, Tsigkas G, et al. 'Missing'' acute coronary syndrome hospitalizations during the COVID-19 era in Greece: medical care avoidance combined with a true reduction in incidence? Clin Cardiol 2020;43:1142–9. 10.1002/clc.23424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv 2020;13:e009438. 10.1161/CIRCINTERVENTIONS.120.009438 Available: https://www.ahajournals.org/doi/10.1161/CIRCINTERVENTIONS.120.009438 [DOI] [PubMed] [Google Scholar]
- 19.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the united states during COVID-19 pandemic. J Am Coll Cardiol 2020;75:2871–2. 10.1016/j.jacc.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centres for Disease Control and Prevention . United States COVID-19 cases and deaths by state over time-archived. 2021. Available: https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36
- 21.Liao J-N, Chao T-F, Liu C-J, et al. Seasonal variation in the risk of ischemic stroke in patients with atrial fibrillation: a nationwide cohort study. Heart Rhythm 2018;15:1611–6. 10.1016/j.hrthm.2018.06.043 [DOI] [PubMed] [Google Scholar]
- 22.Furukawa Y. Meteorological factors and seasonal variations in the risk of acute myocardial infarction. Int J Cardiol 2019;294:13–4. 10.1016/j.ijcard.2019.07.089 [DOI] [PubMed] [Google Scholar]
- 23.Smith EE, Saver JL, Cox M, et al. Increase in endovascular therapy in get with the Guidelines-Stroke after the publication of pivotal trials. Circulation 2017;136:2303–10. 10.1161/CIRCULATIONAHA.117.031097 [DOI] [PubMed] [Google Scholar]
- 24.Saber H, Navi BB, Grotta JC, et al. Real-World treatment trends in endovascular stroke therapy. Stroke 2019;50:683–9. 10.1161/STROKEAHA.118.023967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nogueira RG, Abdalkader M, Qureshi MM, et al. Global impact of COVID-19 on stroke care. Int J Stroke 2021;16:573–84. 10.1177/1747493021991652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueira RG, Qureshi MM, Abdalkader M, et al. Global impact of COVID-19 on stroke care and IV thrombolysis. 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Havenon A, Yaghi S, Majersik JJ, et al. Acute coronary syndrome and ischemic stroke discharges in the united states during the COVID-19 pandemic. Stroke 2021;52:e239–41. 10.1161/STROKEAHA.120.033630 Available: https://www.ahajournals.org/doi/10.1161/STROKEAHA.120.033630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon MD, Nguyen-Huynh M, Leong TK, et al. Changes in patterns of hospital visits for acute myocardial infarction or ischemic stroke during COVID-19 surges. JAMA 2021;326:82–4. 10.1001/jama.2021.8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen DM, Butt JH, Fosbøl E, et al. Nationwide cardiovascular disease admission rates during a second COVID-19 lockdown. Am Heart J 2021;241:35–7. 10.1016/j.ahj.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Leor O, Cid-Álvarez B, Ojeda S, et al. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en españa. RECIC 2021;4059. 10.24875/RECIC.M20000120 [DOI] [Google Scholar]
- 31.De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during covid-19 outbreak in northern italy. N Engl J Med 2020;383:88–9.:NEJMc2009166. 10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in italy in the COVID-19 era. Eur Heart J 2020;41:2083–8. 10.1093/eurheartj/ehaa409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo K-C, Leung WCY, Wong Y-K, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke 2020;51:2228–31. 10.1161/STROKEAHA.120.030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam C-CF, Cheung K-S, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in hong kong, china. Circ Cardiovasc Qual Outcomes 2020;13:CIRCOUTCOMES.120.006631. 10.1161/CIRCOUTCOMES.120.006631 Available: https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.120.006631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roffi M, Guagliumi G, Ibanez B. The obstacle course of reperfusion for ST-segment-elevation myocardial infarction in the COVID-19 pandemic. Circulation 2020;141:1951–3. 10.1161/CIRCULATIONAHA.120.047523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Havenon A, Ney JP, Callaghan B, et al. Characteristics and outcomes among US patients hospitalized for ischemic stroke before vs during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2110314. 10.1001/jamanetworkopen.2021.10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia S, Dehghani P, Grines C, et al. Initial findings from the north american COVID-19 myocardial infarction registry. J Am Coll Cardiol 2021;77:1994–2003. 10.1016/j.jacc.2021.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjmed-2022-000207supp001.pdf (446KB, pdf)
Data Availability Statement
Data are available upon reasonable request.