Abstract
Chagas disease is underappreciated as a health concern in the United States. Approximately 40 000 women of childbearing age living in the United States have chronic Chagas disease. Most of them are unaware that they have an infection that is transmissible to their offspring. The estimated US maternal-to-infant transmission rate of Trypanosoma cruzi is 1% to 5%. Ten percent to 40% of neonates with congenital T cruzi infection have clinical signs consistent with a congenital infection but no findings are unique to Chagas disease. If left untreated, 20% to 40% of infants with Chagas disease will later develop potentially fatal cardiac manifestations. Molecular testing can confirm the diagnosis in neonates. Treatment is well tolerated in infancy and usually results in cure. Screening of at-risk women during pregnancy can identify maternal infection and allow early assessment and treatment for congenital T cruzi infection.
Keywords: Chagas disease, congenital infection, Trypanosoma cruzi
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, has acute and chronic phases. Acute T cruzi infection is typically asymptomatic or can manifest as a mild and self-limited influenza-like illness that lasts 2 to 3 months. After the acute phase, infection enters a chronic phase that, without treatment, persists for life. After years or decades of this quiescent phase, designated the “chronic indeterminate” phase, 20% to 40% of people with untreated infection develop Chagas cardiomyopathy or gastrointestinal disease. Infants born congenitally infected are in the acute phase for their first few months of life and, if untreated, then enter the chronic phase.
Chagas disease is often considered a disease restricted to Latin America and can be underappreciated as a health concern by US healthcare providers (HCPs) [1, 2]. In a survey of US obstetrician-gynecologists, few knew the risk of congenital T cruzi infection, and most never considered Chagas disease in their patients from countries in which it is endemic [1]. Participants in a survey about congenital Chagas disease distributed to Pediatric Infectious Diseases Society members also reported having limited knowledge about congenital T cruzi infection [2].
The Centers for Disease Control and Prevention (CDC) considers Chagas disease a priority for public health action on the basis of the US disease burden, severity of the illness, and the availability of modalities for its treatment and prevention [3]. In this review, we provide updated information for HCPs caring for infants and children regarding the burden of disease and population at risk for Chagas disease in the United States, the features of congenital Chagas disease, and the diagnosis and management of congenital T cruzi infection.
METHODS
We conducted a search of the published literature through the National Library of Medicine (PubMed) using the medical subject heading terms “Chagas disease,” “congenital” and “Trypanosoma cruzi,” and “congenital.” To supplement the search strategy, the reference lists from relevant publications and key articles on Chagas disease epidemiology, diagnosis, treatment, and prevention were reviewed to identify additional publications relevant to congenital T cruzi infection.
Illustrative Case
You are consulted to evaluate a 3.5-month-old infant born to a 40-year-old mother by scheduled repeat cesarean section at term gestation after an uncomplicated pregnancy. The infant’s nursery stay was uncomplicated. The mother donated the infant’s cord for research purposes and was notified that screening by the cord blood bank was positive for Chagas disease. The infant’s mother was born in Guatemala and grew up in a rural area of the country. She has lived in the United States for 15 years, and 5 older children are in the family.
Is this infant at risk for congenital Chagas disease? What evaluation is indicated?
What referral is appropriate for the mother?
Should other children in the family undergo any testing?
What Is the Burden of Chagas Disease in the United States?
Approximately 300 000 persons in the United States are estimated to have T cruzi infection. These people have chronic-phase Chagas disease and acquired their infection in a region of Latin America in which the disease is endemic [4, 5]. Cases of Chagas disease are found among residents of all 50 states and the District of Columbia [4]. Four states (California, Florida, New York, and Texas) each have more than 10 000 persons affected, and 7 other states (Arizona, Georgia, Maryland, Nevada, New Jersey, North Carolina, and Virginia) each have more than 5000 persons affected.
Approximately 85% of T cruzi-infected persons in the United States are estimated to have come to the United States from Mexico, El Salvador, Guatemala, or Honduras. Persons originally from Argentina, Ecuador, Colombia, Brazil, Bolivia, Nicaragua, or Peru account for most of the remainder of those infected [5].
Human cases of domestically acquired Chagas disease in the United States are well documented but uncommon; as of 2018, fewer than 50 cases have been reported. Many of these domestically acquired cases have occurred in persons with an outdoor occupation or leisure activity [6-8]. The vector of T cruzi is the triatomine insect. At least 28 states, many in the southern United States, have T cruzi-infected triatomines or infected mammalian reservoir species, such as armadillos or opossums, or both [9-11].
Approximately 40 000 US women of childbearing age in the United States are estimated to have Chagas disease [5]. Nearly all of these women acquired T cruzi infection while living in a region of endemicity, and most of them are unaware that they have the infection and that it can be transmitted congenitally. An estimated 63 to 315 infants in the United States are born with congenital Chagas disease each year [5]. The total number of children in the United States with Chagas disease was estimated a decade ago to be at least 2000 [12].
Chagas cardiomyopathy contributes to the burden of US heart disease. At least 30 000 to 45 000 persons in the United States have Chagas cardiomyopathy, the majority of whom are undiagnosed [5]. In a cross-sectional study, 13% of 39 patients in New York City with nonischemic dilated cardiomyopathy who were born or had lived for at least 1 year in a country in which Chagas disease is endemic had Chagas disease [13]. Similarly, Chagas disease was diagnosed in 5.2% of 327 patients in Los Angeles who had electrocardiographic conduction abnormalities and who had resided in Latin America for at least 1 year [14]. Ongoing damage can lead to complete heart block, ventricular arrhythmias, or embolic phenomena. Sudden death can result from the rupture of an apical aneurysm, heart failure with dilated cardiomyopathy, or ventricular arrhythmias [15].
What Are the Modes of Chagas Disease Transmission?
The most common mode of transmission is vector-borne, through the bite and subsequent defecation of a blood-sucking triatomine insect known as a kissing bug. Triatomines in areas of endemicity in the Americas can carry T cruzi in their intestinal tracts. Triatomines defecate during or after biting, and T cruzi trypomastigotes, passed in the insect’s feces, can enter the body when the fecal matter contaminates a break in the skin, such as at the bite site, or intact mucous membranes or conjunctivae. The risk of infection in regions of endemicity is greatest for those with repeated, prolonged exposure to the vector [16]. Triatomines are night feeders, and residing in a rural setting and in an adobe or thatched-roofed dwelling in countries in which the disease is endemic increases exposure risk.
Blood transfusion and organ transplantation are also potential modes of transmission. Serologic screening of US blood donors for T cruzi infection was first implemented in 2007 [17]. During 2007–2018, the AABB (formerly known as the American Association of Blood Banks) has received reports of more than 2400 people with confirmed T cruzi infection living in the United States, identified through screening of potential blood donors [18]. Chagas disease can be life-threatening in solid organ transplant recipients who are infected with T cruzi or through the use of an organ(s) from a chronically infected donor [19]. Widespread screening of blood donations and many organ donors has rendered the risks of disease attributable to these modes of transmission rare in the United States [5].
Transmission can occur through the ingestion of food or drink that has been contaminated by an infected triatomine or its fecal material. This mode of transmission is relatively uncommon in regions of endemicity and has not been reported in the United States [20].
Congenital transmission of T cruzi is an increasing public health concern in settings in which such infection is not endemic because of population migrations and in settings of historical endemicity where control measures have reduced vector-borne transmission [21]. The US maternal-to-infant transmission rate of T cruzi is estimated to be 1% to 5% [5]. Countries in which the disease is endemic can have a higher rate of congenital transmission than in those in which it is not endemic [22].
Who Is at Risk for Congenital Chagas Disease?
Infants born to women with Chagas disease are at risk for congenital T cruzi infection. Screening of 4000 women who delivered their infant at a Houston hospital, 85% of whom were non-US born, many from regions in which Chagas disease is endemic, revealed a 1-in-400 rate of chronic previously unrecognized Chagas disease [23]. Screening of 4755 Latin America–born adult residents of Los Angeles County, among whom almost half were childbearing-age women, found an overall 1.24% prevalence of Chagas disease [24]. Transgenerational infection can occur, so women who themselves are infected congenitally can transmit T cruzi to their infants in the absence of direct exposure to the vector [25, 26].
What Is the Pathogenesis of Congenital Chagas Disease?
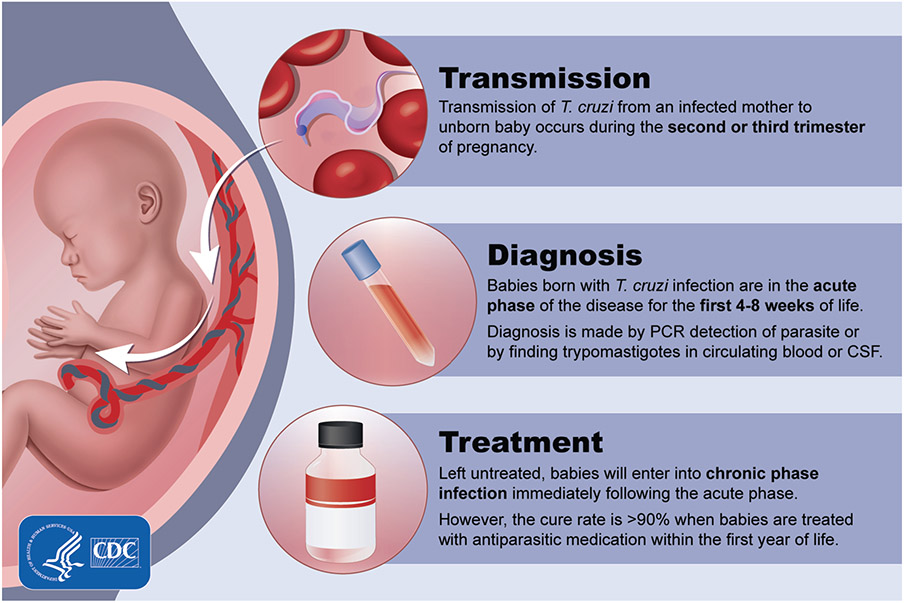
Congenital transmission of T cruzi can occur during acute or chronic maternal infection and throughout the reproductive years. Transmission occurs during the second or third trimester of pregnancy (Figure 1). Congenital infection does not lead to congenital malformation, presumably because transmission occurs after organogenesis is complete [25]. Invasion of the trophoblast with high-grade parasitemia can cause placentitis and villitis and areas of trophoblast destruction and necrosis. When these findings are present, infection can lead to fetal loss, stillbirth, or premature delivery, often with a fatal outcome [25, 27]. Early transmission increases the risk of spontaneous abortion, and transmission after 22 weeks of gestation results in stillbirth or an infected live-born infant [28, 29].
Figure 1.
Time course of congenital Chagas disease
In pregnant women with chronic infection and low-grade parasitemia, villitis is less marked or not observed and parasites are rare or not observed in the extravillous trophoblast. Typical findings include chorioamnionitis and funisitis from infiltration of neutrophils and lymphocytes into the membranes surrounding the fetus and the umbilical cord. The finding of parasites in the intervillous space suggests that infection can also occur by passage through the placental margin zone rather than by invasion of the trophoblast. Transmission of infection can occur near delivery if breaches or tears in the placenta occur [27].
Postnatal transmission of T cruzi occurs rarely, if ever. Although possible in theory, transmission through breastfeeding has not been established, and breastfeeding is generally recommended for infants born to a mother with chronic Chagas disease [30]. As a precaution, infants should not be fed breast-milk from a mother with acute disease, during reactivation of chronic infection caused by immunocompromised status, or if the mother’s nipples are bleeding (until bleeding has resolved).
What Factors Increase the Risk of Congenital Transmission?
Factors known or suspected to enhance transmission are shown in Table 1 [31-39]. Parasite load is the strongest predictor. Young maternal age can increase risk, possibly because young women are more likely than older women to have a recently acquired infection [26, 33]. Vector exposure during pregnancy can also modulate the parasite load [35, 36]. Untreated maternal human immunodeficiency virus coinfection is associated with a high parasite load, which increases the risk of infant T cruzi infection [37]. The transmission rate is higher for twins than for singletons, possibly because of a more intensified downregulation of maternal immunity [36]. Infection can be transmitted during sequential pregnancies.
Table 1.
Factors Associated or Possibly Associated With Increased Risk of Congenital Transmission of Trypanosoma cruzia
| Factor | Comment (Representative Reference[s]) |
|---|---|
| Maternal parasitemia | Parasite load is significantly higher in mothers of infected infants than in those of uninfected infants [32] |
| Maternal age | Younger maternal age is presumed to reflect more recent infection [33]; |
| Vector exposure during pregnancy | Sustained vector exposure might boost maternal immunity and reduce the risk of transmission [35, 36] |
| HIV infection | Increased risk of transmission is attributed to HIV-infected women who have a higher level of parasitemia [37] |
| Maternal immune responses | Congenital transmission is associated with decreased production of interferon-γ by maternal cells in response to parasitic antigens [38, 39] |
| Twin pregnancy | Possibly related to maternal T-cell downregulation in multiple pregnancies [36] |
| Influence of genetic diversity of T cruzi | Can affect transmission, but most data are from regions in which the TcII, V, and VI lineages predominate; additional data are needed from TcI-dominant regions [40, 41] |
Abbreviation: HIV, human immunodeficiency virus.
Modified from Messenger et al [31].
Current consensus recognizes 6 genetic lineages of T cruzi, TcI through TcVI [40]. Genotype can affect transmission, but most data are from South American countries, including Bolivia, Argentina, and Chile, where TcII, TcV, and TcVI predominate in those with congenitally acquired infection [31]. The non-TcI lineages also predominated in a prospective observational study of congenital transmission conducted in 2 hospitals in Mexico [41]. Familial clustering supports a role for parasite genotype or strain in transmission [42]. Additional study is needed from TcI-dominant regions in Central America and Mexico [31].
What Are the Manifestations of Congenital Chagas Disease?
Approximately 10% to 40% of T cruzi congenitally-infected infants have clinical findings of Chagas disease [43, 44] that are detectable at birth or within days or weeks after birth [25]. Low birth weight for gestational age, prematurity, and low Apgar scores are common [26]. Respiratory distress, when present, is thought to be related to prematurity [45]. Table 2 shows the findings in the 91 of 201 infants with congenital Chagas disease who had signs of infection [33, 43, 45, 46]. Hepatomegaly, with or without splenomegaly, was the most common finding and was accompanied in some infants by anemia, thrombocytopenia, or petechiae [46]. Infants with more severe infection presented with meningoencephalitis, pneumonitis, myocarditis, or hydrops fetalis. Ophthalmologic findings can include vitreitis and retinitis [47]. Infants also can have pleural or pericardial effusions and, occasionally, gastrointestinal megasyndromes [26, 48]. Approximately 5% of infants with signs of infection at birth have a fatal outcome, usually as a result of myocarditis or meningoencephalitis [44]. No features are pathognomonic for congenital Chagas disease. The diagnosis should be considered for any infant with clinical signs when the maternal history is consistent with residence in a region of endemicity.
Table 2.
Features of Congenital Chagas Disease Among 91 Neonates With Clinical Findingsa
| Feature | Frequency of Findingb |
|---|---|
| Low birth weight (<2500 g) | ++++ |
| Prematurity | ++ |
| Respiratory distress | +++ |
| Hepatomegaly | ++++ |
| Splenomegaly | +++ |
| Sepsis | ++ |
| Cardiomegaly/heart failure | ++ |
| Myocarditis | ++ |
| Cardiac arrhythmia | ++ |
| Meningoencephalitis | ++ |
| Neurologic signs | ++ |
| Edema/anasarca | ++ |
| Petechiae | ++ |
| Anemia | + |
Reports of 2 confirmed cases of congenital Chagas disease in the United States have been published; each of these children was born to a mother who had immigrated from a country in which the disease is endemic, and each of them presented with hydrops fetalis [49, 50].
Otherwise healthy congenitally infected infants usually do well in infancy. However, 20% to 40% of children with untreated congenital Chagas disease develop irreversible life-threatening, and often fatal, heart disease after years or decades of silent infection. The risk for Chagas cardiomyopathy exists regardless of whether clinical signs of infection were present at birth [28].
What Are Some Challenges to Diagnosing Congenital Chagas Disease?
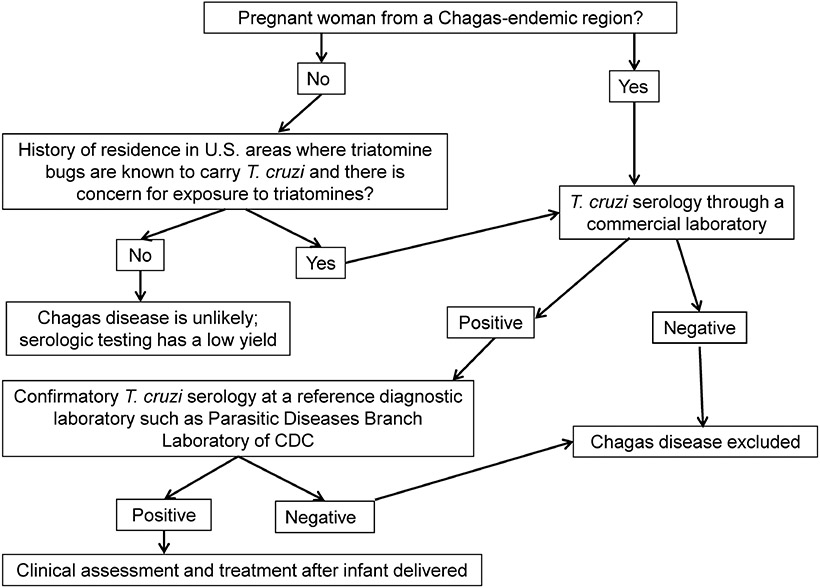
Infants at risk for congenital Chagas disease can be identified before birth through maternal antenatal screening. All women who have lived in a region of endemicity should be tested using a commercially available serologic assay (Figure 2) [51-53]. Targeted maternal screening of women who have lived in a region of endemicity would enable early identification of and appropriate testing for at-risk neonates. Routine antenatal screening for T cruzi is currently not widely implemented in the United States.
Figure 2.
Algorithm for Evaluation of Chagas Disease in Pregnant Women
Because no single serologic test is sufficiently sensitive and specific for establishing the diagnosis, patients with a positive T cruzi antibody screen result require confirmation with 2 or more tests that use different techniques and different antigen preparations at a reference laboratory, such as the Parasitic Diseases Reference Laboratory at the CDC. Requests for CDC testing should be coordinated with the respective state health department, and in many states, including those in which Chagas disease is a reportable condition, routing of specimens through the state health department is required. As of 2018, Chagas disease is reportable in Arizona, Arkansas, Louisiana, Mississippi, Tennessee, Texas, and Utah. Women identified as having Chagas disease should be referred for clinical evaluation and treatment after their infant is delivered.
What Testing Is Needed to Establish the Diagnosis of Congenital Chagas Disease?
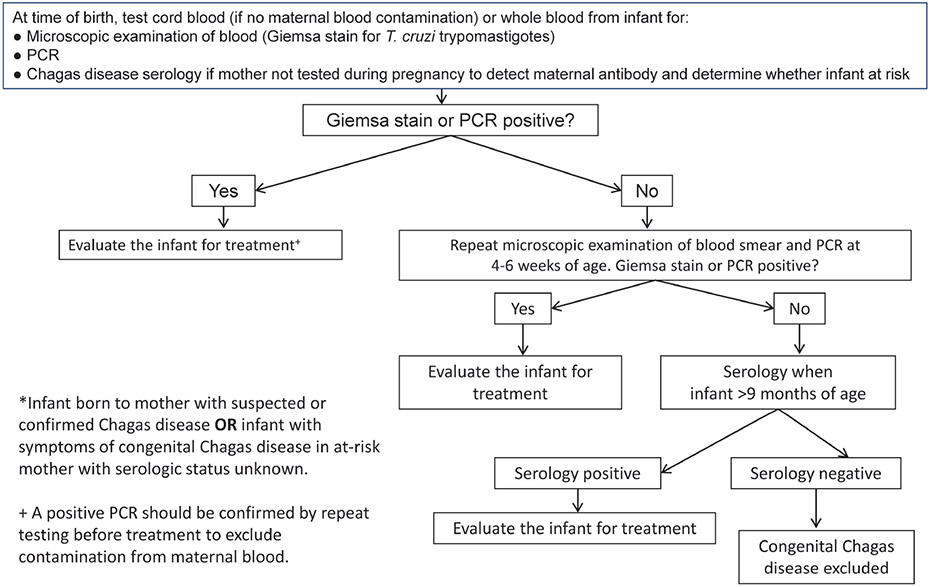
Infants born to a woman known to have T cruzi infection and infants with clinical signs suggestive of Chagas disease should undergo testing as soon as possible after birth (Figure 3) [53]. Serologic testing is appropriate as an initial step if the mother’s infection status is not known. The diagnosis of congenital infection can be confirmed by the detection of motile trypomastigotes through microscopic examination of fresh anticoagulated blood specimens or by polymerase chain reaction (PCR) testing of a whole blood sample. This testing is available through the CDC Parasitic Diseases Reference Laboratory; this laboratory uses a multitargeted PCR testing algorithm using T cruzi minicircle TaqMan real-time PCR and nuclear T cruzi minisatellite TaqMan real-time PCR assays to detect circulating parasite DNA [49, 54]. Results are usually available within 1 week of receipt by the CDC.
Figure 3.
Algorithm for Evaluation of Congenital Chagas Disease: Infant <3 Months of Age*. Abbreviaton: PCR, polymerase chain reaction.
Low levels of maternal DNA can be detected in uninfected infants born to an infected mother; as a consequence, a positive PCR result for an infant must be confirmed by repeat testing as soon as possible (Figure 3). The detection of maternal DNA is unlikely after the first 2 weeks of life. If the result of a second PCR assay is positive, the diagnosis of congenital Chagas disease is confirmed, and the infant should be evaluated clinically and treated. Infants born to a T cruzi-infected mother whose PCR result is negative in the first weeks of life should undergo repeat testing at age 4 to 6 weeks to confirm the absence of infection, because parasite load increases in the weeks after birth and peaks between 1 and 2 months of age [28, 32].
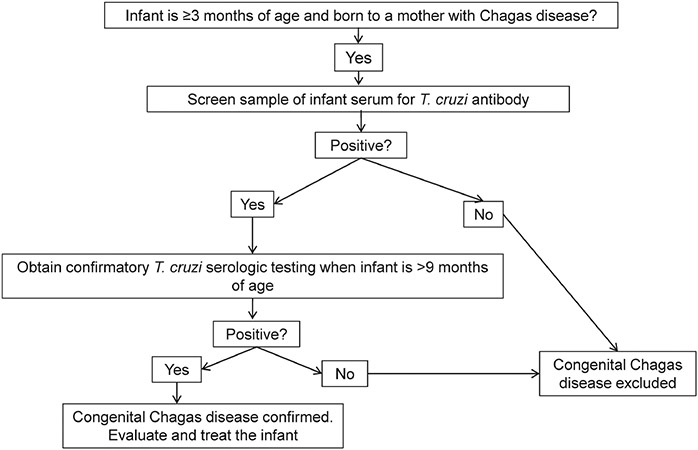
Congenital infection ideally should be detected as early as possible after birth. However, parasitemia levels can vary, and the timing of sample collection in the early postnatal period can be suboptimal. Because of these concerns, the serologic status of an infant born to a mother with chronic Chagas disease should be monitored even if the infant has had negative PCR results at earlier sampling intervals [46]. Passively acquired maternal antibody should no longer be detectable by 9 months after birth. If an infant is evaluated first at 3 months of age or older, serologic testing after 9 months of age is appropriate to document or exclude congenital infection (Figure 4) [52].
Figure 4.
Algorithm for Evaluation of Congenital Chagas Disease for Infants ≥3 Months of Age
Identification of a mother with Chagas disease should trigger screening of other family members. Serologic screening through a commercial laboratory is indicated for siblings of infants born to a mother with Chagas disease. Screening also should be considered for relatives, including the mother’s siblings and the infant’s maternal grandmother, because family clusters of T cruzi infection can occur [55-57]. One family study in a region of endemicity found an average of 3 infected members per nuclear family [57].
What Treatment Is Available for Chagas Disease?
Two medications, benznidazole and nifurtimox, are recommended for the treatment of congenital Chagas disease. For children aged 2 to 12 years, the US Food and Drug Administration–approved dose of benznidazole is 5 to 8 mg/kg per day orally in 2 doses divided every 12 hours for 60 days [58]. The same dosing range has been used to treat infants [16, 50]. Nifurtimox (15–20 mg/kg per day orally in 3 or 4 doses for 90 days) is administered for infants and children aged 10 years or younger. Both medications are well tolerated in neonates and infants [59, 60]. However, on the basis of accumulated clinical experience and a more favorable adverse effect profile, benznidazole is considered first-line treatment. As of 2018, benznidazole can be obtained commercially through Exeltis USA (Florham Park, New Jersey). It is available in a 12.5 mg pediatric formulation and in 100 mg tablets. Information regarding access to benznidazole can be obtained through the company’s website at http://www.benznidazoletablets.com or by contacting the Fast Access Program via Foundation Care at (877) 303–7181. Nifurtimox is available from the CDC under an investigational protocol. Information regarding treatment of T cruzi infection in neonates or young infants with confirmed congenital Chagas disease or release of nifurtimox can be obtained by contacting the CDC’s Parasitic Diseases Branch at (404) 718-4745 or at parasites@cdc.gov.
Who Should Receive Treatment?
Treatment is recommended for all patients with acute or congenital Chagas disease and for chronic T cruzi infection in children younger than 18 years [61]. Pregnant women identified as having Chagas disease should receive treatment after delivery for their benefit and because treatment can prevent transmission of T cruzi during subsequent pregnancies [62-64]. Treatment is contraindicated during pregnancy. Because benznidazole has been detected in breast milk of women undergoing treatment [65] and safety for infants exposed through breastfeeding has not been evaluated, withholding maternal treatment until the cessation of breastfeeding is recommended.
How Can the Diagnosis and Treatment of Congenital Chagas Disease Be Improved?
Enhanced awareness of Chagas disease as a health concern is needed to prompt diagnostic evaluation of at-risk infants and improve long-term outcomes for those with congenital Chagas disease. Knowledge gaps among HCPs should be addressed [1, 66]. Implementation of maternal screening with infant testing and screening could substantially reduce morbidity and death resulting from Chagas disease in the United States. At current costs, such testing would be cost-saving for a maternal prevalence as low as 0.057% and for a mother-to-child transmission probability as low as 0.001% [67].
Improved knowledge of the locales in the United States in which women of childbearing age with chronic T cruzi infection reside could enhance the identification of infants with congenital infection. Improved performance of existing tests is needed as is validation of rapid screening tests for use in infants. Development of effective and well-tolerated drugs for treatment should be a priority [7].
Illustrative Case Dénouement
-
1.
Is this infant at risk for congenital Chagas disease? What evaluation is indicated?
Serologic testing by a cord blood bank is considered a screening procedure. The infant should be considered at risk for congenital Chagas disease if the result of testing, performed at a reference diagnostic laboratory, is positive for T cruzi antibodies. The infant is older than 3 months, so evaluation with serologic testing, rather than molecular testing by PCR, should be performed. Results from a commercial laboratory at the time of the clinic visit revealed a maternal T cruzi immunoglobulin G (IgG) level of 2.1 index value (IV) (≥1.2 IV is a positive result) and an IgM level of <1:16 (negative result). The infant’s T cruzi IgG level was 0.3 IV, and the IgM level was <1:16.
These results suggest that the mother had chronic Chagas disease. Although passively acquired antibodies can persist until 9 months of age, this infant was seronegative at the time of testing, so congenital Chagas disease can be excluded.
-
2.
What referral is appropriate for the mother?
The infant’s mother was referred for further evaluation and treatment to an adult infectious diseases specialist. The result of confirmatory T cruzi serology at a reference diagnostic laboratory was positive. Treatment for Chagas disease with benznidazole will be initiated after she completes breastfeeding her infant.
-
3.
Should other children in the family undergo any testing?
Yes, since T cruzi could have been transmitted during her earlier pregnancies, serologic testing for T cruzi IgG through a commercial laboratory is indicated for the other children. This information was conveyed to the referring pediatrician.
Acknowledgment.
We thank Robin Schroeder for assistance with preparation of the manuscript.
Financial support.
This work was supported by the Centers for Disease Control and Prevention Cooperative Agreement 5NU2GGH001649-03.
Footnotes
Disclaimer. The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention of the Department of Health and Human Services.
Potential conflicts of interest. M. S. E. is the recipient of a research grant from Pfizer, Inc. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Verani JR, Montgomery SP, Schulkin J, Anderson B, Jones JL. Survey of obstetrician-gynecologists in the United States about Chagas disease. Am J Trop Med Hyg 2010; 83:891–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards MS, Abanyie FA, Montgomery SP. Survey of Pediatric Infectious Diseases Society members about congenital Chagas disease. Pediatr Infect Dis J 2018; 37:e24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Parasites—neglected parasitic infections. Available at: https://www.cdc.gov/parasites/npi/index.html. Accessed June 28, 2018.
- 4.Manne-Goehler J, Umeh CA, Montgomery SP, Wirtz VJ. Estimating the burden of Chagas disease in the United States. PLoS Negl Trop Dis 2016; 10:e0005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 2009; 49:e52–4. [DOI] [PubMed] [Google Scholar]
- 6.Dorn PL, Perniciaro L, Yabsley MJ, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 2007; 13:605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery SP, Starr MC, Cantey PT, Edwards MS, Meymandi SK. Neglected parasitic infections in the United States: Chagas disease. Am J Trop Med Hyg 2014; 90:814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia MN, Aguilar D, Gorchakov R, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg 2015; 92:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantey PT, Stramer SL, Townsend RL, et al. The United States Trypanosoma cruzi infection study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion 2012; 52:1922–30. [DOI] [PubMed] [Google Scholar]
- 10.Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis 2009; 9:41–49. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Strutz SE, Frank DM, Rivaldi C-L, Sissel B, Sánchez-Cordero V. Chagas disease risk in Texas. PLoS Negl Trop Dis 2010; 4:e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buekens P, Almendares O, Carlier Y, et al. Mother-to-child transmission of Chagas’ disease in North America: why don’t we do more? Matern Child Health J 2008; 12:283–6. [DOI] [PubMed] [Google Scholar]
- 13.Kapelusznik L, Varela D, Montgomery SP, et al. Chagas disease in Latin American immigrants with dilated cardiomyopathy in New York City. Clin Infect Dis 2013; 57:e7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traina MI, Hernandez S, Sanchez DR, et al. Prevalence of Chagas disease in a U.S. population of Latin American immigrants with conduction abnormalities on electrocardiogram. PLoS Negl Trop Dis 2017; 11:e0005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet 2010; 375:1388–402. [DOI] [PubMed] [Google Scholar]
- 16.Bern C. Chagas’ disease. N Engl J Med 2015; 373:456–66. [DOI] [PubMed] [Google Scholar]
- 17.Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. Chagas disease and the US blood supply. Curr Opin Infect Dis 2008; 21:476–82. [DOI] [PubMed] [Google Scholar]
- 18.AABB. Chagas Disease Biovigilance Network. Available at: http://www.aabb.org/research/hemovigilance/Pages/chagas.aspx. Accessed February 13, 2019.
- 19.Gray EB, La Hoz RM, Green JS, et al. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012-2016. Transpl Infect Dis 2018; 20:e12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alarcón de Noya B, Díaz-Bello Z, Colmenares C, et al. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis 2010; 201:1308–15. [DOI] [PubMed] [Google Scholar]
- 21.Gebrekristos HT, Buekens P. Mother-to-child transmission of Trypanosoma cruzi. J Pediatric Infect Dis Soc 2014; 3(Suppl 1):S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard EJ, Xiong X, Carlier Y, Sosa-Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG 2014; 121:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards MS, Rench MA, Todd CW, et al. Perinatal screening for Chagas disease in southern Texas. J Pediatric Infect Dis Soc 2015; 4:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meymandi SK, Forsyth CJ, Soverow J, et al. Prevalence of Chagas disease in the Latin American-born population of Los Angeles. Clin Infect Dis 2017; 64:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlier Y, Truyens C. Maternal-fetal transmission of Trypanosoma cruzi. In: Telleria J, Tibayrenc M. American Trypanosomiasis-Chagas Disease: One Hundred Years of Research. New York, NY: Elsevier, 2010:539–81. [Google Scholar]
- 26.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev 2011; 24:655–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlier Y, Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop 2015; 151:103–15. [DOI] [PubMed] [Google Scholar]
- 28.Bern C, Martin DL, Gilman RH. Acute and congenital Chagas disease. Adv Parasitol 2011; 75:19–47. [DOI] [PubMed] [Google Scholar]
- 29.Nisida IV, Amato Neto V, Braz LM, Duarte MI, Umezawa ES. A survey of congenital Chagas’ disease, carried out at three health institutions in São Paulo city, Brazil. Rev Inst Med Trop Sao Paulo 1999; 41:305–11. [DOI] [PubMed] [Google Scholar]
- 30.Norman FF, López-Vélez R. Chagas disease and breast-feeding. Emerg Infect Dis 2013; 19:1561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messenger LA, Miles MA, Bern C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev Anti Infect Ther 2015; 13:995–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bern C, Verastegui M, Gilman RH, et al. Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clin Infect Dis 2009; 49:1667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torrico F, Alonso-Vega C, Suarez E, et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg 2004; 70:201–9. [PubMed] [Google Scholar]
- 34.Salas NA, Cot M, Schneider D, et al. Risk factors and consequences of congenital Chagas disease in Yacuiba, south Bolivia. Trop Med Int Health 2007; 12:1498–505. [DOI] [PubMed] [Google Scholar]
- 35.Rendell VR, Gilman RH, Valencia E, et al. Trypanosoma cruzi-infected pregnant women without vector exposure have higher parasitemia levels: implications for congenital transmission risk. PLoS One 2015; 10:e0119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplinski M, Jois M, Galdos-Cardenas G, et al. Sustained domestic vector exposure is associated with increased Chagas cardiomyopathy risk but decreased parasitemia and congenital transmission risk among young women in Bolivia. Clin Infect Dis 2015; 61:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scapellato PG, Bottaro EG, Rodríguez-Brieschke MT. Mother-child transmission of Chagas disease: could coinfection with human immunodeficiency virus increase the risk? Rev Soc Bras Med Trop 2009; 42:107–9. [DOI] [PubMed] [Google Scholar]
- 38.Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol 1991; 146:3626–32. [PubMed] [Google Scholar]
- 39.Hermann E, Truyens C, Alonso-Vega C, et al. Congenital transmission of Trypanosoma cruzi is associated with maternal enhanced parasitemia and decreased production of interferon-gamma in response to parasite antigens. J Infect Dis 2004; 189:1274–81. [DOI] [PubMed] [Google Scholar]
- 40.Zingales B, Andrade SG, Briones MR, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 2009; 104:1051–4. [DOI] [PubMed] [Google Scholar]
- 41.Buekens P, Cafferata ML, Alger J, et al. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: an observational prospective study. Am J Trop Med Hyg 2018; 98:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sánchez Negrette O, Mora MC, Basombrío MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics 2005; 115:e668–72. [DOI] [PubMed] [Google Scholar]
- 43.Freilij H, Altcheh J. Congenital Chagas’ disease: diagnostic and clinical aspects. Clin Infect Dis 1995; 21:551–5. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira I, Torrico F, Muñoz J, Gascon J. Congenital transmission of Chagas disease: a clinical approach. Expert Rev Anti Infect Ther 2010; 8:945–56. [DOI] [PubMed] [Google Scholar]
- 45.Blanco SB, Segura EL, Cura EN, et al. Congenital transmission of Trypanosoma cruzi: an operational outline for detecting and treating infected infants in north-western Argentina. Trop Med Int Health 2000; 5:293–301. [DOI] [PubMed] [Google Scholar]
- 46.Messenger LA, Gilman RH, Verastegui M, et al. Toward improving early diagnosis of congenital Chagas disease in an endemic setting. Clin Infect Dis 2017; 65:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berberian G, Rosanova MT, Kaldzielski C, Paulin P, Castro G, Galina L. Ocular involvement in congenital Chagas disease [in Spanish]. Arch Argent Pediatr 2013; 111:e78–81. [DOI] [PubMed] [Google Scholar]
- 48.Bittencourt AL, Vieira GO, Tavares HC, Mota E, Maguire J. Esophageal involvement in congenital Chagas’ disease. Report of a case with megaesophagus. Am J Trop Med Hyg 1984; 33:30–3. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Congenital transmission of Chagas disease—Virginia, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:477–9. [PubMed] [Google Scholar]
- 50.Alarcón A, Morgan M, Montgomery SP, et al. Diagnosis and treatment of congenital Chagas disease in a premature infant. J Pediatric Infect Dis Soc 2016; 5:e28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlier Y, Torrico F, Sosa-Estani S, et al. Congenital Chagas disease: recommendations for diagnosis, treatment and control of newborns, siblings and pregnant women. PLoS Negl Trop Dis 2011; 5:e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tustin AW, Bowman NM. Chagas disease. Pediatr Rev 2016; 37:177–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Congenital Chagas disease. Available at: https://www.cdc.gov/parasites/chagas/health_professionals/congenital_chagas.html. Accessed June 28, 2018.
- 54.Qvarnstrom Y, Schijman AG, Veron V, Aznar C, Steurer F, da Silva AJ. Sensitive and specific detection of Trypanosoma cruzi DNA in clinical specimens using a multi-target real-time PCR approach. PLoS Negl Trop Dis 2012; 6:e1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plourde PJ, Kadkhoda K, Ndao M. Congenitally transmitted Chagas disease in Canada: a family cluster. CMAJ 2017; 189:E1489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mongeau-Martin G, Ndao M, Libman M, Delage G, Ward BJ. A family cluster of Chagas disease detected through selective screening of blood donors: a case report and brief review. Can J Infect Dis Med Microbiol 2015; 26:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zulantay I, Apt W, Ramos D, et al. The epidemiological relevance of family study in Chagas disease. PLoS Negl Trop Dis 2013; 7:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.US Food and Drug Administration. FDA news release: FDA approves first U.S. treatment for Chagas disease. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm573942.htm. Accessed June 28, 2018.
- 59.Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 2011; 127:e212–8. [DOI] [PubMed] [Google Scholar]
- 60.Chippaux JP, Salas-Clavijo AN, Postigo JR, Schneider D, Santalla JA, Brutus L. Evaluation of compliance to congenital Chagas disease treatment: results of a randomised trial in Bolivia. Trans R Soc Trop Med Hyg 2013; 107:1–7. [DOI] [PubMed] [Google Scholar]
- 61.American Academy of Pediatrics. American trypanosomiasis. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Red Book: 2018 Report of the Committee on Infectious Diseases, 31st ed. Itasca, IL: American Academy of Pediatrics, 2018:826–9. [Google Scholar]
- 62.Murcia L, Simón M, Carrilero B, Roig M, Segovia M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J Infect Dis 2017; 215:1452–8. [DOI] [PubMed] [Google Scholar]
- 63.Álvarez MG, Vigliano C, Lococo B, Bertocchi G, Viotti R. Prevention of congenital Chagas disease by benznidazole treatment in reproductive-age women. An observational study. Acta Trop 2017; 174:149–52. [DOI] [PubMed] [Google Scholar]
- 64.Fabbro DL, Danesi E, Olivera V, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis 2014; 8:e3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.García-Bournissen F, Moroni S, Marson ME, et al. Limited infant exposure to benznidazole through breast milk during maternal treatment for Chagas disease. Arch Dis Child 2015; 100:90–4. [DOI] [PubMed] [Google Scholar]
- 66.Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis 2010; 16:871–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stillwaggon E, Perez-Zetune V, Bialek SR, Montgomery SP. Congenital Chagas disease in the United States: cost savings through maternal screening. Am J Trop Med Hyg 2018; 98:1733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]