Abstract
Non-invasive brain stimulation techniques are popular tools to investigate brain function in health and disease. Although transcranial magnetic stimulation (TMS) is widely used in cognitive neuroscience research to probe causal structure-function relationships, studies often yield inconclusive results. To improve the effectiveness of TMS studies, we argue that the cognitive neuroscience community needs to revise the stimulation focality principle – the spatial resolution with which TMS can differentially stimulate cortical regions. In the motor domain, TMS can differentiate between cortical muscle representations of adjacent fingers. However, this high degree of spatial specificity cannot be obtained in all cortical regions due to the influences of cortical folding patterns on the TMS-induced electric field. The region-dependent focality of TMS should be assessed a priori to estimate the experimental feasibility. Post-hoc simulations allow modeling of the relationship between cortical stimulation exposure and behavioral modulation by integrating data across stimulation sites or subjects.
Keywords: TMS, Cognitive neuroscience, Field simulation, Brain stimulation, NIBS
Graphical Abstract
Non-invasive brain stimulation (NIBS) methods have become increasingly popular tools for studying human brain function in health and disease (Dayan et al., 2013, Polanía et al., 2018). In particular, transcranial magnetic stimulation (TMS; Fig. 1a, left) is a widely-used technique with which the causality of structure-function relationships can be probed (e.g., Hartwigsen and Silvanto, 2022; Pascual-Leone, Walsh and Rothwell, 2000; Walsh and Cowey, 2000). Accordingly, a typical opening sentence of a TMS publication may read as follows: TMS can interfere with neuronal processing in the human cortex with high spatial precision, enabling researchers to draw causal inference about brain functions. Although commonly accepted, this statement is seemingly at odds with the widespread notion of TMS effects on cognitive functions as variable, individual-specific (Hannah et al., 2016), or heterogeneous (Siddiqi et al., 2022). The observed heterogeneity in TMS effects hampers conclusions about the functional relevance of a cortical region: effects are often smaller and less consistent than expected from the simple causality premise stated above (Valero-Cabré et al., 2017). Heterogeneous TMS effects have been attributed to methodological issues (Beynel et al., 2019), individual differences in baseline task-induced brain states (e.g., Silvanto and Cattaneo, 2017), or dependencies between oscillatory brain states and TMS responsiveness (Peters et al., 2020). Here, we argue that the current understanding of stimulation focality is flawed. In our view, this is a major conceptual shortcoming in the cognitive neuroscience community that may hinder progress in using TMS as a neuromodulatory tool. We suggest that the misinterpretation of stimulation specificity at the cortex offers a key explanation for the discrepancy between the expected results of TMS studies and their actual outcome. Below, we discuss why reconceptualising TMS focality is crucial to increase the reliability and validity of TMS experiments in cognitive neuroscience.
Fig. 1.
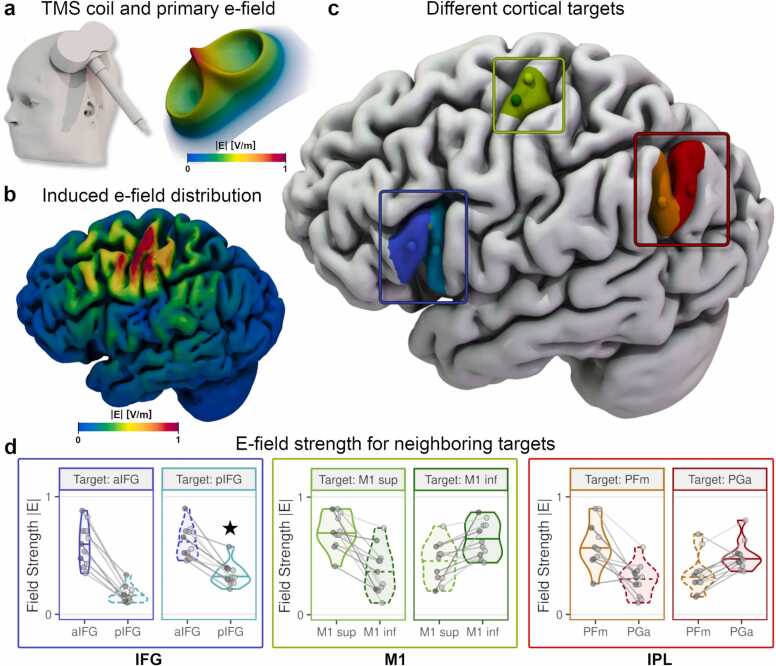
: A functional differentiation between adjacent subregions with TMS is not possible for all cortical areas. a) A model of an MCF-B65 figure-of-eight TMS coil positioned over the left motor cortex (left). Coil model kindly provided by Konstantin Weise. Head model created with SimNIBS/charm. The primary e-field of a figure-of-eight TMS coil shows a well-defined stimulation peak (right; red spike) of the stimulation strength under the centre of the coil concerning the magnitude of E (|E|). b) The effective electric field is shaped by individual-specific factors, such as gyrification patterns and distribution of various tissue types (shown here: grey matter). Superficial regions of neighbouring gyri are exposed to stronger stimulation than deeper regions like sulcal walls and troughs. Stimulation target: inferior parietal lobe (IPL). c, d) Neighbouring cortical targets (spheres) on the same gyrus, e.g., in the M1 region (green area/panels), can be differentially stimulated with TMS if the cortical depth is similar: for both M1 targets, the target area is stimulated more strongly than the off-target area. Likewise, both selected targets in the IPL (PFm/PGa) can be similarly dissociated (red panels). In contrast, targeting the posterior inferior frontal gyrus (pIFG) can yield a stronger stimulation of the anterior IFG (aIFG) than pIFG due to differences in cortical-skin distances (blue panels; marked with a star in d).
Where does the misconception of TMS focality originate? For decades, TMS has successfully been used to stimulate individual finger muscle representations in the motor cortex, leading to the widely-held notion of high spatial focality—in the range of 1–2 cm² (e.g., Ridding and Rothwell, 2007; Ward, 2019). Most TMS researchers will be familiar with a manual search of the motor “hotspot” (Rothwell et al., 1999) for muscle representations in the primary motor cortex (M1). During this procedure, slight shifts of the TMS coil can yield pronounced changes in the muscle response, allowing for a differentiation of cortical muscle representations of adjacent fingers (e.g., Bashir et al., 2013; Raffin et al., 2015). These sharp tuning curves have led to the characterisation of TMS as a relatively high-spatial resolution brain stimulation method. Testimony to this view is the often-used visualisation of the primary electric field (e-field) generated by the TMS coil, which peaks underneath the centre of a figure-of-eight coil (Fig. 1a, right). Importantly, it is often overlooked that cortical stimulation exposure is not equal to this primary e-field, which is a theoretically generated field in free space. Rather, the spatial distribution of the stimulation effect is described by the effective e-field, determined by the primary e-field in combination with the individual brain and head anatomy. Instead of spreading uniformly across the cortex, the effective e-field is shaped by individual tissue distributions (Fig. 1b).
Recently, the NIBS community has capitalised on advancing methods to quantify the cortical stimulation exposure via calculations of the TMS-induced (effective) e-field (Miranda et al., 2003, Opitz et al., 2011, Thielscher et al., 2011, Hartwigsen et al., 2015). Using production-ready toolboxes, such as SimNIBS (Puonti et al., 2020, Thielscher et al., 2015) and ROAST (Huang et al., 2019), individual head models can be constructed from structural magnetic resonance images to compute the induced e-fields for specific TMS coil models and stimulation sites (see Nielsen et al., 2018 for an overview). These e-field estimations have been used to map cortical muscle representations accurately in M1 (Opitz et al., 2013; Bungert et al., 2016; Aonuma et al., 2018; Weise et al., 2020). Moreover, studies from our lab have used e-field strength in cortical regions of interest to explain performance differences in a wide range of cognitive tasks (Kuhnke et al., 2020, van der Burght et al., 2023, Maran et al., 2022). Likewise, simulations have underlined that the stimulation spread is highly dependent on macro-anatomical configurations (e.g., sulcal depth, gyral folding; Thielscher et al., 2011). Currently, efforts are being made to standardise the reporting procedure of NIBS parameters, which might ultimately allow fully automated field calculations (Bertazolli et al., 2023). This dependence on individual brain anatomy prompts important considerations that are currently often ignored in TMS studies of cognition, both at the individual subject and group level.
At the individual level, one should consider that cortical stimulation may not peak directly underneath the coil centre. Rather, the peak may lie at an adjacent, more superficial region, depending on the individual anatomy. For example, targeting a sulcal target A may result in stronger stimulation at an adjacent gyrus B. Likewise, a more superficial neighbouring gyrus D might receive stronger stimulation for a gyral target C. This general principle is illustrated in Fig. 1c & d: when targeting the posterior IFG (pIFG, pars opercularis), the anterior parts of the IFG (aIFG, pars triangularis) receive stronger stimulation exposure on average. However, the conclusion “the target area is causally relevant for function X” critically depends on whether the stimulation effect is confined to the target area. Specifically, to demonstrate causal involvement in a cognitive process, the involvement of a neighbouring area must be ruled out. Similarly, if the aim is to functionally differentiate between two areas and test for a functional-anatomical double dissociation, both areas must be differentially stimulated. E-field simulations may be used to confirm that these key assumptions are met. As such, simulating how the e-field spreads across the cortex of an individual (Gomez-Tames et al., 2018) allows for more valid and more precise conclusions about the causal role of a cortical area for a given cognitive task. Importantly, the spatial profile of the induced e-field is the same for a given coil position/orientation regardless of the stimulator intensity–only the e-field magnitude, that is, the strength of the cortical stimulation, changes. The relative difference in cortical stimulation between two cortical areas (e.g., between aIFG and pIFG, Fig. 1d) remains unchanged when the stimulator output is altered.
At the group level, simple comparisons of behavioural performance (using outcome measures such as response time or accuracy) can only yield robust effects when stimulation consistently reaches the target area in most subjects. In other words, an inference on the functional relevance of a region can only be drawn if the differential stimulation is relatively consistent across the sample. Importantly, this may not be equally feasible in all regions across the cortex. Muscle representations in M1, for example, are often located on the crown and rims of the precentral gyrus (Siebner et al., 2022). At such cortical locations, stimulation may be relatively focal (Romero et al., 2019), thus allowing for differential stimulation of close cortical targets in most subjects (Fig. 1c & d, M1 region). In contrast, differentiating between adjacent regions in areas with stronger cortical folding or higher interindividual variability, such as Broca’s area (Juch et al., 2005), is more challenging (Fig. 2). Functional separation of neighbouring regions with different depth profiles (e.g., van der Burght et al., 2023; Fig. 1c & d, IFG region; Fig. 2) is particularly complex: here, a differential peak stimulation might not be feasible at all, in contrast to the analysis of cortical areas with similar depths (e.g., Fig. 1c & d, IPL region; Fig. 2b, middle row). Group-level effects might be more variable in regions with large inter-subject variance because the e-field distributions will substantially differ. Therefore, large between-subject variability in e-field distribution likely explains the small effect sizes in many TMS studies. In sum, the target region and differences between individual subjects determine the distribution of the TMS-induced e-field. As such, taking both factors into account is key for a valid interpretation of TMS focality. As a side note, we wish to emphasise that it remains currently unknown how differences in microanatomy between motor and non-motor areas shape the response to TMS. Yet, e-field distributions are mostly determined by macroanatomical properties, making e-field modelling an appropriate tool for evaluating the stimulation spread at all parts of the cortex.(Box 1).
Fig. 2.
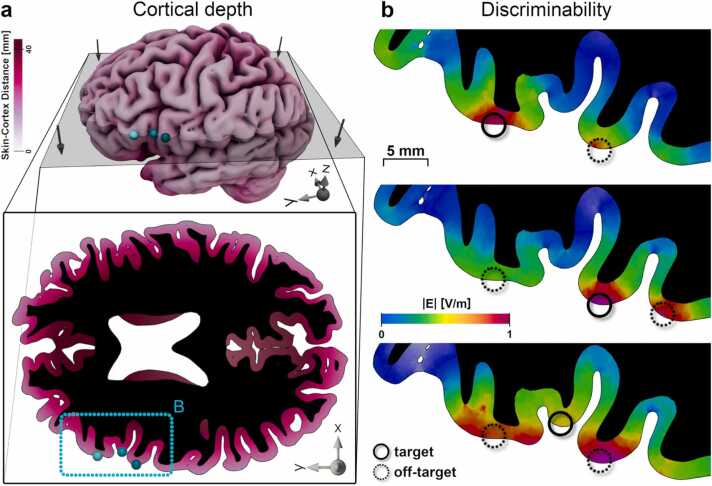
: The cortical folding constraints the discriminability of neighbouring areas by TMS. Differential stimulation is necessary to examine the functional relevance of neighbouring cortical areas with TMS. However, the focality of TMS (the degree to which cortical areas can be differentially stimulated) depends on cortical folding patterns. a) Cortical grey matter for an example subject. Colour: skin-cortex distance. Grey plane: horizontal slice in the bottom panel. Bottom panel: Cortical folding and skin-cortex distances differ across the cortical sheet. Turquoise outline and spheres: Target details in the left inferior frontal gyrus from B. Black area indicates white matter. b) Top: The stimulation is highest at the cortical target (solid circle). This target can be differentiated from the neighbouring gyrus (dashed circle) with TMS. Middle: Stimulation of the main target (solid circle) leads to stimulation of the posterior neighbour (right dashed circle) with a similar strength, whereas the anterior neighbour can be successfully dissociated (left dashed circle). Bottom: Targeting deeper areas (solid circles) exposes superficial, neighbouring gyri (dashed circles) to higher stimulation. The cortical target cannot be differentiated from both neighbouring regions. See Fig. 1 for field simulation details. E-field simulations were done for an arbitrary stimulator intensity (1 A/µs) and normalised across targets to allow for a comparison of stimulation exposures and e-field spread across different cortical targets.
Box 1. Electric field simulations provide important considerations for the focality of TMS.
 |
How can e-field simulations be exploited to attenuate the limited focality of TMS? Although the dependence of the e-field on individual anatomy has been described as a shortcoming above, it also enables researchers to increase focality: by integrating information from multiple e-fields, variations in stimulation strength can be related to variability in motor or cognitive effects. This combination of data can be implemented within-subject, obtaining e-field distributions from multiple stimulations sites or across-subjects, comparing the e-field distributions between various brain and head anatomies. Within-subject modelling has recently been implemented for the motor cortex (Weise et al., 2022, Numssen et al., 2021) and adapted to map spatial attention (Jing et al., 2023), while the analysis of between-subject variation is a promising path for studies on cognition: e-field strengths across subjects within a region of interest are used to explain the TMS modulation of task performance and thus, identify causally involved areas (Kuhnke et al., 2020, van der Burght et al., 2023, Maran et al., 2022). As such, the between-subject variability in e-field strength turns into an advantage and can be used to explain between-subject variability in behaviour. Here, stimulation is understood as a continuous rather than a discrete factor. Whereas behavioural effects have classically been described as driven by active TMS vs sham TMS, we propose to analyse the entire relationship between cortical stimulation strength and behavioural effect. In this way, the functional involvement of neighbouring regions in a certain cognitive process can be demonstrated by directly testing for a functional relationship between local e-field and behaviour—even if visual inspection suggests both regions were similarly affected by stimulation. Currently, a wide range of TMS coil models is available, each with a characteristic (primary) e-field pattern (see Deng et al., 2013 and Drakaki et al., 2022 for overviews on TMS coil designs). Prospectively, multi-channel TMS coils (e.g., Nurmi et al., 2021; Nieminen et al., 2022) will allow for an adjustment of the spatial stimulation profile to specific target variables, e.g., hotspot and spread of the e-field or its spatial orientation. Aside from fine-tuning the spatial focality, this approach enables an increase in temporal resolution to the range of milliseconds by sequentially stimulating several targets (de Lara et al., 2021). The view of TMS-induced effects as part of a continuous dose-response-relationship is generally accepted in motor research (Ridding et al., 1997; Möller et al., 2009; Peterchev et el, 2013; Aberra et al., 2020) but still rarely adopted in the field of cognition. E-field modelling introduces the basis for bio-physiologically plausible TMS dosing metrics. Commonly used dosing strategies based on the resting motor threshold (rMT), e.g., 90% rMT, do not yield the same cortical stimulation exposure across different cortical areas. In contrast, e-field-based dosing (Caulfield et al., 2021, Dannhauer et al., 2022, Weise et al., 2023; Kuhnke et al., 2023) mitigates differences in cortical depths and anatomical properties between M1 (where rMT is established), and the actual target region. However, to leverage its full potential, this dynamic and emerging branch (Gomez-Tames et al., 2020) of applied, simulation-based neurostimulation has to agree on general metrics with which on- and off-target stimulation are defined (c.f. Van Hoornweder et al., 2023), that is, a threshold at which stimulation is considered functionally relevant (Box 2).
Box 2. TMS dosing and its implications for focality.
 |
Which principles can we derive from using e-fields to improve future TMS studies on cognition? First, our understanding of the focality of TMS needs to reflect the induced e-field distribution in the cortex realistically. A correct view of the mode of action of TMS is particularly important for the functional dissociation of neighbouring gyri or when the involvement of neighbouring areas in the task of interest cannot be ruled out. Second, stimulation must be conceptualised as a continuous factor within and across subjects. That is, the stimulation exposure of (neighbouring) cortical areas should be quantified relative to each other. Across subjects, it should be understood that individual anatomy impacts the differential stimulation of neighbouring areas. Third, simulations of the induced e-fields should be used to gain relevant insights into the cortical stimulation exposure before and after data collection (Balderston et al., 2020). A-priori e-field simulations allow for an estimate of the cortical stimulation in the individual subject's brain to identify unfeasible targets or target pairs before the experimental data is acquired. In addition, coil positioning can be optimised to maximise stimulation exposure in a specific cortical area (Weise et al., 2022) or minimise off-target stimulation (Makarov et al., 2021, Lueckel et al., 2022). Post-hoc e-field simulations provide insight into the relationship between stimulation exposure and behaviour for different cortical areas at the group level.
In conclusion, we argue that the conceptualisation of the focality of TMS in cognitive neuroscience requires reconsideration. Instead of distributing uniformly, stimulation spreads to neighbouring cortical areas in a way that is highly dependent on individual brain anatomy. Furthermore, rather than viewing TMS as a binary stimulation method (active or sham stimulation), we emphasise that TMS is better understood as a continuous factor: by analysing variability in stimulation strength in cortical regions and the impact on the behavioural modulation, one can derive their causal relevance for a given cognitive function. Together, these recommendations enable researchers to better select stimulation targets and yield more fine-grained insights into causal structure-function relationships. Ultimately, this should lead to more robust group-level effects in TMS studies on human cognition.
All e-field data were computed with SimNIBS 4.0 (www.simnibs.org). IFG and IPL targets were defined in MNI space based on group results and transformed into single subject spaces for the 11 exemplary individuals shown following standard TMS targeting procedures. M1 targets were manually positioned. Head models were constructed from high-resolution (1 mm³ voxel size) T1- and T2-weighted MRI scans (see Numssen et al., 2021 for details). The simulations were done for a MagVenture MCF-B65 figure-of-eight coil and arbitrary stimulator intensity (1 A/µs). E-field magnitudes (|E|) were extracted from the closest point within grey matter and normalised per subject and target pair to compare stimulation exposures within target pairs. Grey lines and points: single subject data. E-field values are normalised per subject.
References
- Aberra A.S., Wang B., Grill W.M., Peterchev A.V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020;13(1):175–189. doi: 10.1016/j.brs.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston N.L., Roberts C., Beydler E.M., Deng Z.-D., Radman T., Luber B., Lisanby S.H., Ernst M., Grillon C. A generalized workflow for conducting electric field–optimized, fMRI-guided, transcranial magnetic stimulation. Nat. Protoc. 2020 doi: 10.1038/s41596-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir S., Perez J.M., Horvath J.C., Pascual-Leone A. Differentiation of motor cortical representation of hand muscles by navigated mapping of optimal TMS current directions in healthy subjects. J. Clin. Neurophysiol. 2013;30(4):390–395. doi: 10.1097/wnp.0b013e31829dda6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazolli G., Iacovella V., Miniussi C., Bortoletto M. BIDS Extension Proposal NIBS (BEP37 NIBS) Brain Imaging Data Struct. 2023 〈https://bids.neuroimaging.io/bep037〉 [Google Scholar]
- Beynel L., Appelbaum L.G., Luber B., Crowell C.A., Hilbig S.A., Lim W., Deng Z.D. Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: a meta-analysis and recommendations for future studies. Neurosci. Biobehav. Rev. 2019;107:47–58. doi: 10.1016/j.neubiorev.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield K.A., Li X., George M.S. Four electric field modeling methods of dosing prefrontal transcranial magnetic stimulation (TMS): introducing APEX MT dosimetry. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation. 2021;14(4):1032–1034. doi: 10.1016/j.brs.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhauer M., Huang Z., Beynel L., Wood E., Bukhari-Parlakturk N., Peterchev A.V. TAP: Targeting and analysis pipeline for optimization and verification of coil placement in transcranial magnetic stimulation. J. Neural Eng. 2022;19(2) doi: 10.1088/1741-2552/ac63a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E., Censor N., Buch E.R., Sandrini M., Cohen L.G. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat. Neurosci. 2013;16(7):838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.D., Lisanby S.H., Peterchev A.V. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakaki M., Mathiesen C., Siebner H.R., Madsen K., Thielscher A. Database of 25 validated coil models for electric field simulations for TMS. Brain Stimul. 2022;15(3):697–706. doi: 10.1016/j.brs.2022.04.017. [DOI] [PubMed] [Google Scholar]
- Gomez-Tames J., Hamasaka A., Laakso I., Hirata A., Ugawa Y. Atlas of optimal coil orientation and position for TMS: a computational study. Brain Stimul. 2018;11(4):839–848. doi: 10.1016/j.brs.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Gomez-Tames J., Laakso I., Hirata A. Review on biophysical modelling and simulation studies for transcranial magnetic stimulation. Phys. Med. Biol. 2020;65(24):24TR03. doi: 10.1088/1361-6560/aba40d. [DOI] [PubMed] [Google Scholar]
- Hannah R., Rocchi L., Tremblay S., Rothwell J.C. Controllable pulse parameter TMS and TMS-EEG as novel approaches to improve neural targeting with rTMS in human cerebral cortex. Front. Neural Circuits. 2016;10:97. doi: 10.3389/fncir.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G., Silvanto J. Noninvasive Brain Stimulation: Multiple Effects on Cognition. Neuroscientist. 2022 doi: 10.1177/10738584221113806. 107385842211138. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G., Bergmann T.O., Herz D.M., Angstmann S., Karabanov A., Raffin E., Siebner H.R. Modeling the effects of noninvasive transcranial brain stimulation at the biophysical, network, and cognitive level. Prog. Brain Res. 2015;222:261–287. doi: 10.1016/bs.pbr.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Huang Y., Datta A., Bikson M., Parra L.C. Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—a fully automated open-source pipeline. J. Neural Eng. 2019;16(5) doi: 10.1088/1741-2552/ab208d. (doi: 10.1088/1741-2552/ab208d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Numssen O., Weise K., Kalloch B., Haueisen J., Hartwigsen G., Knoesche T. Modeling the effects of transcranial magnetic stimulation on spatial attention. bioRxiv. 2023 doi: 10.1101/2023.01.11.523548. 2023-01. [DOI] [PubMed] [Google Scholar]
- Juch Zimine, Seghier Lazeyras, Fasel Anatomical variability of the lateral frontal lobe surface: implication for intersubject variability in language neuroimaging. NeuroImage. 2005 doi: 10.1016/j.neuroimage.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Kuhnke P., Beaupain M.C., Cheung V.K.M., Weise K., Kiefer M., Hartwigsen G. Left posterior inferior parietal cortex causally supports the retrieval of action knowledge. NeuroImage. 2020;219(May) doi: 10.1016/j.neuroimage.2020.117041. [DOI] [PubMed] [Google Scholar]
- Kuhnke P., Numssen O., Voeller J., Weise K., Hartwigsen G. P-87 Dosage optimization for transcranial magnetic stimulation based on cortical field thresholds. Clinical Neurophysiology. 2023;148:e48. doi: 10.1016/j.clinph.2023.02.104. [DOI] [Google Scholar]
- de Lara L.I.N., Daneshzand M., Mascarenas A., Paulson D., Pratt K., Okada Y., Nummenmaa A. A 3-axis coil design for multichannel TMS arrays. NeuroImage. 2021;224 doi: 10.1016/j.neuroimage.2020.117355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueckel M., Radetz A., Yuen K., Mueller-Dahlhaus F., Kalisch R., Bergmann T.O. TU-176. E-field- and connectivity-optimized TMS targeting: a pilot TMS-fMRI validation at the single-subject level. Clin. Neurophysiol. 2022;141(Supplement) doi: 10.1016/j.clinph.2022.07.080. S30-S3031. [DOI] [Google Scholar]
- Makarov S.N., Wartman W.A., Noetscher G.M., Fujimoto K., Zaidi T., Burnham E.H., Nummenmaa A. Degree of improving TMS focality through a geometrically stable solution of an inverse TMS problem. Neuroimage. 2021;241 doi: 10.1016/j.neuroimage.2021.118437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran M., Numssen O., Hartwigsen G., Zaccarella E. Online neurostimulation of Broca's area does not interfere with syntactic predictions: a combined TMS-EEG approach to basic linguistic combination. Front. Psychol. 2022 doi: 10.3389/fpsyg.2022.968836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda P.C., Hallett M., Basser P.J. The electric field induced in the brain by magnetic stimulation: a 3-D finite-element analysis of the effect of tissue heterogeneity and anisotropy. IEEE Trans. Biomed. Eng. 2003;50(9):1074–1085. doi: 10.1109/TBME.2003.816079. [DOI] [PubMed] [Google Scholar]
- Möller C., Arai N., Lücke J., Ziemann U. Hysteresis effects on the input–output curve of motor evoked potentials. Clin. Neurophysiol. 2009;120(5):1003–1008. doi: 10.1016/j.clinph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Nielsen J.D., Madsen K.H., Puonti O., Siebner H.R., Bauer C., Madsen C.G., Saturnino G.B., Thielscher A. Automatic skull segmentation from MR images for realistic volume conductor models of the head: assessment of the state-of-the-art. NeuroImage. 2018;174:587–598. doi: 10.1016/j.neuroimage.2018.03.001. (doi: 10.1016/j.neuroimage.2018.03.001) [DOI] [PubMed] [Google Scholar]
- Nieminen J.O., Sinisalo H., Souza V.H., Malmi M., Yuryev M., Tervo A.E., Ilmoniemi R.J. Multi-locus transcranial magnetic stimulation system for electronically targeted brain stimulation. Brain Stimul. 2022;15(1):116–124. doi: 10.1016/j.brs.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numssen O., Zier A.L., Thielscher A., Hartwigsen G., Knösche T.R., Weise K. Efficient high-resolution TMS mapping of the human motor cortex by nonlinear regression. NeuroImage. 2021;245 doi: 10.1016/j.neuroimage.2021.118654. [DOI] [PubMed] [Google Scholar]
- Nurmi S., Karttunen J., Souza V.H., Ilmoniemi R.J., Nieminen J.O. Trade-off between stimulation focality and the number of coils in multi-locus transcranial magnetic stimulation. J. Neural Eng. 2021;18(6) doi: 10.1088/1741-2552/ac3207. [DOI] [PubMed] [Google Scholar]
- Opitz A., Windhoff M., Heidemann R.M., Turner R., Thielscher A. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. NeuroImage. 2011;58(3):849–859. doi: 10.1016/j.neuroimage.2011.06.069. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A., Walsh V., Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience – virtual lesion, chronometry, and functional connectivity. Curr. Opin. Neurobiol. 2000;10(2):232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Peterchev A.V., Goetz S.M., Westin G.G., Luber B., Lisanby S.H. Pulse width dependence of motor threshold and input–output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin. Neurophysiol. 2013;124(7):1364–1372. doi: 10.1016/j.clinph.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J.C., Reithler J., Graaf T.A.D., Schuhmann T., Goebel R., Sack A.T. Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity. Commun. Biol. 2020;3(1):1–11. doi: 10.1038/s42003-020-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R., Nitsche M.A., Ruff C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018;21:174–187. doi: 10.1038/s41593-017-0054-4. [DOI] [PubMed] [Google Scholar]
- Puonti O., Van Leemput K., Saturnino G.B., Siebner H.R., Madsen K.H., Thielscher A. Accurate and robust whole-head segmentation from magnetic resonance images for individualized head modeling. Neuroimage. 2020;219 doi: 10.1016/j.neuroimage.2020.117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin E., Pellegrino G., Lazzaro V.D., Thielscher A., Siebner H.R. Bringing transcranial mapping into shape: Sulcus-aligned mapping captures motor somatotopy in human primary motor hand area. NeuroImage. 2015;120:164–175. doi: 10.1016/j.neuroimage.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Ridding M.C., Rothwell J.C. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control. Electroencephal. Clin. Neurophysiol. Electromyogr. Motor Control. 1997;105(5):340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Ridding M.C., Rothwell J.C. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat. Rev. Neurosci. 2007;8(7):559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Romero M.C., Davare M., Armendariz M., Janssen P. Neural effects of transcranial magnetic stimulation at the single-cell level. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-10638-7. 527–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell J.C., Hallett M., Berardelli A., Eisen A., Rossini P.M., Paulus W. Magn. Stimul. Mot. Evoked Potentials Electroencephalogr. Clin. Neurophysiol. 1999;Supplement, 52:97–103. [PubMed] [Google Scholar]
- Siddiqi S.H., Kording K.P., Parvizi J., Fox M.D. Causal mapping of human brain function. Nat. Rev. Neurosci. 2022;23(6):361–375. doi: 10.1038/s41583-022-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner H.R., Funke K., Aberra A.S., Antal A., Bestmann S., Chen R., Ugawa Y. Transcranial magnetic stimulation of the brain: what is stimulated?–a consensus and critical position paper. Clin. Neurophysiol. 2022 doi: 10.1016/j.clinph.2022.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J., Cattaneo Z. Common framework for “virtual lesion” and state-dependent TMS: the facilitatory/suppressive range model of online TMS effects on behavior. BrainCogn. 2017;119:32–38. doi: 10.1016/j.bandc.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A., Opitz A., Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage. 2011;54(1):234–243. doi: 10.1016/j.neuroimage.2010.07.061. [DOI] [PubMed] [Google Scholar]
- Thielscher A., Antunes A., Saturnino G.B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), (2015). doi:10.1109/embc.2015.7318340. [DOI] [PubMed]
- Valero-Cabré A., Amengual J.L., Stengel C., Pascual-Leone A., Coubard O.A. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci. Biobehav. Rev. 2017;83:381–404. doi: 10.1016/j.neubiorev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- van der Burght C.L., Numssen O., Schlaak B., Goucha T., Hartwigsen G. Differential contributions of inferior frontal gyrus subregions to sentence processing guided by intonation. Hum. Brain Mapp. 2023 doi: 10.1002/hbm.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoornweder S., Nuyts M., Frieske J., Verstraelen S., Meesen R., Caulfield K.A. A systematic review and large-scale tES and TMS electric field modeling study reveals how outcome measure selection alters results in a person-and montage-specific manner. bioRxiv. 2023 doi: 10.1016/j.neuroimage.2023.120379. 2023-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V., Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat. Rev. Neurosci. 2000;1(1):73–80. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Ward J. The Student's Guide to Cognitive Neuroscience. 4th ed., Routledge; 2019. [Google Scholar]
- Weise K., Numssen O., Thielscher A., Hartwigsen G., Knösche T.R. A novel approach to localize cortical TMS effects. Neuroimage. 2020;209 doi: 10.1016/j.neuroimage.2019.116486. [DOI] [PubMed] [Google Scholar]
- Weise K., Numssen O., Kalloch B., Zier A.L., Thielscher A., Haueisen J., Hartwigsen G., Knösche T.R. Precise motor mapping with transcranial magnetic stimulation. Nat. Protoc. 2022 doi: 10.1038/s41596-022-00776-6. [DOI] [PubMed] [Google Scholar]
- Weise K., Worbs T., Kalloch B., Numssen O., Hartwigsen G., Knösche T. An efficient and easy-to-use model to determine the stimulation thresholds in transcranial brain stimulation and its application to TMS mapping. Brain Stimul. 2023;16(1) doi: 10.1016/j.brs.2023.01.107. [DOI] [Google Scholar]