Abstract
Purpose
Increased evidence has shown that aerobic exercise reduces airway hyperresponsiveness in asthmatic individuals. However, the underlying mechanisms of action remain elusive. This study aimed to investigate the effect of exercise on airway smooth muscle (ASM) contractile function in asthmatic rats, and uncover the possible involvement of interleukin 4 (IL-4) and the store-operated Ca2+ entry (SOCE) pathway.
Methods
In this study, chicken ovalbumin was used to induce asthma in male Sprague-Dawley rats. The exercise group received moderate-intensity aerobic exercise training for 4 weeks. IL-4 concentrations in bronchoalveolar lavage fluid (BALF) samples were evaluated by enzyme linked immunosorbent assay. The contractile function of the ASM was investigated using tracheal ring tension experiments and intracellular Ca2+ imaging techniques. Western blot analysis was used to evaluate expression levels of calcium-release activated calcium (CRAC) channel protein (Orai) and stromal interaction molecule 1 (STIM1) in ASM.
Results
Our data showed that the carbachol-stimulated, SOCE-mediated contraction of rat ASM was significantly increased in asthmatic rats, which could be abolished by exercise. Pharmacological studies revealed that GSK5498A and BTP-2, selective blockers of CRAC channels significantly inhibited SOCE-induced ASM contraction. In addition, exercise inhibited the up-regulation of IL-4 in BALF as well as STIM1 and Orai expression in the ASM of asthmatic rats. In line with these observations, we demonstrated that pretreatment of the ASM with IL-4 up-regulated the expression level of STIM1, Orai1 and Orai2, thereby promoting SOCE-mediated ASM contraction.
Conclusions
The data in this study reveal that aerobic exercise may improve the ASM contractile function in asthmatic rats by inhibiting IL-4 secretion and by down-regulating the expression of STIM1, Orai1 and Orai2, thus decreasing excessive SOCE-mediated ASM contraction in asthmatic rats.
Keywords: Asthma, exercise, airway, smooth muscle, interleukin 4, calcium release activated calcium channels
INTRODUCTION
Asthma is a long-term breathing disorder characterized by inflammatory responses of the lower respiratory tract, mediated by type 2 helper T (Th2) cells and airway hyperreactivity (AHR).1,2 Asthma affects approximately 300 million individuals worldwide, and the prevalence of asthma has increased in China over the past 10 years.3,4 For patients with asthma, lifestyle modification is an important part of asthma management. It has been well documented that exercise is beneficial to individuals with asthma.5 Moderate physical exercise training has proven to be a safe and effective non-pharmacological means for asthma management.6,7 However, the mechanisms underlying the beneficial effects of aerobic exercise on asthmatic individuals have yet to be elucidated.
Several studies have shown that aerobic exercise can reduce the level of inflammation in asthmatic animals and patients.8,9,10 In line with these studies, a decrease in the expression of Th2 cytokines (interleukin [IL]-4, IL-5 and IL-13) has been observed in asthmatic mice subjected to aerobic exercise.11,12,13 In addition, it has been demonstrated that aerobic exercise reduces AHR in asthmatic mice.14 However, it remains to be investigated whether the inflammatory cytokines participate in the exercise-induced relieve of AHR. Moreover, IL-4 promoted the store-operated Ca2+ entry (SOCE) response by promoting both the expression and activity of store-operated Ca2+ channels (SOCCs).15 Furthermore, in a previous study, it was shown that SOCC blockers could relieve airway inflammation and AHR in ovalbumin (OVA)-sensitized asthmatic mice.16 Together, these observations indicate a possible role for IL-4 in the regulation of airway smooth muscle (ASM) contraction via the SOCE pathway.
As is known, SOCE is stimulated by intracellular sarcoplasmic reticulum/endoplasmic reticulum Ca2+ store depletion. SOCE-mediated variation in intracellular Ca2+ concentration ([Ca2+]i) is one of the main signaling pathways involved in the regulation of ASM contraction.17 At the onset of asthma-induced AHR, smooth muscle spasms are stimulated due to an enhanced ASM contraction response induced by abnormal Ca2+ mobilization.18 Considering that aerobic exercise training reduces airway hyperresponsiveness,19 it is possible that the expression and/or function of the Ca2+ release-activated calcium (CRAC) channel protein, Orai, a key pore-forming unit of SOCCs, and stromal interaction molecule 1 (STIM1), are modified by aerobic exercise.
In the present study, we aimed to investigate the regulatory effect of aerobic exercise on tracheal contraction in asthmatic rats, and to explore whether IL-4 and the SOCE pathway are involved in this process. We hypothesized that aerobic exercise down-regulates protein levels of STIM1/Orai in ASM through an IL-4-mediated pathway and thus reduce symptoms of asthma.
MATERIALS AND METHODS
Animals
A total of 60 male Sprague-Dawley rats at the age of 3–4 months were purchased from the Experimental Animal Center of Southern Medical University (Guangzhou, China). Rats were allowed food and water ad libitum and were housed at a constant room temperature of 24°C and a 12-h light/12-h dark cycle. All animals were maintained in accordance with the guidelines of the US National Institutes of Health (NIH publication No. 8523). Ethical approval for the study was granted by the Animal Experimentation Ethics Committee of Guangzhou Sport University.
Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum, penicillin, streptomycin, Hanks Balanced Salt Solution, bovine serum albumin (BSA), sodium pyruvate, and trypsin were purchased from Gibco (Carlsbad, CA, USA). Collagenase type IA, aluminum hydroxide gel, carbachol (CCH), IL-4, nifedipine, apamin, dimethyl sulfoxide, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, egtazic acid, and OVA were purchased from Sigma-Aldrich (St. Louis, MO, USA). NaCl, KCl, MgSO4, NaHCO3, KH2PO4, CaCl2, and glucose were purchased from Guangzhou Chemical Pharmaceutical Factory (Guangzhou, China). GSK5498A, BTP-2, and thapsigargin (TG) were purchased from MedChem Express (Monmouth Junction, NJ, USA). The IL-4 enzyme-linked immunosorbent assay kit was purchased from NeoBioscience (Shenzhen, China). Fluo8-AM was purchased from Abcam (Cambridge, UK).
OVA sensitization and exercise training protocol
Rats were randomly assigned to the normal group without asthma (control group), asthma group (asthma group) or asthma group with exercise (exercise group). Rats of the asthma and exercise groups were intraperitoneally sensitized to OVA and moderate intensity aerobic exercise training was conducted simultaneously. In brief, rats were sensitized by an intraperitoneal injection of a suspension (1 mL) containing OVA (200 µg), Al (OH)3 (5 mg) and Mg(OH)2 (5 mg) on day 0, 7 and 14, and exposed to aerosolized OVA on days 21–28. Saline was used as the negative control. Meanwhile, rats of the exercise group received exercise training on a treadmill for 1 h/day, 5 days/week for 4 weeks from day 0 of the OVA sensitization. The exercise training protocol consisted of 21 m/min (moderate-intensity, 40 minutes) with 0% grade, and included 20-min warm-up and cool-down periods. Rats were sacrificed after 4 weeks of OVA sensitization and exercise training.
Respiration analysis
Rats were placed into a respiration measurement chamber with a respiration transducer connected to a data acquisition and analysis system (BL-420S; Chengdu Taimeng Technology, China). The chamber was tightened to avoid air leaks, and was kept in a dark environment. The respiratory rate and tidal volume were determined for 10 minutes. Respiratory parameters, including tidal volume and respiration, were collected and analyzed.
Hematoxylin and eosin (H&E) staining
H&E staining of paraffin sections (2 mm) of rat lung tissue was taken according instructions provided with the H&E Staining Kit (SanGong Biotech, Shanghai, China).
Measurement of contractile tension
Contractile tension measurements were performed as previously described.20 Briefly, experimental rats were sacrificed by CO2 asphyxia and tracheal samples were carefully isolated and placed into ice-cold Krebs-Henseleit (K-H) solution, which consisted of 118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25.2 mM NaHCO3, and 11.1 mM glucose, saturated with 95% O2 plus 5% CO2 to maintain a pH of 7.4. After removing the epithelial layer by gentle friction to ensure minimal damage to the smooth muscle cells, tracheal samples were cut into suitable segments (2–3 mm). Tracheal rings were subsequently mounted in a DMT myograph (model 620M; Danish Myo Technology, Aarhus, Denmark) under normalized tension. In each experiment, tracheal rings were stretched to a preload tension of 10 mN and equilibrated for 60 minutes. Next, the contractile function of the tracheal rings was evaluated by replacing the normal K-H solution with 60 mM K+ solution, which was prepared by substitution of NaCl with an equimolar amount of KCl.
To investigate the role of CRAC channels in SOCE-induced contraction, tracheal rings were equilibrated in Ca2+-free Krebs solution for 10 minutes. After pretreatment with both CCH (10 μM) and nifedipine (10 μM) for 10 minutes, 2.5 mM CaCl2 was added to stimulate a SOCE-induced contractile response. The CRAC channel selective blockers, GSK5498A (10 μM) and BPT-2 (3 μM), were applied to characterize the role of CRAC channels in the contractile response induced by SOCE, respectively. In this study, “n” represents the number of rats used for contractile tension measurement.
Collection of bronchoalveolar lavage fluid (BALF)
The neck region of each experimental rat was surgically opened after exsanguination, and the trachea was exposed. Chilled phosphate buffered saline (PBS) was instilled into the lungs using a syringe. PBS (5 mL) was retained in the lungs for 30 seconds, during which the thoracic area was gently massaged. BALF was retrieved and the lungs were instilled again, which was repeated 3 times and a final volume of 10 mL BALF was obtained. BALF was centrifuged (500 g, 4°C) and the supernatant was collected and stored at −80°C until further analysis. The recovery of BALF was > 80%.
Enzyme linked immunosorbent assay (ELISA)
The IL-4 concentration in BALF samples was measured using an IL-4 ELISA kit according to the manufacturer’s instructions (NeoBioscience). The symbol “n” represents the number of BALF samples used for the detection of the IL-4 level.
Measurement of [Ca2+]i
Measurements of [Ca2+]i in the trachea was were taken as previously described with some minor modifications.21 After removal of the rat trachea, the tracheal epithelial layer was carefully removed by gently rubbing with cotton swabs. The trachea was then cut open and fixed onto a plastic block. The tissue was washed 3 times with PBS and incubated with Fluo-8/AM (6 μM) and 0.02% pluronic F-127 in DMEM medium at 37°C for 30 minutes. After washing 3 times with Ca2+-free saline solution, the tissue was fixed in a chamber containing 0.5 mL of Ca2+-free saline solution. A fluorescence microscope system (Nikon, Japan) was used for fluorescence signal detection. After equilibrating for 5 minutes, TG (2 μM) was added to deplete the stored Ca2+. Finally, after 10 minutes, CaCl2 (at a final concentration of 2.5 mM) was added to evoke a Ca2+ influx. [Ca2+]i changes were calculated as a percentage of the initial fluorescence.
Western blot analysis
Total protein was extracted from the smooth muscle layer isolated from the adventitial layer. Equal amounts of protein were resolved by SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane. Membranes were blocked with 5% (wt/vol) BSA solution for 1 hour at room temperature and incubated overnight at 4°C with goat anti-Orai1 (DF7958; Affinity Biosciences, Cincinnati, OH, USA), anti-Orai2 (20592-1-AP; ProteinTech, Wuhan, China), anti-Orai3 (25766-1-AP, ProteinTech), and anti-STIMl (sc-166840; Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies diluted with 5% (wt/vol) BSA solution. Horseradish peroxidase (HRP)-conjugated secondary antibody (Elabscience, Germany), diluted 1:20,000 with 5% (wt/vol) BSA solution, was added to the membranes and incubated for 1 hour at room temperature. The labeled proteins were visualized using an HRP substrate kit (Tanon, Shanghai, China).
Statistical analysis
Statistical analyses were performed using Origin Pro 8.0 (OriginLab Corporation, Northampton, MA, USA). Data are presented as the mean ± SD. Student’s t-test was used to assess differences between the two groups. For three or more groups, data were analyzed using a one-way analysis of variance followed by the Bonferroni test for multiple comparisons. A P < 0.05 was considered statistically significant.
RESULTS
Effect of aerobic exercise on SOCE-induced ASM contraction in asthmatic rats
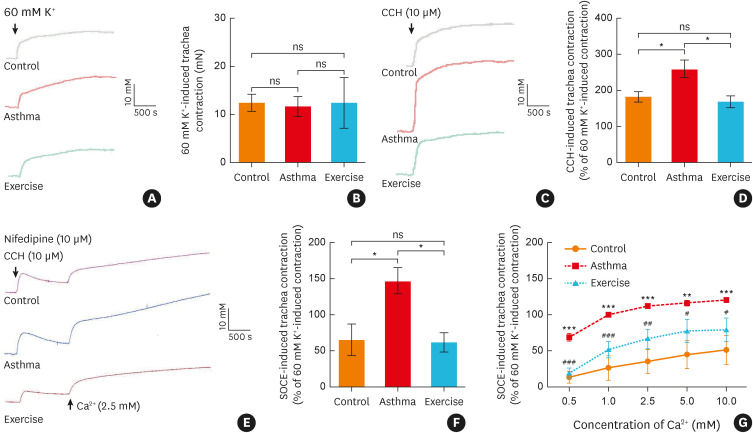
Rat tracheal rings were initially stimulated using a 60 mM K+ solution and no significant differences in contractile responses were observed between the three groups (Fig. 1A and B). However, compared to the control group, the CCH-stimulated ASM contraction was significantly increased in the asthma group, and this effect was mitigated by aerobic exercise training (Fig. 1C and D). Next, the CCH-induced, SOCE-mediated contractile response was evaluated. As illustrated in Fig. 1E, tracheal rings were pretreated with CCH (10 µM) in Ca2+-free K-H solution and incubated with the L-type voltage-gated Ca2+ channel (VGCC) blocker, nifedipine (10 µM), to eliminate the effect of the L-type VGCC-mediated Ca2+ influx. Subsequently, the SOCE-mediated contractile responses were measured in the three groups after being exposed to different extracellular Ca2+ levels. Our data revealed that aerobic exercise attenuated SOCE-mediated excessive contractile responses in the asthmatic rats (Fig. 1E-G).
Fig. 1. Aerobic exercise decreased the SOCE-induced airway smooth muscle contraction of asthmatic rats. (A) Representative traces and (B) statistical analysis (n = 4) of the contractile response induced by a high-concentration K+ solution (60 mM) in rat tracheal rings. (C) Representative traces and (D) statistical analysis of the contractile response induced by CCH (10 μM) in rat tracheal rings (n = 5), *P < 0.05 Asthma vs. Control or Exercise. (E) Representative traces and (F) statistical analysis of the contractile response induced by SOCE in rat tracheal rings (n = 5), *P < 0.05. (G) Statistical curves showing the contractile response induced by SOCE of rat tracheal rings with different extracellular Ca2+ levels (n = 3−4), **P <0.01, ***P < 0.001 Asthma vs. Control; #P < 0.05; ##P < 0.01 Exercise vs. Asthma group. Symbols and bars indicate the mean ± SD.
ns, not significant; CCH, carbachol; SOCE, store-operated Ca2+ entry.
Role of Orai in SOCE-mediated ASM contraction
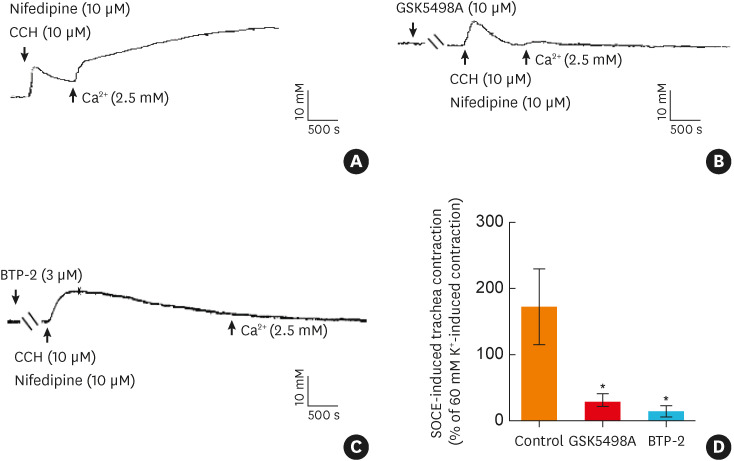
The CRAC channel protein, Orai, has been shown to play a pivotal role in mediating SOCE of ASM.22 As illustrated in Fig. 2, pretreatment of the tracheal rings with GSK5498A (10 μM) or BTP-2 (3 μM), selective blockers of CRAC channels,23,24,25 almost completely abolished SOCE-induced contractile responses of the tracheal rings. These observations demonstrated that Orai was involved in SOCE-mediated ASM contraction and indicating that Orai may be a key mediator of aerobic exercise-induced normalization of the tracheal contractile responses in asthmatic rats.
Fig. 2. Orai is involved in SOCE-induced airway smooth muscle contraction. (A) Representative traces of the contractile response induced by SOCE. (B) Representative traces of the contractile response induced by SOCE when the tracheas were incubated with the selective calcium-release activated Ca2+ channel blocker GSK5498A (10 μM) or BTP-2 (3 μM). (D) Statistical analysis showing the contractile response induced by SOCE under different conditions (n = 4-6). *P < 0.05 vs. the control group. Symbols and bars indicate the mean ± SD.
CCH, carbachol; SOCE, store-operated Ca2+ entry.
Aerobic exercise relieves the SOCE-mediated ASM contraction of asthmatic rats through decreasing the level of IL-4
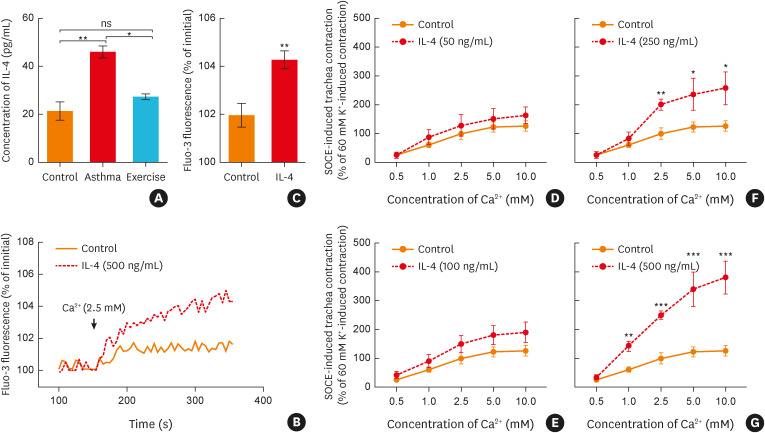
Given the important role of IL-4 in the pathogenesis of asthma,26 the level of IL-4 in BALF was evaluated in the three groups (Fig. 3A). Our data showed that, compared to the control group, the IL-4 level in BALF samples collected from the asthma group was significantly increased. However, this effect was reversed in the exercise group (Fig. 3A). In addition, we found that preincubation with IL-4 (500 ng/mL) for 12 hours significantly increased the SOCE response of ASM (Fig. 3B-C). Subsequently, the effect of IL-4 on the tracheal contractile response was further evaluated. As illustrated in Fig. 3D-G, pretreatment with IL-4 (50–500 ng/mL, 12 hours) remarkably strengthened the CCH-induced, SOCE-mediated tracheal contractile response. Taken together, these data suggested that aerobic exercise may down-regulate the IL-4 concentration and subsequently relieve SOCE-mediated ASM contraction in asthmatic rats.
Fig. 3. Aerobic exercise relieved the SOCE-mediated ASM contraction of asthmatic rats through decreasing the secretion of IL-4. (A) Statistical analysis showing the secreted levels of IL-4 in the bronchoalveolar lavage fluid of each group (n = 5). (B) Representative traces and (C) statistical analysis of the [Ca2+]i response induced by SOCE when the ASM was incubated with IL-4 (500 ng/mL, 12 hours) (n = 5), **P < 0.01 vs. the control group. (D-G) Statistical analysis showing the SOCE-induced ASM contraction when the trachea was incubated with IL-4 (50–500 ng/mL, 12 hours, n = 4–8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group. Symbols and bars indicate the mean ± SD.
IL, interleukin; ns, not significant; SOCE, store-operated Ca2+ entry; ASM, airway smooth muscle.
Aerobic exercise down-regulates the STIM1/Orai protein expression levels of asthmatic rats through an IL-4-mediated pathway
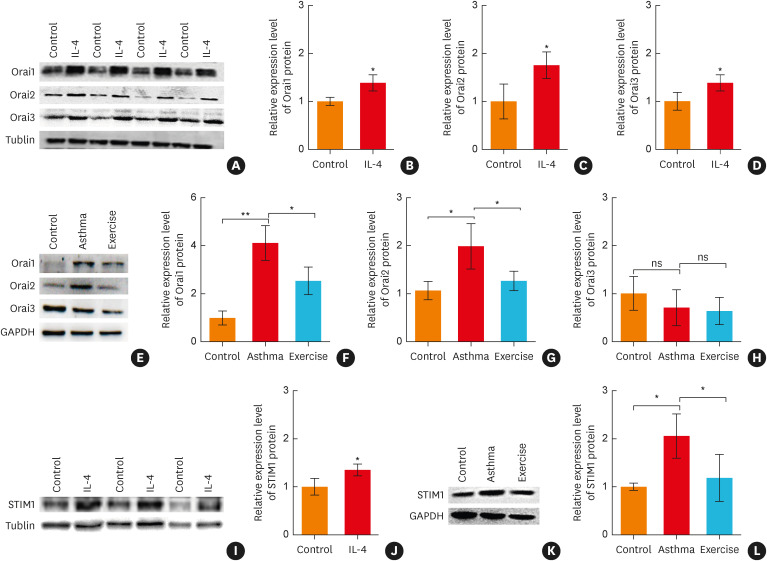
Next, the effect of IL-4 on Orai and STIM protein expression levels in rat ASM was investigated by western blot analysis. As shown in Fig. 4A-D, I and J, the protein expression levels of Orai1, Orai2, Orai3 and STIM1 in ASM were up-regulated when ASM was pretreated with IL-4 (500 ng/mL, 12 hours). In addition, we found that the protein expression levels of Orai1, Orai2 and STIM1, but not Orai3, were up-regulated in the asthma group. Furthermore, aerobic exercise significantly down-regulated Orai1, Orai2, and STIM1 protein levels in asthmatic rats (Fig. 4E-H, K and L).
Fig. 4. Aerobic exercise down-regulated the protein expression levels of Orai and STIM1 of asthmatic rats through the IL-4-related pathway. (A) Representative Western blot images and (B-D) statistical analysis for Orai (subtype 1, 2, 3) protein expression levels of rat ASM after incubation with IL-4 (500 ng/mL, 12 hours) (n = 4), *P < 0.05, **P < 0.01 vs. the control group. (E) Representative western blot images and (F-H) statistical analysis for Orai (subtypes 1, 2 and 3) protein expression levels in the rat ASM of the model rats (n = 3), *P < 0.05, ***P < 0.001. ns indicates no significant difference. (I) Representative western blot images and (J) statistical analysis for STIM1 protein expression levels of rat ASM after incubation with IL-4 (500 ng/mL, 12 hours) (n = 3), *P < 0.05 vs. the control group. (K) Representative western blot images and (L) statistical analysis for STIM1 protein expression levels in the rat ASM of model rats (n = 3), *P < 0.05. Symbols and bars indicate the mean ± SD.
IL, interleukin; ns, not significant; STIM1, stromal interaction molecule 1; ASM, airway smooth muscle.
DISCUSSION
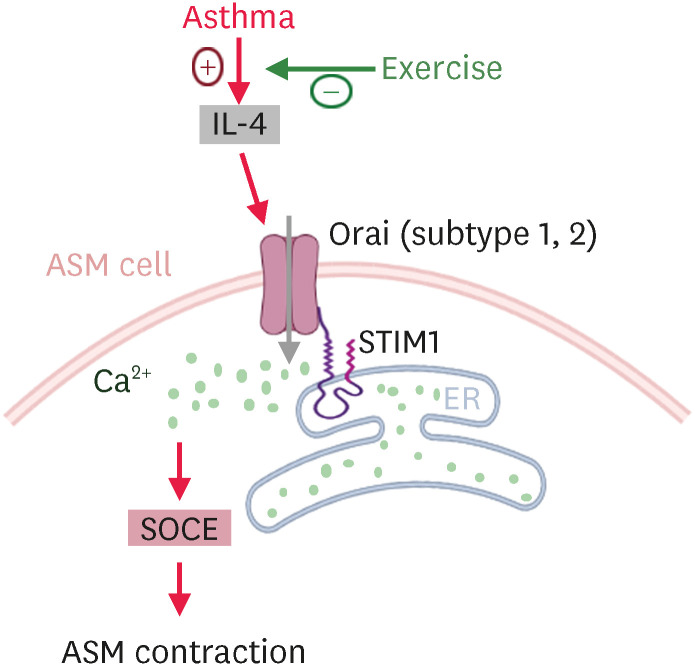
It has previously been demonstrated that appropriate aerobic exercise training is beneficial to asthmatic individuals.7 However, the effect of exercise training on the contractile function of ASM is still unclear. In the present study, we demonstrated that aerobic exercise decreased the Th2 inflammatory cytokine IL-4 level and relieved excessive SOCE-induced ASM contraction in OVA-sensitized asthmatic rats. Based on our observations, exercise has an inhibitory effect on the IL-4-induced up-regulation of STIM1/Orai protein expression levels. The proposed working model of the present study is illustrated in Fig. 5.
Fig. 5. Proposed working model of the aerobic exercise reduces airway smooth muscle contraction in asthmatic rats. Aerobic exercise improved the ASM contractile function in asthmatic rats by inhibiting IL-4 secretion and down-regulating STIM1 and Orai (subtypes 1 and 2) expression levels, thereby decreasing excessive SOCE-mediated ASM contraction in asthmatic rats.
IL, interleukin; ASM, airway smooth muscle; STIM1, stromal interaction molecule 1; SOCE, store-operated Ca2+ entry.
Since the 1970s, asthma has become one of the most prevalent chronic respiratory disorders of developed society. Recently, a rapid increase in the incidence rate of asthma has occurred in the developing world, including China.27,28 It has been recognized that airway inflammation is pivotal for the pathogenesis of asthma. In lung tissue of our asthmatic rat models, infiltration of inflammatory cells and pulmonary alveolar rupture were observed (Supplementary Fig. S1A). The Th2 inflammatory cytokine IL-4 plays an important role in driving eosinophil infiltration. IL-4 induces IgE synthesis by B cells and contributes to mucus production, bronchial fibrosis, and AHR in asthma.15 Moreover, an increased serum level of IL-4 has been confirmed as a major characteristic of asthma.29 Inhibiting the secretion of IL-4 by application of biologics has proven to be an effective treatment of asthma in animal models.30 Moreover, it has previously been reported that aerobic exercise attenuated the asthma phenotype through regulating inflammatory cytokine levels,31 including IL-4 levels, in the BALF of a mouse model of asthma.14 In the present study, our data demonstrated that 4 weeks of aerobic exercise significantly decreased the IL-4 level in BALF of OVA-sensitized asthmatic rats.
As previously reported, Th2 cytokine interleukins play a central role in the pathogenesis of asthma and exhibit a direct effect on ASM, leading to AHR.32,33 For example, IL-13 treatment increased the SOCE response of rat bronchial smooth muscle cells.34 Furthermore, it has been reported that Orai1 mediates the SOCE response of ASM cells.22 However, IL-13 treatment did not influence the expression of Orai1 at the mRNA level.34 In addition, our data showed that IL-13 treatment could not significantly strengthen the SOCE-mediated contraction of trachea rings despite a slight increment of the trachea contraction response (Supplementary Fig. S2). Together, these data suggested that IL-13 may play different pathological roles in bronchial and tracheal smooth muscle cells of asthmatic rats. IL-4 and IL-13 present similar structures and share one receptor subunit (L-4Rα). However, IL-4 and IL-13 have been demonstrated to have some nonredundant pathological functions in allergy and asthma.35 Additionally, in another study, it was demonstrated that IL-4 could induce IL-13-independent AHR.36 However, whether IL-4 affects SOCE in ASM cells has not been reported. In fibrocytes, IL-4 may stimulate fibrocyte differentiation from CD14+ monocytes by up-regulating the expression level of STIM and Orai1.15 Our data demonstrated that IL-4 treatment increased the protein expression levels of STIM1 and Orai1-3. In addition, we found that protein expression levels of both IL-4 and STIM1/Orai1/Orai2 were up-regulated in asthmatic rats and that this could be abolished by a 4-week aerobic exercise. Thus, these results indicate that aerobic exercise reduced the expression levels of STIM1/Orai1/Orai2 in asthmatic rats by inhibiting the secretion of IL-4. Previous studies have suggested that IL-4 predominantly plays a role in the early phase of asthma development through regulating the function of T cells and IgE synthesis. Moreover, IL-13 has been shown to predominantly be involved in allergic reactions, such as mucus hypersecretion.35 However, the findings of our present study indicated that IL-4 may also play roles in the late phases of allergic reactions, such as in airway remodeling., Further investigation is needed to elucidate the underlying molecular mechanisms involved and to uncover the influence of IL-4 on mRNA expression, and transcriptional or post-transcription level of STIM1/Orai1/Orai2.
Moderate exercise increases oxygen consumption, which increases ventilatory demands and thus leads to deeper and more rapid breathing. The improved oxygen uptake capacity together with an increased threshold for getting breathlessness would help those with asthma in coping with their everyday life with a lower effort level.37 These factors have been shown in experimental models to cause stretching of the ASM, bronchodilation and the maintenance of airway calibers.38 A significant increase in the respiratory rate is an important phenotype of asthmatic rats (Supplementary Fig. S1B) and indicates dysfunctional tracheal contractions. In the present study, we found that 4 weeks of aerobic exercise decreased the tracheal contractile response of asthmatic rats. As previously reported, excessive contraction of ASM is a characteristic of AHR in asthmatic individuals.39 The intracellular Ca2+ dynamics of ASM regulate tracheal contraction and thus play a key role in AHR. Several studies have revealed that the abnormal agonist-induced Ca2+ oscillations of ASM result in AHR.18,40 Sustained contraction and the Ca2+ oscillation response require an influx of extracellular Ca2+. It has previously been reported that SOCE, rather than Ca2+ influx via VGCC, provides the major Ca2+ entry pathway in ASM cells.23 As illustrated in Fig. 1, our data showed that VGCC was not involved in the excessive tracheal contraction of OVA-sensitized rats. In another related study, SOCC blockers could attenuate AHR in OVA-sensitized animals.41 In line with these studies, we found that 4 weeks of aerobic exercise decreased the SOCE-induced Ca2+ influx and inhibited the excessive tracheal contraction in asthmatic rats. These data might, at least partially, provide valuable insight into the involvement of an IL-4-regulated, STIM1/Orai-mediated pathway that is responsible for aerobic exercise-induced reversal of AHR in asthma. It should be mentioned that evaluating bronchi contraction would be more appropriate for our study considering the pathophysiology of asthma, and thus in vivo studies on bronchi responses are needed in the future.
In conclusion, our data demonstrated that SOCE was increased in the ASM of OVA-sensitized asthmatic rats, which was accompanied by the significant up-regulation of both IL-4 secretion and STIM1/Orai protein expression. Conversely, a 4-week aerobic exercise protocol decreased the tracheal contractile response of asthmatic rats by suppressing the expression of STIM1/Orai via inhibition of IL-4 secretion. Thus, our results indicate that the SOCE-mediated pathway may serve as a potential therapeutic target for AHR in asthma.
ACKNOWLEDGMENTS
The current work was supported by grants from the Guangdong Basic and Applied Basic Research Foundation (2019A1515012167, 2023A1515012011, 2021A1515011264), the National Natural Science Foundation of China (31600969, 31771315, 31971105), the Anhui Provincial Education Foundation (KJ2020ZD13), and the Macao Science and Technology Development Fund (Project code: 001/2020/ALC).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Pathological profile of the lung tissue and respiration rate from control and asthmatic rats. (A) Hematoxylin and eosin staining showing pathological profile of the lung tissue from control and asthmatic rats, Scar bras: 20 µm. (B) The statistical data of respiratory rate in control and asthmatic rats (n = 4–10), ***P < 0.001 vs. the control group. Symbols and bars indicated the means ± SD.
Effect of IL-13 on the SOCE-induced ASM contraction of rats. Statistical analysis showing the SOCE-induced ASM contraction when incubated the trachea with IL-13 (250 ng/mL, 12 hours, n = 11). Symbols and bars indicated the means ± SD.
References
- 1.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J. 2007;29:179–184. doi: 10.1183/09031936.00087906. [DOI] [PubMed] [Google Scholar]
- 2.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda A, Wasan AN. Asthma in adults. Med Clin North Am. 2020;104:95–108. doi: 10.1016/j.mcna.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Lin J, Wang W, Chen P, Zhou X, Wan H, Yin K, et al. Prevalence and risk factors of asthma in mainland China: the CARE study. Respir Med. 2018;137:48–54. doi: 10.1016/j.rmed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;(9):CD001116. doi: 10.1002/14651858.CD001116.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Ding S, Zhong C. Exercise and asthma. Adv Exp Med Biol. 2020;1228:369–380. doi: 10.1007/978-981-15-1792-1_25. [DOI] [PubMed] [Google Scholar]
- 7.Côté A, Turmel J, Boulet LP. Exercise and asthma. Semin Respir Crit Care Med. 2018;39:19–28. doi: 10.1055/s-0037-1606215. [DOI] [PubMed] [Google Scholar]
- 8.Qin Q, Chen X, Feng J, Qin L, Hu C. Low-intensity aerobic exercise training attenuates airway inflammation and remodeling in a rat model of steroid-resistant asthma. Chin Med J (Engl) 2014;127:3058–3064. [PubMed] [Google Scholar]
- 9.Pastva A, Estell K, Schoeb TR, Atkinson TP, Schwiebert LM. Aerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthma. J Immunol. 2004;172:4520–4526. doi: 10.4049/jimmunol.172.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luks V, Burkett A, Turner L, Pakhale S. Effect of physical training on airway inflammation in animal models of asthma: a systematic review. BMC Pulm Med. 2013;13:24. doi: 10.1186/1471-2466-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira RP, Claudino RC, Duarte AC, Santos AB, Perini A, Faria Neto HC, et al. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176:871–877. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- 12.Vieira RP, Duarte AC, Santos AB, Medeiros MC, Mauad T, Martins MA, et al. Exercise reduces effects of creatine on lung. Int J Sports Med. 2009;30:684–690. doi: 10.1055/s-0029-1224176. [DOI] [PubMed] [Google Scholar]
- 13.Silva RA, Vieira RP, Duarte AC, Lopes FD, Perini A, Mauad T, et al. Aerobic training reverses airway inflammation and remodelling in an asthma murine model. Eur Respir J. 2010;35:994–1002. doi: 10.1183/09031936.00049509. [DOI] [PubMed] [Google Scholar]
- 14.Almeida-Oliveira AR, Aquino-Junior J, Abbasi A, Santos-Dias A, Oliveira-Junior MC, Alberca-Custodio RW, et al. Effects of aerobic exercise on molecular aspects of asthma: involvement of SOCS-JAK-STAT. Exerc Immunol Rev. 2019;25:50–62. [PubMed] [Google Scholar]
- 15.Zhong JN, Lan L, Chen YF, Huang G, He GZ, Yang J, et al. IL-4 and serum amyloid P inversely regulate fibrocyte differentiation by targeting store-operated Ca2+ channels. Pharmacol Rep. 2018;70:22–28. doi: 10.1016/j.pharep.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Ohga K, Takezawa R, Yoshino T, Yamada T, Shimizu Y, Ishikawa J. The suppressive effects of YM-58483/BTP-2, a store-operated Ca2+ entry blocker, on inflammatory mediator release in vitro and airway responses in vivo . Pulm Pharmacol Ther. 2008;21:360–369. doi: 10.1016/j.pupt.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Helli PB, Janssen LJ. Properties of a store-operated nonselective cation channel in airway smooth muscle. Eur Respir J. 2008;32:1529–1539. doi: 10.1183/09031936.00054608. [DOI] [PubMed] [Google Scholar]
- 18.Croisier H, Tan X, Perez-Zoghbi JF, Sanderson MJ, Sneyd J, Brook BS. Activation of store-operated calcium entry in airway smooth muscle cells: insight from a mathematical model. PLoS One. 2013;8:e69598. doi: 10.1371/journal.pone.0069598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.França-Pinto A, Mendes FA, de Carvalho-Pinto RM, Agondi RC, Cukier A, Stelmach R, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax. 2015;70:732–739. doi: 10.1136/thoraxjnl-2014-206070. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YL, Xu JW, Wu XH, Chen PX, Wei F, Gao DD, et al. Relaxant effect of sodium tanshinone IIA sulphonate on mouse tracheal smooth muscle. Planta Med. 2017;83:624–630. doi: 10.1055/s-0042-119950. [DOI] [PubMed] [Google Scholar]
- 21.Peppiatt-Wildman CM, Crawford C, Hall AM. Fluorescence imaging of intracellular calcium signals in intact kidney tissue. Nephron, Exp Nephrol. 2012;121:e49–e58. doi: 10.1159/000342812. [DOI] [PubMed] [Google Scholar]
- 22.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1-mediated store-operated Ca2+ entry in airway smooth muscle cell proliferation. J Appl Physiol. 2011;110:1256–1263. doi: 10.1152/japplphysiol.01124.2010. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Sanderson MJ. Store-operated calcium entry is required for sustained contraction and Ca2+ oscillations of airway smooth muscle. J Physiol. 2017;595:3203–3218. doi: 10.1113/JP272694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derler I, Schindl R, Fritsch R, Heftberger P, Riedl MC, Begg M, et al. The action of selective CRAC channel blockers is affected by the Orai pore geometry. Cell Calcium. 2013;53:139–151. doi: 10.1016/j.ceca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Xu L, Wu Y, Shen H, Lin Z, Fang Y, et al. Parathyroid hormone promotes human umbilical vein endothelial cell migration and proliferation through orai1-mediated calcium signaling. Front Cardiovasc Med. 2022;9:844671. doi: 10.3389/fcvm.2022.844671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izuhara K. Involvement of IL-4 and IL-13 signals in the pathogenesis of bronchial asthma. Arerugi. 2005;54:7–11. [PubMed] [Google Scholar]
- 27.Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alhassan S, Hattab Y, Bajwa O, Bihler E, Singh AC. Asthma. Crit Care Nurs Q. 2016;39:110–123. doi: 10.1097/CNQ.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 29.Chai R, Liu B, Qi F. The significance of the levels of IL-4, IL-31 and TLSP in patients with asthma and/or rhinitis. Immunotherapy. 2017;9:331–337. doi: 10.2217/imt-2016-0131. [DOI] [PubMed] [Google Scholar]
- 30.Walsh GM. Anti-IL-4/-13 based therapy in asthma. Expert Opin Emerg Drugs. 2015;20:349–352. doi: 10.1517/14728214.2015.1050377. [DOI] [PubMed] [Google Scholar]
- 31.Alberca-Custódio RW, Greiffo FR, MacKenzie B, Oliveira-Junior MC, Andrade-Sousa AS, Graudenz GS, et al. Aerobic exercise reduces asthma phenotype by modulation of the leukotriene pathway. Front Immunol. 2016;7:237. doi: 10.3389/fimmu.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devos FC, Pollaris L, Cremer J, Seys S, Hoshino T, Ceuppens J, et al. IL-13 is a central mediator of chemical-induced airway hyperreactivity in mice. PLoS One. 2017;12:e0180690. doi: 10.1371/journal.pone.0180690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boonpiyathad T, Sözener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019;46:101333. doi: 10.1016/j.smim.2019.101333. [DOI] [PubMed] [Google Scholar]
- 34.Gao YD, Zou JJ, Zheng JW, Shang M, Chen X, Geng S, et al. Promoting effects of IL-13 on Ca2+ release and store-operated Ca2+ entry in airway smooth muscle cells. Pulm Pharmacol Ther. 2010;23:182–189. doi: 10.1016/j.pupt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Conde E, Bertrand R, Balbino B, Bonnefoy J, Stackowicz J, Caillot N, et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat Commun. 2021;12:2574. doi: 10.1038/s41467-021-22834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J Allergy Clin Immunol. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Jaakkola JJ, Aalto SA, Hernberg S, Kiihamäki SP, Jaakkola MS. Regular exercise improves asthma control in adults: a randomized controlled trial. Sci Rep. 2019;9:12088. doi: 10.1038/s41598-019-48484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115:925–927. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 39.O’Byrne PM, Inman MD. Airway hyperresponsiveness. Chest. 2003;123(Suppl):411S–6S. doi: 10.1378/chest.123.3_suppl.411s. [DOI] [PubMed] [Google Scholar]
- 40.Moynihan B, Tolloczko B, Michoud MC, Tamaoka M, Ferraro P, Martin JG. MAP kinases mediate interleukin-13 effects on calcium signaling in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L171–L177. doi: 10.1152/ajplung.00457.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohga K, Takezawa R, Yoshino T, Yamada T, Shimizu Y, Ishikawa J. The suppressive effects of YM-58483/BTP-2, a store-operated Ca2+ entry blocker, on inflammatory mediator release in vitro and airway responses in vivo . Pulm Pharmacol Ther. 2008;21:360–369. doi: 10.1016/j.pupt.2007.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pathological profile of the lung tissue and respiration rate from control and asthmatic rats. (A) Hematoxylin and eosin staining showing pathological profile of the lung tissue from control and asthmatic rats, Scar bras: 20 µm. (B) The statistical data of respiratory rate in control and asthmatic rats (n = 4–10), ***P < 0.001 vs. the control group. Symbols and bars indicated the means ± SD.
Effect of IL-13 on the SOCE-induced ASM contraction of rats. Statistical analysis showing the SOCE-induced ASM contraction when incubated the trachea with IL-13 (250 ng/mL, 12 hours, n = 11). Symbols and bars indicated the means ± SD.