Abstract
Purpose
Chronic cough (CC) is associated with health-related quality of life (HRQoL) impairment. However, the determinants of HRQoL are under-investigated.
Methods
Patients aged 19–80 years with CC were prospectively recruited from 10 referral clinics. Comparisons were made with age- and sex-matched controls (1:4 ratio) selected from a Korean general population survey database; 1) a group without current cough (non-cough controls) and 2) another group without major chronic illnesses (healthy controls). HRQoL was assessed using the EuroQoL 5-dimension (EQ-5D) index. In CC patients, cough-specific patient-reported outcomes (PROs) were additionally measured. Cross-sectional analyses were performed to evaluate demographic and clinical parameters associated with the EQ-5D index of CC patients.
Results
A total of 200 CC patients (137 newly referred with CC and 63 refractory or unexplained CC [RUCC] patients), 800 non-cough controls, and 799 healthy controls were analyzed. The EQ-5D index of CC patients was significantly lower than that of non-cough controls or healthy controls (0.82 ± 0.14 vs 0.92 ± 0.14/0.96 ± 0.08; P < 0.001, respectively). The index was also associated with older age (≥ 60 years), female sex, and comorbidities such as asthma or depression. Among the patients with CC, the index was significantly lower in patients with RUCC than in those with newly referred CC, being treated with codeine or cough neuromodulators, or with cough-related fatigue. In Spearman analyses, the EQ-5D index correlated with cough-specific quality of life and cough severity scores, not with throat sensation or cough trigger scores.
Conclusions
The HRQoL impairment of CC patients was associated with older age, female sex, and comorbidities but it was also affected by cough severity, complications, treatments, and treatment responses. Longitudinal studies are warranted to further understand and improve the HRQoL of CC patients.
Keywords: Cough, quality of life, health status, cost of illness, registries
INTRODUCTION
Chronic cough (CC), usually defined as a cough that lasts for more than 8 weeks,1,2,3 is one of the common problems among individuals who seek medical attention.4 CC is a globally prevalent condition affecting about 5%–10% of the general adult population.5 It is often refractory to treatments and frequently persists for years.6,7,8,9,10,11 In a longitudinal study, approximately half of CC patients continued coughing on most days of the week with impairment in cough-related quality of life (QoL), even after 5 years since its initial assessment.10 CC causes substantial impairment in QoL of individuals with or without comorbidities, for whom poor control of cough affects the social, psychological, or physical aspects of QoL.12,13,14,15,16,17 Therefore, cough-specific QoL has been considered as one of the important outcomes in decision making for patients with CC.3,18
Health-related quality of life (HRQoL) is an individual’s perception of the effect of a disease or health status on several aspects of life and well-being.19 Several general population studies have demonstrated that CC is a debilitating condition that impairs the HRQoL of affected individuals.13,14,17,20,21,22,23,24 The EuroQoL 5-dimension (EQ-5D) questionnaire is a generic tool that has been widely used to measure HRQoL under different medical conditions including CC.17,25 In a general population-based study conducted in Korea, the EQ-5D index of CC patients were lower than those of non-CC controls regardless of comorbidities including depression, arthritis, asthma, or chronic obstructive pulmonary disease (COPD).17 Online nationwide studies in several East Asian countries also reported lower EQ-5D index of CC patients compared with that of matched non-cough controls.25,26 Given the long-term persistence of CC and poor treatment response in a large number of CC patients, the extent of HRQoL impairment may not only be confined to cough-specific health issues but also involve indirect or long-term general health consequences of coughing and treatments, including the experience of healthcare journeys, comorbidities, or treatment-related complications. Therefore, the measurement of general HRQoL is likely to be relevant for understanding and quantifying the disease burden of CC. However, the determinants of the HRQoL of CC patients remain under-evaluated as CC was evaluated using rather simple questionnaires in the previous population-based studies.17,25 In this study, we combined two datasets, 1) Korean general population survey and 2) CC patient registry, to compare the HRQoL of CC patients with those of controls and to evaluate clinical parameters associated with the HRQoL impairment.
MATERIALS AND METHODS
Study subjects and clinical parameters
This study was a part of the Korean Chronic Cough Registry study, a prospective ongoing multi-center observational cohort study that was established to investigate the characteristics, clinical course, and health burden of CC. In the Korean Chronic Cough Registry, patients aged 19 to 80 years were recruited from 10 tertiary allergist and pulmonologist referral clinics, who were either 1) newly referred with CC (defined as a cough that lasts more than 8 weeks) as a chief complaint or 2) already diagnosed with refractory or unexplained CC (RUCC) by physicians. The diagnosis of RUCC followed the national and international consensus guidelines1,2,3; a cough with an unknown etiology or refractory to conventional managements of CC-associated conditions including asthma, upper airway diseases, gastroesophageal reflux disease (GERD), lung parenchymal diseases, current smoking, or the use of angiotensin-converting enzyme inhibitor. The exclusion criteria were 1) presence of “red flag signs” such as hemoptysis, severe dyspnea, fever, weight loss, peripheral edema, dysphagia, vomiting, recurrent pneumonia, or other significant signs in physical examination or chest X-rays and 2) any history of significant medical conditions, other than CC, that may considerably impair the physical or psychological aspects of HRQoL, such as active malignancy, heart failure, stroke, or other severe respiratory conditions. The study protocols were approved by the Institutional Review Boards of all participating institutions. All participants provided written informed consent.
To compare with CC cases, age- and sex-matched controls were selected with a 1:4 ratios from the Korean National Health and Nutrition Examination Survey (KNHANES) 2010–2016 databases. The case-control ratio was chosen because ratios greater than 1:4 usually have little additional gain on power.27 Two independent control groups were 1) a group without current cough (non-cough control [NC]) and 2) another group of patients without a history of major chronic illnesses (but regardless of cough status) (healthy control [HC]). NC subjects were randomly sampled among the individuals who responded “no” to a structured question: “Do you have cough currently?”28 HC subjects were those who had no self-reported history of physician diagnosis of the following conditions: hypertension, diabetes mellitus, dyslipidemia, stroke, ischemic heart disease (IHD; angina and myocardial infarction), arthritis (osteoarthritis and rheumatoid arthritis), tuberculosis, asthma, COPD, allergic rhinitis, thyroid diseases, cancer, depression, Alzheimer’s disease, renal failure, viral hepatitis (hepatitis B virus and hepatitis C virus), cataract, glaucoma, or any recent hospitalization within 1 year. The KNHANES did not include data regarding GERD. Further information on the KNHANES can be found online at https://knhanes.kdca.go.kr/knhanes/eng/.
HRQoL measurements
The HRQoL was assessed using the EQ-5D questionnaire, which is validated in Korean.29 The EQ-5D consists of five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The health state is expressed by either three (EQ-5D-3L) or five (EQ-5D-5L) response levels for each of the five health domains. The KNHANES used the original version of EQ-5D (EQ-5D-3L), in which the health status is reported in a range from 11111 (the best health state) to 33333 (the worst health state). The Korean Chronic Cough Registry study used a more recent version, which gives respondents five answer options (EQ-5D-5L) with health states ranging from 11111 (the best health state) to 55555 (the worst health state). The responses were converted into a single index ‘utility’ score using a scoring algorithm; the EQ-5D-3L index was calculated using the Korean valuation set30, whereas the EQ-5D-5L index was calculated by applying an indirect interim mapping method for the comparisons.31 The indexes range from less than 0 (where 0 is a health state equivalent to death; negative values indicate a health state worse than death) to 1 (perfect health). The minimum clinically important difference (MCID) of the EQ-5D index is ≥ 0.05.32 Scores of EQ-VAS (visual analog scale), which captures the self-reported health status from best (100) to worst (0) imaginable health, were also collected. The proportional distribution of problem reporting on each of the five domains within CC group was compared using 5L, while 5L was converted to 3L (i.e., 1 in 5L to 1, 2–4 in 5L to 2, 5 in 5L to 3) to compare the mean value of the response levels for five domains in the CC, RUCC, HC, and NC groups. The correlations of EQ-5D index with cough-specific patient-reported outcomes (PROs) of the CC patients was assessing using 5L.
Clinical parameters of CC patients
Data on cough characteristics, comorbidities, and cough-specific PROs were prospectively collected in the Korean Chronic Cough Registry. Comorbidities were defined by a patient self-reported history of physician diagnosis. PROs included cough severity VAS, throat sensation VAS, Leicester Cough Questionnaire (LCQ), and Cough Hypersensitivity Questionnaire (CHQ) scores.33,34 The Korean version of LCQ is a validated tool to measure cough-specific HRQoL, with total scores ranging from 3 to 21 (lower score indicates worse cough-specific HRQoL status).33 The CHQ completed linguistic validation in Korean language.34 The measurement property of the original CHQ is currently undergoing validation by the developers (Prof. Surinder Birring, personal communication).
Data on recent treatments were retrieved from cough-related drug prescription records for antihistamines, codeine, inhaled corticosteroids, oral corticosteroids, proton pump inhibitors, and neuromodulators including gabapentin, pregabalin, and amitriptyline at the time of recruitment.
Statistical analysis
Categorical variables are expressed as the frequency (%), and differences between groups were assessed by chi-square test. Continuous variables are expressed as the mean ± standard deviation, and comparisons between groups were conducted by Student’s t-test or one-way analysis of variance (ANOVA). P values for between-group comparisons were obtained using a post-hoc Bonferroni test. The relation between the EQ-5D index score among the three groups was also tested with the Jonckheere-Terpstra statistic on trend. Linear regression analysis was performed to identify the determinants of the EQ-5D index in CC and RUCC. Multivariate regression analysis included clinically important variables or statistically significant variables in univariate analysis. Interactions of the variables and CC on the EQ-5D index were evaluated by two-way ANOVA, and the results are presented graphically with marginal predictions and 95% confidence intervals (CIs). Correlations between the index and cough-specific PRO scores were assessed by Spearman’s correlation test. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed using Stata software (Version 16.1, Stata Corp., College Station, TX, USA).
RESULTS
Study population and baseline characteristics
A total of 200 CC patients (137 newly referred with CC and 63 already diagnosed with RUCC) were included as CC cases. Of the 56,632 participants originally enrolled in the KNHANES 2010–2016 who responded to the EQ-5D questionnaire, 800 subjects were selected as the NC group, whereas 799 subjects were selected as the HC group. The number of female HC subjects in their 60s did not precisely match that of CC patients at a ratio of 1:4; thus, only 799 patients were included in the HC group.
The baseline characteristics of the study population are presented in Table 1. In comparison with NC or HC group, there were significantly more CC patients with body mass index ≥ 30 kg/m2 (CC: 9.1%, NC: 3.5%, and HC: 2.8%, P < 0.001) and more CC patients who were never-smokers (CC: 75.5%, NC: 67.4%, and HC: 68.4%, P < 0.001). In comparison with NC group, CC patients had more self-reported diagnosis history of asthma (CC: 11.1% vs. HC: 3.8%, P < 0.001) but less depression (CC: 2.0% vs. NC: 7.3%, P < 0.006). By definition, HC group did not have a comorbidity.
Table 1. Baseline characteristics of each group (n = 1,799).
| Variables | CC cases (n = 200) | NC (n = 800) | HC (n = 799) | P value | |
|---|---|---|---|---|---|
| Age (yr) | 54.9 ± 15.1 | 54.9 ± 15.4 | 54.5 ± 15.4 | 0.908 | |
| Sex | 1.000 | ||||
| Male | 71 (35.5) | 284 (35.5) | 284 (35.5) | ||
| Female | 129 (64.5) | 516 (64.5) | 515 (64.5) | ||
| BMI (kg/m2)* | 24.6 ± 4.8 | 24.1 ± 3.2 | 23.1 ± 3.1 | < 0.001 | |
| < 18.5 kg/m2 | 7 (3.6) | 17 (2.1) | 44 (5.5) | < 0.001 | |
| 18.5–24.9 kg/m2 | 109 (55.3) | 485 (60.8) | 551 (69.1) | ||
| 25.0–29.9 kg/m2 | 63 (32.0) | 268 (33.6) | 180 (22.6) | ||
| ≥ 30 kg/m2 | 18 (9.1) | 28 (3.5) | 22 (2.8) | ||
| Smoking status* | < 0.001 | ||||
| Never | 151 (75.5) | 538 (67.4) | 544 (68.4) | ||
| Ex-smoker | 39 (19.5) | 130 (16.3) | 109 (13.7) | ||
| Current smoker | 10 (5.0) | 130 (16.3) | 142 (17.9) | ||
| Comorbidities | |||||
| Asthma* | 22 (11.1) | 30 (3.8) | N/A | < 0.001† | |
| Allergic rhinitis* | 27 (13.5) | 89 (11.3) | N/A | 0.394† | |
| Pulmonary tuberculosis* | 7 (3.5) | 35 (4.4) | N/A | 0.590† | |
| Hypertension* | 51 (25.8) | 256 (32.0) | N/A | 0.521† | |
| Diabetes mellitus* | 19 (9.6) | 89 (11.1) | N/A | 0.088† | |
| Dyslipidemia* | 20 (10.1) | 120 (15.0) | N/A | 0.072† | |
| IHD* | 5 (2.5) | 30 (3.8) | N/A | 0.396† | |
| Stroke* | 2 (1.0) | 19 (2.4) | N/A | 0.228† | |
| Chronic liver disease* | 2 (1.0) | 15 (1.9) | N/A | 0.396† | |
| Chronic kidney disease* | 2 (1.0) | 4 (0.5) | N/A | 0.343† | |
| Malignancy* | 4 (2.0) | 35 (4.4) | N/A | 0.123† | |
| Depression* | 4 (2.0) | 58 (7.3) | N/A | 0.006† | |
| EQ-5D index | 0.82 ± 0.14 | 0.92 ± 0.14 | 0.96 ± 0.08 | < 0.001 | |
| EQ-VAS | 63.7 ± 18.0 | 77.5 ± 57.6 | 77.5 ± 36.8 | 0.005 | |
CC, chronic cough; NC, non-cough control; HC, healthy control; BMI, body mass index; IHD, ischemic heart disease; EQ-5D, EuroQoL 5-dimension; VAS, visual analog scale.
*Numbers of patients with missing data are as follows: BMI (n = 7), smoking status (n = 6), asthma (n = 1), allergic rhinitis (n = 14), pulmonary tuberculosis (n = 1), hypertension (n = 2), diabetes mellitus (n = 1), dyslipidemia (n = 1), ischemic heart disease (n = 1), stroke (n = 1), chronic liver disease (n = 1), chronic kidney disease (n = 1), malignancy (n = 1), and depression (n = 1).
†P value between CC and NC.
Determinants of a low EQ-5D index: comparison of CC patients and controls
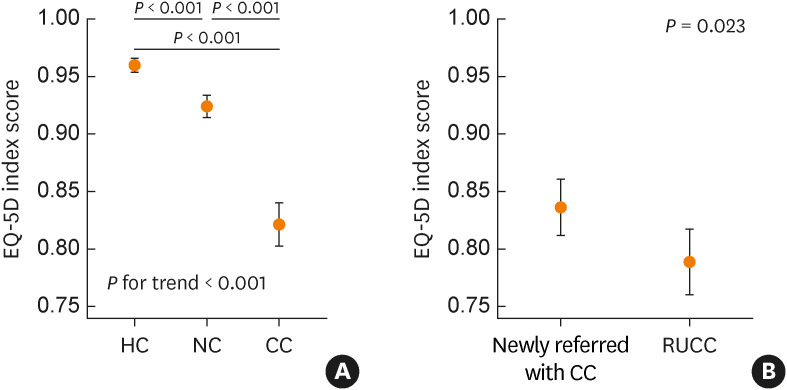
The EQ-5D index was significantly lower in the CC patients than in the NC group or HC group (0.82 ± 0.14 vs 0.92 ± 0.14/0.96 ± 0.08; P < 0.001, respectively), which exceeded the MCID of 0.05 (Fig. 1A, Table 1). Among the five domains of the EQ-5D questionnaire, usual activities, pain/discomfort, and anxiety/depression domains of the CC patients were significantly affected compared with those of NC and HC groups (P < 0.001) (Supplementary Fig. S1). The EQ-VAS score of the CC patients was also significantly lower than that of NC or HC group (63.7 ± 18.0 vs 77.5 ± 57.6/77.5 ± 36.8, P = 0.005, respectively). Of all subgroups, elderly CC patients in their 70s, especially women, had the lowest EQ-5D index (0.75 ± 0.13) (Supplementary Table S1).
Fig. 1. EQ-5D indexes of the (A) HC, NC, and CC groups and (B) newly referred with CC and RUCC patients of the CC group, in means (dots) and 95% confidence intervals (whiskers). P values for between-group comparisons were obtained using a post-hoc Bonferroni test. The relation between EQ-5D index score among the three groups was tested with the Jonckheere-Terpstra statistic on trend (indicated as P for trend).
EQ-5D, EuroQoL 5-dimension; HC, healthy control; NC, non-cough control; CC, chronic cough; RUCC, refractory or unexplained chronic cough.
In multivariate linear regression analyses, CC was significantly associated with the EQ-5D index (correlation coefficient, −0.110; 95% CI, −0.126 to −0.094; P < 0.001), independent of demographic factors and comorbid conditions (Table 2). However, the index was also significantly associated with older age, female sex, and a history of comorbidities such as asthma, hypertension, IHD, stroke, or depression.
Table 2. Multivariate linear regression analyses of factors associated with the EuroQoL 5-dimension index in chronic cough cases and controls (n = 1,799).
| Variables | Coefficient (95% CI) | P value | |
|---|---|---|---|
| Age groups | |||
| ≤ 40s | Reference | ||
| 40s | 0.012 (−0.006 to 0.030) | 0.192 | |
| 50s | 0.002 (−0.015 to 0.020) | 0.787 | |
| 60s | −0.034 (−0.050 to −0.018) | < 0.001 | |
| 70s | −0.067 (−0.085 to −0.049) | < 0.001 | |
| Female (vs. male) | −0.042 (−0.057 to −0.026) | < 0.001 | |
| BMI | |||
| < 18.5 kg/m2 | Reference | ||
| 18.5–24.9 kg/m2 | −0.018 (−0.045 to 0.010) | 0.198 | |
| 25.0–29.9 kg/m2 | −0.023 (−0.050 to 0.005) | 0.113 | |
| ≥ 30 kg/m2 | −0.030 (−0.068 to 0.007) | 0.111 | |
| Smoking status | |||
| Never smoker | Reference | ||
| Ex-smoker | 0.004 (−0.015 to 0.022) | 0.708 | |
| Current smoker | −0.010 (−0.028 to 0.008) | 0.287 | |
| Asthma (vs. none) | −0.063 (−0.095 to −0.031) | < 0.001 | |
| Allergic rhinitis (vs. none) | −0.002 (−0.024 to 0.019) | 0.822 | |
| Pulmonary tuberculosis (vs. none) | 0.001 (−0.032 to 0.036) | 0.927 | |
| Hypertension (vs. none) | −0.027 (−0.043 to −0.011) | < 0.001 | |
| Diabetes mellitus (vs. none) | −0.006 (−0.029 to 0.017) | 0.625 | |
| Dyslipidemia (vs. none) | −0.017 (−0.038 to 0.004) | 0.119 | |
| IHD (vs. none) | −0.045 (−0.083 to 0.009) | 0.020 | |
| Stroke (vs. none) | −0.079 (−0.126 to 0.031) | 0.001 | |
| Chronic liver disease (vs. none) | 0.035 (−0.018 to 0.089) | 0.198 | |
| Chronic kidney disease (vs. none) | −0.009 (−0.097 to 0.079) | 0.847 | |
| Malignancy (vs. none) | −0.018 (−0.053 to 0.017) | 0.313 | |
| Depression (vs. none) | −0.044 (−0.073 to −0.016) | 0.002 | |
| Chronic cough (vs. none) | −0.110 (−0.126 to −0.094) | < 0.001 | |
Adjusted for age, sex, BMI category, smoking, asthma, allergic rhinitis, pulmonary tuberculosis, hypertension, diabetes mellitus, ischemic heart disease, stroke, chronic liver disease, chronic kidney disease, malignancy, and depression.
CI, confidence interval; BMI, body mass index; IHD, ischemic heart disease.
The adjusted predicted indexes of these variables were significantly lower in the CC patients than in the subjects without CC (Supplementary Fig. S2). While the self-reported history of IHD or stroke showed interaction effects on EQ-5D index, age, sex, and other comorbidities such as asthma, hypertension, and depression did not.
Clinical characteristics of CC patients and their associations with the EQ-5D index
In the CC registry, patients already diagnosed with RUCC were significantly older than patients newly referred with CC (60.6 ± 14.2 years vs. 52.2 ± 14.8 years, P < 0.001) and had more self-reported history of asthma (22.6% vs. 5.8%, P = 0.001), hypertension (35.5% vs. 21.3%, P = 0.035), and diabetes mellitus (16.1% vs. 6.6%, P = 0.034) (Supplementary Table S2). Patients with RUCC also had dyspnea more frequently than those newly referred with CC (33.9% vs. 20.7%, P = 0.048). However, newly referred patients had more peripheral blood eosinophilia (18.4% vs. 2.0%, P = 0.006) and chest X-ray abnormalities (14.8% vs. 4.9%, P = 0.050), compared with those with RUCC. Among cough complications, fatigue was significantly more common in RUCC patients (54.8% vs. 35.3%, P = 0.010). In addition, compared with newly referred with CC, more RUCC patients received antihistamines (first-generation) at the time of recruitment (63.6% vs. 47.1%, P = 0.038).
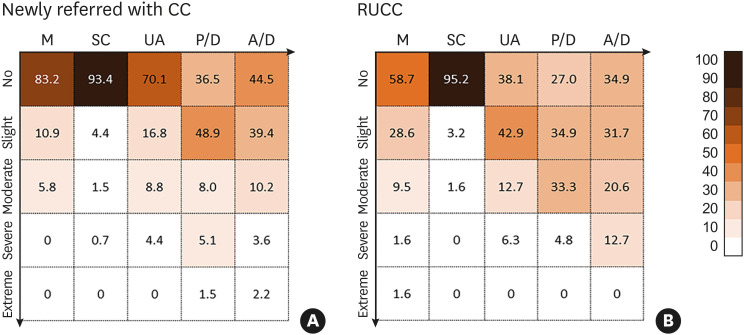
The EQ-5D index of RUCC patients was significantly lower than that of newly referred with CC (0.79 ± 0.11 vs. 0.84 ± 0.14; P = 0.023) (Fig. 1B, Supplementary Table S2). In comparison with newly referred CC patients, significantly more RUCC patients reported problems in the EQ-5D domains except for the self-care domain (mobility, P = 0.001; self-care, P = 1.000; usual activities, P < 0.001; pain/discomfort, P < 0.001 and anxiety/depression, P = 0.021) (Fig. 2).
Fig. 2. Proportional distribution of problem reporting on each of the five domains of the EQ-5D questionnaire between (A) newly referred with CC and (B) RUCC patients.
CC, chronic cough; M, mobility; SC, self-care; UA, usual activities; P/D, pain and discomfort; A/D, anxiety and depression; RUCC, refractory or unexplained chronic cough.
When all the CC patients were sub-grouped according to their recent treatment, the index was significantly lower in patients who were recently treated with codeine (0.81 ± 0.13 vs. 0.85 ± 0.14, P = 0.036) or those treated with cough neuromodulators such as gabapentin or pregabalin than in patients who were not (0.78 ± 0.13 vs. 0.84 ± 0.14, P = 0.034) (Table 3). There was no significant difference in the EQ-5D index according to accompanying symptoms or laboratory results, except for dyspnea (Supplementary Table S3). Among the cough-induced complications, fatigue (0.77 ± 0.14 vs. 0.86 ± 0.12, P < 0.001) and chest pain (0.79 ± 0.16 vs. 0.83 ± 0.13, P = 0.048) but not urinary incontinence (0.81 ± 0.13 vs. 0.83 ± 0.14, P = 0.366) were significantly associated with the index (Table 4).
Table 3. EuroQoL 5-dimension index by recent treatments in chronic cough patients (n = 200).
| Type of treatments | Treated with | Not been treated with | P value |
|---|---|---|---|
| Antihistamine | 0.84 ± 0.12 | 0.81 ± 0.15 | 0.139 |
| Codeine | 0.81 ± 0.13 | 0.85 ± 0.14 | 0.036 |
| Inhaled corticosteroid | 0.83 ± 0.13 | 0.82 ± 0.14 | 0.569 |
| Oral steroid | 0.85 ± 0.15 | 0.82 ± 0.14 | 0.435 |
| PPI | 0.84 ± 0.10 | 0.82 ± 0.15 | 0.481 |
| Neuromodulator | 0.78 ± 0.13 | 0.84 ± 0.14 | 0.034 |
PPI, proton pump inhibitor.
Table 4. EuroQoL 5-dimension index by cough-related complications in chronic cough patients (n = 200).
| Concurrent cough-related complications | (+) | (−) | P value |
|---|---|---|---|
| Fatigue | 0.77 ± 0.14 | 0.86 ± 0.12 | < 0.001 |
| Incontinence | 0.81 ± 0.13 | 0.83 ± 0.14 | 0.366 |
| Chest pain | 0.79 ± 0.16 | 0.83 ± 0.13 | 0.048 |
| Syncope | 0.67 ± 0.14 | 0.82 ± 0.14 | 0.052 |
| Headache | 0.81 ± 0.13 | 0.82 ± 0.14 | 0.581 |
| Others | 0.88 ± 0.80 | 0.82 ± 0.14 | 0.174 |
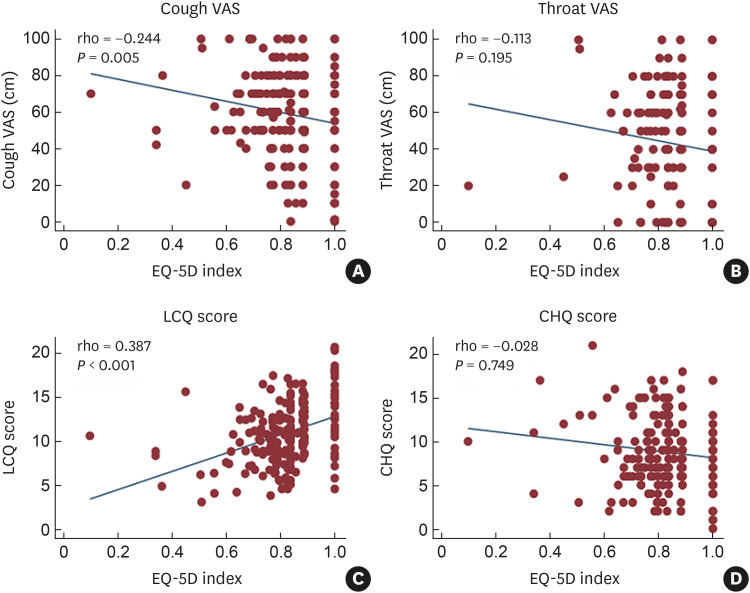
In Spearman’s correlation analyses of PROs, the index modestly correlated with cough severity VAS (r = −0.244, P = 0.005) and LCQ (r = 0.387, P < 0.001) scores but not with throat sensation VAS (r = −0.113, P = 0.195) or CHQ (r = −0.028, P = 0.749) scores (Fig. 3).
Fig. 3. Correlation of the EQ-5D index with cough-specific patient-reported outcomes of CC patients. (A) Cough severity VAS. (B) Throat sensation VAS. (C) LCQ score. (D) CHQ score.
VAS, visual analog scale; EQ-5D, EuroQoL 5-dimension; LCQ, Leicester Cough Questionnaire; CHQ, Cough Hypersensitivity Questionnaire.
In multivariate analyses of factors associated with the EQ-5D index in CC patients, female sex (correlation coefficient, −0.039; 95% CI, −0.078 to −0.001; P = 0.047), lower LCQ score (correlation coefficient, 0.012; 95% CI, 0.006 to 0.018; P < 0.001), and concurrent fatigue as a cough complication (correlation coefficient, −0.052; 95% CI, −0.092 to −0.012; P = 0.011) were significantly associated with low index, when adjusted for age, sex, comorbidities, cough severity, recent treatment history and cough-related complications (Table 5).
Table 5. Multivariate linear regression analyses of factors associated with EuroQoL 5-dimension index in CC patients (n = 200).
| Variables | Coefficient (95% CI) | P value | |
|---|---|---|---|
| Age | −0.001 (−0.001 to 0.001) | 0.838 | |
| Female (vs. male) | −0.039 (−0.078 to −0.001) | 0.047 | |
| Comorbidities | |||
| Asthma (vs. none) | −0.015 (−0.080 to 0.051) | 0.654 | |
| Hypertension (vs. none) | −0.022 (−0.067 to 0.023) | 0.336 | |
| Depression (vs. none) | −0.047 (−0.173 to 0.079) | 0.463 | |
| RUCC (vs. newly referred with CC) | −0.012 (−0.054 to 0.030) | 0.569 | |
| LCQ score | 0.012 (0.006 to 0.018) | < 0.001 | |
| Recent treatment with | |||
| Codeine | 0.006 (−0.035 to 0.047) | 0.775 | |
| Neuromodulators | −0.032 (−0.083 to 0.020) | 0.224 | |
| Complications | |||
| Fatigue | −0.052 (−0.092 to −0.012) | 0.011 | |
| Chest pain | −0.020 (−0.065 to −0.025) | 0.383 | |
Adjusted for age, sex, comorbidities, type of CC, cough severity, recent treatment history and cough-related complications.
CC, chronic cough; CI, confidence interval; RUCC, refractory or unexplained chronic cough; LCQ, Leicester Cough Questionnaire.
DISCUSSION
In this study, we found a considerable decrease in the HRQoL of CC patients in the Korean Chronic Cough Registry. In comparison with controls, CC patients had significantly lower HRQoL after adjusting for confounders, such as age, female sex, and comorbidities such as asthma and depression. This is consistent with the findings of previous population-based studies.17,25 However, we have additionally found that among CC patients, the EQ-5D index was significantly associated with several cough characteristics such as cough severity, cough-specific QoL score, fatigue (as a cough complication), RUCC, or the use of codeine or cough neuromodulators. Although the analyses are cross-sectional, the dose responses with clinical parameters indicate that the relationships are likely causal between CC and HRQoL impairment, and suggest that EQ-5D may be a potentially useful tool for understanding and quantifying the overall disease burden including the long-term health outcomes of CC patients and treatments.
Like the findings of a previous population-based study,17 the EQ-5D index in the elderly CC women was the lowest among the subgroups stratified by age and sex. The index of this subgroup was lower than that in the NC patients with comorbidities, as well as in the HC subjects of the same age group. Several studies that evaluated the HRQoL in the general population have also shown the HRQoL deterioration of elderly women, and this study found that CC patients had even worse HRQoL among the elderly women.35,36,37 Similar to the previous Korean general population study,17 we hypothesized that urinary incontinence, a frequent complication of CC in elderly women, might explain the particularly higher impact of CC in the aged group. However, as shown in the present study, we found no significant relationship between the EQ-5D scores and self-reported urinary incontinence, suggesting that urinary complication itself might not be a major determinant of the HRQoL impairment. Given the significant association between female sex and the HRQoL of CC patients in multivariate analyses (Table 5), there might be undiscovered factors that explain the sex difference in the HRQoL.
The domains of EQ-5D are intended to be used as a general measure rather than to specifically assess the impact of diseases as a disease-specific manner.38 Usually, the generic tools are known to be less sensitive to changes during treatments of respiratory diseases like asthma39; however, we suppose that the generic HRQoL tools such as EQ-5D might have distinct value in the assessment of long-term health outcomes of CC patients, including treatment complications. In the present study, the index was lower among RUCC patients and those treated with codeine or cough neuromodulators such as gabapentin or pregabalin, which are drugs used to inhibit the central pathway in refractory cough. It is difficult to determine the causal relationships of impaired HRQoL, concurrent fatigue (as the complication of coughing), and the use of codeine or neuromodulators in this study due to the cross-sectional nature. HRQoL might be worse due to the adverse or sedative effects of these drugs, which suppress neuronal hyper-excitability, or due to more severe cough, which indicates the use of centrally-acting antitussives. Further longitudinal studies on the improvement of HRQoL after cough treatment are warranted to elucidate this.
Meanwhile, the index showed modest but significant correlations with cough-specific questionnaires, including LCQ and cough severity VAS scores (Fig. 3); however, the index did not show a significant correlation with CHQ score, a measure of cough triggers and throat sensations, which is known to be correlated with LCQ and cough VAS scores.34 These findings suggest that the severity of cough itself, rather than those of cough-associated laryngeal symptoms, may be more relevant to HRQoL of CC patients.
This study has several limitations. First, it was a cross-sectional study, which would be difficult to determine causal relationships. However, the health burden of CC was evaluated by comparing with a non-CC population and a healthy control population without any disease burden, which accounts for differences in demographic and health characteristics. Moreover, the dose relationships of HRQoL with cough-specific PROs such as cough severity or cough-specific QoL were found. Secondly, this study compared the EQ-5D index between the CC patients who were referred for specialty care and agree to participate in the registry study, and the NC subjects. This could have introduced a selection bias in case recruitment toward more severe, long-standing, or treatment-refractory patients. Thirdly, comorbidities, including depression, were defined by a self-reported history of physician diagnosis. Therefore, they are prone to recall and misclassification biases, with no ability to differentiate among degrees of clinical severity. This might be a reason for low prevalence of depression in the CC patients of this study. Fourthly, we chose the 1:4 case-control ratio for matching because we consider the ratio has a good balance between subject number and power gain. The ratio greater than 1:4 does not usually increase power.27 In addition, we could validate our case-control comparison findings at ratios of 1:1 or 1:2 (data presented for review). Finally, socioeconomic factors that can affect HRQoL, such as education, marital/family status, and employment status, were not investigated in the present CC registry study. Despite the limitations, the present study has strength in detailed patient characterization and the findings provide the rationale for further studies to estimate the burden of disease using the EQ-5D questionnaire in CC patients.
In conclusion, the HRQoL was significantly impaired in CC patients, compared to controls. The HRQoL impairment of CC patients was further affected by cough severity, complications, drug treatments and responses. Given the long-term persistence of CC and potential adverse effects of treatments, HRQoL should be one of major outcomes in longitudinal cohort studies of CC.
ACKNOWLEDGMENTS
The study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
Footnotes
Disclosure: W.-J.S. declares grants from Merck Sharp & Dohme Corp. and AstraZeneca, consulting fees from Merck, AstraZeneca, Shionogi and GSK, and lecture fees from Merck, AstraZeneca, GSK and Novartis. Other authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
EuroQoL 5-dimension index of each group stratified by age and sex (n = 1,799)
Comparison of newly referred with CC (n = 137) and patients already diagnosed with RUCC (n = 63)
EQ-5D index according to concurrent symptoms and laboOAratory test results
Mean value of the response levels for five domains of the EQ-5D questionnaire in CC, HC, NC, and RUCC groups. Each domain is scored on a 3-point scale: 1, no problem; 2, some problems; 3, extreme problems.
Adjusted prediction of EQ-5D index.
References
- 1.Irwin RS, French CL, Chang AB, Altman KW, Adams TM, Altman KW, et al. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153:196–209. doi: 10.1016/j.chest.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song DJ, Song WJ, Kwon JW, Kim GW, Kim MA, Kim MY, et al. KAAACI evidence-based clinical practice guidelines for chronic cough in adults and children in Korea. Allergy Asthma Immunol Res. 2018;10:591–613. doi: 10.4168/aair.2018.10.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55:55. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An J, Lee JH, Won HK, Kang Y, Kwon HS, Lee JS, et al. Cough presentation and cough-related healthcare utilization in tertiary care: analysis of routinely collected academic institutional database. Lung. 2022;200:431–439. doi: 10.1007/s00408-022-00555-w. [DOI] [PubMed] [Google Scholar]
- 5.Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45:1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 6.Poe RH, Harder RV, Israel RH, Kallay MC. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723–728. doi: 10.1378/chest.95.4.723. [DOI] [PubMed] [Google Scholar]
- 7.McGarvey LP, Heaney LG, Lawson JT, Johnston BT, Scally CM, Ennis M, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53:738–743. doi: 10.1136/thx.53.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127:1710–1713. doi: 10.1378/chest.127.5.1710. [DOI] [PubMed] [Google Scholar]
- 9.Kastelik JA, Aziz I, Ojoo JC, Thompson RH, Redington AE, Morice AH. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25:235–243. doi: 10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 10.Koskela HO, Lätti AM, Purokivi MK. Long-term prognosis of chronic cough: a prospective, observational cohort study. BMC Pulm Med. 2017;17:146. doi: 10.1186/s12890-017-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SY, Song WJ, Won HK, Chung SJ, Kim JY, Park HW, et al. Cough persistence in adults with chronic cough: a 4-year retrospective cohort study. Allergol Int. 2020;69:588–593. doi: 10.1016/j.alit.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 12.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158:1657–1661. doi: 10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 13.Ternesten-Hasséus E, Larsson S, Millqvist E. Symptoms induced by environmental irritants and health-related quality of life in patients with chronic cough: a cross-sectional study. Cough. 2011;7:6. doi: 10.1186/1745-9974-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain SA, Garrod R, Douiri A, Masefield S, Powell P, Bücher C, et al. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193:401–408. doi: 10.1007/s00408-015-9701-2. [DOI] [PubMed] [Google Scholar]
- 15.Kanemitsu Y, Niimi A, Matsumoto H, Iwata T, Ito I, Oguma T, et al. Gastroesophageal dysmotility is associated with the impairment of cough-specific quality of life in patients with cough variant asthma. Allergol Int. 2016;65:320–326. doi: 10.1016/j.alit.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Kang SY, Won HK, Lee SM, Kwon JW, Kim MH, Jo EJ, et al. Impact of cough and unmet needs in chronic cough: a survey of patients in Korea. Lung. 2019;197:635–639. doi: 10.1007/s00408-019-00258-9. [DOI] [PubMed] [Google Scholar]
- 17.Won HK, Lee JH, An J, Sohn KH, Kang MG, Kang SY, et al. Impact of chronic cough on health-related quality of life in the Korean adult general population: the Korean National Health and Nutrition Examination Survey 2010–2016. Allergy Asthma Immunol Res. 2020;12:964–979. doi: 10.4168/aair.2020.12.6.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung KF, McGarvey L, Song WJ, Chang AB, Lai K, Canning BJ, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers. 2022;8:45. doi: 10.1038/s41572-022-00370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61:975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voll-Aanerud M, Eagan TM, Wentzel-Larsen T, Gulsvik A, Bakke PS. Changes in respiratory symptoms and health-related quality of life. Chest. 2007;131:1890–1897. doi: 10.1378/chest.06-2629. [DOI] [PubMed] [Google Scholar]
- 22.Adams RJ, Appleton SL, Wilson DH, Taylor AW, Ruffin RE. Associations of physical and mental health problems with chronic cough in a representative population cohort. Cough. 2009;5:10. doi: 10.1186/1745-9974-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheaton AG, Ford ES, Thompson WW, Greenlund KJ, Presley-Cantrell LR, Croft JB. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States--National Health and Nutrition Examination Survey 2007–2010. BMC Public Health. 2013;13:854. doi: 10.1186/1471-2458-13-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virchow JC, Li VW, Fonseca E, Salmen H, Martin A, Brady J, et al. Chronic cough in Germany: results from a general-population survey. ERJ Open Res. 2022;8:00420-2021. doi: 10.1183/23120541.00420-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo T, Tobe K, Okuyama K, Kikuchi M, Chen Y, Schelfhout J, et al. Disease burden and quality of life of patients with chronic cough in Japan: a population-based cross-sectional survey. BMJ Open Respir Res. 2021;8:8. doi: 10.1136/bmjresp-2020-000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu CJ, Song WJ, Kang SH. The disease burden and quality of life of chronic cough patients in South Korea and Taiwan. World Allergy Organ J. 2022;15:100681. doi: 10.1016/j.waojou.2022.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol. 1992;135:1042–1050. doi: 10.1093/oxfordjournals.aje.a116398. [DOI] [PubMed] [Google Scholar]
- 28.Kang MG, Song WJ, Kim HJ, Won HK, Sohn KH, Kang SY, et al. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: The Korean National Health and Nutrition Examination Survey 2010–2012. Medicine (Baltimore) 2017;96:e6486. doi: 10.1097/MD.0000000000006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SH, Ahn J, Ock M, Shin S, Park J, Luo N, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res. 2016;25:1845–1852. doi: 10.1007/s11136-015-1205-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee YK, Nam HS, Chuang LH, Kim KY, Yang HK, Kwon IS, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009;12:1187–1193. doi: 10.1111/j.1524-4733.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Kim HJ, Lee SI, Jo MW. Comparing the psychometric properties of the EQ-5D-3L and EQ-5D-5L in cancer patients in Korea. Qual Life Res. 2012;21:1065–1073. doi: 10.1007/s11136-011-0018-1. [DOI] [PubMed] [Google Scholar]
- 32.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kwon JW, Moon JY, Kim SH, Song WJ, Kim MH, Kang MG, et al. Reliability and validity of a Korean version of the Leicester cough questionnaire. Allergy Asthma Immunol Res. 2015;7:230–233. doi: 10.4168/aair.2015.7.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Won HK, Kang SY, Kang Y, An J, Lee JH, Lee SM, et al. Cough-related laryngeal sensations and triggers in adults with chronic cough: symptom profile and impact. Allergy Asthma Immunol Res. 2019;11:622–631. doi: 10.4168/aair.2019.11.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20:205–216. doi: 10.1007/s10198-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park B, Ock M, Lee HA, Lee S, Han H, Jo MW, et al. Multimorbidity and health-related quality of life in Koreans aged 50 or older using KNHANES 2013–2014. Health Qual Life Outcomes. 2018;16:186. doi: 10.1186/s12955-018-1016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong EL, Cheung AW, Wong AY, Xu RH, Ramos-Goñi JM, Rivero-Arias O. Normative profile of health-related quality of life for Hong Kong general population using preference-based instrument EQ-5D-5L. Value Health. 2019;22:916–924. doi: 10.1016/j.jval.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Whalley D, Globe G, Crawford R, Doward L, Tafesse E, Brazier J, et al. Is the EQ-5D fit for purpose in asthma? Acceptability and content validity from the patient perspective. Health Qual Life Outcomes. 2018;16:160. doi: 10.1186/s12955-018-0970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyland ME, Lanario JW, Menzies-Gow A, Mansur AH, Dodd JW, Fowler SJ, et al. Comparison of the sensitivity of patient-reported outcomes for detecting the benefit of biologics in severe asthma. Chron Respir Dis. 2021;18:14799731211043530. doi: 10.1177/14799731211043530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EuroQoL 5-dimension index of each group stratified by age and sex (n = 1,799)
Comparison of newly referred with CC (n = 137) and patients already diagnosed with RUCC (n = 63)
EQ-5D index according to concurrent symptoms and laboOAratory test results
Mean value of the response levels for five domains of the EQ-5D questionnaire in CC, HC, NC, and RUCC groups. Each domain is scored on a 3-point scale: 1, no problem; 2, some problems; 3, extreme problems.
Adjusted prediction of EQ-5D index.