Abstract
Objective:
We propose a risk-tailored approach for management of lung cancer screening results. This approach incorporates individual risk factors and LDCT image features into calculations of immediate and next-screen (1-year) risks of lung cancer detection, which in turn can recommend short-interval imaging or 1-year or 2-year screening intervals.
Methods:
We first extended the “LCRAT+CT” individualized risk calculator to predict lung cancer risk after either a negative or abnormal LDCT screen. To develop the abnormal screens portion, we analyzed 18,129 abnormal LDCTs in the National Lung Screening Trial (NLST), including lung cancers detected immediately (n=649) or at the next screen (n=235). We estimated the potential impact of this approach among NLST participants with any screen result (negative or abnormal).
Results:
Applying the draft National Health Service (NHS) England protocol for lung screening to NLST participants referred 76% of participants to a 2-year interval, but delayed diagnosis for 40% of detectable cancers. The LCRAT+CT risk model, with a threshold of <0.95% cumulative lung-cancer risk, would also refer 76% of participants to a 2-year interval, but would delay diagnosis for only 30% of cancers, a 25% reduction versus the NHS protocol. Alternatively, LCRAT+CT, with a threshold of <1.7% cumulative lung-cancer risk, would also delay diagnosis for 40% of cancers, but would refer 85% of participants for a 2-year interval, a 38% further reduction in the number of required 1-year screens beyond the NHS protocol.
Conclusions:
Using individualized risk models to determine management in lung cancer screening could substantially reduce the number of screens or increase early detection.
Keywords: lung-cancer screening, LDCT screening, false-positive LDCT, abnormal LDCT, precision medicine, risk-based medicine, risk modeling, precision prevention, precision screening, risk-tailored screening
Introduction
Building on the success of two large randomized trials,1,2 screening by low-dose computed tomography (LDCT) is currently recommended in the USA for people aged 50–80 who have smoked at least 20 pack-years and who currently smoke or have quit within the last 15 years.3 A targeted programme is planned by the National Health Service (NHS) of England4,5 that will employ risk prediction models to decide who is eligible for screening. Use of formal risk calculators is consistent with “Equal Management of Equal Risks”, a principle that ensures consistent, efficient, and fair guidelines.6
To date, most lung cancer screening studies and programs worldwide have offered annual screening to all participants. However, people with a negative LDCT screen have reduced lung cancer risk during future screening,7–10 and studies support implementing a tailored approach to extend the screening interval to 2 years for some of these individuals.10–13 However, there is no risk-based approach for deciding between 1-year and 2-year intervals. One approach is to recommend annual screening only for the subset of screen-negative people who have the highest 1-year lung cancer risk calculated by the previously published “LCRAT+CTneg” risk model.13 LCRAT+CTneg combines pre-screening risk from the Lung Cancer Risk Assessment Tool (LCRAT) with information from a recent negative LDCT screen. If a 2-year return had been recommended for screen-negative people with LCRAT+CT 1-year risk under 0.3%, then 58% of screen-negatives would have qualified for a 2-year interval in the National Lung Screening Trial (NLST), with the trade-off of delayed diagnosis for 24% of lung cancers that were detectable in one year.
However, for individuals who do not have a negative screen but instead have an abnormal LDCT that does not lead to a lung cancer diagnosis (a “return-to-screening” abnormal LDCT), it is unclear whether biennial screening could be acceptable. Abnormal LDCTs, which are usually abnormal because of a nodule with longest diameter 4mm or greater, are common1,14 and increase future lung-cancer risk.15 Many individuals with abnormal results are recommended a surveillance CT, and the vast majority become “return-to-screening” abnormal LDCTs because they do not lead to immediate cancer diagnosis.1,16,17
For prediction of lung cancer risk during screening, it is useful to distinguish between ‘immediate’ and ‘next-screen’ risk of lung cancer detection in relation to a screening result of interest. Immediate risk refers to lung cancer that is detected after diagnostic follow-up prompted by the result of interest. In contrast, next-screen risk refers to when an individual has a screening result of interest that is believed to be non-malignant, is returned to usual screening, and has another screen after a specified interval (e.g., 1 year) that leads to lung cancer diagnosis. A person’s immediate risk could serve as the basis for recommending short-interval (3–6 month) surveillance scans or further diagnostic procedures. A person’s combination of immediate and next-screen risk could serve as the basis for longer-term action, in particular for choosing the length of the interval before the next screen.
Current calculators for immediate risk of lung cancer do not incorporate pre-screening risk, and some apply only to the baseline screen.18–21 Further, no method is available to calculate next-screen or future risk following a return-to-screening abnormal LDCT. Here, we propose a unified risk-based approach for identifying appropriate, individualized lung screening management and intervals. We first developed a prediction model for immediate risk of lung cancer after an abnormal LDCT, which incorporates individual pre-screening risk and LDCT image features. Unlike other models for immediate risk, it is formally valid for both prevalence (T0) and incidence (T1+) screens. Second, we selected among nodule and non-nodule features to develop a model for risk at the screen 1 year following a return-to-screening abnormal LDCT. Together, these two models comprise a new portion of LCRAT+CT that predicts risk for abnormal LDCTs, called “LCRAT+CTpos”.
Finally, we combined the results from LCRAT+CTneg and LCRAT+CTpos, denoted the LCRAT+CT model, and analyzed NLST participants with any screening result (negative or abnormal). We examined outcomes of the LCRAT+CT model including the fraction of participants referred for a 1-year vs 2-year interval and the fraction of lung cancers with delayed diagnosis. We compared our risk-based proposal to the NHS England Targeted Lung Health Checks Programme, where 2-yearly screening is recommended except for individuals with baseline nodules with volume>80mm3 (or diameter>5mm when volumetry is unreliable) or new nodules with volume>30mm3 (or diameter>4mm), who receive annual screening.5 The Lung-RADS protocol is widely used in the USA, but a meaningful comparison was not possible since Lung-RADS does not recommend 2-yearly screening for anyone.
Methods
Data source and analysis cohort for development of the LCRAT+CTpos model
The NLST randomized 53,452 current and former smokers to 3 annual screens (denoted T0, T1, and T2) with either LDCT or radiography.1 Eligibility required age 55–74 years, ≥30 pack-years of smoking, and ≤15 quit-years. A positive (“abnormal”) CT required a non-calcified nodule with longest diameter ≥4mm, or rarely, non-nodule suspicious abnormalities.1,22 The NLST recorded the presence, size, location, attenuation, and margins of nodules; nodule changes in comparison with the prior year’s screening LDCT; and non-nodule features such as emphysema. We used nodule data to identify new or growing nodules at the T1 and T2 screens (see Supplement).
To develop LCRAT+CTpos, we restricted to LDCT-arm participants who completed the baseline questionnaire and had at least one abnormal LDCT, and then analyzed immediate risk of lung cancer detection. We use ‘immediate’ to refer to screen-detected lung cancers diagnosed after follow-up from an abnormal result and before the next screen. Among the abnormal LDCTs not classified as lung cancer (i.e., return-to-screening abnormal LDCTs, labelled “false-positives” in the NLST), we also calculated risk of cancer detection at the next screen. We defined “next-screen” cancers as those detected due to a positive result at the next annual screen after the return-to-screening abnormal LDCT. A “linked-year method” identified next-screen cancers as those occurring within 1 year of a diagnostic follow-up initiated within 1 year after a positive screen.23 We did not consider interval cancers, which by NLST definition occurred after negative screens.
Specifically, for the next-screen risk model, we analyzed risk at the T1-screen among participants who a) had a return-to-screening abnormal LDCT at T0-screen and b) attended the T1-screen and had a valid result. For the T2-screen, we analyzed analogous participants who had a return-to-screening abnormal LDCT at the T1-screen. We calculated LCRAT+CTpos cumulative risk of both immediate (denoted ri) and 1-year next-screen risk (rn) as cumulative risk=ri+(1-ri)rn. This expression denotes that each participant begins with immediate risk ri, and then for those without immediate lung cancer (1-ri), they contribute their 1-year next-screen risk (rn). We based decisions for 1-year vs. 2-year screening on cumulative risk because it accounts for potentially missed immediate cancers that would remain over the next year.
Statistical analysis
LCRAT+CT is a discrete-time Markov risk model24 for the binary indicator of lung-cancer status.13 The existing model for screen-negatives, LCRAT+CTneg, was developed in the NLST and successfully validated using data from the German LUSI trial.13,25 LCRAT+CT first calculates 1-year pre-screening risk using the Lung Cancer Risk Assessment Tool (LCRAT), a model for risk of incident lung cancer in the absence of screening.26 The LCRAT includes demographics, smoking, and other lung cancer risk factors and was successfully validated in 7 cohorts after development in the PLCO community care arm,26,27 including 3 cohorts from the UK.28 Here, we developed LCRAT+CTpos, which includes two components. The immediate risk component calculates lung cancer risk based on the current LDCT, accounting for pre-screening LCRAT risk, nodules and other features on the LDCT, and whether the screen is a prevalence or incidence screen. The next-screen risk component predicts next-screen risk after a return-to-screening abnormal LDCT. LCRAT+CTpos accounts for features on an abnormal LDCT image by fitting log-binomial regression models.13,29 Therefore, risk equals LCRAT 1-year pre-screening risk raised to an exponent, where the exponent is calculated as the sum of the regression coefficients corresponding to features of the abnormal LDCT. We re-examined whether 4 properties of NLST screening, which hold for negative screens, also hold for abnormal screens (Supplement).
Among 29 features of abnormal LDCTs that were routinely collected in the NLST, we selected a reduced set of features for inclusion in LCRAT+CTpos. We separately applied a) least absolute shrinkage and selection operator or lasso30 and b) backwards-stepwise selection to minimize the Akaike Information Criterion. We fit a model including all features selected by either approach, and then excluded those that no longer contributed to the model or had low potential to be consistently identified in clinical practice. Using the final model, we assessed calibration using 10-fold cross-validation, and discrimination using the optimism-corrected AUC statistic.31 We re-fit the model using generalized estimating equations to confirm that residual intra-individual correlation did not affect estimates (data not shown).
Following the development of LCRAT+CTpos, we then examined outcomes of the full LCRAT+CT model (LCRAT+CTneg and LCRAT+CTpos) by analyzing NLST participants regardless of screening result (negative or abnormal).13 We calculated the potential impact of using the full LCRAT+CT to choose management strategies between short-interval imaging (with potential clinical work-up), a 1-year interval, or a 2-year interval. These analyses used risks calculated during 10-fold cross-validation to avoid using the same data in both the prediction and evaluation. We focused on a 2-year interval as an alternative to annual screening because it was supported by the results of the NELSON trial, whereas a 2.5-year interval led to a potentially higher proportion of late-stage cancers.11
Results
Table 1 shows characteristics of NLST participants with at least one abnormal LDCT result. Variation in baseline lung cancer risk factors led to wide variation in 1-year pre-screening risks calculated by LCRAT (median 0.35%).
Table 1:
National Lung Screening Trial LDCT-arm participants with at least one abnormal screening result who were analyzed to develop the LCRAT+CTpos model, by characteristics included in the Lung Cancer Risk Assessment Tool (LCRAT)
| Characteristic (at T0 screen) | Immediate-screen analysis | Next-screen analysis |
|---|---|---|
| N (%) or median (IQR) | N (%) or median (IQR) | |
|
| ||
| Total number of unique individuals | 10,149 | 8,299 |
| Total number of LDCT screens | 18,129 | 12,993 |
| Total number of lung cancers | 649 | 235 |
| Included in analysis of immediate-screen cancer risk | ||
| One screen | 4,254 (41.9) | N/A |
| Two screens | 3,810 (37.5) | N/A |
| Three screens | 2,085 (20.5) | N/A |
| Included in analysis of next-screen cancer risk | ||
| Once (at either T1 or T2) | N/A | 3,605 (43.4) |
| Twice (at both T1 and T2) | N/A | 4,694 (56.6) |
| 1-year pre-screening risk at T0 | 0.35% (0.21%−0.59%) | 0.35% (0.21%−0.58%) |
| Sex | ||
| Male | 5,925 (58.4) | 4,858 (58.5) |
| Female | 4,244 (41.6) | 3,441 (41.5) |
| Age | ||
| 55–59 | 3,892 (38.3) | 3,205 (38.6) |
| 60–64 | 3,140 (30.9) | 2,566 (30.9) |
| 65–69 | 2,041 (20.1) | 1,660 (20.0) |
| 70–74 | 1,076 (10.6) | 868 (10.5) |
| Race/ethnicity | ||
| Non-Hispanic White | 9,292 (91.6) | 7,628 (91.9) |
| Non-Hispanic Black | 369 (3.6) | 272 (3.3) |
| Hispanic | 147 (1.4) | 127 (1.5) |
| Asian/Other | 341 (3.4) | 272 (3.3) |
| Education | ||
| No high school diploma | 638 (6.3) | 511 (6.2) |
| High school graduate/GED | 2,736 (27.0) | 2,210 (26.6) |
| Post-high school training other than college | 1,422 (14.0) | 1,171 (14.1) |
| Associate degree or some college | 2,323 (22.9) | 1,896 (22.8) |
| Bachelor’s degree | 1,647 (16.2) | 1,361 (16.4) |
| Graduate school | 1,383 (13.6) | 1,150 (13.9) |
| Body mass index | ||
| Underweight | 101 (1.0) | 84 (1.0) |
| Normal weight | 3,029 (29.8) | 2,451 (29.5) |
| Overweight | 2,746 (27.1) | 3,487 (42.0) |
| Obese | 4,273 (42.1) | 2,277 (27.4) |
| Family history of lung cancer | ||
| No first-degree relatives | 8,523 (84.0) | 6,979 (84.1) |
| 1 first-degree relative | 1,518 (15.0) | 1,233 (14.9) |
| 2 or more first-degree relatives | 108 (1.1) | 87 (1.0) |
| Years since quitting smoking | ||
| Current smoker | 5,475 (53.9) | 4,381 (52.8) |
| 1 to 5 | 1,751 (17.3) | 1,465 (17.7) |
| 6 to 10 | 1,505 (14.8) | 1,205 (14.5) |
| 11 or more | 1,418 (14.0) | 1,248 (15.0) |
| Total pack-years | ||
| 30–39 | 2,342 (23.1) | 1,972 (23.8) |
| 40–49 | 2,740 (27.0) | 2,241 (27.0) |
| 50 or more | 5,069 (49.9) | 4,086 (49.2) |
| Total years smoked | ||
| <30 | 609 (6.0) | 514 (6.2) |
| 30–39 | 3,686 (36.3) | 3,090 (37.2) |
| 40–49 | 4,620 (45.5) | 3,730 (44.9) |
| 50 or more | 1,234 (12.2) | 965 (11.6) |
| Cigarettes per day | ||
| <20 | 511 (5.0) | 416 (5.0) |
| 20–29 | 4,856 (47.8) | 3,911 (48.1) |
| 30–39 | 2,386 (23.5) | 1,956 (23.6) |
| 40 or more | 2,396 (23.6) | 1,936 (23.3) |
| Self-reported emphysema | ||
| No | 9,186 (90.5) | 7,544 (90.9) |
| Yes | 963 (9.5) | 755 (9.1) |
To develop LCRAT+CTpos, we restricted to LDCT-arm participants who completed the baseline questionnaire and had at least one abnormal LDCT, and then analyzed immediate risk of lung cancer detection. We use ‘immediate’ to refer to screen-detected lung cancers diagnosed after follow-up from an abnormal result and before the next screen. Among the abnormal LDCTs not classified as lung cancer (i.e., return-to-screening abnormal LDCTs, labelled “false-positives” in the NLST), we also calculated risk of cancer detection at the next screen. We defined “next-screen” cancers as those detected due to a positive result at the next annual screen after the return-to-screening abnormal LDCT. Missing values were imputed as previously described.26
Risk of immediate lung cancer diagnosis given a current abnormal LDCT – immediate risk portion of LCRAT+CT pos
For risk at the current screen, 649 lung cancer cases were detected immediately following 18,129 abnormal screens (3.6% risk) occurring among 10,149 individuals (Table 1).
Detailed results of model building are in the Supplement. The final model included terms for the longest diameter among all nodules, the number of nodules, presence of nodules with ground-glass opacity, presence of micronodules, nodule location, nodules with indeterminate margins, nodules showing growth between screens, new nodules, and nodules showing a suspicious change in attenuation (Supplementary Table 1). Among model predictors (Table 2), the distinction between prevalence and incidence screens was accounted for by a strong interaction with spiculation (p<0.0001). Median risk at the prevalence (T0) screen, if any nodule was spiculated, was 11.1%. This reduced to 5.8% median risk at incidence (T1-T2) screens. Also, female gender (p=0.0075) and overweight/obesity (p=0.019) increased risk (beyond their effect on pre-screening LCRAT risk).
Table 2:
Effect of features noted on an abnormal LDCT screen on risk of immediate lung cancer among participants in the National Lung Screening Trial: the immediate risk component of the LCRAT+CTpos model
| Immediate lung cancer detection risk = r0(x)y with exponent y for pre-screening risk r0 calculated as follows: |
Prevalence of feature | Median risk among individuals with the feature (IQR) | |
|---|---|---|---|
|
| |||
| What was the longest diameter among all nodules? | This gives the initial value: | ||
| N/A* | 0.60 (0.52–0.69) | 2.5% | 4.1% (3.0–5.4%) |
| 4–5mm | 1.01 (0.92–1.10) | 35.7% | 0.26% (0.14–0.47%) |
| 6–7mm | 0.82 (0.75–0.89) | 28.3% | 0.72% (0.39–1.4%) |
| 8–10mm | 0.64 (0.58–0.70) | 17.2% | 2.4% (1.3–4.3%) |
| 11–13mm | 0.50 (0.44–0.57) | 6.6% | 6.2% (3.7–11.0%) |
| 14mm or greater | 0.40 (0.35–0.46) | 9.8% | 9.8% (8.2–25.8%) |
| Was any nodule in the upper lobes? | If yes, subtract 0.08 (0.05–0.11) | 47.0% | 1.2% (0.43–4.6%) |
| Was any nodule in the right middle lobe or lingula? | If yes, add 0.05 (0.01–0.09) | 25.5% | 0.55% (0.21–1.8%) |
| Prevalence screen: did any nodule have spiculated margins? | If yes, subtract 0.27 (0.22–0.31) | 13.7% | 11.1% (3.3–27.6%) |
| Incidence screen(s), did any nodule have spiculated margins? | If yes, subtract 0.12 (0.08–0.16) | 10.8% | 5.8% (1.6–17.1%) |
| Did any nodule have indeterminate margins? | If yes, subtract 0.07 (0.04–0.11) | 30.3% | 2.0% (0.92–3.9%) |
| If incidence screen(s), were any new nodules present? | If yes, subtract 0.05 (0.02–0.09) | 41.6% | 1.1% (0.38–4.1%) |
| If incidence screen(s), did any nodule show interval growth? | If yes, subtract 0.17 (0.13–0.20) | 9.8% | 8.8% (3.6–20.0%) |
| Were there any ground-glass nodules? | If yes, add 0.11 (0.07–0.15) | 18.8% | 1.0% (0.31–3.5%) |
| Were there any micronodules? | If yes, add 0.05 (0.02–0.08) | 38.6% | 0.59% (0.23–2.0%) |
| Is the participant female? | If yes, subtract 0.04 (0.01–0.06) | 41.5% | 0.83% (0.30–3.0%) |
| Is the participant overweight or obese (BMI>25)? | If yes, subtract 0.03 (0.01–0.06) | 69.7% | 0.75% (0.27–2.7%) |
| Compute the natural log of one plus the number of nodules | Multiply by 0.15 (0.10–0.19) and then add to total exponent | Mean # of nodules: 1.68 | For screen with 1 nodule†: 0.72% (0.28–2.5%) |
The formula r0(x)y calculates immediate lung cancer detection risk as the pre-screening risk r0 based on individual covariates x (i.e., r0(x)), raised to an exponent y which is calculated as described in the table. Note that lower numbers imply higher risk and subtracting increases risk. Pre-screening risk r0 refers to 1-year lung cancer risk calculated by LCRAT. Number of nodules and micronodules appear to reduce risk because many predictors increase risk if any nodule has that feature, so these factors “standardize” for multiple chances to have a deleterious feature. The prevalence and risks for nodule features are calculated using data from all screens.
Screen was positive for a reason (i.e., suspicious abnormalities) other than a nodule ≥4 mm
Median number of nodules found on a screen was 1 (59.5% of screens had 1 nodule only).
The model showed good cross-validated internal calibration (649 cases observed vs. 650 predicted, p>0.99) and discrimination (optimism-corrected AUC=0.86).
Table 2 outlines calculations for immediate risk by LCRAT+CTpos. For example, consider a normal-BMI male at median pre-screening risk by LCRAT (0.35%) whose abnormal LDCT shows a single 7-mm solid nodule (initial exponent value of 0.82) in the left upper lobe (contribution of −0.08 to exponent) with only smooth margins (no contribution to exponent). The exponent for pre-screening risk is 0.82 (initial value) – 0.08 (upper lobe) = 0.74. The predicted immediate detection risk is 0.35%0.74 = 1.5% (n.b. risk increases with exponents less than 1, or coefficients less than zero).
Risk of next-screen lung cancer after a return-to-screening abnormal LDCT – next-screen risk portion of LCRAT+CTpos
For risk at the next screen following a return-to-screening abnormal LDCT, 235 lung cancer cases were detected following 12,993 return-to-screening abnormal LDCTs, giving an average risk of 1.8% (Table 1). This is 4.7 times higher than the 0.4% average next-screen risk after a negative screen13,15 (p<0.001). These 12,993 LDCTs occurred among 8,299 participants.
For the next-screen risk model, we confirmed our previous observations13 that (1) inclusion of pre-screening risk improved prediction, (2) prior screen results were not informative beyond the current one, and (3) the pre-screening risk exponent was similar at the T1 vs. T2 screen (Supplement). Non-Hispanic black race increased next-screen risk, but we did not include this effect in the model (see Discussion).
The final model accounted for the longest diameter among all nodules and the presence of any nodule(s) in the upper lobe(s), in the right middle lobe or lingula, with part-solid attenuation, with spiculated margins, with indeterminate margins, showing growth between screens, appearing as a new nodule, and showing a suspicious change in attenuation (Supplementary Table 2). The model showed good cross-validated internal calibration (235 cases observed vs. 238.7 predicted, p=0.79) and discrimination (optimism-corrected AUC=0.78).
Table 3 outlines calculations for next-screen risk by LCRAT+CTpos. In the previous example of a single 7mm left-upper-lobe solid-nodule with smooth margins with immediate risk=1.5%, now the exponent for pre-screening risk is 0.91 (7mm nodule) – 0.06 (upper lobe) + 0 (smooth margins) = 0.85. Therefore, the predicted next-screen detection risk is 0.35%0.85 = 0.82%. One-year cumulative risk equals 1.5%+(1–1.5%)*0.82%=2.3%.
Table 3:
Effect of features noted on a return-to-screening abnormal LDCT screen on risk of next-screen lung cancer among participants in the National Lung Screening Trial: the next-screen risk component of the LCRAT+CTpos model
| Next-screen lung cancer detection risk = r0(x)y with exponent y for pre-screening risk r0 calculated as follows: |
Prevalence of feature | Median risk among individuals with the feature (IQR) | |
|---|---|---|---|
|
| |||
| What was the longest diameter among all nodules? | This gives the initial value: | ||
| N/A* | 0.79 (0.65–0.98) | 1.9% | 1.4% (0.93–2.2%) |
| 4–5mm | 0.98 (0.91–1.06) | 39.0% | 0.53% (0.29–0.92%) |
| 6–7mm | 0.91 (0.85–0.99) | 29.8% | 0.84% (0.46–1.6%) |
| 8–10mm | 0.79 (0.73–0.86) | 16.4% | 2.1% (1.2–3.7%) |
| 11–13mm | 0.70 (0.62–0.77) | 5.7% | 5.4% (3.0–8.2%) |
| 14mm or greater | 0.76 (0.68–0.84) | 7.2% | 4.5% (2.6–7.7%) |
| Was any nodule in the upper lobes? | If yes, subtract 0.06 (0.01–0.12) | 44.9% | 1.5% (0.73–3.4%) |
| Was any nodule in the right middle lobe or lingula? | If yes, add 0.08 (0.02–0.15) | 26.4% | 0.62% (0.30–1.5%) |
| Did any nodule have part-solid attenuation? | If yes, subtract 0.07 (0.002–0.13) | 5.3% | 3.7% (1.6–7.4%) |
| Did any nodule have spiculated margins? | If yes, subtract 0.16 (0.10–0.22) | 9.6% | 4.4% (2.3–8.0%) |
| Did any nodule have indeterminate margins? | If yes, subtract 0.09 (0.04–0.14) | 28.1% | 2.0% (0.92–3.9%) |
| If incidence screen(s), were any new nodules present? | If yes, subtract 0.04 (−0.01–0.10) | 39.8% | 1.3% (0.60–3.0%) |
| If incidence screen(s), did any nodule show interval growth? | If yes, subtract 0.08 (0.003–0.16) | 7.1% | 3.9% (1.9–7.7%) |
| If incidence screen(s), did any nodule show a suspicious change in attenuation? | If yes, subtract 0.18 (0.08–0.27) | 2.0% | 8.3% (2.9–15.4%) |
The formula r0(x)y calculates next-screen detection risk as the pre-screening risk r0 based on individual covariates x (i.e., r0(x)), raised to an exponent y which is calculated as described in the table. Note that lower numbers imply higher risk and subtracting increases risk. Pre-screening risk r0 refers to 1-year lung cancer risk calculated by LCRAT. The prevalence and risks for nodule features are calculated among return-to-screening abnormal LDCTs at the T1 screen (N=6,510).
Screen was positive for a reason (i.e., suspicious abnormalities) other than a nodule ≥4 mm.
Potential impact of individualized risk calculators for management during lung cancer screening – evaluation of the full LCRAT+CT model
Compared with the NLST, the NHS England protocol would have referred 36% fewer people with abnormal screens for short-interval (e.g., 3-month) or fast-track surveillance imaging, while delaying diagnosis for only 4% of cancers. If the LCRAT+CTpos model for immediate risk had been used to identify people for short-interval or fast-track surveillance imaging, a ≥0.60% immediate risk-threshold would also delay diagnosis for 4% of cancers, yet would refer 42% fewer people with abnormal screens for short-interval/fast-track imaging compared with the NLST. This is a 9% additional reduction versus the NHS England protocol.
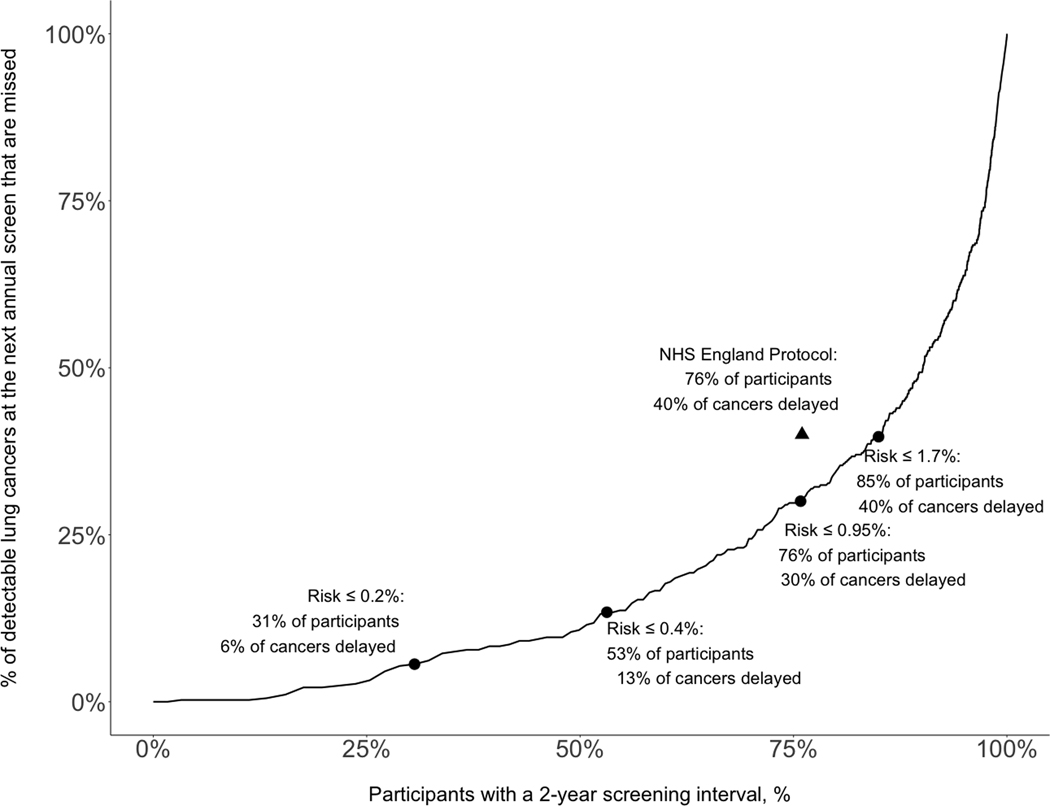
Figure 1 estimates outcomes from using the full LCRAT+CT model, including both LCRAT+CTneg and LCRAT+CTpos, to choose between 1-year and 2-year screening intervals. The Figure analyses participants with both negative and abnormal screen results, and accompanying data is provided in Table 4. By the principle of “equal management of equal risks”,6 individuals at the same cumulative risk of cancer should be managed equally, regardless of whether their recent screen was deemed abnormal or negative. Figure 1 therefore evaluates the full LCRAT+CT model by including individuals with either a recent negative or recent return-to-screening abnormal LDCT result, and the analysis allows individuals to be recommended a 1-year or 2-year screening interval regardless of whether they had a recent negative or abnormal result. Predictions for those with recent negative LDCTs are calculated by LCRAT+CTneg as previously described, using an equation in which pre-screening risk is raised to an exponent calculated based on the presence or absence of emphysema and/or consolidation on the negative LDCT.13
Figure 1: Potential impact of using the full LCRAT+CT risk model to refer the lowest-risk participants for a 2-year screening interval in the National Lung Screening Trial, among participants with any screening result (negative or abnormal).
The figure includes both individuals with a recent abnormal screen, with risks calculated by LCRAT+CTpos as described in the manuscript, as well as individuals with a recent negative screen, whose risks were calculated by LCRAT+CTneg as previously described.13 The triangle represents the performance of the NHS England protocol; because it is not on the line, a risk-based program should be superior. The risk used is cumulative risk of both immediate (denoted ri) and 1-year next-screen risk (rn); then cumulative risk = ri+(1-ri)rn. This cumulative risk expression denotes that each participant begins with immediate risk ri, then for those who do not have immediate cancer (1-ri), they contribute their 1-year next-screen risk (rn). 1-year LCRAT+CT risks were calculated by 10-fold cross-validation, so that no record contributes to its own prediction. The data in this figure are also presented, together with additional information, in Table 4.
Table 4:
Detailed results describing the potential impact of using the full LCRAT+CT risk model to choose between 1-year and 2-year intervals during lung cancer screening in the National Lung Screening Trial, among participants with any screening result (negative or abnormal).
| Risk threshold for a 1-year interval | Participants referred for a 2-year interval, N (%) | Cancers missed that are detectable immediately or at the next annual screen, N (%) | Participants with a recent negative result referred for a 2-year interval, N (%) | Participants with a recent abnormal result referred for a 2-year interval, N (%) |
|---|---|---|---|---|
| 0% A | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 0.10% | 5443 (11.2) | 1 (0.3) | 5407 (15.2) | 36 (0.3) |
| 0.20% | 14849 (30.6) | 21 (5.6) | 14548 (40.9) | 301 (2.3) |
| 0.40% | 25781 (53.1) | 50 (13.4) | 24447 (68.8) | 1334 (10.3) |
| 0.95% B | 36796 (75.8) | 112 (30.0) | 32679 (92.0) | 4117 (31.7) |
| 1.70% C | 41227 (85.0) | 148 (39.7) | 34835 (98.0) | 6392 (49.2) |
| 100% D | 48523 (100) | 373 (100) | 35530 (100) | 12993 (100) |
The figure includes both individuals with a recent abnormal screen, with risks calculated by LCRAT+CTpos as described in the manuscript, as well as individuals with a recent negative screen, whose risks were calculated by LCRAT+CTneg as previously described.13 The total sample size of 48,523 is the number of NLST screens, with either a negative result (n=35,530) or a return-to-screening abnormal result (n=12,993) and a known lung cancer outcome at the following screen. The risk used is cumulative risk of both immediate (denoted ri) and 1-year next-screen risk (rn); then cumulative risk = ri+(1-ri)rn. This cumulative risk expression denotes that each participant begins with immediate risk ri, then for those who do not have immediate cancer (1-ri), they contribute their 1-year next-screen risk (rn). LCRAT+CT risks were calculated by 10-fold cross-validation, so that no record contributes to its own prediction. The NHS England protocol, when applied to these data (using diameter thresholds), would recommend a 2-year interval for approximately 76% of individuals and delay diagnosis by 1 year for 40% of next-screen lung cancers.
A 0% risk threshold is equivalent to annual screening (1-year interval) for all participants.
This threshold results in referring the same number of NLST participants to a 2-year interval (76%) as the NHS England protocol, but decreases the number of cancers delayed 1-year in diagnosis by 25% (from 40% to 30%).
This threshold results in delayed detection for approximately the same number of cancers as the NHS England protocol (40%), but further reduces the number of required screens at a 1-year interval. The NHS England protocol reduced 1-year screens by 76% and this LCRAT+CT threshold would reduce them by 85% (a further reduction of 38%).
A 100% risk threshold is equivalent to 2-yearly screening for all participants.
LCRAT+CT can be used to determine a threshold to recommend a 1-year interval for a subset of higher-risk individuals. For example, the NHS England protocol was designed with this intention, and when applied to the NLST data (using diameter thresholds), it would recommend a 2-year interval for 76% of individuals and delay diagnosis by 1 year for 40% of next-screen lung cancers. In contrast, a risk-based approach using LCRAT+CT with a cumulative risk-threshold of <0.95% would also refer 76% of people for a 2-year interval but delay diagnosis for only 30% of cancers, a 25% reduction versus the NHS protocol (Figure 1, Table 4). Alternatively, a risk-based approach that matches the NHS protocol in delaying diagnosis for 40% of cancers (<1.7% cumulative risk threshold for a 2-year interval) would reduce the number of screens by 85% vs. the NLST (Figure 1, Table 4). This is an additional 38% reduction in the number of screens 1 year later compared with the NHS England protocol (which reduced by 76% compared with the NLST). At this <1.7% threshold, 98% of individuals with a recent negative result would be offered a 2-year interval, along with 49% of individuals with a recent return-to-screening abnormal result (Table 4). To further reduce the percentage of delayed diagnosis, a lower threshold for annual screening could be applied, such as 0.20% which would refer 30.6% of participants for a 2-year interval while delaying diagnosis for only 5.6% of cancers.
Discussion
We developed a risk-based approach to lung cancer screening allowing continual, individualized risk assessment, starting from pre-screening risk to decide eligibility for screening, then updating risk with LCDT results to refer for short-interval imaging (with potential clinical work-up) and then to tailor screening intervals. Considering participants with any screening result, our approach could maintain the sensitivity of the protocol for the NHS England Targeted Lung Health Checks yet further reduce the number of CTs by 38% (LCRAT+CT cumulative risk<1.7%). Alternatively, our approach could refer the same proportion of participants for a 2-year interval as the NHS England protocol, yet reduce by 25% the fraction of cancers delayed in diagnosis (LCRAT+CT cumulative risk<0.95%). Clearly, in some contexts, it would be desirable to further reduce the number of delayed diagnoses, which could be easily achieved by lowering the LCRAT+CT threshold required for annual screening. In practice, our approach could be automated, calculating LCRAT 1-year pre-screening risk using an online risk calculator32 and then subsequently updating immediate and next-screen risk with LCRAT+CT using information routinely collected from LDCTs.
To maximize efficiency, our approach draws on detailed information from standard risk factors such as age and smoking, as well as LDCT image features. In contrast, protocols such as Lung-RADS and the NHS England protocol rely almost exclusively on information about nodule diameter or volume, which is categorized for alignment with management strategies.5,17 This approach, although simple and practical for routine use, considers neither traditional risk factors (age, smoking) nor other LDCT image features. It can only recommend closer follow-up for individuals with nodules exceeding size thresholds on the recent LDCT, and cannot prioritize people who have high future risk for other reasons. Unlike other risk-based approaches, such as the Brock model20, LCRAT+CT accounts for all pre-screening risk-factors, accounts for the distinction between the initial (prevalence) screen and future (incidence) screens, and calculates cumulative risk using models optimized for immediate risk of cancer and 1-year risk of lung-cancer detection.
When an indeterminate nodule is detected on a screening LDCT, it is commonly followed up with a surveillance low-dose CT performed at 3 or 6 months to assess nodule growth.5,17 The most important limitation of our analysis is that we could not account for data from these surveillance scans because they were not collected in the NLST. In practice, when deciding what action to take after a surveillance scan, the LCRAT+CT risk score could be considered together with information from the new LDCT. In the future, we hope to update our model to additionally account for information from surveillance scans. We also note that when we refer to suggested follow-up for people with immediate risk >0.60%, we have not distinguished between the majority who should receive short-interval imaging and the smaller, highest-risk group who might be referred directly to ‘fast-track’ clinical work-up. In principle, a higher immediate-risk threshold for fast-track work-up could be established, such as ≥10%, which would refer only 10% of screen-positives to ‘fast-track’ work-up yet immediately detect 59% of prevalent cancers.
We found that some pre-screening risk factors, although included in LCRAT, needed to be separately accounted for in LCRAT+CTpos. For immediate risk, risk increased with female sex, as also seen in the Brock model,20 and with overweight/obesity. Risk of next-screen detection was higher in non-Hispanic blacks after adjusting for nodule characteristics (Supplement). We did not include this effect in our model because we believe it is an artifact of lower rates of follow-up among non-Hispanic blacks after an abnormal LDCT.33 An adjustment for race remains in LCRAT, which increases lung cancer risk for non-Hispanic blacks. However, this finding warrants investigation in datasets with larger minority populations, because if a genuine effect, it could exacerbate existing racial/ethnic disparities in lung cancer.34–38
NLST data do not link nodules on LDCT images with subsequent cancer diagnoses. Therefore, when cancer was detected one year after an abnormal LDCT, we could not ascertain whether the cancer was “missed” at the prior abnormal LDCT or had arisen de novo in the interval. Though our model appears internally valid, it requires validation in external populations. We caution that our impact estimates could be optimistic since our model was both developed and applied in the NLST, albeit with appropriate adjustments. The NLST also did not record volumetric information for nodules, which might improve models for risk during screening, and may have affected our results pertaining to the NHS England protocol.2,39 Finally, additional research is needed to understand precisely how many of the diagnoses delayed by a 2-year screening interval would result in a lost opportunity to prevent lung cancer death. It is possible that delayed diagnosis for small-nodule cancers may have a relatively small impact on prognosis.40
One model conceptually similar to LCRAT+CT was previously proposed by Schreuder et al.41 The Schreuder model accounts for multiple pre-screening risk factors and LDCT findings, but was developed and is formally valid only for baseline LDCT findings and outcomes at the T1 screen. In contrast, our model is formally valid for use during continuous screening. A prior validation study in the German LUSI trial found similar performance of the Schreuder model and LCRAT+CTneg.25 Before LCRAT+CT can be implemented in a clinical setting, a key next step will be to independently validate the full LCRAT+CT model in lung screening studies, including those with large sample sizes.
Although the LCRAT+CT model has a relatively straightforward formulation, it would be most efficiently implemented using automated processes within electronic health records (EHRs). The first step would be to calculate individual pre-screening risk by the LCRAT model. This can be done using an online tool,32 but we are currently integrating it into an EHR system which will make it easier for clinicians to use. If this step is done at screening initiation, then the LCRAT risk score could be used to define the population eligible for screening using a previously proposed threshold, or to identify additional individuals who do not meet current criteria (e.g. USPSTF) but have high likelihood of screening benefit.26,42 The second step would be to classify a person’s screen as either negative or abnormal based on the modified Fleischner criteria used in the NLST, solely to decide whether to use LCRAT+CTneg or LCRAT+CTpos. The third step would be to implement risk calculations for immediate (if abnormal) and next screen risk, according to the risk models described herein (for abnormal screens, LCRAT+CTpos) or previously (for negative screens, LCRAT+CTneg).13 This step would use previously recorded information describing the features and nodules on the LDCT image. Finally, EHR systems could be programmed to return the suggested management based on pre-defined risk thresholds for short-term surveillance, a 1-year interval, or a 2-year interval. In the future, an important enhancement will be to modify our models to incorporate information from radiomics analysis of LDCT scans,43,44 and this approach could be automated in a similar fashion. We are currently incorporating LCRAT into an EHR system and intend to incorporate LCRAT+CT in the future.
In conclusion, using individualized risk models to decide who should be referred for short-interval imaging and to choose the length of lung cancer screening intervals could substantially increase the efficiency of lung cancer screening programs. It is likely that risk-based approaches to patient management in screening would improve cost-effectiveness, though this remains to be observed. Programs recommending biennial screening for most participants could substantially improve sensitivity by using risk models to identify high-risk individuals to be referred for annual screening.
Supplementary Material
Highlights.
The LCRAT+CT prediction model for lung cancer risk during screening offers a unified, risk-tailored approach to recommend short-interval imaging, a 1-year interval, or a 2-year interval.
Immediate and future lung cancer risk after an abnormal LDCT screen depends on pre-screening traditional risk factors as well as features of lung nodules including size, location, margins, attenuation, new appearance, and interval change.
Compared with the draft protocol for the NHS England Targeted Lung Health Checks, the LCRAT+CT model with a threshold of <0.95% cumulative risk could reduce the number of cancers missed at 1 year by 25%, while offering a 2-year interval to the same proportion of participants (76%).
Funding:
This study was supported in part by the Intramural Research Program of the U.S. National Institutes of Health/National Cancer Institute. H. Robbins was supported in part by the Cancer, Epidemiology, Prevention, and Control Training Grant (NCI T32 CA009314), an individual National Research Service Award (NCI F31 CA210660), the INTEGRAL program (NCI U19 CA203654), and NCI R03 CA245979.
Footnotes
Disclaimer: The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. The NIH approved the final version of the manuscript but had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation of the manuscript.
Conflicts of interest: HAR, LCC, AKC, and HAK report no conflict of interest. CDB receives consulting fees from Medial Early Sign, LLC and GRAIL, Inc. D.R. Baldwin reports personal fees from AstraZeneca, MSD, BMS, and Roche, outside the submitted work. The Lung Cancer Risk Assessment Tool (LCRAT) was previously proposed in a manuscript co-authored by Drs. Cheung, Berg, Chaturvedi, and Katki.
References
- 1.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325(10):962–70. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160(5):330–8. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Programme. Targeted Screening for Lung Cancer with Low Radiation Dose Computed Tomography. Standard Protocol prepared for the NHS England Targeted Lung Health Checks Programme. Version 1. Available at https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-lu. 2019.
- 6.Castle PE, Katki HA. Screening: A risk-based framework to decide who benefits from screening. Nat Rev Clin Oncol 2016;13(9):531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patz EF, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol 2016;17(5):590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15(12):1332–41. [DOI] [PubMed] [Google Scholar]
- 9.Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol 2017;18(11):1523–31. [DOI] [PubMed] [Google Scholar]
- 10.Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computed tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187(8):848–54. [DOI] [PubMed] [Google Scholar]
- 11.Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72(1):48–56. [DOI] [PubMed] [Google Scholar]
- 12.Sverzellati N, Silva M, Calareso G, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26(11):3821–9. [DOI] [PubMed] [Google Scholar]
- 13.Robbins HA, Berg CD, Cheung LC, Chaturvedi AK, Katki HA. Identification of candidates for longer lung cancer screening intervals following a negative low-dose computed tomography result. J Natl Cancer Inst 2019;111(9):996–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosbie PA, Balata H, Evison M, et al. Implementing lung cancer screening: baseline results from a community-based “Lung Health Check” pilot in deprived areas of Manchester. Thorax 2018;74(4):405–9. [DOI] [PubMed] [Google Scholar]
- 15.Pinsky P, Gierada DS. Long-term cancer risk associated with lung nodules observed on low-dose screening CT scans. Lung Cancer 2020;139:179–84. [DOI] [PubMed] [Google Scholar]
- 16.Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: accredited by NICE. Thorax 2015;70(Suppl 2):ii1 LP-ii54. [DOI] [PubMed] [Google Scholar]
- 17.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). Accessed 2020 Sep 22. Available at https://www.acr.org/Quality-Safety/Resources/LungRADS.
- 18.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157(8):849–55. [PubMed] [Google Scholar]
- 19.Gould MK, Ananth L, Barnett PG, Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131(2):383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369(10):910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128(4):2490–6. [DOI] [PubMed] [Google Scholar]
- 22.MacMahon H, Austin JHM, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237(2):395–400. [DOI] [PubMed] [Google Scholar]
- 23.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369(3):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diggle P, Heagerty P, Liang K-Y, Zeger S. Chapter 10: Transition Models. In: Analysis of Longitudinal Data, Second Edition. 2013. [Google Scholar]
- 25.Maldonado SG, Hynes LC, Motsch E, et al. Validation of multivariable lung cancer risk prediction models for the personalized assignment of optimal screening frequency: A retrospective analysis of data from the German Lung Cancer Screening Intervention Trial. Transl Lung Cancer Res 2021;10(3):1305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA 2016;315(21):2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katki HA, Petito LC, Cheung LC, et al. Implications of 9 risk prediction models for selecting ever-smokers for CT lung-cancer screening. Ann Intern Med 2018;169(1):10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins HA, Alcala K, Swerdlow AJ, et al. Comparative performance of lung cancer risk models to define lung screening eligibility in the United Kingdom. Br J Cancer 2021;124(12):2026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wacholder S Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol 1986;123(1):174–84. [DOI] [PubMed] [Google Scholar]
- 30.Tibshirani R Regression shrinkage and selection via the lasso. J R Stat Soc Ser B 1996;58(1):267–88. [Google Scholar]
- 31.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4):361–87. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute. Risk-based NLST Outcomes Tool (RNOT). Accessed 2018 Apr 17. Available at https://analysistools.nci.nih.gov/lungCancerScreening.
- 33.Sesti J, Sikora TJ, Turner DS, et al. Disparities in follow-up after low-dose lung cancer screening. Semin Thorac Cardiovasc Surg 2019;32(4):1058–63. [DOI] [PubMed] [Google Scholar]
- 34.Robbins HA, Engels EA, Pfeiffer RM, Shiels MS. Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst 2015;107(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016;66(4):290–308. [DOI] [PubMed] [Google Scholar]
- 36.Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: A review. Ann Am Thorac Soc 2020; 10.1513/AnnalsATS.201907-556CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol 2019;5(9):1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins HA, Johansson M. Defining equity in eligibility for cancer screening. JAMA Oncol 2020;6(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 2018;73(8):779–81. [DOI] [PubMed] [Google Scholar]
- 40.Henschke CI, Yip R, Yankelevitz DF, Smith JP. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158(4):246–52. [DOI] [PubMed] [Google Scholar]
- 41.Schreuder A, Schaefer-Prokop CM, Scholten ET, Jacobs C, Prokop M, van Ginneken B. Lung cancer risk to personalise annual and biennial follow-up computed tomography screening. Thorax 2018;73:626–33. [DOI] [PubMed] [Google Scholar]
- 42.Landy R, Young CD, Skarzynski M, et al. Using prediction models to reduce persistent racial/ethnic disparities in draft 2020 USPSTF lung cancer screening guidelines. J Natl Cancer Inst 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillies RJ, Schabath MB. Radiomics improves cancer screening and early detection. Cancer Epidemiol Biomarkers Prev 2020;29(12):2556 LP – 2567. [DOI] [PubMed] [Google Scholar]
- 44.Massion PP, Antic S, Ather S, et al. Assessing the accuracy of a deep learning method to risk stratify indeterminate pulmonary nodules. Am J Respir Crit Care Med 2020;202(2):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.