Summary
Background
Household air pollution (HAP) from solid fuel use is associated with adverse birth outcomes, but data for exposure–response relationships are scarce. We examined associations between HAP exposures and birthweight in rural Guatemala, India, Peru, and Rwanda during the Household Air Pollution Intervention Network (HAPIN) trial.
Methods
The HAPIN trial recruited pregnant women (9–<20 weeks of gestation) in rural Guatemala, India, Peru, and Rwanda and randomly allocated them to receive a liquefied petroleum gas stove or not (ie, and continue to use biomass fuel). The primary outcomes were birthweight, length-for-age, severe pneumonia, and maternal systolic blood pressure. In this exposure–response subanalysis, we measured 24-h personal exposures to PM2·5, carbon monoxide, and black carbon once pre-intervention (baseline) and twice post-intervention (at 24–28 weeks and 32–36 weeks of gestation), as well as birthweight within 24 h of birth. We examined the relationship between the average prenatal exposure and birthweight or weight-for-gestational age Z scores using multivariate-regression models, controlling for the mother's age, nulliparity, diet diversity, food insecurity, BMI, the mother's education, neonate sex, haemoglobin, second-hand smoke, and geographical indicator for randomisation strata.
Findings
Between March, 2018, and February, 2020, 3200 pregnant women were recruited. An interquartile increase in the average prenatal exposure to PM2·5 (74·5 μg/m3) was associated with a reduction in birthweight and gestational age Z scores (birthweight: –14·8 g [95% CI –28·7 to –0·8]; gestational age Z scores: –0·03 [–0·06 to 0·00]), as was an interquartile increase in black carbon (7·3 μg/m3; –21·9 g [–37·7 to –6·1]; –0·05 [–0·08 to –0·01]). Carbon monoxide exposure was not associated with these outcomes (1·7; –3·1 [–12·1 to 5·8]; –0·003 [–0·023 to 0·017]).
Interpretation
Continuing efforts are needed to reduce HAP exposure alongside other drivers of low birthweight in low-income and middle-income countries.
Funding
US National Institutes of Health (1UM1HL134590) and the Bill & Melinda Gates Foundation (OPP1131279).
Introduction
Household air pollution (HAP) exposures from the use of solid cooking fuels such as wood, coal, charcoal, dung, and agricultural residues are a leading risk factor for ill health in low-income and middle-income countries (LMICs), accounting globally for an estimated 2·3 million premature deaths annually and 91·5 million disability-adjusted life-years.1 Systematic reviews have summarised the evidence for an association between HAP exposure and adverse health effects, including child pneumonia, chronic obstructive lung disease, lung cancer, and cataracts.2 Few studies or reviews have focused on adverse perinatal outcomes including low birthweight.3, 4, 5, 6
LMICs bear a disproportionate share of low birthweight (defined as <2500 g regardless of gestational age), accounting for nearly 91% of the global burden.7 The aetiology of low birthweight is complex, and despite ongoing efforts to address known risk factors such as maternal malnutrition, malaria, and smoking,8 progress has been slow towards the ambitious global nutrition target of a 30% reduction of low birthweight by 2025.7 As nearly 3·8 billon people worldwide rely on solid fuels,9 a strengthened understanding of the relationship between HAP and low birthweight would be extremely valuable for prioritising efforts to decrease HAP exposures during pregnancy to improve birth outcomes.
Research in context.
Evidence before this study
Household air pollution (HAP) exposures from the use of solid cooking fuels such as wood, coal, charcoal, dung, and agricultural residues are a leading risk factor for ill health in low-income and middle-income countries (LMICs), accounting for an estimated 2·3 million premature deaths annually and 91·5 million disability-adjusted life-years. Several systematic reviews have summarised the evidence for associations between HAP exposures and adverse health effects, including child pneumonia, chronic obstructive lung disease, lung cancer, and cataracts but few have focused on adverse perinatal outcomes including low birthweight. Furthermore, most previous studies examining HAP exposure related health effects have used categorical indicators of exposure, based on primary fuel use. Only three HAP studies to date have reported quantitative exposure–response relationships for birthweight and PM2·5 or carbon monoxide and none have examined associations with black carbon. Most studies also did not rely on longitudinal personal exposures during pregnancy or separate the direct effects of these pollutants on birthweight from that mediated through gestational age. Quantitative exposure–response relationships for HAP-associated exposures and birthweight thus remain poorly characterised.
Added value of this study
The Household Air Pollution Intervention Network (HAPIN) trial is among the largest household energy intervention trials directed at reducing HAP exposures for pregnant women and their neonates. The study provides one of the largest and most diverse datasets on pregnancy period 24-h personal exposures for PM2·5, black carbon, and carbon monoxide, together with measurements of birthweight and ultrasound-assessed gestational age from rural communities in four countries (Guatemala, India, Peru, and Rwanda). Furthermore, primary information on multiple confounders or strong risk factors for birthweight allowed the development of robust models to estimate the strength and shape of exposure–response relationships. The study provides some of the first estimates of exposure–response relationships for gestational black carbon exposures from HAP and birthweight as well as exposure–response relationships for PM2·5, black carbon, and carbon monoxide exposures with weight-for-gestational-age Z scores. As the results are consistent across four diverse countries, they are widely generalisable. The personal exposure estimates for pregnant women also provide some of the largest numbers of measurements to the WHO global household air pollution database.
Implications of all the available evidence
The study provides important new information about exposure–response relationships for gestational HAP exposures and birthweight as well as weight-for-gestational-age Z scores on a multicountry scale. The positive exposure–response results for PM2·5 and black carbon, in conjunction with measured exposure levels, can be used to inform clean household fuel policy scenarios targeting the reduction of HAP exposures. The results provide further support for continuing efforts to reduce HAP exposure alongside other drivers of low birthweight in LMICs.
Most previous studies that examine the association between HAP exposures and low birthweight have used categorical indicators of exposure based on primary fuel use, with only a handful reporting quantitative exposure–response relationships for PM2·5 or smaller10, 11 or carbon monoxide.12, 13, 14 These exposure–response studies report significant associations between prenatal PM2·5 or carbon monoxide exposures and low birthweight, but also report many limitations: small sample sizes, an inability to measure multiple pollutants, and the use of single personal exposure measures during pregnancy and longitudinal kitchen area measurements as proxies of longer-term personal exposure. Randomised control trials of HAP interventions in Nepal,15 Nigeria,12 and Ghana16 have reported null effects from intention-to-treat analyses for effects on birthweight, but exposure–response analyses within these studies have been scarce.13 To our knowledge, no studies have examined exposure–response relationships between prenatal black carbon exposures and birthweight.
The multicountry Household Air Pollution Intervention Network (HAPIN) trial was designed to assess health effects after the replacement of biomass cookstoves with liquefied petroleum gas cookstoves in rural Guatemala, India, Peru, and Rwanda, with the goal of reducing HAP in LMICs. The trial was shown to have high fidelity (ie, delivery of the intervention as intended) and adherence to the intervention and also led to a substantial reduction in personal exposure to PM2·5 and black carbon exposures during pregnancy.17, 18 Intention-to-treat analyses on infant birthweight (the first of the primary outcomes reported) were negative, with no significant difference in birthweight between infants born to women who used liquefied petroleum gas cookstoves and those born to women who used biomass cookstoves.19 The mean birthweight was 2921 g in the intervention group and 2898 g in the control group, for an adjusted mean difference of 19·5 g (95% CI –10·1 to 49·2). Here we present results from secondary exposure–response analyses for birthweight performed in the HAPIN trial. We hypothesised that high exposure to PM2·5, black carbon, and carbon monoxide during pregnancy would result in low birthweight among infants born to women enrolled in the HAPIN trial in each of—and across—the four countries.
Methods
Study participants and settings
Participants were pregnant women enrolled in the HAPIN trial, details of which have been published previously20, 21, 22 and are summarised in the trial registration (NCT02944682). The specific study areas in each country (Jalapa Municipality, Guatemala; Villupuram and Nagapatinam districts of Tamil Nadu, India; Department of Puno, Peru; and Eastern Province, Rwanda) were selected based on a high prevalence of cooking with biomass, low background ambient PM2·5 concentrations, and acceptable field feasibility as assessed during an 18-month period of planning and formative research.23, 24 Between March, 2018, and February, 2020, we recruited 3200 (800 per country) non-smoking women who were pregnant, between 18 and 35 years of age, 9 weeks to less than 20 weeks of gestation (determined via ultrasound), and who used biomass as a primary fuel. In accordance with the trial protocol, half of the participants in each country were randomly assigned to an intervention group that received a liquefied petroleum gas stove and a continuous supply of liquefied petroleum gas fuel following enrolment and throughout their pregnancy, whereas the remaining participants acted as controls and continued to rely chiefly on solid biomass for cooking.
The study protocol was reviewed and approved by institutional review boards or ethics committees at: Emory University, Atlanta, GA, USA (00089799), Johns Hopkins University, Baltimore, MD, USA (00007403), Sri Ramachandra Institute of Higher Education and Research, Chennai, India (IEC-N1/16/JUL/54/49), the Indian Council of Medical Research–Health Ministry Screening Committee, New Delhi, India (5/8/4-30/(Env)/Indo-US/2016-NCD-I), Universidad del Valle de Guatemala, Guatemala City, Guatemala (146-08-2016/11-2016), the Guatemalan Ministry of Health National Ethics Committee, Guatemala City, Guatemala (11-2016), A B PRISMA, Lima, Peru, the London School of Hygiene & Tropical Medicine, London, UK (11664-5), the Rwandan National Ethics Committee, Kigali, Rwanda (357/RNEC/2018), and Washington University, St Louis, MO, USA (201611159).
Personal exposure monitoring during pregnancy
Prenatal personal exposure monitoring protocols and results have been described previously.22, 25 Briefly, at each study site, women who were pregnant participated in three 24-h personal exposure assessments, once at baseline (<20 weeks of gestation) and twice after randomisation (at 24–28 weeks and 32–36 weeks of gestation). During each session, women wore customised vests or aprons fitted so that the instrumentation was situated close to their breathing zone. PM2·5 monitoring was performed using the Enhanced Children's MicroPEM (RTI International, Research Triangle Park, NC, USA), which collects gravimetric samples on pre-weighed 15 mm Teflon filters (Measurement Technologies Laboratories, Minneapolis, MN, USA) using a 2·5 μm impactor at a flow rate of 0·3 L per minute and real-time nephelometric data.26 Black carbon was estimated post-sampling on the Enhanced Children's MicroPEM filters using the SootScan Model OT-21 Optical Transmissometer (Magee Scientific, Berkeley, CA, USA). Carbon monoxide monitoring was done using the Lascar EL-CO-USB-300 DataLogger (Lascar Electronics, Erie, PA, USA). Participants were instructed to always wear the vest or apron during the 24-h measurement period, except when sleeping, bathing, or when conducting other activities during which the equipment could not be safely worn. During these times, they were instructed to keep the vest or apron nearby. Additionally, data were collected on sociodemographic and household characteristics and activity patterns that might influence exposure.
Procedures for assuring data quality, weighing filters, and estimating missing gravimetric data based on nephelometry have been described previously.25 Briefly, gravimetric data quality assurance involved a combination of threshold values for flow rates, inlet pressure, and sampling duration, as well as visual inspection of damaged filters by the room technicians doing the weighing. In cases for which nephelometric but not gravimetric data were available, PM2·5 exposure was estimated based on nephelometric data, using an instrument-specific regression coefficient for the association between nephelometric and gravimetric data for that specific Enhanced Children's MicroPEM instrument as described previously.25 Carbon monoxide quality assurance protocols included calibrations with zero air and span gas and a visual inspection system similar to what was applied in the Ghana Randomized Air Pollution and Health Study (GRAPHS)27 in Ghana.
For exposure–response analyses, gestational exposures were defined for the intervention group as the average of the pre-intervention and post-intervention exposures, weighted by the amount of gestational time spent in each period. The pre-intervention period exposure was estimated using the baseline measurement, whereas the post-intervention exposure was estimated using one or both personal measurements done after the intervention. This method allowed for exposure changes resulting from the introduction of the intervention to be weighted according to the length of time participants had the intervention during gestation. An unweighted average of the baseline and other available (1–2) gestational period measurements was used for controls, as they continued using biomass as the primary cooking fuel throughout gestation.
Birthweight outcome measurements
Following a standard protocol, birthweight was measured within 24 h of birth by a trained field worker or nurse using a Seca 334 mobile digital baby scale. Neonates were weighed naked to the nearest 10 g and duplicate measurements were recorded on tablet-based REDCap forms. If the first two measured birthweights differed by more than 10 g, a third measurement was taken. The average of the measurements was used in the data analysis. Neonates were typically assessed at the health facilities where they were born. Each scale was calibrated weekly in the field offices before deployment using standard 5 lb and 10 lb weights; scales not within a 2·5% SD of the standard weight were recalibrated. When we were unable to reach the neonate during the prescribed 24-h window—due mainly to COVID-19 restrictions or critically ill neonates admitted to intensive care units or referral hospitals—we used measurements provided by the facility, if available, but conducted sensitivity analyses to compare results.
As gestational age is a potential mediator in the causal pathway between HAP exposure and birthweight, we did not adjust for it in the exposure–response models; had we done so, its inclusion would not allow estimation of the total effect of exposure.28 However, we additionally estimated Z scores for weight adjusted by gestational age defined using INTERGROWTH tables as a secondary analysis. These weight-for-gestational-age Z scores were derived by subtracting off the standard INTERGROWTH sex-specific weight for a given gestational age and dividing by the INTERGROWTH SD of that weight. Measurements were considered invalid if the gestational age at birth was greater than 300 days or if the birthweight-for-gestational-age Z score did not fall between –6 and 5.
Statistical analysis
The statistical analysis plan was agreed upon in advance and published with the trial registration before unblinding. Analyses were independently replicated by a member of the HAPIN Investigators team (LM). Exposure–response analyses were modelled separately for each pollutant (PM2·5, black carbon, and carbon monoxide) and birthweight or birthweight-for-gestational-age Z score.
Covariate selection for models was guided by a directed acyclic graph (appendix p 8). A minimal set of potential confounders or strong risk factors (eg, neonate sex) were identified in systematic reviews of birthweight,3, 6 and from previous studies of HAP and birthweight.10, 11, 12, 27, 29 We used 5% change-in-estimate methods as outlined by Greenland30 to evaluate and determine covariates included in the model. Final models included the following covariates: mother's age (categorical: <20, 20–24, 25–29, or 30–35 years), nulliparity (categorical: yes or no), diet diversity (categorical: low, medium, or high), food insecurity score (categorical: secure, mildly secure [1, 2, or 3], or moderately [4, 5, or 6] or severely [7 or 8] secure), baseline BMI (continuous), mother's education (categorical), neonate sex (categorical), baseline haemoglobin concentration (continuous), and exposure to second-hand smoke (categorical: yes or no). We also included a variable for ten geographical randomisation strata (one in Rwanda, one in Guatemala, two in India, and six in Peru). We created a category of missing for women with missing BMI or haemoglobin data so that they were not excluded from the analysis.
For both birthweight and Z scores, we first fitted linear models with different exposure metrics (ie, linear and log linear). We then evaluated non-linear categorical (quartile modes), as well as quadratic, two-piece linear, and restricted cubic spline model with three knots31 models, and assessed model fit using Akaike's Information Criterion. The knots for the two-piece spline were chosen based on Akaike's Information Criterion (using quartile cut points initially and then narrowing down), while knots for restricted cubic splines were placed at the 5th, 50th, and 75th percentiles of exposure. We also used thin plate smoothing splines via generalised additive models, with penalisation determined by generalised cross-validation scores, using R package mgcv. We also examined effect modification by country, as well as by neonate sex, via interaction terms between our exposure metrics and these variables.
We did not run multi-pollutant models because the two pollutants that showed some effect on birthweight, black carbon, and PM2·5 were highly correlated indicating that the inclusion of both in a model would diminish the effect of each (Spearman coefficient of 0·79). The third pollutant, carbon monoxide, showed no effect on birthweight and its addition to a multi-pollutant model would have had little or no effect.
Role of the funding source
The funders were not involved in the study design, data collection, data analysis, or interpretation of the data, or the decision to submit the paper for publication.
Results
3200 women were enrolled in the study, of whom five were determined to be ineligible after randomisation and exited the study. After accounting for miscarriages, stillbirths, and withdrawals, 3195 pregnancies yielded 3060 livebirths (table 1). Of these, 3018 had valid birthweights (others had birthweights measured outside the 24-h window or the study team was unable to obtain any birthweight measurement; appendix p 7). 16 additional births were excluded because of a gestational age of more than 300 days; weight-for-gestational-age Z scores are unavailable in the INTERGROWTH database beyond 300 days of gestation. 3002 mother–child pairs were thus eligible for inclusion in exposure–response analyses. These were further restricted by the availability of exposure data for each of the three pollutants of interest. 18 participants were missing BMI data and 26 were missing haemoglobin data but were included in the analyses as part of the category for missing BMI or haemoglobin data.
Table 1.
Summary of the number of observations used in the exposure–response analysis
| Number of pregnant women enrolled | Number with valid birthweights* | Number with valid PM2·5exposure measures | Number with valid black carbon exposure measures | Number with valid carbon monoxide exposure measures | |
|---|---|---|---|---|---|
| Guatemala | 800 | 750 | 703 | 677 | 727 |
| India | 799 | 773 | 710 | 698 | 735 |
| Peru | 798 | 730 | 609 | 567 | 600 |
| Rwanda | 798 | 749 | 695 | 618 | 710 |
| Overall | 3195 | 3002 (94%) | 2717 (91%)† | 2560 (85%)† | 2772 (92%)† |
Data are n or n (%).
Women whose neonates had valid birthweights, excluding those whose birth with gestational age was greater than 300 days (Z scores unavailable from the INTERGROWTH database).
Percentage estimates obtained using 3002 as denominator.
The mean age was 25·36 years (SD 4·46; n=3002), with 1151 (38%) of 3002 participants reporting nulliparity (table 2). The average gestational age at recruitment was 15·3 weeks (SD 3·1). 987 (33%) of 3002 women had secondary or higher levels of education. India had the lowest BMI, haemoglobin, and diet diversity scores, whereas Peru had the highest. India had the highest proportion of smokers in the household. Mobile phone ownership was uniformly high across all countries.
Table 2.
Trial-wide and country-specific maternal characteristics
| Guatemala (n=750) | India (n=773) | Peru (n=730) | Rwanda (n=749) | Overall (n=3002) | ||
|---|---|---|---|---|---|---|
| Age, years | ||||||
| <20 | 115 (15%) | 122 (16%) | 93 (13%) | 46 (6%) | 376 (13%) | |
| 20–24 | 303 (40%) | 373 (48%) | 261 (36%) | 187 (25%) | 1124 (37%) | |
| 25–29 | 221 (29%) | 223 (29%) | 232 (32%) | 280 (37%) | 956 (32%) | |
| 30–35 | 111 (15%) | 55 (7%) | 144 (20%) | 236 (32%) | 546 (18%) | |
| Gestational age at recruitment, weeks | 14·3 (3·0) | 16·0 (3·0) | 15·7 (3·3) | 15·4 (2·8) | 15·3 (3·1) | |
| Nulliparous | ||||||
| Yes | 213 (28%) | 442 (57%) | 278 (38%) | 218 (29%) | 1151 (38%) | |
| No | 537 (72%) | 337 (44%) | 448 (61%) | 529 (71%) | 1851 (62%) | |
| Highest level of education completed | ||||||
| No formal education or some primary school | 358 (48%) | 275 (36%) | 32 (4%) | 316 (42%) | 981 (33%) | |
| Primary school or some secondary school | 298 (40%) | 219 (28%) | 224 (31%) | 299 (40%) | 1040 (35%) | |
| Secondary, vocational, or some university | 100 (13%) | 279 (36%) | 474 (65%) | 134 (18%) | 987 (33%) | |
| Height, cm | 148 (5·3) | 151 (5·6) | 152 (4·5) | 156 (5·8) | 152 (6·2) | |
| BMI, kg/m2 | 23·7 (3·3) | 19·7 (3·1) | 26·0 (3·5) | 23·4 (3·4) | 23·2 (4·1) | |
| Haemoglobin, g/dL | 12·7 (1·04) | 10·3 (1·2) | 14·0 (1·2) | 12·4 (1·5) | 12·4 (1·9) | |
| Minimum dietary diversity score | ||||||
| Low (<4) | 514 (69%) | 600 (78%) | 73 (10%) | 505 (67%) | 1692 (56%) | |
| Medium (4–5) | 206 (27%) | 149 (19%) | 403 (55%) | 208 (28%) | 966 (32%) | |
| High (>5) | 30 (4%) | 24 (3%) | 254 (35%) | 35 (5%) | 343 (11%) | |
| Household food insecurity score | ||||||
| Food secure | 415 (55%) | 628 (81%) | 378 (52%) | 276 (37%) | 1697 (57%) | |
| Mild (1, 2, or 3) | 238 (32%) | 108 (14%) | 251 (34%) | 212 (28%) | 809 (27%) | |
| Moderate (4, 5, or 6) or severe (7 or 8) | 88 (12%) | 33 (4%) | 91 (12%) | 243 (32%) | 455 (15%) | |
| Number of people sleeping in the house | 5·1 (2·6) | 3·7 (1·5) | 4·5 (1·7) | 3·4 (1·4) | 4·3 (2) | |
| Someone in the household smokes | ||||||
| Yes | 39 (5%) | 244 (32%) | 7 (1%) | 28 (4%) | 318 (11%) | |
| No | 711 (95%) | 529 (68%) | 722 (99%) | 719 (96%) | 2681 (89%) | |
| Owns household assets | ||||||
| Colour television | 344 (46%) | 577 (75%) | 470 (64%) | 98 (13%) | 1489 (50%) | |
| Radio | 283 (38%) | 105 (14%) | 540 (74%) | 420 (56%) | 1348 (45%) | |
| Mobile phone | 687 (92%) | 635 (82%) | 699 (96%) | 594 (79%) | 2615 (87%) | |
| Bicycle | 94 (13%) | 120 (16%) | 278 (38%) | 229 (31%) | 721 (24%) | |
| Bank account | 186 (25%) | 695 (90%) | 172 (24%) | 221 (30%) | 1274 (42%) | |
Data are n (%) or mean (SD). Descriptive statistics summary based on 3002 pregnant women included in the final analyses, which includes women with livebirths, valid birthweights, and gestational age at birth less than 300 days.
We obtained 2717 valid 24-h prenatal personal PM2·5 exposure measurements, 2560 black carbon measurements, and 2772 carbon monoxide measurements (appendix p 4). Mean weighted exposures during pregnancy were 92·2 μg/m3 (SD 83·9) for PM2·5, 10·0 μg/m3 (7·4) for black carbon, and 2·0 ppm (2·9) for carbon monoxide. PM2·5 and black carbon exposures were highly correlated (Spearman's p=0·79), but correlations between exposure to PM2·5 and carbon monoxide (p=0·34) as well as black carbon and carbon monoxide (p=0·39) were relatively weak. The intervention resulted in reductions in exposure. Post-intervention mean personal PM2·5 was 24·0 μg/m3 in the intervention group and 70·7 μg/m3 in the control group. Similar reductions of exposure were seen for black carbon (2·8 μg/m3 vs 9·6 μg/m3) and carbon monoxide (0·2 ppm vs 1·1 ppm).
Details on exposure settings and additional sociodemographic characteristics are reported elsewhere.25 Missing exposure data were largely due to equipment failure and were likely to be missing at random.25
The mean birthweight of liveborn neonates was 2909 g (SD 471) with mean gestational age at delivery of 39·3 weeks (1·5); 163 (5·3%) of 3002 births were classified as preterm and 531 (17·7%) as low birthweight (figure 1). Mean birthweight was 2921 g (SD 474·3) in the intervention group and 2898 g (467·9) in the control group, a difference of 23 g (95% CI –10·1 to 49·2).
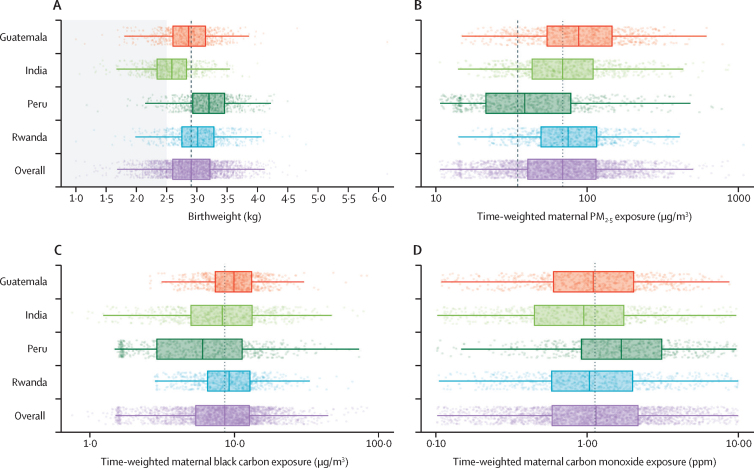
Figure 1.
Distribution of (A) birthweight and time-weighted (B) PM₂·₅, (C) black carbon, and (D) carbon monoxide
The corresponding numeric data are provided in the appendix (p 4). Results are presented separately for each study site and in combination for the entire trial. Dots are individual datapoints. X-axes are log transformed in panels B, C, and D. Thick solid lines inside the boxes are the medians. The lower and upper hinges (ie, the ends of the boxes) correspond to the 25th and 75th percentiles. The whiskers (ie, the lines beyond the boxes) extend from the hinge to 1·5 IQR. The panel-wide dotted vertical lines are study-wide medians. In panel A, the shaded area indicates low birthweight (<2500 g). In panel B, the dashed line is the WHO interim target level one annual guideline value of 35 μg/m3.
In linear models, an interquartile increase in gestational exposure for PM2·5 (74·51 μg/m3) was associated with a change in birthweight of –14·8 g (95% CI –28·7 to –0·8) and for black carbon (7·30 μg/m3) –21·9 g (–37·7 to –6·1; table 3). For weight-for-gestational-age Z scores, the same exposure increases were associated with a decrease of 0·03 (95% CI –0·06 to 0·00) and 0·05 (–0·08 to –0·01) SDs, respectively (table 4). No associations were apparent between carbon monoxide exposures and birthweight in the linear models or between any of the measured pollutants and low birthweight prevalence. Quartile analyses (appendix p 2) showed that the decrease in birthweight and Z scores were not monotonic for PM2·5, whereas decreases were monotonic for Z scores but not birthweight for black carbon.
Table 3.
Change in birthweight for an IQR increase in PM2·5, black carbon, and carbon monoxide
| Model type | Estimate (95% CI) | p value | Akaike's Information Criterion | |
|---|---|---|---|---|
| Adjusted associations | ||||
| PM2·5 | Linear | −14·8 (−28·7 to −0·8) | 0·038 | 40 211 |
| PM2·5 | Log linear | −11·2 (−33·6 to 11·2) | 0·327 | 40 215 |
| Black carbon | Linear | −21·9 (−37·7 to −6·1) | 0·007 | 37 876 |
| Black carbon | Log linear | −19·2 (−40·1 to 1·7) | 0·071 | 37 880 |
| Carbon monoxide | Linear | −3·1 (−12·1 to 5·8) | 0·499 | 41 017 |
| Carbon monoxide | Log linear | 10·6 (−7·2 to 28·4) | 0·245 | 41 017 |
| Crude associations* | ||||
| PM2·5 | Linear | −19·5 (−33·8 to −5·2) | 0·008 | 40 458 |
| PM2·5 | Log linear | −21·6 (−44·4 to 1·3) | 0·064 | 40 462 |
| Black carbon | Linear | −25·5 (−41·7 to −9·4) | 0·002 | 38 070 |
| Black carbon | Log linear | −25·2 (−46·4 to −4·0) | 0·019 | 38 074 |
| Carbon monoxide | Linear | −3·5 (−12·7 to 5·7) | 0·451 | 41 268 |
| Carbon monoxide | Log linear | 8·2 (−10·2 to 26·6) | 0·384 | 41 268 |
Both models were adjusted for mother's education, baseline BMI, nulliparity, diet diversity, food insecurity score, second-hand smoke, baseline haemoglobin, age, neonate sex, and ten randomisation strata. For the linear model, the IQR for PM2·5 was 74·51, for black carbon was 7·30, and for carbon monoxide was 1·68. On the log scale, IQRs for were 1·04 for PM2.5, 0·85 for black carbon, and 1·40 for carbon monoxide.
Estimates for change per IQR for crude models were adjusted only for randomisation strata.
Table 4.
Change in weight-for-gestational age Z scores with an IQR increase in PM2·5, black carbon, and carbon monoxide
| Model | Estimate (95% CI) | p value | Akaike's Information Criterion | |
|---|---|---|---|---|
| Adjusted associations | ||||
| PM2·5 | Linear | −0·03 (−0·06 to 0·00) | 0·380 | 7021 |
| PM2·5 | Log linear | −0·04 (0·09 to 0·01) | 0·100 | 7023 |
| Black carbon | Linear | −0·05 (−0·08 to −0·01) | 0·006 | 6591 |
| Black carbon | Log linear | −0·06 (−0·10 to −0·01) | 0·019 | 6593 |
| Carbon monoxide | Linear | −0·003 (−0·023 to 0·017) | 0·780 | 7215 |
| Carbon monoxide | Log linear | 0·02 (−0·02 to 0·06) | 0·239 | 7214 |
| Crude associations* | ||||
| PM2·5 | Linear | −0·04 (−0·07 to −0·01) | 0·013 | 7161 |
| PM2·5 | Log linear | −0·06 (−0·11 to −0·01) | 0·020 | 7162 |
| Black carbon | Linear | −0·05 (−0·09 to −0·02) | 0·003 | 6708 |
| Black carbon | Log linear | −0·06 (−0·11 to −0·02) | 0·007 | 6710 |
| Carbon monoxide | Linear | −0·003 (−0·024 to 0·017) | 0·739 | 7342 |
| Carbon monoxide | Log linear | 0·02 (−0·02 to 0·06) | 0·323 | 7341 |
All models adjusted for mother's education, baseline BMI, nulliparity, diet diversity, food insecurity score, second-hand smoke, baseline haemoglobin, age, neonate sex, and ten randomisation strata. For the linear model, the IQR for PM2·5 was 74·51, for black carbon was 7·30, and for carbon monoxide was 1·68. On the log scale, IQRs were 1·04 for PM2.5, 0·85 for black carbon, and 1·40 for carbon monoxide.
Estimates for change in IQR from a crude model adjusting only for randomisation strata.
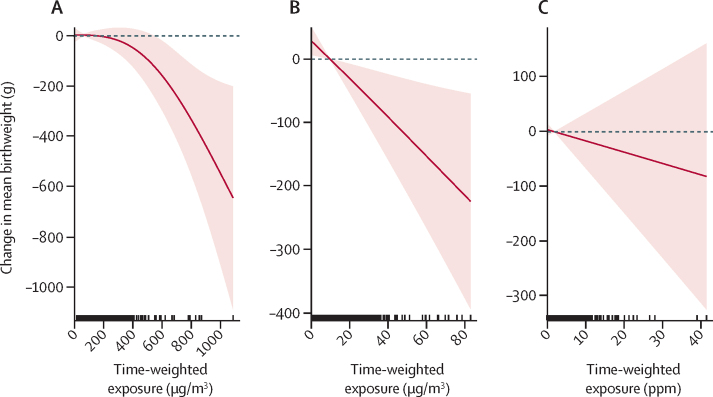
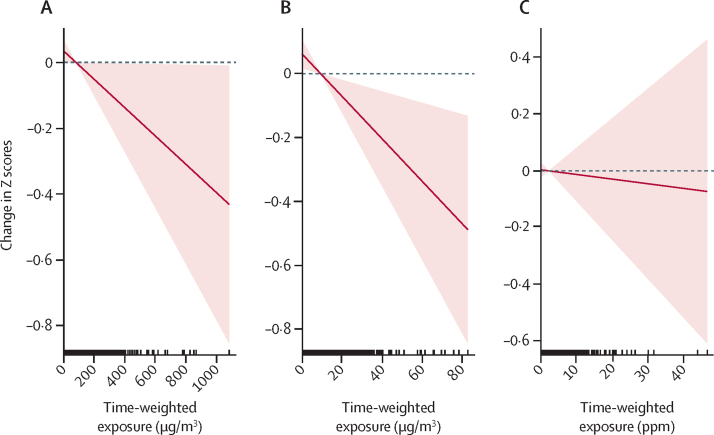
Evaluation of different models indicated that the linear was appropriate to model the relationships between the birthweight outcomes and black carbon. For PM2·5, however, a quadratic (non-linear) fit was better suited to the birthweight outcome (appendix pp 3, 8), with a positive linear coefficient (0·2325) and a negative quadratic coefficient (–0·009), indicating an initial increase in birthweight with higher PM₂·₅ followed by a subsequent decrease at the higher exposures. Both categorical and cubic spline models supported this relationship (appendix p 8). Linear models fit best for black carbon for both birthweight and Z scores, as well as PM2·5 and Z scores. Smoothed exposure–response curves for PM2·5 and black carbon and birthweight and weight-for-gestational-age Z scores can be seen in figure 2 and figure 3.
Figure 2.
Exposure–response relationships between birthweight and prenatal (A) PM2·5, (B) black carbon, and (C) carbon monoxide personal exposures
Dashed lines correspond to the 95% CI and vertical dashes along the x-axis are observed measurements.
Figure 3.
Exposure–response relationships between weight-for-gestational age Z scores and prenatal (A) PM2·5, (B) black carbon, and (C) carbon monoxide personal exposures
Dashed lines correspond to the 95% CI and vertical dashes along the x-axis are observed measurements.
Trends for full-term births (2839 of 3002, 94% of births) were similar to trends for all births (appendix p 3). No statistically significant interactions were observed with neonate sex, but female births showed a larger effect than male births for birthweight, and for Z scores (appendix p 3). Trends were reasonably consistent across countries for the association between PM2·5 and black carbon with both birthweight and Z scores (appendix p 5). We also ran separate models for our three exposure measurements during gestation—ie, for baseline, midpoint, and end of gestation measurements (these corresponding roughly to early second trimester, end of second trimester, and end of third trimester). These models, for both birthweight and Z score, showed no pattern whereby early or later exposures had stronger effects on the outcome (appendix p 6). Indeed all time-specific exposure–response coefficients were weaker than those coefficients using average exposure. This outcome might occur because single measurements involve more measurement error than average exposure across gestation, biasing results to the null.
Discussion
The findings of this study suggest that reducing prenatal HAP exposure could yield modest potential benefits for birthweight that are not consistent across all pollutants. To our knowledge, ours is the first study reporting on exposure–response relationships between gestational black carbon exposures from HAP and birthweight. Notably, a 7·3 μg/m3 reduction in prenatal black carbon exposure was associated with an increase in birthweight of about 22 g, which could have positive implications for populations with a high prevalence of low birthweight. The results from the recently reported intention-to-treat analyses in the HAPIN trial19 were negative but showed a 20 g higher birthweight for the intervention group than the control group (albeit not statistically significant). The secondary exposure–response analysis from the trial reported here is consistent with these results, showing a decrease in birthweight for those with higher exposure.
Only three previous studies have published quantitative exposure–response results for birth outcomes in relation to HAP exposure, focusing on PM2·5 or carbon monoxide. In a cohort of 239 pregnant women in Tanzania, there was a negative association between carbon monoxide exposure and newborn birthweight, but results were not statistically significant.32 The Tanzania study also reported a 150 g (95% CI –300 to 0) reduction in birthweight per 23·0 μg/m3 increase in PM2·5. The second study, among 1285 women in the Tamil Nadu region of India, reported a 4 g (95% CI 1·08–6·76) decrease in birthweight and a 2% increase in the prevalence of low birthweight (0·05–4·1) for each 10 μg/m3 increase in kitchen area PM2·5 measured during pregnancy.10 The third study, conducted as part of the GRAPHS trial in Ghana,13 observed the effects of carbon monoxide on birthweight, birth length, and gestational age that were modified by placental malarial status. Among infants from pregnancies without evidence of placental malaria, each 1 ppm increase in carbon monoxide was associated with reduced birthweight (−53·4 g, 95% CI −84·8 to −21·9), birth length (−0·3 cm, −0·6 to −0·1), gestational age (−1·0 days, −1·8 to −0·2), and weight-for-gestational-age Z score (−0·08 SD, −0·16 to −0·01). These associations were not observed in pregnancies with evidence of placental malaria. PM2·5 measurements were, however, scarce in the GRAPHS trial and no association between PM2·5 exposure and birthweight was observed.
The negative associations between PM2·5 exposures and birthweight in our study are consistent with previous studies, but at the lower end of reported estimates. In contrast, the lack of an association between prenatal carbon monoxide exposure and birthweight was unexpected. However, this finding is not entirely surprising as the correlations between PM2·5 and carbon monoxide have not been uniform across HAP settings. A systematic review examining this relationship33 found inconsistent correlation with slightly stronger correlation among exclusive biomass users relative to mixed fuel users (R2=0·29 vs 0·18). The relatively modest correlations between either PM2·5 or black carbon and carbon monoxide observed in our study could be attributable to our study conditions of exclusive biomass and liquefied petroleum gas use.
Although the kind of effect on birthweight seen by decreasing pollutant exposure (eg, an increase of 20 g with a reduction of pollutant equal to the IQR) might not be clinically significant for an individual, at a population level shifting the distribution of birthweight by a small amount can have important benefits for population health.34 We estimate that a shift from average HAP levels in LMICs to the WHO interim target level one of 35 μg/m3 of PM2·5 could decrease infant mortality by about 170 000 deaths per year (appendix p 9).
Trials of cookstove interventions to improve birth outcomes have had mixed outcomes: an improved biomass cookstove in a cohort of 174 infants in Guatemala was associated with 89 g (95% CI –27 to 204) higher birthweight in adjusted analysis,14 and a clean-burning ethanol stove intervention in Nigeria was associated with 128 g (20 to 236) higher birthweight among 258 infants in adjusted analysis.12 Meanwhile, neither an improved biomass nor a liquefied petroleum gas stove improved birth outcomes in two linked trials covering almost 3000 individuals in southern Nepal.15 These trials have not reported quantitative exposure–response relations. In the GRAPHS trial,13 although there was a significant exposure–response relationship between carbon monoxide exposures and birthweight, neither prenatally introduced liquefied petroleum gas nor improved biomass cookstoves improved birthweight. The investigators in all previous trials hypothesised that these findings are perhaps due to lower-than-expected exposure reductions in the intervention groups.
The HAP exposure levels associated with biomass use (such as at baseline and in the control group) in our study are at the lower end of what has been reported in previous trials, with the possible exception of the GRAPHS trial. On the basis of pilot phase exposure reductions23, 24 and estimated supra-linear exposure–response relationships for HAP and birthweight,35 we hypothesised that the levels observed during pilot work implied that exposure reductions would occur on the steep part of the response curve for birthweight. Given the relative paucity of studies on quantitative exposure–response analyses for HAP based on personal exposures, the shape of the exposure–response curve could possibly be different than what was previously estimated. Our study contributes important information regarding this relationship based on high-quality personal HAP exposure and birthweight measurements from four diverse settings that can inform future development of pooled exposure–response coefficients spanning the range of experienced HAP exposures and could inform future exposure–response curves that integrate across air pollution sources.
Following our original statistical analysis plan for the exposure–response analyses, we did not adjust p values for multiple comparisons. We note, however, that if we had used a Benjamini-Hochberg false discovery rate adjustment for six comparisons (eg, two outcomes and three pollutants), we would have used a p value of 0·03 instead of 0·05 as a significance level, and the inverse associations between black carbon and both birthweight and Z scores would have remained significant.36
There were several limitations to our study. We note that other unmeasured factors including placental malaria, water and sanitation, and nutritional deficiencies could have outweighed the effects of HAP on birthweight outcomes. We measured personal exposure only three times during pregnancy. Although this number is more than most studies, such measurements undoubtebly involve some error. However, as this error is most likely to be non-differential (ie, not differing by birthweight or Z score), it is likely to have biased our exposure–response findings to the null.
In this study population drawn from diverse sociodemographic settings across four countries, exposure to HAP—particularly to black carbon and to a lesser extent to PM2·5—during pregnancy was associated with reduced birthweight and weight-for-gestational-age Z scores. To our knowledge, ours is the first study reporting on exposure–response relationships between gestational black carbon exposures from HAP and birthweight. The association, although modest, provides strong support for continuing efforts to address HAP exposures alongside other drivers of impaired fetal growth in LMICs.
Data sharing
De-identified individual participant data, as well as the study protocol, statistical analysis plan, informed consent form, and analytics code will be available to anyone who wishes to access the data for any purpose, from 6 months post publication, via DataVerse.
For more on INTERGROWTH see https://intergrowth21.tghn.org
For more on DataVerse see https://dataverse.unc.edu/dataverse/Emory
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The HAPIN trial was funded by the US National Institutes of Health (cooperative agreement 1UM1HL134590) in collaboration with the Bill & Melinda Gates Foundation (OPP1131279). We thank the members of the advisory committee—Patrick Brysse, Donna Spiegelman, and Joel Kaufman—for their valuable insight and guidance throughout the trial. We also thank all research staff and study participants for their dedication to and participation in this important trial. A multidisciplinary, independent data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute (NHLBI) monitors the quality of the data and protects the safety of patients enrolled in the HAPIN trial. The board included Nancy R Cook, Stephen Hecht, Catherine Karr (Chair), Joseph Millum, Nalini Sathiakumar, Paul K Whelton, Gail Weinmann, and Thomas Croxton (Executive Secretaries). Programme coordination was by: Gail Rodgers, Bill & Melinda Gates Foundation; Claudia L Thompson, National Institute of Environmental Health Science; Mark J Parascandola, National Cancer Institute; Marion Koso-Thomas, Eunice Kennedy Shriver, National Institute of Child Health and Human Development; Joshua P Rosenthal, Fogarty International Center; Conception R Nierras, National Institutes of Health Office of Strategic Coordination Common Fund; and Katherine Kavounis, Dong-Yun Kim, Antonello Punturieri, and Barry S Schmetter, NHLBI. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the US National Institutes of Health or Department of Health and Human Services.
HAPIN Investigators
Vigneswari Aravindalochanan, Kalpana Balakrishnan, Gloriose Bankundiye, Dana Boyd Barr, Vanessa Burrowes, Alejandra Bussalleu, Devan Campbell, Eduardo Canuz, Adly Castañaza, Howard H Chang, William Checkley, Yunyun Chen, Marilú Chiang, Maggie L Clark, Thomas F Clasen, Rachel Craik, Mary Crocker, Victor G Davila-Roman, Lisa de las Fuentes, Oscar De León, Anaité Diaz-Artiga, Ephrem Dusabimana, Lisa Elon, Juan Gabriel Espinoza, Irma Sayury Pineda Fuentes, Sarada Garg, Ahana Ghosh, Dina Goodman, Savannah Gupton, Sarah Hamid, Stella Hartinger, Steven A Harvey, Mayari Hengstermann, Ian Hennessee, Phabiola Herrera, Shakir Hossen, Marjorie Howard, Penelope P Howards, Lindsay Jaacks, Shirin Jabbarzadeh, Michael A Johnson, Katherine Kearns, Miles A Kirby, Jacob Kremer, Margaret A Laws, Pattie Lenzen, Jiawen Liao, Amy E Lovvorn, Jane Mbabazi, Eric McCollum, John P McCracken, Julia N McPeek, Rachel Meyers, J Jaime Miranda, Erick Mollinedo, Libny Monroy, Lawrence Moulton, Alexie Mukeshimana, Krishnendu Mukhopadhyay, Bernard Mutariyani, Luke Naeher, Abidan Nambajimana, Durairaj Natesan, Florien Ndagijimana, Laura Nicolaou, Azhar Nizam, Jean de Dieu Ntivuguruzwa, Aris Papageorghiou, Jennifer Peel, Ricardo Piedrahita, Ajay Pillarisetti, Naveen Puttaswamy, Elisa Puzzolo, Ashlinn Quinn, Karthikeyan Dharmapuri Rajamani, Sarah Rajkumar, Usha Ramakrishnan, Rengaraj Ramasami, Alexander Ramirez, Ghislaine Rosa, Joshua Rosenthal, P Barry Ryan, Sudhakar Saidam, Zoe Sakas, Sankar Sambandam, Jeremy A Sarnat, Suzanne Simkovich, Sheela S Sinharoy, Kirk R Smith, Kyle Steenland, Damien Swearing, Gurusamy Thangavel, Lisa M Thompson, Ashley Toenjes, Lindsay Underhill, Jean Damascene Uwizeyimana, Viviane Valdes, Amit Verma, Lance A Waller, Jiantong Wang, Megan Warnock, Kendra N Williams, Wenlu Ye, Bonnie N Young, and Ashley Younger.
Contributors
KB contributed to the conceptualisation, methodology, formal analysis, and writing of the original draft. KS contributed to the methodology, formal analysis, and writing of the original draft. TC, WC, and JLP contributed to the investigation and review and editing of subsequent drafts. HC contributed to the methodology, formal analysis, visualisation of data, and validation of data analysis. MJ, LPN, AD-A, MAK, GT, SS, KM, NP, VA, SG, FN, SH, LJU, KAK, DC, and JK contributed to the resources and data curation. AP and WY contributed to the visualisation of data, investigation, and review and editing of subsequent drafts. JPM, LMT, and GR contributed to the resources and review and editing of subsequent drafts. LW, SJ, JW, and YC contributed to the data curation. JR, AQ, and PPH contributed to the review and editing of subsequent drafts. ATP and UR contributed to the resources. KB, KS, and HC accessed and verified the data used in the study and all authors had access to the underlying data.
Supplementary Material
References
- 1.Bennitt FB, Wozniak SS, Causey K, Burkart K, Brauer M. Estimating disease burden attributable to household air pollution: new methods within the Global Burden of Disease Study. Lancet Glob Health. 2021;9:S18. [Google Scholar]
- 2.Smith KR, Bruce N, Balakrishnan K, et al. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 3.Amegah AK, Quansah R, Jaakkola JJK. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: a systematic review and meta-analysis of the empirical evidence. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh R, Causey K, Burkart K, Wozniak S, Cohen A, Brauer M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: a meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope DP, Mishra V, Thompson L, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010;32:70–81. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- 6.Younger A, Alkon A, Harknett K, Jean Louis R, Thompson LM. Adverse birth outcomes associated with household air pollution from unclean cooking fuels in low- and middle-income countries: a systematic review. Environ Res. 2022;204 doi: 10.1016/j.envres.2021.112274. [DOI] [PubMed] [Google Scholar]
- 7.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7:e849–e860. doi: 10.1016/S2214-109X(18)30565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Accrombessi M, Zeitlin J, Massougbodji A, Cot M, Briand V. What do we know about risk factors for fetal growth restriction in Africa at the time of Sustainable Development Goals? A scoping review. Paediatr Perinat Epidemiol. 2018;32:184–196. doi: 10.1111/ppe.12433. [DOI] [PubMed] [Google Scholar]
- 9.Stoner O, Lewis J, Martínez IL, Gumy S, Economou T, Adair-Rohani H. Household cooking fuel estimates at global and country level for 1990 to 2030. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balakrishnan K, Ghosh S, Thangavel G, et al. Exposures to fine particulate matter (PM2.5) and birthweight in a rural-urban, mother-child cohort in Tamil Nadu, India. Environ Res. 2018;161:524–531. doi: 10.1016/j.envres.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 11.Wylie BJ, Kishashu Y, Matechi E, et al. Maternal exposure to carbon monoxide and fine particulate matter during pregnancy in an urban Tanzanian cohort. Indoor Air. 2017;27:136–146. doi: 10.1111/ina.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander DA, Northcross A, Karrison T, et al. Pregnancy outcomes and ethanol cook stove intervention: a randomized-controlled trial in Ibadan, Nigeria. Environ Int. 2018;111:152–163. doi: 10.1016/j.envint.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Quinn AK, Adjei IA, Ae-Ngibise KA, et al. Prenatal household air pollutant exposure is associated with reduced size and gestational age at birth among a cohort of Ghanaian infants. Environ Int. 2021;155 doi: 10.1016/j.envint.2021.106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect. 2011;119:1489–1494. doi: 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz J, Tielsch JM, Khatry SK, et al. Impact of improved biomass and liquid petroleum gas stoves on birth outcomes in rural Nepal: results of 2 randomized trials. Glob Health Sci Pract. 2020;8:372–382. doi: 10.9745/GHSP-D-20-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack DW, Ae-Ngibise KA, Gould CF, et al. A cluster randomised trial of cookstove interventions to improve infant health in Ghana. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson M, Pillarisetti A, Piedrahita R, et al. Exposure contrasts of pregnant women during the Household Air Pollution Intervention Network randomized controlled trial. Environ Health Perspect. 2022;130 doi: 10.1289/EHP10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn AK, Williams KN, Thompson LM, et al. Fidelity and adherence to a liquefied petroleum gas stove and fuel intervention during gestation: the multi-country Household Air Pollution Intervention Network (HAPIN) randomized controlled trial. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph182312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clasen TF, Chang HH, Thompson LM, et al. Liquefied petroleum gas or biomass for cooking and effects on birth weight. N Engl J Med. 2022;387:1735–1746. doi: 10.1056/NEJMoa2206734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr DB, Puttaswamy N, Jaacks LM, et al. Design and rationale of the biomarker center of the Household Air Pollution Intervention Network (HAPIN) trial. Environ Health Perspect. 2020;128 doi: 10.1289/EHP5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clasen T, Checkley W, Peel JL, et al. Design and rationale of the HAPIN study: a multicountry randomized controlled trial to assess the effect of liquefied petroleum gas stove and continuous fuel distribution. Environ Health Perspect. 2020;128 doi: 10.1289/EHP6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MA, Steenland K, Piedrahita R, et al. Air pollutant exposure and stove use assessment methods for the Household Air Pollution Intervention Network (HAPIN) trial. Environ Health Perspect. 2020;128 doi: 10.1289/EHP6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao J, Kirby MA, Pillarisetti A, et al. LPG stove and fuel intervention among pregnant women reduce fine particle air pollution exposures in three countries: pilot results from the HAPIN trial. Environ Pollut. 2021;291 doi: 10.1016/j.envpol.2021.118198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambandam S, Mukhopadhyay K, Sendhil S, et al. Exposure contrasts associated with a liquefied petroleum gas (LPG) intervention at potential field sites for the multi-country household air pollution intervention network (HAPIN) trial in India: results from pilot phase activities in rural Tamil Nadu. BMC Public Health. 2020;20 doi: 10.1186/s12889-020-09865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson M, Pillarisetti A, Piedrahita R, et al. Exposure contrasts of pregnant women during the Household Air Pollution Intervention Network randomized controlled trial. Environ Health Perspect. 2022;130 doi: 10.1289/EHP10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrowes VJ, Piedrahita R, Pillarisetti A, et al. Comparison of next-generation portable pollution monitors to measure exposure to PM2.5 from household air pollution in Puno, Peru. Indoor Air. 2020;30:445–458. doi: 10.1111/ina.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chillrud SN, Ae-Ngibise KA, Gould CF, et al. The effect of clean cooking interventions on mother and child personal exposure to air pollution: results from the Ghana Randomized Air Pollution and Health Study (GRAPHS) J Expo Sci Environ Epidemiol. 2021;31:683–698. doi: 10.1038/s41370-021-00309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox RR. Academic Press; Cambridge, MA: 2011. Introduction to robust estimation and hypothesis testing. [Google Scholar]
- 29.Tielsch JM, Katz J, Thulasiraj RD, et al. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. Int J Epidemiol. 2009;38:1351–1363. doi: 10.1093/ije/dyp286. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 32.Wylie BJ, Matechi E, Kishashu Y, et al. Placental pathology associated with household air pollution in a cohort of pregnant women from Dar es Salaam, Tanzania. Environ Health Perspect. 2017;125:134–140. doi: 10.1289/EHP256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter E, Norris C, Dionisio KL, et al. Assessing exposure to household air pollution: a systematic review and pooled analysis of carbon monoxide as a surrogate measure of particulate patter. Environ Health Perspect. 2017;125 doi: 10.1289/EHP767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 35.Steenland K, Pillarisetti A, Kirby M, et al. Modeling the potential health benefits of lower household air pollution after a hypothetical liquified petroleum gas (LPG) cookstove intervention. Environ Int. 2018;111:71–79. doi: 10.1016/j.envint.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data, as well as the study protocol, statistical analysis plan, informed consent form, and analytics code will be available to anyone who wishes to access the data for any purpose, from 6 months post publication, via DataVerse.
For more on INTERGROWTH see https://intergrowth21.tghn.org
For more on DataVerse see https://dataverse.unc.edu/dataverse/Emory