Introduction
The opportunity to provide continuous care to patients between office visits using digital technologies holds tremendous potential to improve health care quality and patient outcomes. In 2019, the Center for Medicare and Medicaid Services (CMS) launched the remote physiologic monitoring (RPM) program that provided reimbursement for using technology to monitor patients between visits [1]. RPM delivers continuous or periodic digital data to a central location. These data typically are reviewed by clinical staff (eg, nurses, medical assistants) whose time is billed “incident to” the supervising physician. RPM offers an intuitive complement to remote care delivered via telehealth. In part related to the COVID-19 public health emergency, RPM subsequently was expanded by CMS to improve coverage and reduce barriers to access.
The RPM program requires that a biosensor be used to monitor patients between visits, often but not always in conjunction with a smartphone app. For many health conditions, a biosensor device is a logical component to chronic disease management. Examples include continuous glucose monitoring (diabetes), daily weights via a smart scale (heart failure), dysrhythmia detection (cardiac conditions), and ambulatory blood pressure monitoring (hypertension). Early evidence supporting RPM use appears favorable [2]. Outside of isolated published examples that have largely been confined to a single chronic illness, the extent to which RPM has been deployed on a national scale is unknown. Using US Medicare data, we examined the uptake of RPM in the United States from 2019 (its inception year) to 2021.
Methods
We examined publicly available Medicare Part B National Summary Data File data from January 2019 to December 2021 [3]. We extracted Medicare payment amounts and the associated services allowed based on relevant Current Procedural Terminology (CPT) codes; individual patient information is not available in this data source. RPM services were grouped as setup (CPT 99453), data transmission (CPT 99454), and monitoring time, which is billed in 20-minute increments (CPT 99457,99458). Results were stratified by calendar year and analyzed in R version 4.2.3 (R Foundation for Statistical Computing).
Results
In 2019, the total amount paid by CMS for RPM was US $5.5 million. In 2020, RPM payments increased almost 9-fold to US $41.5 million, followed by a further 2.5-fold increase in 2021, totaling more than US $101 million annually (Figure 1).
Figure 1.
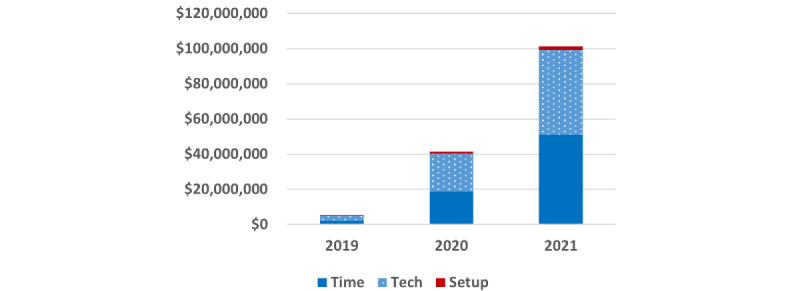
Medicare payments for remote patient monitoring services, 2019-2021.
Assuming providers initiate the program and bill setup fees only once per patient, the number of new patients increased from 20,640 (2019) to 90,149 (2020) and further increased to 123,476 (2021). The total payments made by CMS for the technical service (data transmission) were comparable to the payment for the time spent monitoring. Most (69%) monthly reimbursement for patient monitoring was for 20 minutes; only 31% was for monitoring beyond 20 minutes.
Discussion
Based on national data from the Medicare program, RPM grew approximately 19-fold over 3 years, suggesting rapid uptake. However, some have raised concerns about the potential for overuse of RPM without clinical benefit [3]. Moreover, the use of RPM appears to be confined to a small group of physicians, predominantly primary care providers focused on hypertension or diabetes management [4]. In addition to Medicare, both commercial insurance programs and many states’ Medicaid programs also cover RPM services [4,5]. Importantly, in 2022 CMS further expanded remote monitoring for certain medical specialties (musculoskeletal [rheumatology, orthopedics], respiratory medicine). Under this new program called remote therapeutic monitoring (RTM), a software app alone can be used for monitoring, and patients provide data through the app without a biosensor [6]. The software itself is the medical device and would be registered and cleared by the US Food and Drug Administration as a class 1 (or higher) device.
Thus beginning in 2022, RTM widens the spectrum of health domains available for monitoring, since any patient-reported outcome (eg, disease activity) or clinically relevant information (eg, medication adherence) now is reimbursable. We eagerly await the evaluation of the impact on patient outcomes offered by RPM and RTM, recognizing that much work to optimize their use remains.
Acknowledgments
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P30AR072583). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- CMS
Center for Medicare and Medicaid Services
- CPT
Current Procedural Terminology
- RPM
remote physiologic monitoring
- RTM
remote therapeutic monitoring
Data Availability
Data can be shared and are publicly available (see manuscript for source).
Footnotes
Authors' Contributions: JRC contributed toward the concept and design of the study; drafting the manuscript; statistical analysis; administrative, technical, or material support; and supervision. JW made critical revisions to the manuscript for important intellectual content. JRC and JW contributed toward the acquisition, analysis, or interpretation of data and obtained funding for the study.
Conflicts of Interest: JRC receives consulting fees from and owns stock in TNacity Blue Ocean. JRC is a part-time employee of Illumination Health (a health care research organization). JW has no conflicts of interest.
References
- 1.Payment policies for CY 2019. Federal Register. [2022-10-26]. https://www.federalregister.gov/documents/2018/11/23/2018-24170/medicare-program-revisions-to-payment-policies-under-the-physician-fee-schedule-and-other-revisions#h-81 .
- 2.Taylor ML, Thomas EE, Snoswell CL, Smith AC, Caffery LJ. Does remote patient monitoring reduce acute care use? A systematic review. BMJ Open. 2021 Mar 02;11(3):e040232. doi: 10.1136/bmjopen-2020-040232. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=33653740 .bmjopen-2020-040232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mecklai K, Smith N, Stern AD, Kramer DB. Remote patient monitoring - overdue or overused? N Engl J Med. 2021 Apr 15;384(15):1384–1386. doi: 10.1056/NEJMp2033275. [DOI] [PubMed] [Google Scholar]
- 4.Tang M, Mehrotra A, Stern AD. Rapid growth of remote patient monitoring is driven by a small number of primary care providers. Health Aff (Millwood) 2022 Sep;41(9):1248–1254. doi: 10.1377/hlthaff.2021.02026. [DOI] [PubMed] [Google Scholar]
- 5.Remote patient monitoring. Center for Connected Health Policy. [2023-04-03]. https://www.cchpca.org/topic/remote-patient-monitoring/
- 6.Medicare Program; CY 2022 payment policies under the physician fee schedule and other changes to part B payment policies; Medicare Shared Savings program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Federal Register. [2022-10-25]. https://public-inspection.federalregister.gov/2021-23972.pdf .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be shared and are publicly available (see manuscript for source).


