Abstract
Mounting evidence shows the great promise of nanoparticle drug delivery systems (nano-DDSs) to improve delivery efficiency and reduce off-target adverse effects. By tracking drug delivery and distribution, monitoring nanoparticle degradation and drug release, aiding and optimizing treatment planning, and directing the design of more robust nano-DDSs, image guidance has become a vital component of nanomedicine. Recently, chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) has emerged as an attempting imaging method for achieving image-guided drug delivery. One of the unbeatable advantages of CEST MRI is its ability to detect diamagnetic compounds that cannot be detected using conventional MRI methods, making a broad spectrum of bioorganic agents, natural compounds, even nano-carriers directly MRI detectable in a high-spatial-resolution manner. To date, CEST MRI has become a versatile and powerful imaging technology for non-invasive in vivo tracking of nanoparticles and their loaded drugs. In this review, we will provide a concise overview of different forms of recently developed, CEST MRI trackable nano-DDSs, including liposomes, polymeric nanoparticles, self-assembled drug-based nanoparticles, and carbon dots. The potential applications and future perspectives will also be discussed.
Keywords: CEST MRI, nanoparticles, image-guided drug delivery, label-free MRI detection
1. Introduction
Despite the recent tremendous accomplishments in our fundamental understanding of the causes of cancer as well as the pre-clinical and clinical development of new treatments, cancer still remains a leading cause of death worldwide. Currently, treatment of unresectable cancers relies mainly on chemotherapy, but its clinical outcome is not satisfactory, and the quality of life for patients is poor due to severe systemic side effects. As such, extensive research efforts have been dedicated to the development of nanomedicine, especially the nanoparticle-mediated drug delivery systems (nano-DDSs) [1–4]. Preclinical studies have shown that nanoparticle systems can significantly improve the therapeutic index of drugs and reduce systemic toxicity. This is largely attributed to the notable enhanced permeability and retention (EPR) effect [5–8] in solid tumors as a result of their hyper-and defective vascular architecture and impaired lymphatic drainage. Besides for treating cancer, many nano-DDSs are developed for other diseases. To date, there are 27 Food and Drug Administration (FDA) or European Medicines Agency (EMA) approved nanoparticle therapeutics [2–4]. However, many clinical studies also showed that the improvement in the overall survival rate of cancer patients remains modest [9–13]. It is now well accepted that, compared to animal tumor models, the vascular anatomies and functions in solid human tumors are much more heterogeneous, preventing the EPR effect from acting effectively [14, 15]. Thus, it is highly desirable to have clinical image-guidance tools for characterizing the pathophysiological parameters of human tumors, tracking the delivery of drugs (location and quantity) and predicting the therapeutic outcome in patients. To achieve imaging abilities, many nano-DDSs are labeled with contrast agents or imaging probes that can be non-invasively detected by medical imaging modalities, such as optical imaging [16], nuclear imaging [17], computed tomography (CT) [18], or magnetic resonance imaging (MRI) [19]. Many studies have shown that imaging guidance is essential for ensuring nano-DDSs are working as expected in vivo, allowing real-time assessing and adjusting treatment plan in each individual patient, and directing the development and optimization of more robust nano-DDSs [20]. Systems in which therapeutic and diagnostic capabilities are integrated are also called theranostics [21, 22].
Chemical exchange saturation transfer (CEST) is a recently emerging but rapidly developing MRI technology [23–25]. In conventional 1H MRI, water protons are utilized to generate MRI signal. Generally speaking, the observable difference in MR image intensities between different tissues is called contrast, and an agent that can cause MRI signal change and, hence, increase tissue contrast, is called contrast agent. The change in MR image intensities in most scenarios is due to the changes in the magnetic properties of water protons, such as T1 or T2 relaxation times. In CEST MRI detection, the ability to generate MRI contrast is achieved by exchangeable protons, named by their physical properties of consistently exchanging with surrounding water protons. The exchange rates of these protons have to be in the slow-to-intermediate range to make them distinguishable from water protons at a specific frequency off set(Δω) in an NMR time scale. The CEST contrast is produced when these exchange protons are magnetically modulated, such as saturation, an MRI term referring to attenuating MR signal of a proton using RF pulses. Followed by the relocation of these exchangeable protons from CEST agents to their surrounding water molecules, the modulated magnetic signals carried by the exchangeable protons are transferred from CEST agents to water molecules, resulting changes in water MR signal. For soluble small molecular agents, macromolecules, and agents incorporated on the surface of well-dispersed nanoparticles, exchangeable protons are directly exposed to bulky water, saturation transfer follows the pathways of chemical exchange in the form of proton exchange [23, 26, 27] (figure 1(a)), water ligand exchange [27, 28] (figure 1(b)), or their combination (figure 1(c)). For those entrapped inside nanoparticles such as liposomes, saturation transfer takes place in the form of compartmental exchange [29–32] (figure 1(d)) or a combination of compartmental and proton exchange [33, 34] (figure 1(e)).
Figure 1.
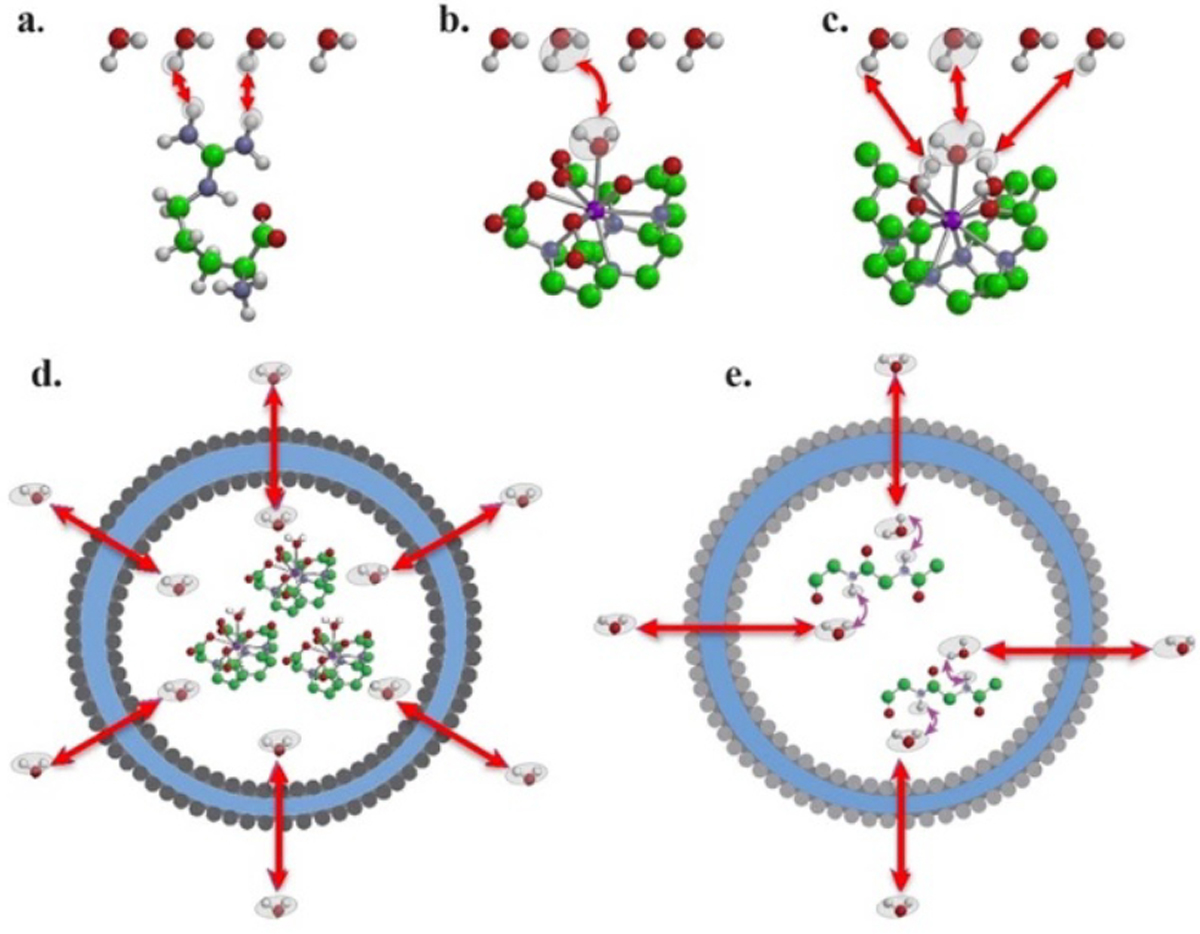
The exchange pathways that can result in chemical exchange saturation transfer (CEST) contrast: (a) proton exchange; (b) molecule exchange; (c) proton + molecule; (d) compartment exchange; and (e) molecule-mediated compartment exchange. Reprinted with permission from [24].
Given exchangeable protons exist in many biological compounds, unlike conventional T1 and T2 MRI contrast agents that are mainly paramagnetic compounds and thus have to be synthesized, CEST agents can be diamagnetic as long as exchangeable protons such as hydroxyl, amine, and amide protons are present (figure 2). As a result, many bioorganic compounds and natural agents can be directly used as CEST contrast agent, providing a practical and easy way to construct image-guided nano-DDSs.
Figure 2.
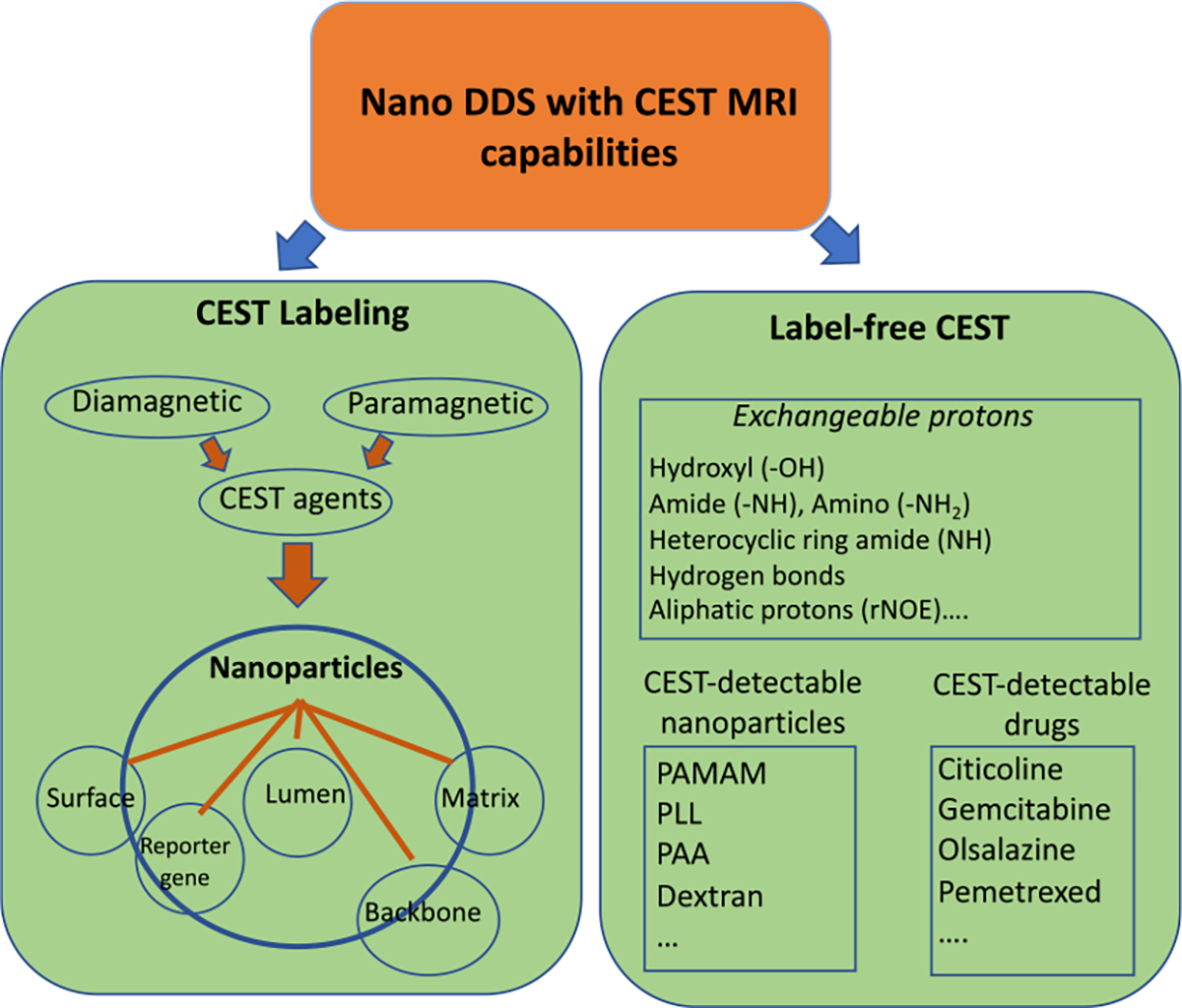
Classification of CEST nano-DDS. Nano-DDSs can be endowed with CEST MRI capability either by a conventional labeling strategy or in a label-free manner. For the labeling strategy, CEST contrast agents, including both diamagnetic and paramagnetic agents, can be incorporated into nanoparticles either physically or chemically. For those drugs (payload) or drug carriers with intrinsic CEST contrast, they may be directly CEST-detectable.
According to the labeling strategy used to achieve CEST signal, CEST-guided nano-DDSs can be classified to labeled and label-free systems. In a labeled CEST nano-DDSs, specific CEST agents are incorporated into nanoparticles, either physically or chemically, to achieve imaging capability. These labeling agents can be diamagnetic agents (diaCEST) or paramagnetic CEST (paraCEST) agents. paraCEST agents are often complexes containing lanthanide paramagnetic metal to obtain highly shifted chemical shifts to enhance detection limits substantially [35, 36]. These CEST agents can be integrated with the nano-DDS using the similar way as for loading drugs, such as intra-particle space (intra-luminal) loading and surface binding.
In contrast, nano-DDSs may be inherently CEST MRI detectable in a label-free manner. To date, a number of drugs, such as gemcitabine [37], citicoline [38], olsalazine (Olsa) [39, 40], etc, as well as drug carriers, such as polyaminoamine (PAMAM) [26], poly-L-lysine (PLL) [26], and dextrans [41], have been demonstrated for constructing nano-DDSs with CEST MRI capability (figure 2). Such a label-free strategy provides unprecedented advantages because requiring no additional chemical labeling can make future translation much easier.
2. Liposomes
Liposomes are phospholipid bilayer spherical particles formed spontaneously through self-assembling in water [42]. Liposomes are one of the most commonly used nanoparticulate drug carriers, and several liposome formulations have been approved for the clinical use [43]. Since the first report of CEST MRI of liposomes in 2005 [44], many studies have been reported to construct CEST detectable liposomes and demonstrate their applications in drug delivery.
Aime and his colleagues first developed the approach to use liposomes as a platform nanoparticle to achieve a high number of exchangeable protons so as to obtain ultra-high sensitivity, namely lipoCEST [44]. In this pioneering study, paramagnetic shift reagent Tm-DOTMA was used to shift the chemical shift of the water protons in the aqueous inner cavities to 3.1 ppm downfield from outside water. The intra-liposomal water can continuously exchange water molecules outside, producing a detectable CEST signal by compartmental exchange (figure 1(d)). As a result, the MRI detectability was strikingly increased with an in vitro detection limit of 90 pM (per liposomes). This approach was further improved by using non-spherical liposomes [45]. By loading liposomes with hypo-osmotic solution, liposomes undergo osmotic shrinkage and deform to a non-spherical shape. As a result, the CEST offsets of lipoCEST signal were extended from several ppm for spherical liposomes to a much large range (up to 30–45 ppm) for non-spherical liposomes [46–49]. A recent study also showed the possibility of using transition metals, such as CoII, to construct lipoCEST agent [50]. Besides liposomes, this approach has also been applied to block copolymer vesicles [51]. Of note, paramagnetic lipoCEST can also be accomplished using non-water shift agents such as TmDOTA-Tetraglycinate. Lanthanide DOTA-tetraglycinate (LnDOTA-(gly)4−) complexes contain four exchangeable amide protons at very large offsets (up to 77 ppm). The liposomes loaded with TmDOTA-Tetraglycinate showed a much higher CEST sensitivity, i.e. by a factor of 104 compared to the un-encapsulated counterparts [52].
A wide array of diamagnetic agents has been explored for labeling liposomes, with most of them following a combined compartmental and proton exchange pathway (figure 1(e)). While most diamagnetic agents have CEST offsets close to water resonance, many are naturally available and, hence, have a much lower translational barrier compared to synthetic compounds. It has been shown that glycogen (1 ppm) [53], L-arginine (2 ppm) [53, 54], or PLL (3.6 ppm) [53], glucose (1.2 ppm) [55], barbituric acid (5 ppm) [56], and iodixanol (4.3 ppm) [57] could be used to construct CEST MRI trackable liposomes. The potential applications include in vivo tracking of the intradermally-administered liposomes in lymph nodes [53], monitoring drug delivery and release [56], and in vivo pH sensing [54]. Taking advantage of the different chemical shifts(MR frequencies) of exchangeable protons, liposomes loaded with different diaCEST [53, 58] agents of distinctive chemical shifts can be identified in a single CEST MRI acquisition. By assigning a pseudo color to each pixel based on the peak frequency offsets of CEST MRI signal, liposomes with different payloads can be simultaneously visualized (figure 3), namely ‘multi-color’ CEST MRI, in a high spatial resolution manner.
Figure 3.
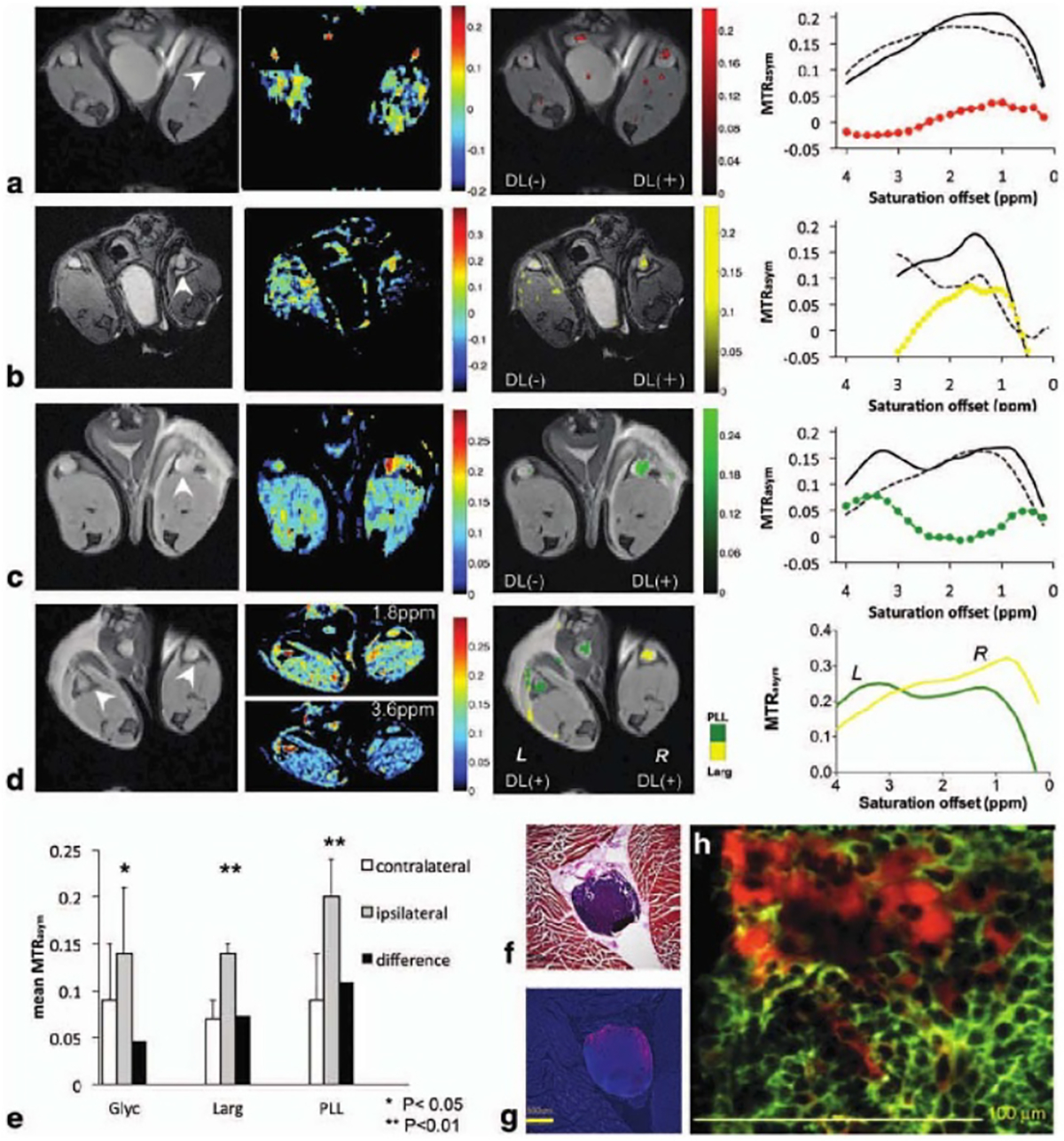
Representative color MR images using different digmagnetic liposomes (DL) combinations: (a) unilateral glyc liposomes, (b) unilateral L-arg liposomes (c) unilateral PLL liposomes, and (d) bilateral L-arg and PLL liposomes. Arrowheads in (a)–(d) indicate the location of PLNs. For (a)–(c), shown are from left to right: T2-weighted anatomical image, MTRasym image at the frequency of interest with CEST contrast displayed using the jet color map in Matlab, MTRasym/T2w image overlay with CEST contrast highlighted using a 64-bit scaled single color (i.e. red, yellow or green) map, and mean MTRasym plot. In the mean MTRasym plot, the values shown are of DL(+) PLN (solid line), DL(−) PLN (dashed line), and their difference (colored line). (d) Representative Two-color CEST image demonstrating simultaneous detection and visualization of PLL (green, left PLN) and L-arg (yellow, right PLN), with the mean MTRasym values of the left (L) and right (R) PLN plotted in green and yellow, respectively. (e) Comparison of CEST contrast in DL(+) nodes (grey columns) and in DL(−) nodes (white columns) for all three animal groups studied (n = 3 mice for glyc and n = 4 mice for L-arg, PLL) with the black columns showing the difference between them. The p values were calculated using a student t test (type II, two tails with SD marked by error bars); (f) H&E stain and (g) fluorescent image of a PLN with rhodamine-labeled liposomes in red and cell nuclei in blue (DAPI stain); (h) merged immunofluorescent image of anti-CD45.1-allophycocyanin (pan-leukocyte marker, green) and rhodamine-labeled liposomes (red) demonstrates extracellular location of DLs without uptake by dendritic cells or macrophages. Reprinted with permission from [53].
When the encapsulated drugs are released in vivo, the co-encapsulated CEST agents are also released, resulting in changes in CEST signal, which in turn can be utilized to monitor drug release. For example, Langereis et al [59] showed that the temperature-sensitive liposomes loaded with [Tm(HPDO3A)(H2O)] and NH4PF6 have a switchable 1H CEST and 19F MRI signal, i.e. below melting temperature (Tm), liposomes had detectable CEST signals at 11 and −17 ppm but no 19F signal due to the spectral broadening effect caused by the high concentration of paramagnetic shift agents inside the liposomes; when temperature reaches Tm, CEST MRI signal is eliminated as the result of releasing paramagnetic shift agents from the intra-liposomal space, whereas 19F MRI is turned on as the result of the release of the paramagnetic agents. This provides a way to detect not only the location of liposomes but also the subsequent drug release.
On the other hand, CEST-trackable liposomes can also be constructed in a label-free manner if the encapsulated drugs are directly CEST MRI detectable. Our group has been exclusively working on the label-free strategy to develop CEST-guided, liposome-based DDSs. For example, we have demonstrated the intrinsic CEST MRI contrast of gemcitabine, a first-line chemo-drug for pancreatic cancer, can be utilized for in vivo tracking of gemcitabine-loaded liposomes in a CT26 tumor murine model [37]. The same strategy was later used to construct label-free, theranostic citicoline-loaded liposomes. Citicoline is a promising neuroprotective drug and can promote the synthesis of brain phospholipids, inhibit phospholipase activation, and alleviate oxidative stress [60]. Mounting evidence demonstrates liposomes can significantly improve the bioavailability of citicoline in the regions undergoing ischemic stroke and consequently enhance its therapeutic efficacy [61, 62]. Exploiting the amine and hydroxy exchangeable protons of citicoline (figure 4(a)), our group has demonstrated that liposomal citicoline (figure 4(b)), could be directly detected using CEST MRI without any synthetic labeling [38]. The improvement in citicoline delivery to the ischemia-damaged regions by means of intra-arterially injected, non-targeted liposomes or intravenously injected, VCAM-1-targeted liposomes were observed by CEST MRI (figure 4(c)).
Figure 4.
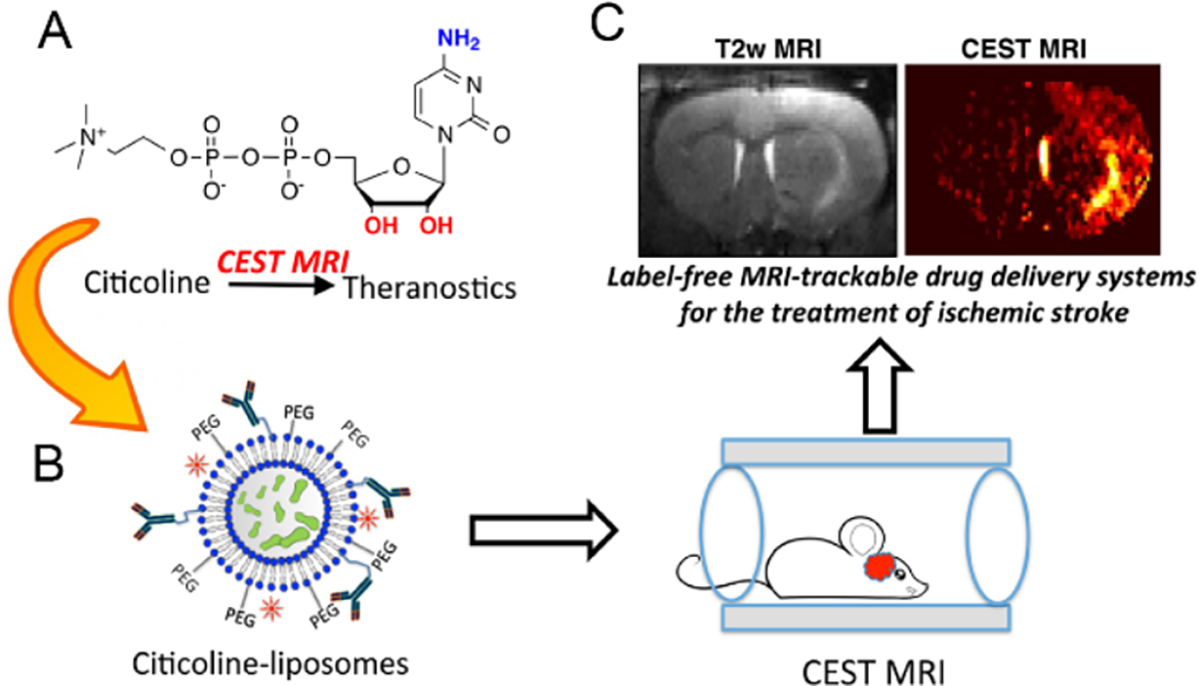
In vivo CEST MRI detection of the delivery of citicoline-liposomes in rat brain after acute ischemic injury. (a) Chemical structure of citicoline, with CEST-generating exchangeable amine and hydroxyl protons are labeled in blue and red, respectively. (b) Citicoline is loaded into liposomes for simultaneous drug delivery and CEST MRI. (c) CEST MRI detection of citicoline in the regions undergoing ischemic stroke is accomplished in a label-free manner. Reprinted with permission from [38].
Several studies revealed that both the size and formulation of liposomes have a substantial impact on lipoCEST signals by affecting water permeation across the liposomal membrane and thus, the exchange rate between intra- and extra-liposomal water. Zhao et al [30] showed that the lipoCEST signal of TmDOTA-liposomes increased almost six-fold due to the markedly increased exchange rate (from 0.059 s−1 to 1.02 s−1, ~17 folds) when the size of liposomes was decreased from 536 nm to 99 nm, attributed to the increase of surface–volume ratio. The properties of phospholipid also play an important role in lipoCEST by affecting water permeability. Interestingly, Chan et al reported that DSPC-formulated liposomes boosted the lipoCEST signal of barbituric acid by 20% than that of DPPC (30% versus 24%) [56]. Contrarily, Demetriou et al recently reported that DPPC-formulated liposomes increased the lipoCEST signal of 2-DG by more than 50% [55]. In the latter study, the cross-membrane water exchange rates were determined to be 66 and 95 Hz for DSPC- and DPPC-liposomes, respectively, at pH 7 and 37 °C, indicating that DSPC is a more rigid lipid and can slow down water exchange. The discrepancy between the observations in the two studies can be explained by the different exchange rates of entrapped CEST agents, i.e. 900 Hz for barbituric acid and ~6000 Hz for glucose. Hence, to maximize the lipoCEST signal, the lipid formulation has to optimize according to the agents to be incorporated.
3. Polymeric nanoparticles
Both natural and synthetic polymers have been used to synthesize a variety of solid colloidal nanoparticles, including hyperbranched dendrimers, nanocapsules, and core–shell structured polymeric micelles [63]. Polymeric nanoparticles have been widely exploited to deliver drugs, proteins, genes, vaccines, polypeptides, nucleic acids, etc [64].
Dendrimers are an important class of nano-DDSs. Dendrimers are a unique class of polymers, with hyperbranched, tree-like structures and well-defined size and shape. To date, a number of dendrimers have been developed, including PLL, PAMAM, phenoxymethyl(methylhydrazone), 2,2-bis-methylolpropionic acid (bis-MPA) and polyprolimine (PPI), and at least five types of dendrimers are commercially available, including Tomalia-type PAMAM dendrimers, Denkewalter-type PLL dendrimers, Hult-type bis-MPA dendrimers, Majoral/Caminade-type phosphorous dendrimers, and Vogtle/Meijer/Multhaupe-type PPI dendrimers [35]. In one of the earliest CEST studies in 2001 [26], Goffeney and colleagues explored the CEST properties of five commonly used gene carriers, including PAMAM-G5 dendrimers. The study showed that PAMAM-G5 dendrimer have the highest CEST signal at 3.5 ppm (neutral pH, 37 °C) among the five cationic polymer-based gene carriers studied (i.e. PLL, PLE, PAA, and PEI). Interestingly, the CEST signal stems from amide protons but not amine protons because the latter has an exchange rate that is too fast to generate a detectable CEST signal at neutral pH. To improve the MRI detectability further, both PARACEST or DIACEST agents have been used to label dendrimers. Pikkemaat et al reported labeling poly(propylene imine) dendrimers with Yb-DOTAM complexes, which exhibited pH-sensitive CEST contrast at Δω=−15 ppm [65]. Ali and Pagel later reported Eu-DOTAGly-conjugated PAMAM dendrimers, which have a much larger frequency offset at −55 ppm, and demonstrated in vivo CEST MRI detection of the accumulation of intravenously injected dendrimers in both breast cancer [36] and glioma [66] models. Similarly, diamagnetic CEST agents can also be used to develop CEST MRI detectable dendrimers. In this context, Lesniak and colleagues synthesized salicylic acid (SA)-conjugated dendrimers [67]. SA has a favorable chemical shift at 9.4 ppm [68], much larger than most commonly used diamagnetic CEST agents. As shown in figure 5, CEST MRI has been successfully used to detect SA-conjugated dendrimers and revealed dendrimers’ accumulation in brain tumors.
Figure 5.
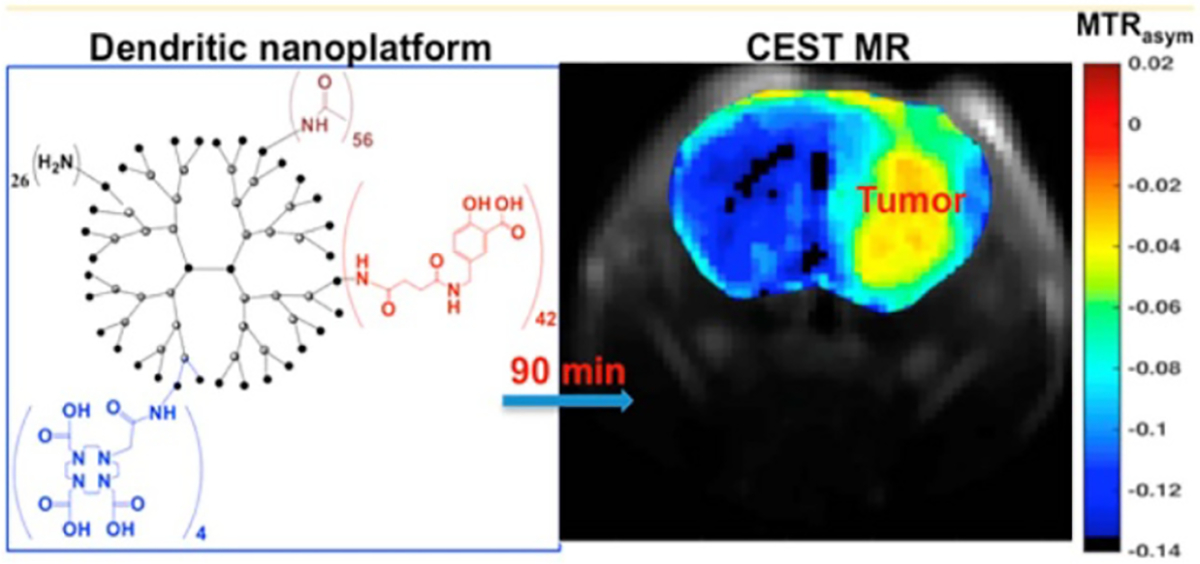
PAMAM-SA conjugate as a high-performance nanoplatform with CEST capabilities. Left panel: chemical structure of PAMAM-SA conjugate (G5-SA-D-Ac). Black: PAMAM-G5, red: SA. The presence of DOTA on PAMAM dendrimers will allow for dual or multi-modality PET/SPECT/MR imaging. Right panel: in vivo CEST images were calculated as an average of MTRasym from 8.7 to 9.9 ppm from water and were acquired pre-intratumoral infusion and 30 and 60 min postintratumoral infusion of 500 μM solutions of G5-SA-D-Ac conjugates into U87 glioblastoma xenografts in SCID mice. MRI data, G5-SA-D-Ac dendrimer conjugates can be detected via CEST contrast within the tumor. The yellow arrow highlights tumor location. Reprinted with permission from [67].
A wide variety of natural polymers, such as proteins, peptides, glycans, and starches, have been utilized as building blocks for constructing nano-DDSs. Many of them possess intrinsic CEST signals. For example, polysaccharides, including dextran and glycogen, have been extensively studied as nano-DDSs for delivering drugs and genes [69]. We have demonstrated that dextran [70–73] and glycogen [74] can be directly visualized in CEST MRI by their abundant OH protons, which has allowed in vivo tracking of their delivery and distribution in the liver and tumors.
Importantly, as the exchange rate and, thus, CEST signal of labile protons can be strongly affected by environmental factors, CEST MRI can be integrated with responsive nano-DDSs for sensing these environmental factors (e.g. enzyme or pH). For example, poly-L-glutamate (PLG), a widely used polymeric drug carrier, has no detectable CEST signal in its polymeric form [75], whereas its monomer glutamate is CEST MRI detectable at 3 ppm. Using this difference in CEST signal, Harris et al demonstrated that PLG could be used as a contrast agent for CEST MRI detection of the overexpressed cathepsin in tumors, a lysosomal protease that can degrade PLG to glutamate. In another study, Zhang et al [76] have developed pH-responsive tertiary-amine-based block copolymer poly(ethylene glycol)-b-poly[2-(diisopropylamino)ethyl methacrylate] micelles. When the polymers form micelles near physiological pH, there is no CEST signal; when the micelles dissociate in an acidic environment, the CEST signal is turned on as a result of exchangeable protons are exposed to water (figure 6).
Figure 6.
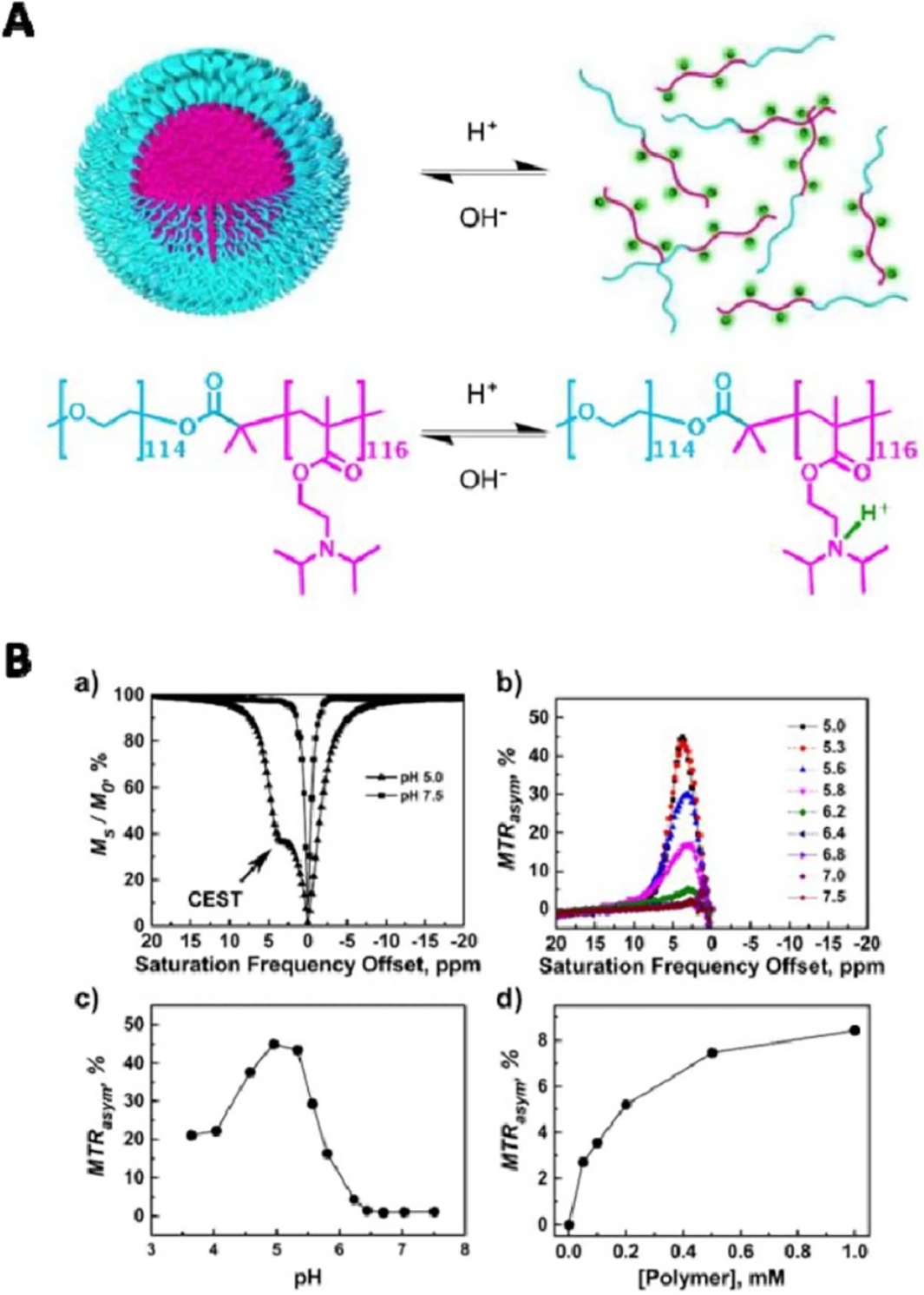
The use of CEST for monitoring the pH-responsive behaviors of PEG114-b-PDPA116 polymers. (A) The micelle/unimer equilibrium in the block copolymer, PEG114-b-PDPA116 is exquisitely sensitive to pH. (B) (a) Representative CEST spectra of PEG-b-PDPA at pH 5.0 and 7.5, respectively; (b) a plot of MTRasym versus the saturation frequency offset at pH range between 5.0 and 7.5; (c) a plot of MTRasym (±3.8 ppm) versus pH and (d) a plot of MTRasym versus PEG-b-PDPA copolymer concentration at a fixed pH of 5.8. All data were acquired on a B0 = 9.4 T NMR spectrometer (400 MHz for 1H). Reprinted with permission from [76].
It should be noted that the signal of a CEST agent may change after being loaded in nano-DDSs. For example, Snoussi et al showed that mixing positivelycharged PLL with negatively charged poly-uridylic acid (poly[rU]) could enhance the CEST signal of PLL, and the degree of enhancement is dependent on poly[rU] concentration [77]. On the other hand, the CEST signal of poly[rU] was attenuated after complexation. Therefore, one has to take this into account when designing CEST-guided nano-DDSs.
4. Self-assembled, drug-based nanoparticles
Self-assembly is the process of association of unit molecules into highly arranged, ordered nanostructures. Recent advancements in nanomedicine make it possible to use drugs as the basis for constructing well-defined supramolecular nanostructures through spontaneous self-assembly. Self-assembled, drugbased nanoparticles represent an emerging class of nano-DDS with the ability to provide a much higher and more reproducible drug loading efficiency than conventional methods [78]. As many drugs can be CEST MRI detectable [37], self-assembled, drug-based nanoparticles are possibly also CEST MRI detectable without the need for additional imaging agents. To date, two studies have reported using self-assembled, drug-based nanoparticles for CEST MRI guided nano-DDSs. In the first study by Lock et al [79], an amphiphilic prodrug was synthesized by conjugating pemetrexed (Pem) with a short peptide containing two glutamic acids and two phenylalanines. Pem is an FDA-approved anticancer drug with an intrinsic CEST MRI signal at 5.2 ppm. The amphiphilic prodrug can spontaneously associate into filamentous assemblies (namely nanofiber hydrogel) under physiological conditions without compromising the CEST signal of Pem. Consequently, the CEST MRI assessment of the delivery, distribution, and release of Pem-based assemblies was demonstrated in a mouse glioma model. In the second study, Yue et al developed a new in vivo self-assembling drug conjugate (Olsa-RVRR) composed of Olsa, an anticancer drug with CEST signal at 9.8 ppm and a cell-penetrating peptide (RVRR) [40]. The peptide sequence was designed to be responsive to furin, an enzyme highly expressed in many cancers. In the cytoplasm of cancer cells, Olsa-RVRR is reduced by glutathione and cleaved by furin, resulting in the cleaved Olsa that would spontaneously self-assemble to nanostructures through intermolecular interactions (figure 7). The self-assembled nanostructures can prevent Olsa from rapid efflux and consequently increase the therapeutic effect significantly. The extended accumulation of nanostructures could be detected by CEST MRI, making the system a tumor-specific, enzyme-responsive theranostic nano-DDS.
Figure 7.
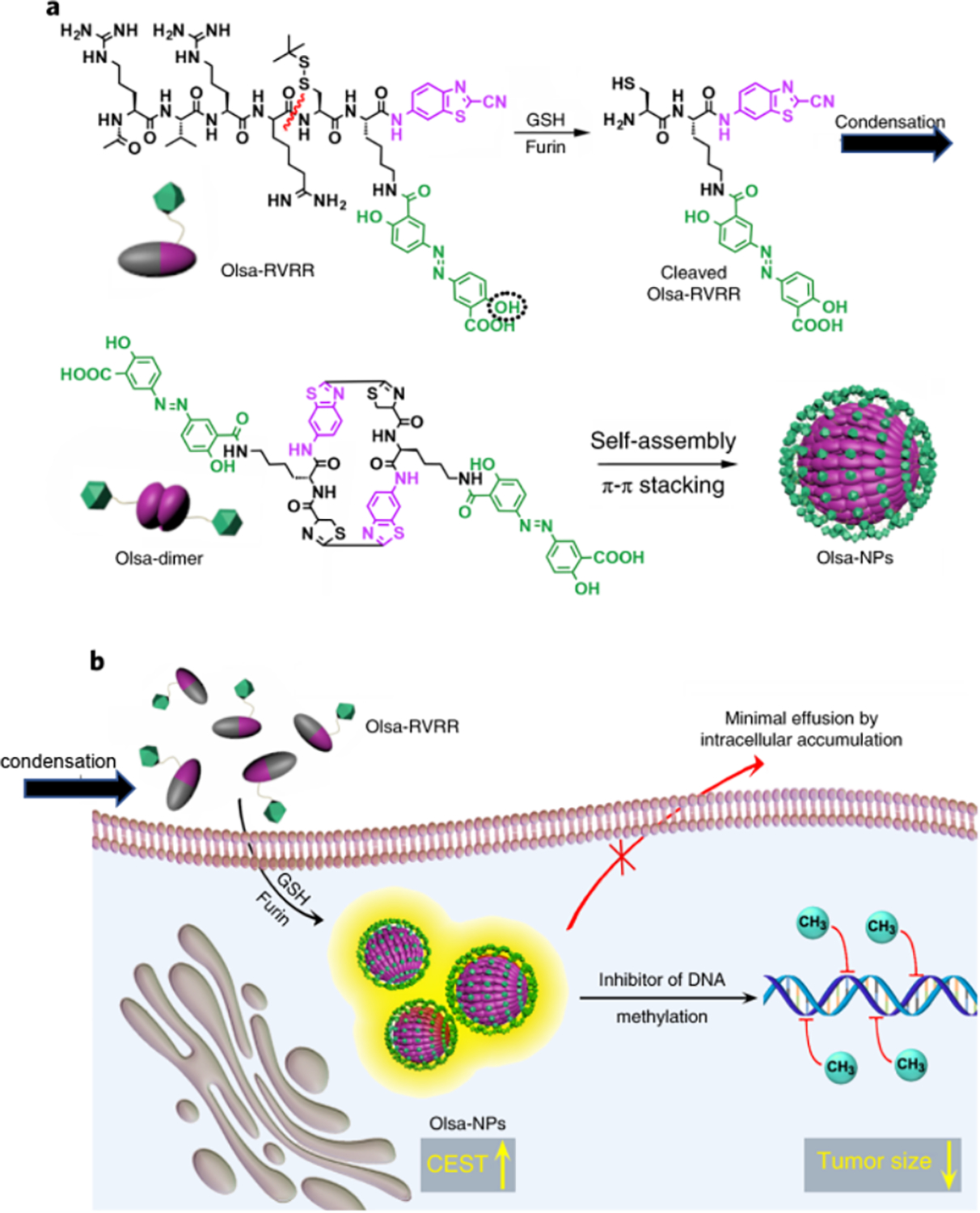
(a) Self-assembly of Olsa-RVRR into Olsa-NPs through a series of steps. Red line indicates the site of furin cleavage, and the circled hydroxyl group indicates the exchangeable hydroxyl proton that provides OlsaCEST signal at 9.8 ppm from the water frequency. (b) After Olsa-RVRR enters the cytoplasm of high furin-expressing cells (HCT116 cells in this study), it undergoes reduction by GSH and cleavage of the peptide by furin near the Golgi complex where cleaved Olsa-RVRR is generated. Amphiphilic oligomers (mostly dimers) are then formed from the click reaction between two cleaved Olsa-RVRR molecules, followed by self-assembly into Olsa-NPs as a result of intermolecular π–π stacking. The intracellular accumulation of Olsa-NPs then serves as a reservoir of Olsa molecule-enhancing CEST contrast and inhibiting DNA methylation for tumor therapy. Reprinted with permission from [40].
5. Carbon-dots
Carbon dots (C-dots) are an emerging class of fluorescent carbogenic nano-materials with an outer shell composed of carboxylic or other chemical functional groups and an inner graphitic core containing covalently bound oxygen and nitrogen atoms [80]. C-dots typically have a quasi-spherical shape and size of several nanometers, with a size-dependent near-infrared fluorescence behavior. As a type of organic, hard nanoparticles, C-dots are considered to be more biocompatible than the more widely used heavy metal-based quantum dots [81]. Thanks to the abundant functional groups on the surface, C-dots can be easily conjugated with drugs and imaging agents to construct image-guided nano-DDS. In a recent proof-of-concept study, C-dots were decorated with arginine to achieve an enhanced CEST signal at 2 ppm in addition to the intrinsic CEST signal at 1 ppm stemming from the surface hydroxyl groups. The synthesized arginine-modified C-dots were applied to label cells, allowing using CEST MRI to track the implanted cells in the mouse brain, with the intrinsic fluorescence signal of C-dots was used to validate the CEST MRI findings (figure 8) [82]. The study implied that C-dots could be easily modified to have noninvasive MR imaging ability. Similarly, other organic nanostructures, such as carbon nanotubes and graphene, may also be modified using the similar methods to obtain CEST MRI capacity, which however has not been explored yet.
Figure 8.
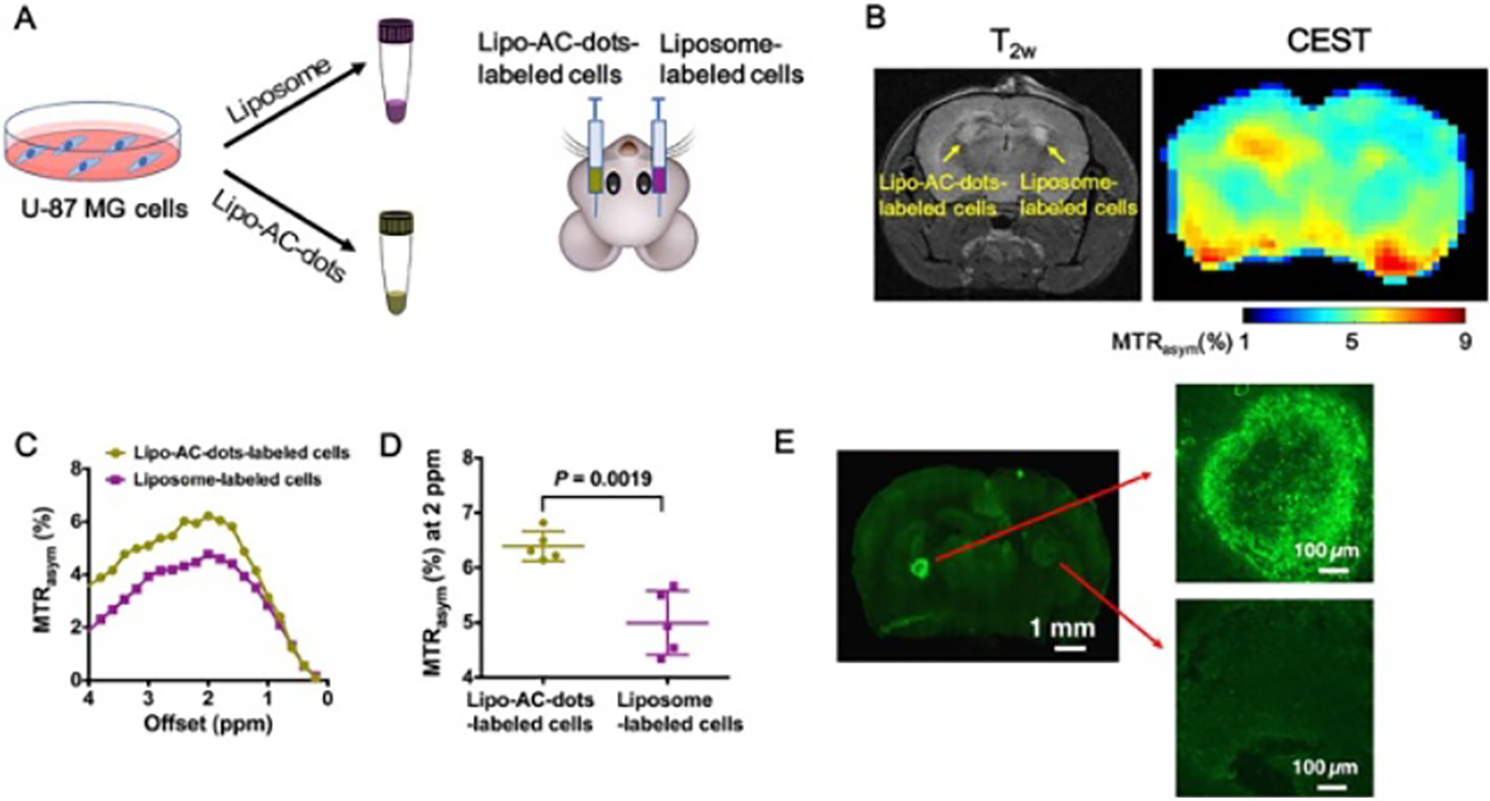
In vivo CEST MRI of mouse brain 1 d after AC-dots-labeled U-87 MG cells implantation in the striatum. (a) Illustration of the preparation and implantation of Lipo-AC-dots-labeled cells, with empty liposome-labeled cells as the control. (b) T2-weighted image and the corresponding CEST image at 2 ppm of a mouse brain at 24 h after the implantation. (c) MTRasym plots of C-dots-labeled cells and control cells using manually drawn ROIs based on the T2w image. (d) Comparison of CEST contrast at 2 ppm between Lipo-AC-dots-labeled cells and liposome-labeled cells. (e) Ex vivo fluorescence imaging of the brain slice. Excitation wavelength = 495 nm; emission wavelength = 519 nm. Reprinted with permission from [82].
Finally, the abovemenioned CEST MRI trackable nano-DDSs are summarized in table 1 for a quick overview of the types, sources of CEST MRI contrast along with examples and offsets, and potential applications of these CEST MRI trackable nano-DDSs.
Table 1.
List of CEST MRI trackable nano-DDSs.
| Types ofDDS | Source(s) of CEST contrast | Examples and offsets | Potential applications |
|---|---|---|---|
|
| |||
| Liposomes | Paramagnetic shifting agents + intra-liposomal water | Tm-DOTMA in liposomes of regular shapes (3.1 ppm) [44] Tm-DOTMA in nonspherical liposomes (up to 30–45 ppm) [46–49]. [Tm(HPDO3A)(H2O)] (11 and −17 ppm) [59] |
Monitoring of drug delivery and temperaturetriggered drug release in tumors [59] |
| Paramagnetic agents | CoII macrocyclic complexes (−13 ppm) [50] Tm-DOTA-(gly)4−) complexes (−51 ppm) [52] |
pH sensing [52] | |
| Diamagnetic agents | Glycogen or glucose (~1 ppm) [53, 55] L-arginine (2 ppm) [53, 54] poly-L-lysine (3.6 ppm) [53] Barbituric acid (5 ppm) [56] Iodixanol (4.3 ppm) [57] |
In vivo tracking of the intradermallyadministered liposomes in lymph nodes [53] Monitoring of drug delivery and release in the tumor [56, 57] pH sensing [54] |
|
| Drugs | Anticancer drug gemcitabine (2.3 ppm) [37]. Neuroprotective drug citicoline (2 ppm) [38]. |
MRI detection of drug distrubution in tumors [37] and stroke [38] | |
| Polymeric nanoparticles | Dendrimers | Amide protons of PAMAM dendrimer (3.5 ppm) [26] Yb-DOTAM (−15 ppm) [65] Eu-DOTAGly (−55 ppm) [36, 66] Salicylic acid (8.7–9.9 ppm) [67] |
In vivo CEST MRI detection of the accumlation of Eu-DOTAGly-conjugated PAMAM dendrimers in breast cancer [36] and glioma [66] |
| Natural polymers and polymeric drug carrier | Dextran [70–73] and glycogen [74] poly-L-glutamate (PLG) (3 ppm after enzyme digestion) [75] Aimes on block copolymer PEG-b-PDPA (3.9 ppm) [76] |
PSMA receptor imaging [70] MRI detection of tumorspecific enzymes [75] Image-guided, pHresponsive drug delivery systems [76] |
|
| Selfassembled, drug-based nanoparticles | Drugs | Pemetrexed (5.2 ppm) [79] Olsalazine (9.8 ppm) [40] |
Monitoring of the location of hydrogel and release of pemetrexed in brain tumors [79] Theanostic systems for targeted imaging and treating furin-expressing tumor cells using self-assembling drug conjugate [40] |
| Carbon-dots | Bioogranic molecules | Intrisic hydroxyl groups (1 ppm) and decorated arginines (2 ppm) on the surface [82] | Cell tracking [82] |
6. Conclusion and future directions
In the last decades, significant progress has been made in the field of nanomedicine. There are 27 FDA or EMA approved nanoparticle-based therapeutics, and many others are being tested in clinical trials. Many new forms of imageable nanomedicines have been synthesized, investigated, and validated for precise diagnosis and treatment monitoring of a variety of diseases. As a cutting-edge MRI technology, CEST MRI has become an attempting technology to construct image-guided nano-DDS. As demonstrated by the aforementioned examples, CEST MRI has been applied to many different types of nanoparticles, including liposomes, polymeric nanoparticles, self-assembled drug-based nanoparticles, and carbon dots. Several studies also demonstrated the possibility to use the drug to be delivered directly or even the nano-carriers as the CEST MRI agents, allowing the construction of label-free theranostics without the need for chemical labeling. Importantly, even small-molecule-based CEST MRI has a relatively low sensitivity (on the order of mM), the nanoparticulate forms of CEST agents often have a substantially improved sensitivity because a high concentration of CEST agents is present. Indeed, some studies implied the sensitivity of nanoparticulate forms of CEST agents (e.g. lipoCEST) is sufficiently high for some very challenging biomedical applications, e.g. receptor imaging. As a quickly developing field, the arsenal of CEST-imageable agents, drugs, and drug carriers are being expanded, making it possible to create more sophisticated CEST-guided nano-DDSs. Hence, CEST MRI provides a unique opportunity to develop theranostic nano-DDSs, paving a new pathway for constructing highly clinically translatable, image-guided nano-DDSs.
However, it should be noted that CEST-guided nano-DDS is still in its infancy. Almost all of the reported studies were carried out in preclinical animal models, with most of them demonstrated in tumor models. In addition, the majority of reported CEST MRI studies were acquired using high field small animal MRI scanners (7T or above). Therefore, the clinical translation of these CEST-guided nano-DDSs will require extensive validation to ensure acceptable accuracy, specificity, repeatability, reproducibility, and quantifiability. The validation methods include invasive pathohistological assays and noninvasive imaging modalities such as fluorescence imaging, bioluminescence imaging, CT, nuclear medicine, or other well-established MRI or MRS methods. Another technical challenge is the accurate quantification of CEST MRI in different in vivo scenarios, where many environmental factors (e.g. pH and temperature) may be altered due to pathological changes. As many of these environmental factors can confound the CEST contrast, caution must be taken to interpret the CEST contrast correctly. Theoretically, simultaneously quantifying CEST agent and detecting pH is possible by the means of advanced MRI acquisition and analysis methods [83]. For example, several recent studies have shown the ability to measure pH by measuring the B1 dependence of a single CEST agent [83, 84]. Such an approach, in turn, can be used to improve the accuracy of CEST quantification in different compartments where pHs are different. On the other hand, the unique pH dependence of the CEST signal can be utilized for sensing the pH. For example, given the pH of the lysosome and endosome is much lower than the extracellular matrix, CEST MRI may be useful to discriminate the internalized nano-DDSs at the target sites, a potential area that has not been explored yet. Finally, a number of technical hurdles have to be solved in order to move CEST MRI from high field small animal scanners to clinical scanners and translate from animals to humans. To speed up the CEST acquisition and thus reduce motion artifacts, advanced fast imaging techniques, such as compressed sensing and parallel imaging, may be used, which have been investigated in several recent studies [85–87]. For the application at 3T clinical scanners, agents with exchangeable protons of large chemical shifts such as SA may be more favorable because the frequency separation between the CEST agent and water is proportional to the field strength. Compared to diamagnetic CEST agents, the clinical translation of paramagnetic agents may be more difficult, even not totally impossible.
Acknowledgments
This work was supported by NIH/NCI R01CA211087 and National Multiple Sclerosis Society (NMSS) PP1908-34973.
References
- [1].Wang AZ, Langer R and Farokhzad OC 2012. Nanoparticle delivery of cancer drugs Annu. Rev. Med. 63 185–98 [DOI] [PubMed] [Google Scholar]
- [2].Jain RK and Stylianopoulos T 2010. Delivering nanomedicine to solid tumors Nat. Rev. Clin. Oncol. 7 653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS and Farokhzad OC 2008. Nanoparticles in medicine: therapeutic applications and developments Clin. Pharmacol. Ther. 83 761–9 [DOI] [PubMed] [Google Scholar]
- [4].Davis ME, Chen ZG and Shin DM 2008. Nanoparticle therapeutics: an emerging treatment modality for cancer Nat. Rev. Drug Discovery 7 771–82 [DOI] [PubMed] [Google Scholar]
- [5].Matsumura Y and Maeda H 1986. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs Cancer Res. 46 6387–92 [PubMed] [Google Scholar]
- [6].Maeda H, Wu J, Sawa T, Matsumura Y and Hori K 2000. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review J. Control. Release 65 271–84 [DOI] [PubMed] [Google Scholar]
- [7].Maeda H 2001. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting Adv. Enzyme Regul. 41 189–207 [DOI] [PubMed] [Google Scholar]
- [8].Maeda H 2010. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects Bioconjugate Chem. 21 797–802 [DOI] [PubMed] [Google Scholar]
- [9].Gill PS et al. 1996. Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma J. Clin. Oncol. 14 2353–64 [DOI] [PubMed] [Google Scholar]
- [10].Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du Mond C, Mamelok RD and Henry DH 1998. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial J. Clin. Oncol. 16 2445–51 [DOI] [PubMed] [Google Scholar]
- [11].Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME and Lacave AJ 2001. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan J. Clin. Oncol. 19 3312–22 [DOI] [PubMed] [Google Scholar]
- [12].O’brien M, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback D, Tomczak P and Ackland S 2004. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer Ann. Oncol. 15 440–9 [DOI] [PubMed] [Google Scholar]
- [13].Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M and O’Shaughnessy J 2005. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer J. Clin. Oncol. 23 7794–803 [DOI] [PubMed] [Google Scholar]
- [14].Tredan O, Galmarini CM, Patel K and Tannock IF 2007. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 99 1441–54 [DOI] [PubMed] [Google Scholar]
- [15].Olive KP et al. 2009. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer Science 324 1457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shen J, Zhao L and Han G 2013. Lanthanide-doped upconverting luminescent nanoparticle platforms for optical imaging-guided drug delivery and therapy Adv. Drug. Deliv. Rev. 65 744–55 [DOI] [PubMed] [Google Scholar]
- [17].Zhu W, Yang Y, Jin Q, Chao Y, Tian L, Liu J, Dong Z and Liu Z 2019. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemo-photodynamic cancer therapy Nano Res. 12 1307–12 [Google Scholar]
- [18].Zhu J, Wang G, Alves CS, Tomas H, Xiong Z, Shen M, Rodrigues J and Shi X 2018. Multifunctional dendrimer-entrapped gold nanoparticles conjugated with doxorubicin for pH-responsive drug delivery and targeted computed tomography imaging Langmuir 34 12428–35 [DOI] [PubMed] [Google Scholar]
- [19].Xu Y, Shan Y, Zhang Y, Yu B, Shen Y and Cong H 2019. Multifunctional Fe3O4@C-based nanoparticles coupling optical/MRI imaging and pH/photothermal controllable drug release as efficient anti-cancer drug delivery platforms Nanotechnology 30 425102. [DOI] [PubMed] [Google Scholar]
- [20].Janib SM, Moses AS and MacKay JA 2010. Imaging and drug delivery using theranostic nanoparticles Adv. Drug. Deliv. Rev. 62 1052–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Funkhouser J 2002. Reinventing pharma: the theranostic revolution Curr. Drug Discovery 2 17–19 [Google Scholar]
- [22].Kelkar SS and Reineke TM 2011. Theranostics: combining imaging and therapy Bioconjugate Chem. 22 1879–903 [DOI] [PubMed] [Google Scholar]
- [23].Ward KM, Aletras AH and Balaban RS 2000. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J. Magn. Reson. 143 79–87 [DOI] [PubMed] [Google Scholar]
- [24].Liu G, Song X, Chan KW and McMahon MT 2013. Nuts and bolts of chemical exchange saturation transfer MRI NMR Biomed. 26 810–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Zijl P C and Yadav NN 2011. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn. Reson. Med. 65 927–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goffeney N, Bulte JW, Duyn J, Bryant LH Jr. and van Zijl P C 2001. Sensitive NMR detection of cationic-polymer-based gene delivery systems using saturation transfer via proton exchange J. Am. Chem. Soc. 123 8628–9 [DOI] [PubMed] [Google Scholar]
- [27].Aime S, Barge A, Delli Castelli D, Fedeli F, Mortillaro A, Nielsen FU and Terreno E 2002. Paramagnetic lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications Magn. Reson. Med. 47 639–48 [DOI] [PubMed] [Google Scholar]
- [28].Zhang S, Winter P, Wu K and Sherry AD 2001. A novel europium(III)-based MRI contrast agent J. Am. Chem. Soc. 123 1517–8 [DOI] [PubMed] [Google Scholar]
- [29].Aime S, Delli Castelli D and Terreno E 2005. Highly sensitive MRI chemical exchange saturation transfer agents using liposomes Angew. Chem., Int. Ed. 117 5649–51 [DOI] [PubMed] [Google Scholar]
- [30].Zhao JM, Har-el YE, McMahon MT, Zhou J, Sherry AD, Sgouros G, Bulte JW and van Zijl P C 2008. Size-induced enhancement of chemical exchange saturation transfer (CEST) contrast in liposomes J. Am. Chem. Soc. 130 5178–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flament J et al. 2013. In vivo CEST MR imaging of U87 mice brain tumor angiogenesis using targeted LipoCEST contrast agent at 7 T Magn. Reson. Med. 69 179–87 [DOI] [PubMed] [Google Scholar]
- [32].Veshtort M and Griffin RG 2006. SPINEVOLUTION: a powerful tool for the simulation of solid and liquid state NMR experiments J. Magn. Reson. 178 248–82 [DOI] [PubMed] [Google Scholar]
- [33].Swanson S 1998. Protein mediated magnetic coupling between lactate and water protons J. Magn. Reson. 135 248–55 [DOI] [PubMed] [Google Scholar]
- [34].Estilaei M, Matson G and Meyerhoff D 2003. Indirect imaging of ethanol via magnetization transfer at high and low magnetic fields Magn. Reson. Med. 49 755–9 [DOI] [PubMed] [Google Scholar]
- [35].McMahon MT and Bulte JWM 2018. Two decades of dendrimers as versatile MRI agents: a tale with and without metals WIREs Nanomed. Nanobiotechnol 10 e1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ali MM, Yoo B and Pagel MD 2009. Tracking the relative in vivo pharmacokinetics of nanoparticles with PARACEST MRI Mol. Pharm. 6 1409–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li Y et al. 2016. CEST theranostics: label-free MR imaging of anticancer drugs Oncotarget 7 6369–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu H et al. 2016. Label-free CEST MRI detection of citicoline-liposome drug delivery in ischemic stroke Theranostics 6 1588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cortez-Maya S, Pedro-Hernandez LD, Martinez-Klimova E, Ramirez-Apan T and Martinez-Garcia M 2019. Anticancer activity of water-soluble olsalazine-PAMAM-dendrimer-salicylic acid-conjugates Biomolecules 9 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yuan Y, Zhang J, Qi X, Li S, Liu G, Siddhanta S, Barman I, Song X, McMahon MT and Bulte JWM 2019. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy Nat. Mater 18 1376–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Akbey S, Ehses P, Stirnberg R, Zaiss M and Stocker T 2019. Whole-brain snapshot CEST imaging at 7 T using 3D-EPI Magn. Reson. Med. 82 1741–52 [DOI] [PubMed] [Google Scholar]
- [42].Lamichhane N, Udayakumar TS, D’Souza WD, Simone CB 2nd, Raghavan SR, Polf J and Mahmood J 2018. Liposomes: clinical applications and potential for image-guided drug delivery Molecules 23 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chang HI and Yeh MK 2012. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy Int. J. Nanomed 7 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Aime S, Delli Castelli D and Terreno E 2005. Highly sensitive MRI chemical exchange saturation transfer agents using liposomes Angew. Chem., Int. Ed. Engl. 44 5513–5 [DOI] [PubMed] [Google Scholar]
- [45].Aime S, Castelli DD, Crich SG, Gianolio E and Terreno E 2009. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications Acc. Chem. Res. 42 822–31 [DOI] [PubMed] [Google Scholar]
- [46].Castelli DD, Terreno E, Longo D and Aime S 2013. Nanoparticle-based chemical exchange saturation transfer (CEST) agents NMR Biomed. 26 839–49 [DOI] [PubMed] [Google Scholar]
- [47].Terreno E, Delli Castelli D, Violante E, Sanders HM, Sommerdijk NA and Aime S 2009. Osmotically shrunken LIPOCEST agents: an innovative class of magnetic resonance imaging contrast media based on chemical exchange saturation transfer Chemistry 15 1440–8 [DOI] [PubMed] [Google Scholar]
- [48].Terreno E, Cabella C, Carrera C, Delli Castelli D, Mazzon R, Rollet S, Stancanello J, Visigalli M and Aime S 2007. From spherical to osmotically shrunken paramagnetic liposomes: an improved generation of LIPOCEST MRI agents with highly shifted water protons Angew. Chem., Int. Ed. 46 966–8 [DOI] [PubMed] [Google Scholar]
- [49].Tripepi M, Ferrauto G, Bennardi PO, Aime S and Delli Castelli D 2020. Multilamellar LipoCEST agents obtained from osmotic shrinkage of paramagnetically loaded giant unilamellar vescicles (GUVs) Angew. Chem., Int. Ed. 59 2279–83 [DOI] [PubMed] [Google Scholar]
- [50].Abozeid SM, Asik D, Sokolow GE, Lovell JF, Nazarenko AY and Morrow JR 2020. Co(II) complexes as liposomal CEST agents Angew. Chem., Int. Ed. Engl. 59 12093–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Grüll H, Langereis S, Messager L, Castelli DD, Sanino A, Torres E, Terreno E and Aime S 2010. Block copolymer vesicles containing paramagnetic lanthanide complexes: a novel class of T1- and CEST MRI contrast agents Soft Matter 6 4847–50 [Google Scholar]
- [52].Opina AC, Ghaghada KB, Zhao P, Kiefer G, Annapragada A and Sherry AD 2011. TmDOTA-tetraglycinate encapsulated liposomes as pH-sensitive LipoCEST agents PLoS One 6 e27370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu G et al. 2012. In vivo multicolor molecular MR imaging using diamagnetic chemical exchange saturation transfer liposomes Magn. Reson. Med. 67 1106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chan KW et al. 2013. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability Nat. Mater 12 268–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Demetriou E, Story HE, Bofinger R, Hailes HC, Tabor AB and Golay X 2019. Effect of liposomal encapsulation on the chemical exchange properties of diamagnetic CEST agents J. Phys. Chem. B 123 7545–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chan KW et al. 2014. A diaCEST MRI approach for monitoring liposomal accumulation in tumors J. Control. Release 180 51–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen Z et al. 2019. CT and CEST MRI bimodal imaging of the intratumoral distribution of iodinated liposomes Quant. Imaging Med. Surg 9 1579–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McMahon MT, Gilad AA, DeLiso MA, Berman SM, Bulte JW and van Zijl P C 2008. New ‘multicolor’ polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI Magn. Reson. Med. 60 803–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Langereis S, Keupp J, van Velthoven J L, de Roos I H, Burdinski D, Pikkemaat JA and Grull H 2009. A temperature-sensitive liposomal 1H CEST and 19F contrast agent for MR image-guided drug delivery J. Am. Chem. Soc. 131 1380–1 [DOI] [PubMed] [Google Scholar]
- [60].Grieb P 2014. Neuroprotective properties of citicoline: facts, doubts and unresolved issues CNS Drugs 28 185–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bogdanov P, Sampedro J, Sola-Adell C, Simo-Servat O, Russo C, Varela-Sende L, Simo R and Hernandez C 2018. Effects of liposomal formulation of citicoline in experimental diabetes-induced retinal neurodegeneration Int. J. Mol. Sci. 19 2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fukuta T, Ishii T, Asai T and Oku N 2019. Applications of liposomal drug delivery systems to develop neuroprotective agents for the treatment of ischemic stroke Biol. Pharm. Bull. 42 319–26 [DOI] [PubMed] [Google Scholar]
- [63].Cho K, Wang X, Nie S, Chen ZG and Shin DM 2008. Therapeutic nanoparticles for drug delivery in cancer Clin. Cancer Res. 14 1310–6 [DOI] [PubMed] [Google Scholar]
- [64].Castro KCD, Costa JM and Campos MGN 2020. Drug-loaded polymeric nanoparticles: a review Int. J. Polym. Mater. Polym. Biomater 1–13 [Google Scholar]
- [65].Pikkemaat JA et al. 2007. Dendritic PARACEST contrast agents for magnetic resonance imaging Contrast Media Mol. Imaging 2 229–39 [DOI] [PubMed] [Google Scholar]
- [66].Ali MM, Bhuiyan MP, Janic B, Varma NR, Mikkelsen T, Ewing JR, Knight RA, Pagel MD and Arbab AS 2012. A nano-sized PARACEST-fluorescence imaging contrast agent facilitates and validates in vivo CEST MRI detection of glioma Nanomedicine 7 1827–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lesniak WG, Oskolkov N, Song X, Lal B, Yang X, Pomper M, Laterra J, Nimmagadda S and McMahon MT 2016. Salicylic acid conjugated dendrimers are a tunable, high performance CEST MRI nanoplatform Nano Lett. 16 2248–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yang X, Song X, Li Y, Liu G, Banerjee SR, Pomper MG and McMahon MT 2013. Salicylic acid and analogues as diaCEST MRI contrast agents with highly shifted exchangeable proton frequencies Angew. Chem., Int. Ed. 52 8116–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Huh MS, Lee EJ, Koo H, Yhee JY, Oh KS, Son S, Lee S, Kim SH, Kwon IC and Kim K 2017. Polymeric Gene Delivery Systems (New York: Springer; ) pp 65–83 [Google Scholar]
- [70].Liu G, Banerjee SR, Yang X, Yadav N, Lisok A, Jablonska A, Xu J, Li Y, Pomper MG and van Zijl P 2017. A dextran-based probe for the targeted magnetic resonance imaging of tumours expressing prostate-specific membrane antigen Nat. Biomed. Eng 1 977–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Li Y et al. 2018. Characterization of tumor vascular permeability using natural dextrans and CEST MRI Magn. Reson. Med. 79 1001–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chen H, Liu D, Li Y, Xu X, Xu J, Yadav NN, Zhou S, van Zijl PCM and Liu G 2019. CEST MRI monitoring of tumor response to vascular disrupting therapy using high molecular weight dextrans Magn. Reson. Med. 82 1471–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Han Z, Zhang S, Fujiwara K, Zhang J, Li Y, Liu J, van Zijl PCM, Lu ZR, Zheng L and Liu G 2019. Extradomain-B fibronectin-targeted dextran-based chemical exchange saturation transfer magnetic resonance imaging probe for detecting pancreatic cancer Bioconjugate Chem. 30 1425–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhou Y, van Zijl PCM, Xu X, Xu J, Li Y, Chen L and Yadav NN 2020. Magnetic resonance imaging of glycogen using its magnetic coupling with water Proc. Natl Acad. Sci. USA 117 3144–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Haris M et al. 2014. In vivo magnetic resonance imaging of tumor protease activity Sci. Rep. 4 6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang S, Zhou K, Huang G, Takahashi M, Sherry AD and Gao J 2013. A novel class of polymeric pH-responsive MRI CEST agents Chem. Commun. 49 6418–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Snoussi K, Bulte JW, Gueron M and van Zijl P C 2003. Sensitive CEST agents based on nucleic acid imino proton exchange: detection of poly(rU) and of a dendrimer-poly(rU) model for nucleic acid delivery and pharmacology Magn. Reson. Med. 49 998–1005 [DOI] [PubMed] [Google Scholar]
- [78].Cheetham AG, Chakroun RW, Ma W and Cui H 2017. Self-assembling prodrugs Chem. Soc. Rev. 46 6638–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lock LL et al. 2017. One-component supramolecular filament hydrogels as theranostic label-free magnetic resonance imaging agents ACS Nano 11 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sun YP et al. 2006. Quantum-sized carbon dots for bright and colorful photoluminescence J. Am. Chem. Soc. 128 7756–7 [DOI] [PubMed] [Google Scholar]
- [81].Luo PG, Sahu S, Yang ST, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L and Sun YP 2013. Carbon ‘quantum’ dots for optical bioimaging J. Mater. Chem. B 1 2116–27 [DOI] [PubMed] [Google Scholar]
- [82].Zhang J, Yuan Y, Gao M, Han Z, Chu C, Li Y, van Zijl PCM, Ying M, Bulte JWM and Liu G 2019. Carbon dots as a new class of diamagnetic chemical exchange saturation transfer (diaCEST) MRI contrast agents Angew. Chem., Int. Ed. 58 9871–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Longo DL, Sun PZ, Consolino L, Michelotti FC, Uggeri F and Aime S 2014. A general MRI-CEST ratiometric approach for pH imaging: demonstration of in vivo pH mapping with iobitridol J. Am. Chem. Soc. 136 14333–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Han X, Huang J, To AKW, Lai JHC, Xiao P, Wu EX, Xu J and Chan KWY 2020. CEST MRI detectable liposomal hydrogels for multiparametric monitoring in the brain at 3T Theranostics 10 2215–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Heo HY, Zhang Y, Lee DH, Jiang S, Zhao X and Zhou J 2017. Accelerating chemical exchange saturation transfer (CEST) MRI by combining compressed sensing and sensitivity encoding techniques Magn. Reson. Med. 77 779–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhang Y, Heo HY, Jiang S, Lee DH, Bottomley PA and Zhou J 2016. Highly accelerated chemical exchange saturation transfer (CEST) measurements with linear algebraic modeling Magn. Reson. Med. 76 136–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhang Y, Heo HY, Lee DH, Jiang S, Zhao X, Bottomley PA and Zhou J 2017. Chemical exchange saturation transfer (CEST) imaging with fast variably-accelerated sensitivity encoding (vSENSE) Magn. Reson. Med. 77 2225–38 [DOI] [PMC free article] [PubMed] [Google Scholar]


