Key Points
Question
Is Parkinson disease risk increased in military service members who were stationed at Marine Corps Base Camp Lejeune, North Carolina, during 1975-1985 when the water supply was contaminated with trichloroethylene and other volatile organic compounds?
Findings
This cohort study of 340 489 service members found that the risk of Parkinson disease was 70% higher in Camp Lejeune veterans compared with veterans stationed at a Marine Corps base where water was not contaminated. In veterans without Parkinson disease, risk was also significantly higher for several prodromal features of Parkinson disease.
Meaning
The study’s findings suggest that exposure to trichloroethylene in water may increase the risk of Parkinson disease; millions worldwide have been and continue to be exposed to this ubiquitous environmental contaminant.
This cohort study assesses the association between the contaminated water supply at Camp Lejeune and incidence of Parkinson disease among veterans who resided there between 1975 and 1985.
Abstract
Importance
An increased risk of Parkinson disease (PD) has been associated with exposure to the solvent trichloroethylene (TCE), but data are limited. Millions of people in the US and worldwide are exposed to TCE in air, food, and water.
Objective
To test whether the risk of PD is higher in veterans who served at Marine Corps Base Camp Lejeune, whose water supply was contaminated with TCE and other volatile organic compounds (VOCs), compared with veterans who did not serve on that base.
Design, Setting, and Participants
This population-based cohort study examined the risk for PD among all Marines and Navy personnel who resided at Camp Lejeune, North Carolina (contaminated water) (n = 172 128), or Camp Pendleton, California (uncontaminated water) (n = 168 361), for at least 3 months between 1975 and 1985, with follow-up from January 1, 1997, until February 17, 2021. Veterans Health Administration and Medicare databases were searched for International Classification of Diseases diagnostic codes for PD or other forms of parkinsonism and related medications and for diagnostic codes indicative of prodromal disease. Parkinson disease diagnoses were confirmed by medical record review.
Exposures
Water supplies at Camp Lejeune were contaminated with several VOCs. Levels were highest for TCE, with monthly median values greater than 70-fold the permissible amount.
Main Outcome and Measures
Risk of PD in former residents of Camp Lejeune relative to residents of Camp Pendleton. In those without PD or another form of parkinsonism, the risk of being diagnosed with features of prodromal PD were assessed individually and cumulatively using likelihood ratio tests.
Results
Health data were available for 158 122 veterans (46.4%). Demographic characteristics were similar between Camp Lejeune (5.3% women, 94.7% men; mean [SD] attained age of 59.64 [4.43] years; 29.7% Black, 6.0% Hispanic, 67.6% White; and 2.7% other race and ethnicity) and Camp Pendleton (3.8% women, 96.2% men; mean [SD] age, 59.80 [4.62] years; 23.4% Black, 9.4% Hispanic, 71.1% White, and 5.5% other race and ethnicity). A total of 430 veterans had PD, with 279 from Camp Lejeune (prevalence, 0.33%) and 151 from Camp Pendleton (prevalence, 0.21%). In multivariable models, Camp Lejeune veterans had a 70% higher risk of PD (odds ratio, 1.70; 95% CI, 1.39-2.07; P < .001). No excess risk was found for other forms of neurodegenerative parkinsonism. Camp Lejeune veterans also had a significantly increased risk of prodromal PD diagnoses, including tremor, anxiety, and erectile dysfunction, and higher cumulative prodromal risk scores.
Conclusions and Relevance
The study’s findings suggest that the risk of PD is higher in persons exposed to TCE and other VOCs in water 4 decades ago. Millions worldwide have been and continue to be exposed to this ubiquitous environmental contaminant.
Introduction
Occupational exposure to the industrial solvent trichloroethylene (TCE) was previously associated with a 6-fold increased risk of Parkinson disease (PD) in a small study of twin pairs discordant for PD.1 Animal studies provide biological plausibility for this association by recapitulating key pathologic characteristics of PD, including mitochondrial impairment, intraneuronal aggregation of phosphorylated α-synuclein protein, and regionally specific degeneration of nigrostriatal dopaminergic neurons.2,3,4,5,6 Yet, despite decades of widespread industrial and commercial usage, the human epidemiology supporting a causal link between TCE and PD comprises the aforementioned analytic study, several case reports,7,8 and a cluster of industrial workers in a small manufacturing facility.2 Furthermore, despite the fact that TCE and the similar compound tetrachloroethylene (PCE) are present in up to one-third of US drinking water supplies,9,10,11,12 only a single underpowered mortality study has assessed the risk of PD from TCE or PCE in drinking water.13
In one of the best-documented large-scale contaminations in US history, the drinking water supplied to residents of Marine Corps Base Camp Lejeune in North Carolina was contaminated with TCE, PCE, and several other volatile organic compounds (VOCs) from approximately 1953 until 1987.14,15,16,17 Wells that provided water to the base during this period were contaminated by on-base sources, including leaking underground storage tanks, industrial spills, and waste disposal sites (largely TCE) and an off-base dry cleaning business (largely PCE). The wells were taken offline in the mid-1980s after testing mandated by the Safe Drinking Water Act15,17,18 discovered the contamination. During 1975-1985, the period of maximal contamination, the estimated monthly median TCE level was 366 μg/L, more than 70-fold the US Environmental Protection Agency (EPA) maximum contaminant level (MCL) of 5 μg/L.14,19 Maximum contaminant levels were also exceeded for PCE (median, 15.4 μg/L; MCL, 5 μg/L) and vinyl chloride (median, 22.2 μg/L; MCL 2 μg/L). Despite relatively limited human epidemiology, in light of this contamination, on January 13, 2017, the US Congress and Veterans Administration (VA) designated PD a presumptive service-connected condition for veterans who served at Camp Lejeune between August 1, 1953, and December 31, 1987, making them eligible for benefits.20
For these reasons, we conducted a study to compare the risk of PD among veterans who resided at Camp Lejeune during 1975-1985 with those who resided at Marine Corps Base Camp Pendleton, a large California base that did not have contaminated water.21 Because PD pathology and associated clinical features begin years before diagnosis,22 we additionally compared the prevalence of diagnoses that may precede a PD diagnosis, termed prodromal PD.23
Methods
This cohort study was approved by the institutional review boards of the University of California, San Francisco; San Francisco VA Health Care System; and Edward Hines, Jr VA Hospital, with a waiver of requirement for individual informed consent because the study posed no more than a minimal risk to patients. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Cohort Assembly
Study cohorts were previously assembled by the US Agency for Toxic Substances and Disease Registry (ATSDR) as reported by Bove et al.14 Marine and Navy personnel stationed at Camp Lejeune and Camp Pendleton between April 1975 and December 1985 were identified from the Defense Manpower Data Center Active Duty Military Personnel Master File and US Marine Corps. This time frame was chosen because it was the period of maximal contamination at Camp Lejeune and because the Defense Manpower Data Center file did not contain information on unit location until April 1975. Personnel who served at both locations were assigned to the Camp Lejeune cohort. The cohorts included 172 128 individuals who served at Camp Lejeune and 168 361 who served at Camp Pendleton. Within these, we identified an analytic cohort that included all individuals who ever used Veterans Health Administration (VHA) or Medicare health care services.
Parkinson Disease Ascertainment
For veterans who used VHA services, we searched Corporate Data Warehouse24 Outpatient, Inpatient, and Community Care (care in the community paid for by VHA) files for all PD diagnostic codes (International Classification of Diseases, Ninth Revision [ICD-9] code 332.0 and Tenth Revision [ICD-10] code G20) between January 1, 1999, and February 17, 2021. We also identified ICD codes for other forms of neurodegenerative parkinsonism, including multiple system atrophy, progressive supranuclear palsy, dementia with Lewy bodies (DLB), and corticobasal degeneration (eTable 1 in Supplement 1), as well as prescriptions for dopaminergic medications in the Corporate Data Warehouse Pharmacy file. For each diagnostic code instance, we extracted the date, VA site, clinic type, provider type, and specialty. To facilitate diagnostic reviews, we also searched for diagnostic codes likely to be inconsistent with a diagnosis of idiopathic PD, such as secondary parkinsonism, Huntington disease, and motor neuron diseases.
We reviewed the medical record notes of all individuals identified to validate diagnosis and diagnosis date. The reviewer (S.M.G.) was unaware of camp status. To apply standard diagnostic criteria for PD,25 we recorded the presence or absence of rest tremor, bradykinesia, cogwheel rigidity, postural reflex impairment, asymmetry of motor signs, and response to levodopa. We classified individuals as having probable or possible PD based on the totality of available information. We assigned a diagnosis of probable PD when PD was consistently coded by physicians and nurse practitioners over time and was well supported by the clinical data without other data suggesting an alternative diagnosis. We assigned a diagnosis of possible PD when follow-up time was limited and/or clinical features and response to medications were less well documented.
We used a parallel approach to ascertain cases in Medicare files, including outpatient claims (Part B) and inpatient and skilled nursing facilities claims (Part A) from January 1, 1997, through December 31, 2018, and pharmacy claims (Part D) from January 1, 2006, through December 31, 2018. We also extracted every iteration of an ICD code for PD, neurodegenerative parkinsonism, and PD medication and codes potentially inconsistent with idiopathic PD.
We reviewed all available Medicare-derived information and VHA medical records for veterans who also obtained VHA care but were not directly ascertained through VHA diagnostic codes. We assigned a diagnosis of probable PD for veterans ascertained via Medicare if they had at least 2 PD codes by a neurologist at least 6 months apart, had been prescribed a PD medication, and had no conflicting diagnostic information. We assigned a diagnosis of possible PD if they had (1) 1 PD code by a neurologist and at least 2 medication dates, (2) at least 2 codes by a neurologist but without a medication, or (3) at least 2 codes by nonneurologist physicians and 2 medication dates and had no conflicting diagnostic information.
Ascertainment of Prodromal PD Features
We identified ICD-9 and ICD-10 diagnostic codes for rapid eye movement sleep behavior disorder (RBD), disorders of smell or taste, tremor, constipation, depression, anxiety, erectile dysfunction, urinary dysfunction, and seborrheic dermatitis in VHA and Medicare files during the same follow-up period as for PD (eTable 2 in Supplement 1). We validated RBD diagnoses by review of available medical record notes and sleep study reports.
Covariate Data
We collected covariate data for use in adjusted analytic models. Sex and race and ethnicity were determined from VA data if available or from Medicare or ATSDR files if not. We determined smoking status using VA health factors data.26 Rank and duration of service at a camp were obtained from ATSDR files.
Statistical Analysis
We compared participant characteristics using Pearson χ2 statistic for categorical variables or Student t tests for continuous variables. We evaluated associations between camp and PD using unconditional logistic regression to derive odds ratios (ORs) and 95% CIs. To control for potential confounding, we included age, sex, race (Black, White, other), and ethnicity (Hispanic, non-Hispanic) in all models. We also tested models that included rank (officer, enlisted) and smoking status (ever, never), though smoking status was unknown for a substantial proportion of the cohort. We repeated analyses in subgroups defined by sex and by race and ethnicity. We conducted several analyses to explore possible biased ascertainment of PD in Camp Lejeune veterans due to potential awareness of the contamination and presumptive service connection that entitles them to VA benefits. Because the government ruling was published January 13, 2017, we performed a sensitivity analysis that excluded individuals with PD ascertained after this date. We also conducted an analysis that included only those who were active VHA users prior to their PD diagnosis. Finally, although power was limited, we explored associations between camp and other forms of neurodegenerative parkinsonism and conducted a sensitivity analysis that pooled diagnoses of PD and DLB given their overlapping pathologic characteristics.27
We evaluated associations of each prodromal PD feature and camp in individuals who did not have PD or another neurodegenerative parkinsonism using logistic regression models as above. We additionally constructed 2 cumulative prodromal PD risk scores. An internal model was derived using the observed associations between each feature and a validated PD diagnosis in our study cohort. We calculated positive and negative likelihood ratios for each feature as described by Berg et al28 and Schwartz29 (eTable 3 in Supplement 1). An external model applied Movement Disorders Society (MDS) prodromal PD research criteria likelihood ratio weightings for the available features.23 As recommended, we calculated summary risk scores by multiplying positive and negative likelihood ratios, but ignored negative likelihood ratios when prevalence of a feature was less than 10%.28 We log-transformed prodromal risk scores and tested associations of each with camp in multiple linear regression models. We also dichotomized risk scores at the 99th percentile and tested associations with camp in logistic models as above. We additionally tested associations in men and women separately and conducted sensitivity analyses that adjusted for total number of years of VHA health care usage.
Statistical analyses were performed using SAS, version 9.4 software (SAS Institute Inc). A 2-sided P < .05 was considered significant.
Results
The analytic cohort included 84 824 veterans from Camp Lejeune (49.3%; mean [SD] age, 59.6 [4.4] years) and 73 298 from Camp Pendleton (43.5%; mean [SD] age, 59.8 [4.6] years) who used VHA or Medicare health care services (Table 1). Demographic characteristics of veterans who did and did not use VHA or Medicare services are summarized in eTables 4 to 6 in Supplement 1. Populations were demographically similar, although the Camp Lejeune cohort had slightly more female (4491 [5.3%] vs 2811 [3.8%] for Camp Pendleton; male, 80 333 [94.7%] vs 70 487 [96.2%], respectively) and Black veterans (25 196 [29.7%] vs 17 141 [23.4%]) and fewer Hispanic veterans (5068 [6.0%] vs 6904 [9.4%]). The remainder of the cohorts comprised 57 331 (67.6%) vs 52 082 (71.1%) White and 2291 (2.7%) vs 4050 (5.5%) other race and ethnicity for Camp Lejeune and Camp Pendleton, respectively. Duration of residence at Camp Lejeune was slightly longer than at Camp Pendleton (mean [SD], 25.0 [17.4] vs 22.7 [16.6] months).
Table 1. Demographic Characteristics.
| Variable | No. (%) | ||
|---|---|---|---|
| Camp Lejeune | Camp Pendleton | Total | |
| No. of veterans | 172 128 | 168 361 | 340 489 |
| VHA usage (% of total) | 79 420 (46.1) | 67 356 (40.0) | 146 776 (43.1) |
| Medicare usage (% of total) | |||
| VHA and Medicare | 10 281 (6.0) | 8003 (4.8) | 18 284 (5.4) |
| Medicare only | 5404 (3.1) | 5942 (3.5) | 11 346 (3.3) |
| VHA or Medicare usage (% of total)a | 84 824 (49.3) | 73 298 (43.5) | 158 122 (46.4) |
| Sex | |||
| Female | 4491 (5.3) | 2811 (3.8) | 7302 (4.6) |
| Male | 80 333 (94.7) | 70 487 (96.2) | 150 820 (95.4) |
| Race | |||
| Black | 25 196 (29.7) | 17 141 (23.4) | 42 337 (26.8) |
| White | 57 331 (67.6) | 52 082 (71.1) | 109 413 (69.2) |
| Otherb | 2291 (2.7) | 4050 (5.5) | 6341 (4.0) |
| Ethnicity | |||
| Hispanic | 5068 (6.0) | 6904 (9.4) | 11 972 (7.6) |
| Non-Hispanic | 79 756 (94.0) | 66 394 (90.6) | 146 150 (92.4) |
| Deceased | 1600 (1.9) | 1745 (2.4) | 3345 (2.1) |
| Age at end of follow-up | |||
| Mean (SD), y | 59.6 (4.4) | 59.8 (4.6) | 59.7 (4.5) |
| <50 y | 2310 (2.7) | 2287 (3.7) | 4597 (2.9) |
| 50-59 y | 36 461 (43.0) | 29 812 (40.7) | 66 273 (41.9) |
| 60-69 y | 45 310 (53.4) | 40 368 (55.1) | 85 678 (54.2) |
| ≥70 y | 743 (0.9) | 832 (1.1) | 1575 (1.0) |
| Age when residence at the camp began, mean (SD), y | 20.0 (2.5) | 20.1 (2.7) | 20.03 (2.6) |
| Duration lived on base during 1975-1985, mean (SD), mo | 25.0 (17.4) | 22.7 (16.6) | 23.89 (17.1) |
| Rank | |||
| Officers | 2570 (3.0) | 2800 (3.8) | 5370 (3.4) |
| Enlisted | 82 254 (97.0) | 70 498 (96.2) | 152 752 (96.6) |
| Smoking status | |||
| Ever smoked | 40 420 (47.7) | 34 596 (47.2) | 75 016 (47.4) |
| Never smoked | 21 891 (25.8) | 17 612 (24.0) | 39 504 (25.0) |
| Unknown | 22 513 (26.5) | 21 090 (28.8) | 43 603 (27.6) |
Abbreviation: VHA, Veterans Health Administration.
Statistics below this row refer to those with VHA or Medicare usage. See eTables 4 to 6 in Supplement 1 for additional descriptive data.
Other race included Asian, Native American, Native Hawaiian, and other.
Parkinson Disease and Parkinsonism
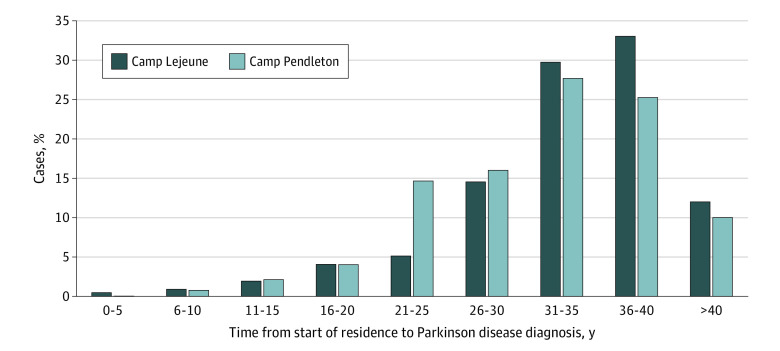
A total of 725 individuals had at least 1 ICD code for PD, 376 (87.4%) of whom were determined to have probable PD and 54 (12.6%) possible PD (Table 2). A total of 398 cases of PD (92.6%) were identified through VHA data and 32 (7.4%) through Medicare. The crude prevalence rate of PD was higher in Camp Lejeune (0.33% vs 0.21% in Camp Pendleton). Mean (SD) age at PD diagnosis was 54.2 (7.4) years, reflecting the relatively young age of the cohort, and slightly older in the Camp Lejeune than in the Camp Pendleton cohort (mean [SD], 54.7 [7.2] vs 53.2 [7.7] years). The duration from start of camp residence until PD diagnosis was 1.7 years longer for Camp Lejeune (mean [SD], 33.9 [7.1] years vs 32.2 [7.3] years in Camp Pendleton) (Table 2, Figure). A total of 257 veterans with PD (59.7%) were ascertained before 2017, and 260 veterans with PD (60.5%) were active VHA health care users prior to their diagnosis.
Table 2. Parkinson Disease (PD) and Other Neurodegenerative Parkinsonism Diagnoses.
| Variable | No. (%) | P value | ||
|---|---|---|---|---|
| Camp Lejeune (n = 279) | Camp Pendleton (n = 151) | Total (n = 430) | ||
| VHA records (% total PD) | 263 (94.3) | 135 (89.4) | 398 (92.6) | .07 |
| Medicare only (% total PD) | 16 (5.7) | 16 (10.6) | 32 (7.4) | |
| Crude PD prevalence, % | 0.33 | 0.21 | 0.27 | <.001 |
| Probable PD (% total PD) | 249 (89.2) | 127 (84.1) | 376 (87.4) | .12 |
| Possible PD (% total PD) | 30 (10.8) | 24 (15.9) | 54 (12.6) | |
| PD diagnosis age, mean (SD); range, y | 54.7 (7.2); 30.0-75.0 | 53.2 (7.7); 29.0-70.0 | 54.2 (7.4); 29.0-75.0 | .04 |
| Years from start of camp residence until PD diagnosis, mean (SD); range | 33.9 (7.1); 4.5-45.0 | 32.2 (7.3); 10.5-44.7 | 33.3 (7.2); 4.5-45.0 | .02 |
| PD before January 13, 2017 | 148 (53.0) | 109 (72.2) | 257 (59.7) | .19 |
| PD with VHA usage before PD incidence date | 173 (62.0) | 87 (57.6) | 260 (60.5) | <.001 |
| Other neurodegenerative parkinsonism | ||||
| DLB | 12 (0.014) | 7 (0.010) | 19 (0.012) | .41 |
| MSA | 4 (0.005) | 10 (0.014) | 14 (0.009) | .11 |
| PSP | 5 (0.006) | 5 (0.007) | 10 (0.006) | .82 |
| CBD | 1 (0.001) | 1 (0.001) | 2 (0.001) | .92 |
| Indeterminate | 7 (0.008) | 5 (0.007) | 12 (0.008) | .74 |
Abbreviations: CBD, corticobasal degeneration; DLB, dementia with Lewy bodies; MSA, multiple system atrophy; PSP, progressive supranuclear palsy; VHA, Veterans Health Administration.
Figure. Duration From Start of Camp Residence Until Parkinson Disease Diagnosis.
Risk of PD was 70% higher in Camp Lejeune veterans (OR, 1.70; 95% CI, 1.39-2.07) (Table 3). Inclusion of smoking or military rank in logistic models had minimal impact. Risk was attenuated when restricted to cases ascertained before 2017 (OR, 1.28; 95% CI, 1.00-1.64), but was slightly higher when including only those who were active VHA users prior to diagnosis (OR, 1.82; 95% CI, 1.41-2.36) or those with probable PD (OR, 1.81; 95% CI, 1.46-2.24). Parkinson disease risk was substantially lower among Black veterans (OR, 0.33; 95% CI, 0.25-0.45) and ever-smokers (OR, 0.49; 95% CI, 0.40-0.61). Though not statistically significant, risk was also lower in Hispanic (OR, 0.68; 95% CI, 0.45-1.02) and female (OR, 0.79; 95% CI, 0.49-1.28) veterans. Pooling PD and DLB diagnoses did not affect risk estimates, and no significant associations were found for other forms of neurodegenerative parkinsonism.
Table 3. Risk of Parkinson Disease (PD) in Residents of Camp Lejeune vs Camp Pendleton.
| Modela | Camp Lejeune, OR (95% CI) | P value |
|---|---|---|
| Possible or probable PD (total with PD n = 430) | 1.70 (1.39-2.07) | <.001 |
| Smoking added to model | 1.69 (1.39-2.06) | <.001 |
| Rank added to model | 1.71 (1.40-2.08) | <.001 |
| PD ascertained before January 13, 2017 (n = 257) | 1.28 (1.00-1.64) | .052 |
| VHA patient before PD diagnosis (n = 260) | 1.82 (1.41-2.36) | <.001 |
| Probable PD (n = 376) | 1.81 (1.46-2.24) | <.001 |
| PD or DLB (n = 449) | 1.70 (1.40-2.07) | <.001 |
Abbreviations: DLB, dementia with Lewy bodies; OR, odds ratio; VHA, Veterans Health Administration.
All models adjusted for age, sex, and race and ethnicity.
Prodromal Features
In models restricted to cohort members without PD or another neurodegenerative parkinsonism, a diagnosis of anxiety, tremor, or erectile dysfunction was significantly associated with Camp Lejeune. Risk of depression and risk of olfactory impairment were also higher in Camp Lejeune but were not statistically significant (Table 4). A diagnosis of RBD was not associated with camp, but the sample size was very small (n = 134 [0.085%]).
Table 4. Prodromal Feature Associations With Camp Lejeune in Veterans Without PD or Neurodegenerative Parkinsonism (n = 157 637)a.
| Feature | No. (%) | Lejeune, OR (95% CI) | P value |
|---|---|---|---|
| Tremor | 2318 (1.5) | 1.19 (1.09-1.29) | <.001 |
| Olfactory impairment | 686 (0.4) | 1.12 (0.96-1.30) | .14 |
| Erectile dysfunction | 32 424 (20.6) | 1.12 (1.09-1.14) | <.001 |
| Anxiety | 48 149 (30.5) | 1.08 (1.05-1.10) | <.001 |
| Seborrheic dermatitis | 5771 (3.7) | 1.04 (0.98-1.09) | .20 |
| Depression | 68 974 (43.8) | 1.02 (1.00-1.04) | .07 |
| Constipation | 15 860 (10.1) | 1.01 (0.98-1.04) | .63 |
| RBD | 134 (0.1) | 0.99 (0.70-1.39) | >.99 |
| Urinary dysfunction | 8699 (5.5) | 0.98 (0.94-1.03) | .39 |
| Internal prodromal risk score ≥99th percentileb | 1501 (1.0) | 1.14 (1.03-1.26) | .01 |
| MDS prodromal risk score ≥99th percentilec | 1332 (0.8) | 1.18 (1.06-1.32) | .003 |
Abbreviations: OR, odds ratio; RBD, rapid eye movement sleep behavior disorder.
Logistic regression models adjusted for age, sex, and race and ethnicity.
Prodromal risk score using internally derived likelihood ratios.
Prodromal risk score using MDS likelihood ratios.
Internal and MDS-prodromal risk scores were highly skewed and similarly distributed. Median internal score was 0.68 (range, 0.30-23 268.42; 99th percentile, 36.53). Median MDS risk score was 1.14 (range, 0.63-16 972.80; 99th percentile, 27.20). After log-transformation, residence at Camp Lejeune was significantly associated with both risk scores overall and in men only (Table 4; eTable 7 in Supplement 1). Inclusion of a variable for total years of VHA usage strengthened the association of both prodromal risk scores with residence at Camp Lejeune (eTable 7 in Supplement 1). In logistic models, residence at Camp Lejeune was associated with a statistically significant increased risk of prodromal risk scores in the 99th percentile (internal: OR, 1.14 [95% CI, 1.03-1.26]; MDS: OR, 1.18 [95% CI, 1.06-1.32]) (Table 4). In sensitivity analyses, risk was increased 14% to 20% overall and among men (eTable 7 in Supplement 1). Risk estimates in women were similar but imprecise.
Discussion
This study is the first in our knowledge to assess the association of PD and exposure to TCE-contaminated water in a large, well-powered, population-based cohort. We found a 70% higher risk of PD in veterans who resided at Camp Lejeune relative to those who resided at Camp Pendleton during 1975-1985, a period when monthly median levels of TCE in the Camp Lejeune water supply exceeded the EPA maximum contaminant level by some 70-fold. Remarkably, among veterans without PD, residence at Camp Lejeune was associated with a higher risk of several clinical diagnoses that are well-established prodromal features of PD. Former Camp Lejeune residents had higher cumulative prodromal risk scores and were 15% more likely to score in the top 1%, suggesting they may be in a prediagnostic phase of evolving PD pathology. This observation is especially important in this relatively young cohort given the long pathologic evolution of PD22 and the long latencies that have been reported for many environmental risk associations with PD,30,31,32,33,34 including a prior study of TCE in a more elderly twin cohort by Goldman et al.1
Animal studies have supported a causal association of TCE with PD.4 Mirroring the histopathologic hallmarks of PD, TCE administered chronically to rodents causes selective loss of nigrostriatal dopaminergic neurons, increases intraneuronal phosphorylated α-synuclein, and activates microglia with concomitant increases in markers of oxidative stress and associated motor deficits.3,5,6 Oral administration reduces activity of mitochondrial complex I,2,3,35 the site of action of the PD-associated pesticide rotenone and the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.36,37,38 Other potential mechanisms of toxicity may involve induction of leucine-rich repeat kinase 2 activity, endolysosomal dysfunction, and microbiome perturbation, all also implicated in PD pathogenesis.39,40 Proposed proximate toxicants include the potent mitochondrial complex I inhibitor 1-trichloromethyl-1,2,3,4-tetrahydro-β-carboline (also referred to as TaClo),41 which formed in vivo in the brains of mice fed TCE for 8 months,6 and S-(1,2-dichlorovinyl)-L-cysteine, a byproduct of hepatic and renal TCE metabolism.42,43
Despite being a well-established carcinogen,44 global consumption of TCE continues to increase.45 Historically, TCE has been used in a wide range of industrial and commercial applications since the 1920s. It was the predominant dry cleaning solvent from the 1930s until the 1950s when it was supplanted by PCE. It was used as a surgical anesthetic and to decaffeinate coffee into the 1970s and was present in carpet cleaners, spot removers, office products, and many other home-use products. Its predominant usage in industry and the military has been degreasing and cleaning of metal parts, although workers in textile industries, oils and fats production, and pharmaceutical and chemical manufacturing may also be exposed. Today, TCE’s primary uses are in vapor degreasing and as an intermediate in the production of hydrofluorocarbon refrigerants and other chemicals.10,46,47
Between 9% and 34% of US water supplies have measurable amounts of TCE.10 In addition to the many legacy sources of exposure, environmental releases continue to accrue.48 Highly persistent in soil and groundwater, large subsurface TCE plumes exist throughout the world, often unbeknownst to those who live and work above them.49,50 Vapor intrusion into homes and businesses from contaminated soil is a common source of inhalational exposure and may have compounded the exposures sustained by Camp Lejeune residents through their water supply. Trichloroethylene is lipophilic and readily absorbed through respiratory, dermal, and gastrointestinal routes.10,12 Exposures may occur through occupational usage, environmental point sources, vapor intrusion, or ingestion of contaminated food and water.9,51 Dermal and respiratory exposures may also occur during cooking and bathing. Reflecting its environmental ubiquitousness, TCE has been broadly detected in human breast milk, blood, and urine.52
It should be noted that in addition to the exposed service members studied here, hundreds of thousands of family members and civilian workers exposed to contaminated water at Camp Lejeune may also be at increased risk of PD, cancers, and other health consequences.13,53,54,55 Continued prospective follow-up of this population is essential.
Strengths and Limitations
Our study has many strengths. The cohorts were population based, including all service members who resided at Camp Lejeune or Camp Pendleton during a 10-year period. We did not rely on potentially biased self-report to determine exposure; rather, we inferred exposure based on camp. We validated PD diagnoses by review of medical record notes and applied accepted diagnostic criteria. The 0.27% prevalence we observed closely matches the expected prevalence for a population with this age distribution, suggesting relatively complete ascertainment.56 We explored potential confounding by a range of variables and performed sensitivity analyses that found consistent associations of both PD and prodromal PD with residence at Camp Lejeune.
Our study also had several limitations. We only had diagnostic information for cohort members who received health care through the VHA or Medicare, which could have resulted in biased ascertainment of Camp Lejeune veterans because of their awareness of the contamination and the presumption that qualifies them for VA benefits if diagnosed with PD. We addressed this potential bias in multiple ways. We performed analyses that excluded PD ascertained after the 2017 federal announcement of the diagnostic presumption or that considered only cases that occurred in veterans who were already receiving VA health care at the time of their diagnosis. Importantly, we assessed risk of PD prodromal features both individually and in 2 cumulative risk models. None of these features would qualify veterans for benefits, and their association with a future PD diagnosis would not be known to most, reducing the risk for biased ascertainment among Camp Lejeune veterans. Furthermore, adjustment for duration of VHA health care usage did not attenuate associations between prodromal risk scores and residence at Camp Lejeune; rather, it strengthened them, arguing against differential VHA usage as a possible confounder. One would also expect biased ascertainment to exist for all forms of parkinsonism, yet we did not observe any increased risk of progressive supranuclear palsy, corticobasal degeneration, or multiple system atrophy at Camp Lejeune, disorders that likely have differing etiologies and have not been linked to TCE.
A highly plausible explanation for the association of residence at Camp Lejeune and risk of PD or prodromal PD is exposure to contaminated water. However, we cannot be certain that everyone who resided at Camp Lejeune between 1975 and 1985 was in fact exposed to biologically meaningful levels of contaminants, and we are unable to account for other environmental exposures that individuals from either camp may have sustained before, during, or after military service. However, inclusion of unexposed individuals in the Camp Lejeune cohort would tend to bias results toward the null. Differential exposure to environmental toxicants outside the camps could have occurred if former residents of Camp Lejeune or Camp Pendleton were more or less likely to be exposed to toxicants while deployed or if they were differentially exposed to toxicants where they lived and worked after separation from the military. We considered adjusting analyses for potential exposure to Agent Orange, but because its usage ended in the early 1970s, very few members of the cohort would have had the opportunity for exposure. Finally, although TCE was the VOC present in the Camp Lejeune water supply at the highest concentrations, the water also contained high levels of PCE, vinyl chloride, and benzene. These other compounds, or mixtures of compounds, could have contributed to the associations we observed.
Conclusions
This cohort study’s findings suggest that the risk of PD is 70% higher in veterans who were exposed to TCE and other VOCs 40 years ago. Trichloroethylene is a ubiquitous environmental contaminant used throughout the world since the 1920s. Many millions have been and continue to be exposed.
eTable 1. Neurodegenerative Parkinsonism Diagnostic Codes
eTable 2. Prodromal Feature Diagnostic Codes
eTable 3. Prodromal Feature Frequencies and Likelihood Ratios (LRs)
eTable 4. Full ATSDR Cohort Characteristics
eTable 5. Characteristics of Those With VHA and/or Medicare Usage (in Current Study)
eTable 6. Characteristics of Those Without VHA or Medicare Usage (Not in Current Study)
eTable 7. Prodromal Risk Score Associations With Camp in Persons Without PD or Other Neurodegenerative Parkinsonism
Data Sharing Statement
References
- 1.Goldman SM, Quinlan PJ, Ross GW, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71(6):776-784. doi: 10.1002/ana.22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gash DM, Rutland K, Hudson NL, et al. Trichloroethylene: parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63(2):184-192. doi: 10.1002/ana.21288 [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Choi DY, Hunter RL, et al. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem. 2010;112(3):773-783. doi: 10.1111/j.1471-4159.2009.06497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Miranda BR, Greenamyre JT. Trichloroethylene, a ubiquitous environmental contaminant in the risk for Parkinson’s disease. Environ Sci Process Impacts. 2020;22(3):543-554. doi: 10.1039/C9EM00578A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keane PC, Hanson PS, Patterson L, et al. Trichloroethylene and its metabolite TaClo lead to degeneration of substantia nigra dopaminergic neurones: effects in wild type and human A30P mutant α-synuclein mice. Neurosci Lett. 2019;711:134437. doi: 10.1016/j.neulet.2019.134437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Shin EJ, Dang DK, et al. Trichloroethylene and Parkinson’s disease: risk assessment. Mol Neurobiol. 2018;55(7):6201-6214. doi: 10.1007/s12035-017-0830-x [DOI] [PubMed] [Google Scholar]
- 7.Guehl D, Bezard E, Dovero S, Boraud T, Bioulac B, Gross C. Trichloroethylene and parkinsonism: a human and experimental observation. Eur J Neurol. 1999;6(5):609-611. doi: 10.1046/j.1468-1331.1999.650609.x [DOI] [PubMed] [Google Scholar]
- 8.Kochen W, Kohlmüller D, De Biasi P, Ramsay R. The endogeneous formation of highly chlorinated tetrahydro-beta-carbolines as a possible causative mechanism in idiopathic Parkinson’s disease. Adv Exp Med Biol. 2003;527:253-263. doi: 10.1007/978-1-4615-0135-0_29 [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Schaum J. Exposure assessment of trichloroethylene. Environ Health Perspect. 2000;108(Suppl 2)(suppl 2):359-363. doi: 10.1289/ehp.00108s2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toxicological profile for trichloroethylene. Agency for Toxic Substances and Disease Registry ; 2019. Accessed April 1, 2023. https://www.atsdr.cdc.gov/toxprofiles/tp19.pdf [PubMed] [Google Scholar]
- 11.Toxicological profile for tetrachloroethylene. Agency for Toxic Substances and Disease Registry ; 2019. Accessed April 1, 2023. https://www.atsdr.cdc.gov/ToxProfiles/tp18.pdf [PubMed] [Google Scholar]
- 12.Committee on Human Health Risks of Trichloroethylene . Assessing the Human Health Risks of Trichloroethylene. The National Academy of Sciences; 2006. [Google Scholar]
- 13.Bove FJ, Ruckart PZ, Maslia M, Larson TC. Mortality study of civilian employees exposed to contaminated drinking water at USMC Base Camp Lejeune: a retrospective cohort study. Environ Health. 2014;13:68. doi: 10.1186/1476-069X-13-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bove FJ, Ruckart PZ, Maslia M, Larson TC. Evaluation of mortality among marines and navy personnel exposed to contaminated drinking water at USMC Base Camp Lejeune: a retrospective cohort study. Environ Health. 2014;13(1):10. doi: 10.1186/1476-069X-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maslia ML, Sautner JB, Faye RE, et al. Chapter A, summary of findings. In: Analyses of Groundwater Flow, Contaminant Fate and Transport, and Distribution of Drinking Water at Tarawa Terrace and Vicinity, U.S. Marine Corps Base Camp Lejeune, North Carolina: Historical Reconstruction and Present-Day Conditions. Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services; 2007. [Google Scholar]
- 16.Agency for Toxic Substances and Disease Registry . Analyses and Historical Reconstruction of groundwater Flow, Contaminant Fate and Transport, and Distribution of Drinking Water Within the Service Areas of the Hadnot Point and Holcomb Boulevard Water Treatment Plants and Vicinities, U.S. Marine Corps Base Camp Lejeune, North Carolina. Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services; 2012. [Google Scholar]
- 17.Maslia ML, Suárez-Soto RJ, Sautner JB, et al. Analyses and Historical Reconstruction of Groundwater Flow, Contaminant Fate and Transport, and Distribution of Drinking Water Within the Service Areas of the Hadnot Point and Holcomb Boulevard Water Treatment Plants and Vicinities, U.S. Marine Corps Base Camp Lejeune, North Carolina. Agency for Toxic Substances and Disease Registry; 2013. [Google Scholar]
- 18.Safe Drinking Water Act (SDWA). US Environmental Protection Agency . Accessed April 1, 2023. https://www.epa.gov/sdwa
- 19.National primary drinking water regulations. Environmental Protection Agency ; 2009. Accessed April 1, 2023. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Organic
- 20.Department of Veterans Affairs . Diseases associated with exposure to contaminants in the water supply at Camp Lejeune, Final Rule. Fed Reg. 2017;82:4173-4185. [PubMed] [Google Scholar]
- 21.Agency for Toxic Substances and Disease Registry . Public health assessment for Marine Corps Base (MCB) Camp Pendleton. Camp Pendleton, San Diego County, California. Agency for Toxic Substances and Disease Registry; 2008. [Google Scholar]
- 22.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13 [DOI] [PubMed] [Google Scholar]
- 23.Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB; MDS Task Force on the Definition of Parkinson’s Disease . Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464-1470. doi: 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- 24.Corporate Data Warehouse (CDW). US Department of Veterans Affairs Health Services Research & Development . Updated March 16, 2022. Accessed December 29, 2022. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- 25.Gibb WR. Accuracy in the clinical diagnosis of parkinsonian syndromes. Postgrad Med J. 1988;64(751):345-351. doi: 10.1136/pgmj.64.751.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett PG, Chow A, Flores NE. Using Tobacco Health Factors Data for VA Health Services Research. Health Economics Resource Center; 2014. [Google Scholar]
- 27.Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018;16(1):34. doi: 10.1186/s12916-018-1016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611. doi: 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz A. Diagnostic test calculator. University of Illinois Chicago. Accessed November 7, 2022. http://araw.mede.uic.edu/cgi-bin/testcalc.pl [Google Scholar]
- 30.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006;60(1):65-72. doi: 10.1002/ana.20882 [DOI] [PubMed] [Google Scholar]
- 31.Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73(9):1062-1069. doi: 10.1001/jamaneurol.2016.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46(4):598-605. doi: [DOI] [PubMed] [Google Scholar]
- 33.Abbott RD, Ross GW, White LR, et al. Environmental, life-style, and physical precursors of clinical Parkinson’s disease: recent findings from the Honolulu-Asia Aging Study. J Neurol. 2003;250(suppl 3):III30-III39. doi: 10.1007/s00415-003-1306-7 [DOI] [PubMed] [Google Scholar]
- 34.Rocha E, Laar AV, Webb K, et al. Transient exposure of rats to rotenone causes delayed, progressive, parkinsonian motor deficits associated with nigrostriatal degeneration, progressive neuroinflammation and synucleinopathy. Research Square. Preprint posted online November 7, 2022. doi: 10.21203/rs.3.rs-2203458/v1 [DOI]
- 35.Sauerbeck A, Hunter R, Bing G, Sullivan PG. Traumatic brain injury and trichloroethylene exposure interact and produce functional, histological, and mitochondrial deficits. Exp Neurol. 2012;234(1):85-94. doi: 10.1016/j.expneurol.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherer TB, Richardson JR, Testa CM, et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100(6):1469-1479. doi: 10.1111/j.1471-4159.2006.04333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866-872. doi: 10.1289/ehp.1002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson JR, Caudle WM, Guillot TS, et al. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicol Sci. 2007;95(1):196-204. doi: 10.1093/toxsci/kfl133 [DOI] [PubMed] [Google Scholar]
- 39.De Miranda BR, Castro SL, Rocha EM, Bodle CR, Johnson KE, Greenamyre JT. The industrial solvent trichloroethylene induces LRRK2 kinase activity and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. Neurobiol Dis. 2021;153:105312. doi: 10.1016/j.nbd.2021.105312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Banerjee N, Liang Y, Wang G, Hoffman KL, Khan MF. Gut microbiome-host interactions in driving environmental pollutant trichloroethene-mediated autoimmunity. Toxicol Appl Pharmacol. 2021;424:115597. doi: 10.1016/j.taap.2021.115597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riederer P, Foley P, Bringmann G, Feineis D, Brückner R, Gerlach M. Biochemical and pharmacological characterization of 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline: a biologically relevant neurotoxin? Eur J Pharmacol. 2002;442(1-2):1-16. doi: 10.1016/S0014-2999(02)01308-0 [DOI] [PubMed] [Google Scholar]
- 42.Elkin ER, Bridges D, Loch-Caruso R. The trichloroethylene metabolite S-(1,2-dichlorovinyl)-L-cysteine induces progressive mitochondrial dysfunction in HTR-8/SVneo trophoblasts. Toxicology. 2019;427:152283. doi: 10.1016/j.tox.2019.152283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanai Y, Endou H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J Toxicol Sci. 2003;28(1):1-17. doi: 10.2131/jts.28.1 [DOI] [PubMed] [Google Scholar]
- 44.Toxicological review of trichloroethylene. US Environmental Protection Agency ; 2011. Accessed April 1, 2023. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0199tr/0199tr.pdf
- 45.C2 chlorinated solvents. S&P Global Commodity Insights ; August 2020. Accessed April 1, 2023. https://www.spglobal.com/commodityinsights/en/ci/products/c2-chlorinated-chemical-economics-handbook.html
- 46.International Agency for Research on Cancer . Trichloroethylene, Tetrachloroethylene, and Some Other Chlorinated Agents. International Agency for Research on Cancer, World Health Organization; 2014. [Google Scholar]
- 47.Bakke B, Stewart PA, Waters MA. Uses of and exposure to trichloroethylene in U.S. industry: a systematic literature review. J Occup Environ Hyg. 2007;4(5):375-390. doi: 10.1080/15459620701301763 [DOI] [PubMed] [Google Scholar]
- 48.TRI Explorer. US Environmental Protection Agency ; 2022. Accessed December 29, 2022. https://enviro.epa.gov/triexplorer/tri_release.chemical
- 49.National priorities list and superfund alternative approach sites. US Environmental Protection Agency ; 2022. Accessed December 29, 2022. https://www.epa.gov/superfund/search-superfund-sites-where-you-live
- 50.Nieves E. The superfund sites of Silicon Valley. New York Times. March 26, 2018.
- 51.Fleming-Jones ME, Smith RE. Volatile organic compounds in foods: a five year study. J Agric Food Chem. 2003;51(27):8120-8127. doi: 10.1021/jf0303159 [DOI] [PubMed] [Google Scholar]
- 52.Beamer PI, Luik CE, Abrell L, Campos S, Martínez ME, Sáez AE. Concentration of trichloroethylene in breast milk and household water from Nogales, Arizona. Environ Sci Technol. 2012;46(16):9055-9061. doi: 10.1021/es301380d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruckart PZ, Bove FJ, Maslia M. Evaluation of exposure to contaminated drinking water and specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: a case-control study. Environ Health. 2013;12:104. doi: 10.1186/1476-069X-12-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruckart PZ, Bove FJ, Maslia M. Evaluation of contaminated drinking water and preterm birth, small for gestational age, and birth weight at Marine Corps Base Camp Lejeune, North Carolina: a cross-sectional study. Environ Health. 2014;13:99. doi: 10.1186/1476-069X-13-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruckart PZ, Bove FJ, Shanley E III, Maslia M. Evaluation of contaminated drinking water and male breast cancer at Marine Corps Base Camp Lejeune, North Carolina: a case control study. Environ Health. 2015;14:74. doi: 10.1186/s12940-015-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marras C, Beck JC, Bower JH, et al. ; Parkinson’s Foundation P4 Group . Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21. doi: 10.1038/s41531-018-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Neurodegenerative Parkinsonism Diagnostic Codes
eTable 2. Prodromal Feature Diagnostic Codes
eTable 3. Prodromal Feature Frequencies and Likelihood Ratios (LRs)
eTable 4. Full ATSDR Cohort Characteristics
eTable 5. Characteristics of Those With VHA and/or Medicare Usage (in Current Study)
eTable 6. Characteristics of Those Without VHA or Medicare Usage (Not in Current Study)
eTable 7. Prodromal Risk Score Associations With Camp in Persons Without PD or Other Neurodegenerative Parkinsonism
Data Sharing Statement