This study assesses cardiac morphology and function at midgestation in fetuses of mothers prior to development of gestational diabetes and compares them with those of unaffected controls.
Key Points
Question
Is fetal cardiac remodeling evident prior to exposure to gestational diabetes (GD)?
Findings
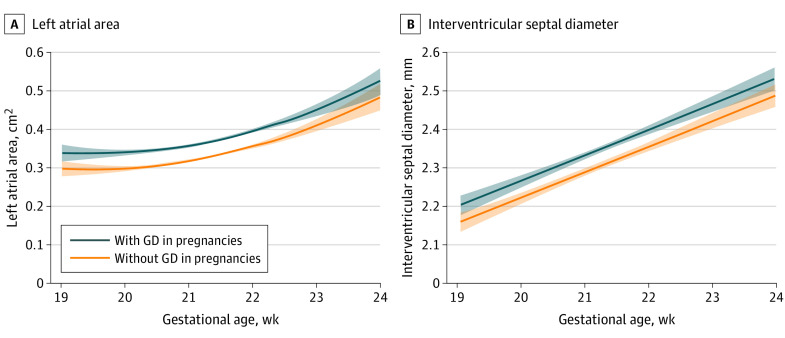
In this observational study, in pregnancies at risk for development of GD compared with those that were not, fetal interventricular thickness and left atrial area at midgestation were increased by 2.2% and 14%, respectively.
Meaning
In this study, prior to development of GD, there was evidence of fetal cardiac remodeling, suggesting that the adverse maternal underlying risk factor profile and not only exposure to glycemia contribute to fetal cardiac changes.
Abstract
Importance
Fetuses in women with gestational diabetes (GD) compared with those without GD show evidence of subclinical cardiac functional and morphological changes. However, it is uncertain whether glycemia or the adverse maternal underlying risk factor profile is the main driver for fetal cardiac remodeling.
Objective
To assess cardiac morphology and function at midgestation in fetuses of mothers prior to development of GD and compare them with those of unaffected controls.
Design, Setting, and Participants
During this prospective nonintervention screening study at 19 to 23 weeks’ gestation, fetal cardiac morphology and function were assessed in all participants. Pregnancy complications were obtained from the medical records of the women. Fetal cardiac morphology and function were assessed in all participants at Harris Birthright Research Institute at King’s College Hospital, London, United Kingdom. Participants included pregnant women with singleton pregnancy who attended their routine fetal ultrasound examination at midgestation and agreed to participate in the Advanced Cardiovascular Imaging Study in pregnancy.
Main Outcome and Measures
Comparison of fetal cardiac morphology and function between mothers who subsequently developed GD and those who did not develop GD.
Methods
This was a prospective nonintervention screening study of 5620 women with singleton pregnancies at 19 to 23 weeks’ gestation. Conventional and more advanced echocardiographic modalities, such as speckle tracking, were used to assess fetal cardiac function in the right and left ventricle. The morphology of the fetal heart was assessed by calculating the right and left sphericity index.
Results
The 5620 included patients had a mean age of 33.6 years. In 470 cases, the women were diagnosed with GD after the midgestation echocardiographic assessment (8.4%). Women with GD, compared with the non-GD group, were older, had higher BMI, higher prevalence of family history of diabetes, non-White ethnicity, chronic hypertension, and GD in a previous pregnancy. In fetuses of the GD group compared with the non-GD group, there was mild increase in interventricular millimeter thickness (0.04; 95% CI, 0.03-0.06 mm) and left atrial area (0.04; 95% CI, 0.04-0.05), whereas left and right functional indices were comparable between groups with the exception of left ventricular ejection fraction, which was marginally improved in the GD group (0.02; 95% CI, 0.03-0.03).
Conclusions and Relevance
This study demonstrates that prior to development of GD, there was mild alteration in fetal cardiac morphology without affecting cardiac function. This suggests that the adverse maternal risk factor profile and not only the glycemia might contribute to cardiac remodeling noted in fetuses of women with GD.
Introduction
Epidemiological studies have consistently demonstrated that exposure to maternal diabetes in utero increases the risk of cardiovascular disease in the offspring and cardiovascular changes appear from fetal life and extend to adolescence and adulthood.1,2 For example, our group has shown that fetuses of mothers with gestational diabetes (GD) have more globular hearts with an increase in right and left ventricular sphericity index and subclinical systolic cardiac dysfunction that persists in infancy.2 Other groups have documented an increase in left ventricular mass and left ventricular wall thickness in fetuses from mothers with diabetes and, in particular when diabetes was poorly controlled, the cardiovascular changes persisted in infancy and childhood.3,4,5 Higher systolic and mean arterial blood pressure has also been reported in children and adolescents in association with exposure to maternal diabetes and higher rates of premature cardiovascular heart disease have been shown in offspring of mothers with diabetes in a 40-year follow-up study in Denmark.6,7,8
The mechanisms of programming of cardiovascular alterations by maternal diabetes remain speculative.9 It is possible that exposure to glycemia could lead to increased secretion of fetal insulin, which can have adverse effects on fetal vascular gene expression and result in vascular and cardiac changes.10 Inflammatory and oxidative processes may also be involved to modify both gene expression and the vasculature.11 However, apart from glycemia, offspring of mothers with diabetes tend to be at increased risk of in utero exposure to many other classical cardiovascular risk factors, such as obesity and higher maternal blood pressure, which are commonly prevalent in mothers at risk of developing GD and these may also contribute to fetal cardiovascular remodeling.12,13 The objective of this screening study was to assess cardiac morphology and function in fetuses of mothers prior to development of GD and compare findings with a group of fetuses whose mothers did not develop GD, with the aim to determine whether maternal programming impact on the fetal heart is initiated prior to clinical development of GD.
Methods
Study Design and Participants
This was a prospective observational study in women attending a routine hospital visit at 19+1 to 23+3 weeks’ gestation at King’s College Hospital, London, United Kingdom, between August 2019 and December 2021. This visit included recording of maternal demographic characteristics and medical history and maternal echocardiography for assessment of cardiovascular function. Gestational age was determined by the measurement of fetal crown-rump length at 11 to 13 weeks or the fetal head circumference at 19 to 23 weeks.14,15 The women gave written informed consent to participate in the Advanced Cardiovascular Imaging Study (REC No. 18/NI/0013, IRAS ID:237936), which was approved by the National Health Service Research ethics committee. The reporting of the data follows Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Patient characteristics included maternal age, weight, height (which were measured at the time of screening), self-reported race (Black, East Asian, South Asian, White, and multiracial), method of conception (natural or assisted conception requiring in vitro fertilization or the use of ovulation drugs), history of chronic hypertension, diabetes, systemic lupus erythematosus or antiphospholipid syndrome, family history of diabetes (first- and second-degree relative) and obstetric history including parity (parous or nulliparous if no previous pregnancies at 24 or more weeks), previous pregnancy with GD, gestational age at delivery, and birth weight of the neonate in the last pregnancy.
The inclusion criteria for this study were singleton pregnancies delivering a nonmalformed liveborn or stillborn. We excluded pregnancies with aneuploidies and major fetal abnormalities, including congenital heart defects. We also excluded pregnancies where diagnosis of GD was made prior to fetal cardiac screening.
Screening Diagnosis of Gestational Diabetes
The diagnosis of GD in our hospital is based on the results of the oral glucose tolerance test (OGTT) with administration of 75 g of glucose; the diagnosis is made if the fasting plasma glucose level is 5.6 mmol/L or higher and/or the 2-hour plasma glucose level is 7.8 mmol/L or higher.16 The OGTT is carried out in 3 groups of women. First, women with any 1 risk factor (BMI higher than 30, previous birth of macrosomic baby weighing more than 4.5 kg, previous GD, first-degree relative with diabetes, or persistent glucosuria) are offered measurement of hemoglobin A1C at booking and if the value is 6.5% or higher, then they have an OGTT, usually at 12 weeks’ gestation. Second, in all women at 26 to 28 weeks’ gestation, a plasma glucose level is measured 1 to 2 hours after eating 50 g or more of carbohydrate and if the concentration is 6.7 mmol/L or higher, then OGTT is carried out. Third, after 28 weeks’ gestation, OGTT is performed if there is polyhydramnios or the fetus becomes macrosomic.
Fetal Cardiac Functional Analysis
A comprehensive fetal cardiac functional assessment was carried out using Canon Aplio i900 machines with a convex transducer (i8CX1; Canon Medical Systems Europe BV). Measurements were performed using conventional pulsed wave Doppler (PW-Doppler) and M-Mode, as well as more advanced imaging modalities, such as tissue Doppler imaging and speckle tracking echocardiography, as previously described.2 Right systolic ventricular function was assessed by measuring tricuspid annular plane systolic excursion and right ventricular global-longitudinal strain using speckle tracking. Left ventricular systolic function was assessed by calculating myocardial performance index and left ventricular global longitudinal strain (Figure 1). Image acquisition for speckle tracking analysis was performed in a 4-chamber view at an apex up or down projection.17 A clip of 3 to 5 seconds with a minimum of 100 frames per second was obtained for each case in accordance with recent guidelines and analysis was carried out using proprietary software (Vitrea; Canon Medical Systems), as previously described.17 Peak longitudinal strain for the left ventricle was obtained at the time of the aortic valve closure without obtaining measurements of postsystolic strain. Left ventricular diastolic function was assessed by calculating E/A ratio by measuring the mitral valve early (E) and late (A) diastolic filling peak Doppler velocities and E/e′ from tissue Doppler, as previously described.2
Figure 1. Fetal Cardiac Function and Structure.
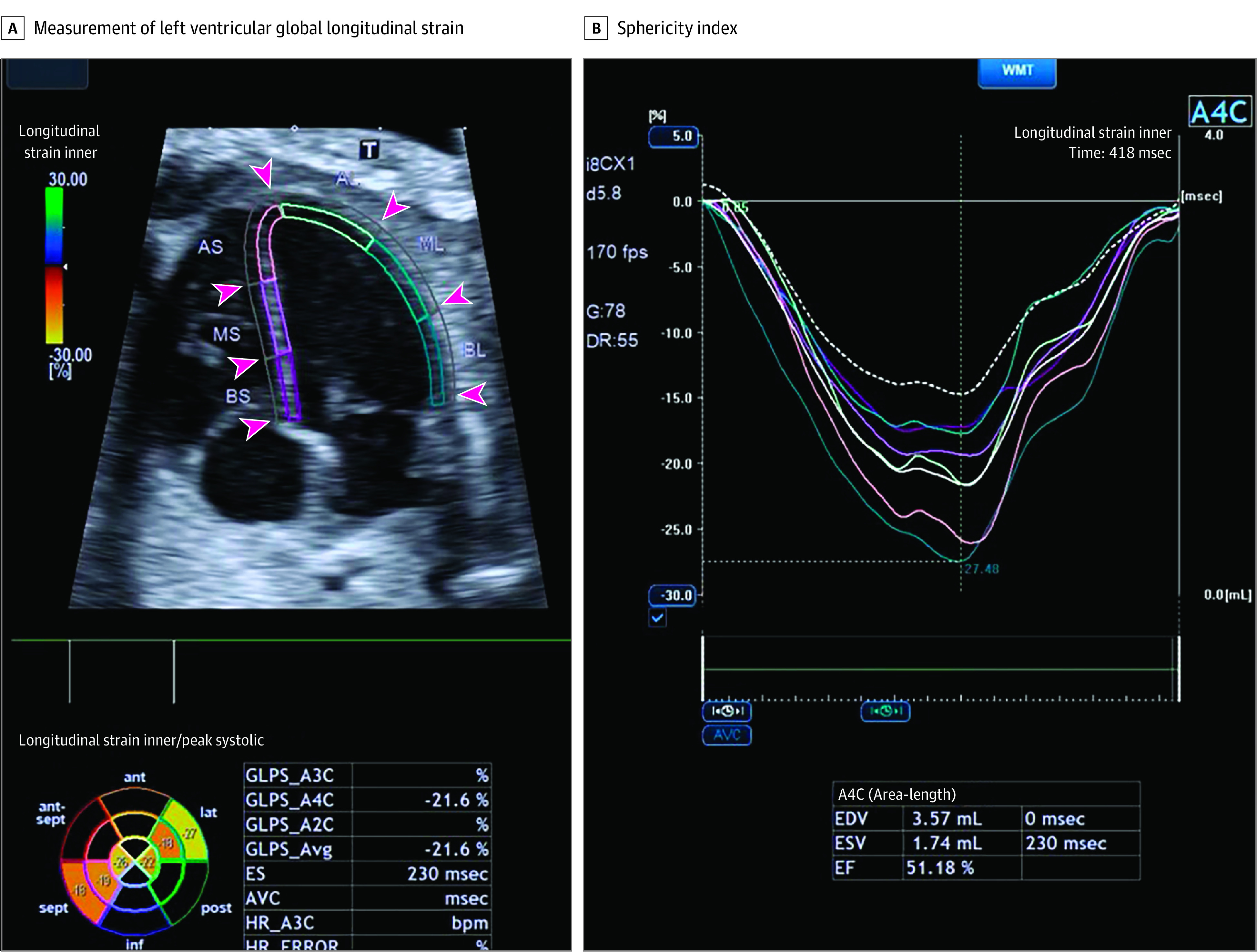
The endocardial borders are traced in the 4-chamber view and the mean peak longitudinal strain is calculated at the time of the aortic valve closure. The curves show the segmental deformation analysis and the different colors provide information about different segments. Additional measurements of end diastolic volume (EDV), end systolic volume (ESV), and ejection fraction (EF) are also performed. AL indicates anterior lateral; AS, anterior septal; BS, basal septal; BL, basal lateral; ES, end systole; GLPS, global longitudinal peak strain; HR, heart rate; ML, midlateral; MS, midseptal.
The morphology of the left and right ventricle was assessed in an apical or basal 4-chamber view, and the length and width of the left and right ventricles were measured in end-diastole (Figure 2). The sphericity index was calculated by dividing base-to-apex length by transverse diameter. The left atrial area was assessed in end diastole in the 4-chamber view and interventricular diastolic thickness in the septal view. Fetal cardiac examinations were carried out by 7 trained fetal medicine fellows (I.H. and L.M.C.) who also performed the analysis of the Doppler indices. Analysis of speckle tracking was carried out by 2 operators (I.H. and L.M.C.). Inter- and intra-analyzer reproducibility for the Doppler indices was assessed in 20 fetuses. We have previously reported on reproducibility of speckle tracking analysis.17
Figure 2. Sphericity Index.
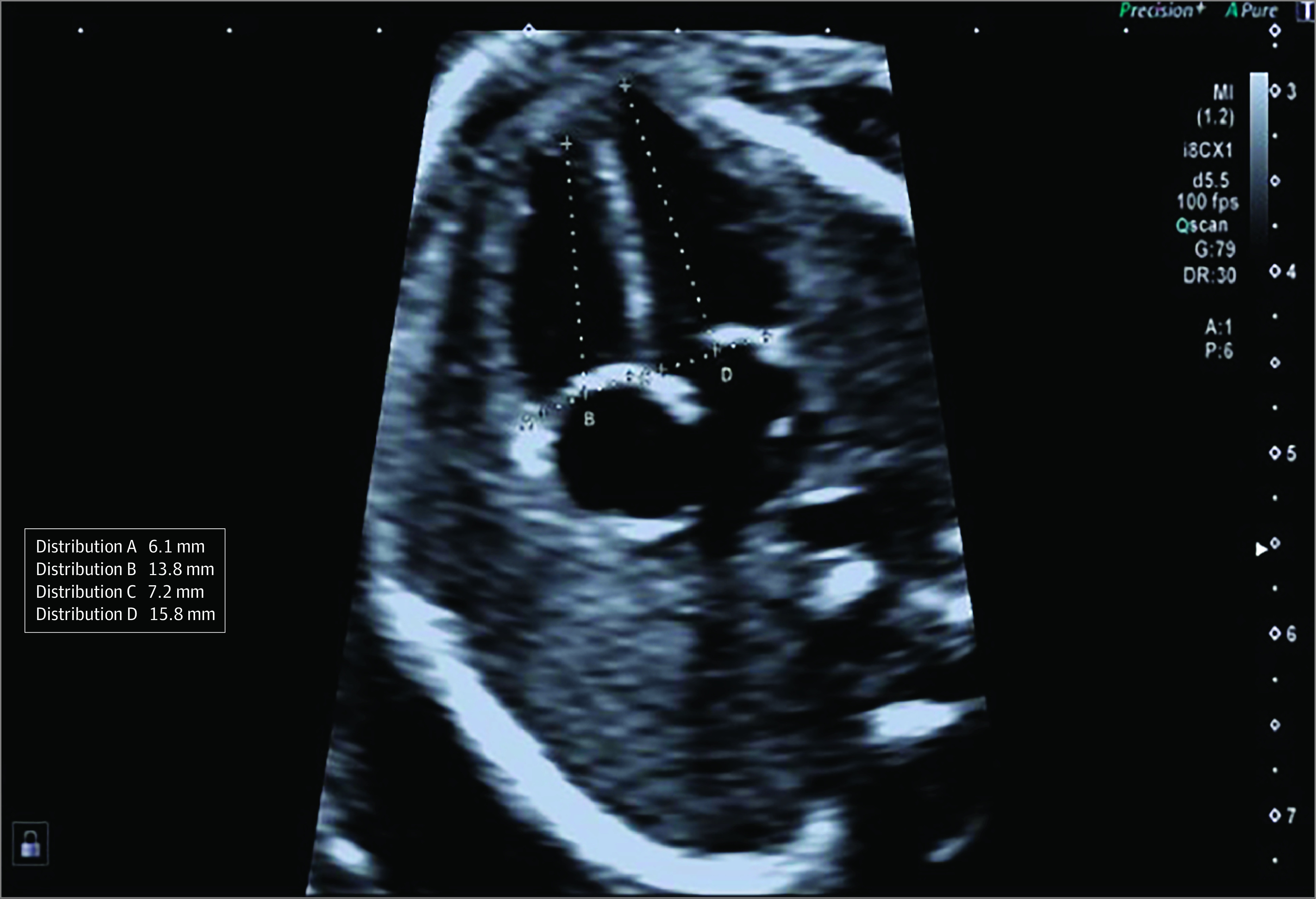
The length and the width of each ventricle was calculated in end diastole and sphericity index is calculated as base-to-apex length by transverse diameter.
Statistical Analysis
Data were expressed as median (IQR) for continuous variables and number (percentage) for categorical variables. t and χ2 Tests or Fisher exact test were used for comparing outcome groups for continuous and categorical data, respectively.
Multiple linear regression models were fitted to each of the 21 cardiac indices with terms for gestational age at measurement, maternal age, weight, height, race, heart rate, method of conception, diabetes, history of GD, history of chronic hypertension, systemic lupus erythematosus or antiphospholipid syndrome, and GD. These terms were prespecified on the basis of exploratory analysis and previous publication.18,19 Histograms were used to identify suitable transformations where appropriate and backward elimination was used for model selection.
These regression models were first used to assess the association between gestational age, maternal characteristics, and medical history of GD and preeclampsia, and each of the cardiac markers. Second, the partial residuals from the fitted models, after excluding the contribution of GD and preeclampsia, comprised either the deviations from the median (δ) or the log10 multiple of the median (MoM) values, depending on the transformation of the cardiac outcome variable in the original model fitting.20,21 Standardizing the indices into δ or MoMs allows us to observe the contribution of GD to each of the indices over and above gestational age and maternal characteristics and medical history. Median δ or MoMs with 95% CIs by GD status were calculated and compared. As this was an exploratory observational study, no adjustments for multiple comparisons were made. The statistical software package R version 4.1.2 (The R Project) was used for all data analyses.22
Results
Study Population
The study population of 5620 women with singleton pregnancies included 470 patients who were diagnosed with GD after the midgestation echocardiographic studies (8.4%). The GD group, compared with the non-GD group, had higher mean maternal age (33.9; 95% CI, 30.5-37.4) and BMI (28.2; 95% CI, 25.2-32.8) and a higher proportion of family history of diabetes (107 patients [11.3%]); Black (94 patients [20.0%]), East Asian (30 patients [6.4%]), and South Asian women (45 patients [9.6%]), chronic hypertension (19 patients [4.0%]); and GD in a previous pregnancy (41 patients [8.7%]) (Table 1). In women with GD, 237 (50%) were treated with insulin and/or metformin, while the remaining were treated with diet alone.
Table 1. Maternal and Pregnancy Characteristics of the Study Population.
| Characteristic | Unaffected by GD (n = 5150) | GD (n = 470) | P value |
|---|---|---|---|
| Maternal age, mean, y (95% CI) | 33.2 (30.1-36.2) | 33.9 (30.5-37.4) | .001 |
| Maternal weight, mean, kg (95% CI) | 70.1 (63.2-79.0) | 76.8 (67.0-88.9) | <.001 |
| Maternal height, mean, cm (95% CI) | 166 (162.0-170.0) | 165 (160.0-169.0) | <.001 |
| BMIa (95% CI) | 25.4 (23.0-28.6) | 28.3 (25.2-32.8) | <.001 |
| Gestational age, mean, wk (95% CI) | 21.3 (20.9-21.6) | 21.3 (20.9-21.6) | .94 |
| Race, No. (%)b | |||
| Black | 710 (13.8) | 94 (20.0) | <.001 |
| East Asian | 119 (2.3) | 30 (6.4) | |
| South Asian | 289 (5.6) | 45 (9.6) | |
| White | 3853 (74.8) | 283 (60.2) | |
| Multiracial | 179 (3.5) | 18 (3.8) | |
| Medical history, No. (%) | |||
| Chronic hypertension | 70 (1.4) | 19 (4.0) | <.001 |
| SLE/APS | 16 (0.3) | 0 | .45 |
| Smoker, No. (%) | 63 (1.2) | 2 (0.4) | .19 |
| Family history of preeclampsia, No. (%) | 165 (3.2) | 17 (3.6) | .08 |
| Family history of diabetes, No. (%) | |||
| 1st Degree | 504 (9.8) | 65 (13.8) | .004 |
| 2nd Degree | 343 (6.7) | 42 (8.9) | |
| Method of conception, No. (%) | |||
| Spontaneous | 4813 (93.5) | 442 (94.0) | .01 |
| In vitro fertilization, fresh | 104 (2.0) | 5 (1.1) | |
| In vitro fertilization, frozen | 199 (3.9) | 23 (4.9) | |
| Ovulation drugs | 34 (0.7) | 0 | |
| Parity, No. (%) | |||
| Nulliparous | 2854 (55.4) | 235 (50.0) | <.001 |
| Parous, no previous GD | 2255 (43.8) | 194 (41.3) | |
| Parous, previous GD | 41 (0.8) | 41 (8.7) |
Abbreviations: APS, antiphospholipid syndrome; GD, gestational diabetes; SLE, systemic lupus erythematosus.
Calculated as weight in kilograms divided by height in meters squared.
Race was self-reported.
Maternal Characteristics and Fetal Cardiac Changes in the GD and Non-GD Groups
In fetuses of women with GD compared with the non-GD group, interventricular thickness and left atrial area were increased (Figure 3), whereas functional indices were comparable between groups with the exception of left ventricular ejection fraction, which was marginally increased in the GD group (Table 2). Maternal weight was significantly associated with right ventricular sphericity index and global myocardial functional index of myocardial performance index, as well as the more sensitive systolic cardiac markers of left ventricular global longitudinal systolic strain (eTable in Supplement 1). Maternal age, in vitro fertilization, and blood pressure were also associated with fetal cardiac morphological and functional indices (eTable in Supplement 1). Fetuses of mothers with GD who developed more severe disease, as shown by the use of pharmacological treatment, ie, insulin or metformin, had no significant differences in fetal cardiac structural or functional changes compared with fetuses of mothers with GD treated with diet alone (Table 2).
Figure 3. Fetal Cardiac Function in Gestational Diabetes (GD).
The association and 95% CIs between left trial area (A) and interventricular septal diameter (B) with GD in pregnancies and without GD is illustrated.
Table 2. δ Values of Fetal Cardiac Indices in the Pregnancies With And Without Gestational Diabetes (GD) and in the GD Group According To Treatment For Hyperglycemia.
| Mean, δ (95% CI) | |||||
|---|---|---|---|---|---|
| Characteristic | No GD (n = 5150) | GD (n = 470) | GD diet (n = 233) | GD metformin (n = 170) | GD insulin (n = 67) |
| Morphometry | |||||
| Right ventricular sphericity index | −0.01 (−0.01 to 1.01) | 0.01 (−0.03 to 0.04) | 0.01 (−0.04 to 0.06) | 0.02 (−0.05 to 0.08) | −0.03 (−0.11 to 0.05) |
| Left ventricular sphericity index | 0 (−0.01 to 0.02) | −0.04 (−0.08 to −0) | −0.04 (−0.09 to 0.01) | −0.01 (−0.09 to 0.06) | −0.10 (−0.19 to −0.01) |
| Longitudinal left ventricular diameter, mm | 0.01 (−0.05 to 0.06) | −0.01 (−0.2 to 0.2) | 0.04 (−0.2 to 0.30) | −0.02 (−0.3 to 0.30) | −0.15 (−0.60 to 0.30) |
| Transverse left ventricular diameter, mm | −0.01 (−0.04 to 0.02) | 0.08 (−0.02 to 0.18) | 0.09 (−0.05 to 0.22) | 0.03 (−0.1 to 0.20) | 0.20 (−0.05 to 0.45) |
| Longitudinal right ventricular diameter, mm | .02 (−0.04 to 0.05) | 0.12 (−0.04 to 0.27) | 0.2 (−0.03 to 0.41) | 0.03 (−0.22 to 0.29) | 0.07 (−0.34 to 0.47) |
| Transverse right ventricular diameter, mm | −0.01 (−0.04 to 0.02) | 0.06 (−0.04 to 0.16) | 0.08 (−0.07 to 0.22) | −0.02 (−0.15 to 0.12) | 0.17 (−0.12 to 0.47) |
| Interventricular septal diameter in diastole, mm | 0 (−0.01 to 0.01) | 0.04 (0.03-0.05)a | 0.04 (0.03-0.05) | 0.05 (0.04-0.06) | 0.04 (0.03-0.05) |
| Diastolic indices | |||||
| Mitral valve, early, cm/s | 0 (−0.15 to 0.15) | −0.01 (−0.55 to 0.53) | −0.5 (−1.3 to 0.2) | 0.6 (−0.2 to 1.4) | 0.26 (−1.33 to 1.84) |
| Mitral valve, late, cm/s | 0.04 (−0.17 to 0.25) | −0.42 (−1.12 to 0.28) | −0.8 (−1.7 to 0.1) | 0.4 (−0.6 to 1.5) | −1.27 (−3.72 to 1.18) |
| Mitral valve early/late | .04 −.04 to .03) | 0.01 (0-0.01) | −0.02 (−0.02 to 0.02) | 0.01 (−0.01 to 0.03) | 0.02 (−0.01 to 0.05) |
| E/e′ ratio | 0.01 (−0.06 to 0.07) | −0.18 (−0.38. 0.03) | −0.4 (−0.6 to −0.1) | 0.1 (−0.2 to 0.4) | −0.28 (−1.00 to 0.44) |
| Left atrial area, cm2 | 0.0 (−.02 to .02) | 0.04 (0.04-0.05)a | 0.04 (0.03-0.05) | 0.04 (0.03-0.04) | 0.05 (0.04-0.06) |
| Isovolumic relaxation time, m | 0.02 (−0.04 to 0.04) | −0.02 (−0.03 to −0.02)a | −0.002 (−0.03 to −0.01) | −0.02 (−0.04 to −0.01) | −0.04 (−0.06 to −0.02) |
| Systolic indices | |||||
| Myocardial performance index | 0.03 (−0.02 to 0.03) | −0.04 (−0.01 to 0.03) | 0.02 (−0.01 to 0.01) | −0.01 (−0.02 to 0.01) | −0.01 (−0.03 to 0.02) |
| Tricuspid annular plane systolic excursion, mm | −0.01 (−0.03 to 0.02) | 0.06 (−0.01 to 0.13) | 0.1 (0.01-0.19) | −0.03 (−0.14 to 0.09) | 0.11 (−0.08 to 0.30) |
| Isovolumic contraction time, m | −0.03 (−0.02 to 0.01) | 0.03 (−0.02 to 0.01) | 0.02 (0.01-0.03) | −0.01 (−0.02 to 0) | −0.02 (−0.03 to −0.05) |
| Ejection time, s | 0.01 (−0.03 to 0.04) | 0.01 (−0.01 to 0.04) | −0.02 (−0.03 to −0.04) | 0.01 (−0.04 to 0.02) | −0.01 (−0.03 to 0.02) |
| Speckle tracking | |||||
| Right ventricular global longitudinal strain, % | −0.02 (−0.16 to 0.12) | 0.22 (−0.24 to 0.68) | 0.1 (−0.6 to 0.8) | 0.3 (−0.4 to 1.1) | 0.32 (−0.81 to 1.45) |
| Left ventricular global longitudinal strain, % | −0.03 (−0.25 to 0.19) | 0.3 (−0.4 to 1.0) | 0.2 (−0.9 to 1.3) | 0.1 (−1.01 to 1.29) | 1.09 (−0.58 to 2.75) |
| Right ventricular ejection fraction, % | −0.01 (−0.02 to 0.01) | 0.07 (−0.01 to 0.02) | 0.02 (−0.01 to 0.01) | 0.01 (0.02-0.02) | 0.02 (−0.01 to 0.04) |
| Left ventricular ejection fraction, % | −0.01 (−0.01 to 0.04) | 0.02 (0.01-0.03)a | 0.02 (−0.01 to 0.04) | 0.02 (−0.04 to 0.04) | 0.04 (−0.03 to 0.04) |
Significantly different from the no GD group.
Discussion
Main Findings of the Study
This cross-sectional, observational study demonstrates that there is evidence of cardiac remodeling at midgestation in fetuses of women who subsequently develop GD with a mild increase in interventricular thickness and left atrial area. Fetal ventricular morphology and systolic and diastolic right and left ventricular function in the group that developed GD were similar to those in the non-GD group with the exception of left ventricular ejection fraction, which was marginally improved in the GD group. These findings suggest that the adverse maternal risk factor profile rather than glycemia alone contribute to fetal cardiac programming in women with GD.
Comparison With Results of Previous Studies
In this study, we elected to perform detailed fetal cardiac assessment using conventional and more advanced techniques, such as speckle tracking, to identify early preclinical cardiac changes. The reason for this choice is that we and others have previously shown that in GD fetal cardiac changes are subtle and are not detectable by conventional Doppler techniques.2,23,24
Most of the previous studies were performed in the third trimester and reported mostly right ventricular systolic dysfunction in fetuses of mothers with GD, whereas contradictory results were reported for the left ventricle.2,3,23,24,25 Gestational age and glycemic control appeared to be important determinants for cardiac alterations, which suggests that longer and greater exposure to a glycemic insult can adversely affect fetal cardiac function.23
Earlier in gestation, Wang et al26 reported impaired contractility in both the left and right ventricle at 24 to 27 weeks’ gestation. Atiq et al27 reported at 19 to 24 weeks’ gestation that the functional cardiac variables myocardial performance index and left ventricular mitral E/A ratios were adversely altered in the GD group.27 Our study was performed at 19 to 23 weeks’ gestation, prior to the diagnosis of GD. From the various fetal cardiac indices, only interventricular thickness and left atrial area were marginally increased in the GD group. Current knowledge suggests that fetal myocardial hypertrophy is likely to be the consequence of both fetal hyperinsulinemia and also due to increased expression and affinity of insulin receptors, which subsequently lead to proliferation and hypertrophy of cardiac myocytes.28,29 Additionally, it has been postulated that fluctuations in glucose values, rather than basal state, may also be important determinants of fetal cardiac alterations in maternal diabetes.30 Considering that none of the women in our study had the diagnosis of GD at the time of fetal cardiac screening, our data suggest that maternal characteristics might drive the noted cardiac alterations. In addition, a marginal increase in left ventricular ejection fraction was noted in the GD group; however, no clear explanation for this finding could be identified. We also examined whether fetuses of mothers who developed more severe GD, using as proxy diabetic treatment, have a different cardiac phenotype at midgestation but this could not be confirmed from our study.
Women at risk of GD often have a constellation of adverse cardiovascular risk factors, including being older, obese, and with higher blood pressure, and there is often a family history of diabetes.12,31 Exposure to these cardiovascular risk factors has been associated with metabolic abnormalities and increased inflammation, which may adversely affect placental physiology and impact the fetal heart. Consistent with previous reports, in our study, women at risk of GD had increased weight and BMI and were older than those in the non-GD group. The proportion of women of Black and Asian race was also higher in women with GD. We found that several maternal characteristics had an adverse effect on a variety of fetal cardiac functional indices irrespective of whether the women developed GD or not.
Strengths and Limitations
The main strengths of our study are (1) unselected population of women who attended routine antenatal scan at 19 to 23 weeks’ gestation, (2) fetal cardiac functional assessment was performed using the same strict protocol to minimize variability of results, and (3) adjustment of results for maternal characteristics to be able to identify the presence of unknown pathways that might contribute to fetal cardiac remodeling prior to GD development and demonstrated that exposure to hyperglycemia is key to stimulate fetal cardiac alterations. Our main limitation is that our study is cross-sectional; thus, we can only speculate from our studies and from those from other groups that exposure to hyperglycemia is key to stimulate fetal cardiac alterations. In addition, it is possible that longer exposure to maternal adverse risk factors may also differentially affect fetal cardiac function later in gestation in pregnancies of mothers with GD compared with non-GD mothers. In addition, we followed NICE guidelines for diabetes screening during pregnancy; however, it is possible that some women may have developed GD before their GD screening and this might have contributed to small anatomical fetal cardiac changes that were identified in this study.
Conclusions
In this cross-section, the offspring of women with GD were at high risk for development of cardiovascular disease in childhood and early adulthood. Our study demonstrated that, at midgestation, there were mild changes in fetal cardiac morphology prior to GD diagnosis whereas functional alterations are evident with exposure to glycemia. This finding suggests that maternal characteristics, as well as possible fluctuations in maternal glucose levels, might contribute to fetal cardiac remodeling process.
eTable. Fitted regression models for each of the studied cardiac indices
Data sharing statement
References
- 1.Yu Y, Arah OA, Liew Z, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. doi: 10.1136/bmj.l6398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera J, Semmler J, Anzoategui S, Zhang H, Nicolaides KH, Charakida M. Cardiac function in gestational diabetes mellitus: a longitudinal study from fetal life to infancy. BJOG. 2021;128(2):272-279. doi: 10.1111/1471-0528.16434 [DOI] [PubMed] [Google Scholar]
- 3.Miranda JO, Cerqueira RJ, Ramalho C, Areias JC, Henriques-Coelho T. Fetal cardiac function in maternal diabetes: a conventional and speckle-tracking echocardiographic study. J Am Soc Echocardiogr. 2018;31(3):333-341. doi: 10.1016/j.echo.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Do V, Al-Hashmi H, Ojala T, et al. Cardiovascular health of offspring of diabetic mothers from the fetal through late-infancy stages. JACC Cardiovasc Imaging. 2019;12(5):932-934. doi: 10.1016/j.jcmg.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 5.Do V, Eckersley L, Lin L, et al. Persistent aortic stiffness and left ventricular hypertrophy in children of diabetic mothers. CJC Open. 2020;3(3):345-353. doi: 10.1016/j.cjco.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr. 2000;136(5):587-592. doi: 10.1067/mpd.2000.105129 [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Zhang S, Li W, et al. Maternal gestational diabetes is associated with offspring’s hypertension. Am J Hypertens. 2019;32(4):335-342. doi: 10.1093/ajh/hpz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathirana MM, Lassi ZS, Roberts CT, Andraweera PH. Cardiovascular risk factors in offspring exposed to gestational diabetes mellitus in utero: systematic review and meta-analysis. J Dev Orig Health Dis. 2020;11(6):599-616. doi: 10.1017/S2040174419000850 [DOI] [PubMed] [Google Scholar]
- 9.Higa R, Leonardi ML, Jawerbaum A. Intrauterine programming of cardiovascular diseases in maternal diabetes. Front Physiol. 2021;12:760251. doi: 10.3389/fphys.2021.760251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146(12):5341-5349. doi: 10.1210/en.2005-0938 [DOI] [PubMed] [Google Scholar]
- 11.de Mendonça ELSS, Fragoso MBT, de Oliveira JMX, Xavier JA, Goulart MOF, de Oliveira ACM. Gestational diabetes mellitus: the crosslink among inflammation, nitroxidative stress, intestinal microbiota and alternative therapies. Antioxidants (Basel). 2022;11(1):129. doi: 10.3390/antiox11010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart. 2013;99(15):1118-1121. doi: 10.1136/heartjnl-2013-303945 [DOI] [PubMed] [Google Scholar]
- 13.Meyer K, Zhang, L. Fetal programming of cardiac function and disease. Reprod Sci. 2007;14(3):209-216. doi: 10.1177/1933719107302324 [DOI] [PubMed] [Google Scholar]
- 14.Robinson HP, Fleming JE. A critical evaluation of sonar “crown-rump length” measurements. Br J Obstet Gynaecol. 1975;82(9):702-710. doi: 10.1111/j.1471-0528.1975.tb00710.x [DOI] [PubMed] [Google Scholar]
- 15.Lesmes C, Gallo DM, Panaiotova J, Poon LC, Nicolaides KH. Prediction of small-for-gestational-age neonates: screening by fetal biometry at 19-24 weeks. Ultrasound Obstet Gynecol. 2015;46(2):198-207. doi: 10.1002/uog.14826 [DOI] [PubMed] [Google Scholar]
- 16.Walker JD. NICE guidance on diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabet Med. 2008;25(9):1025-1027. doi: 10.1111/j.1464-5491.2008.02532.x [DOI] [PubMed] [Google Scholar]
- 17.Semmler J, Day TG, Georgiopoulos G, et al. Fetal speckle-tracking: impact of angle of insonation and frame rate on global longitudinal strain. J Am Soc Echocardiogr. 2020;33(9):1141-1146.e2. doi: 10.1016/j.echo.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 18.Semmler J, Abdel-Azim S, Anzoategui S, Zhang H, Nicolaides KH, Charakida M. Influence of birth weight on fetal cardiac indices at 35-37 weeks’ gestation. Ultrasound Obstet Gynecol. 2021;57(2):266-272. doi: 10.1002/uog.23522 [DOI] [PubMed] [Google Scholar]
- 19.Semmler J, Garcia-Gonzalez C, Sanchez Sierra A, Gallardo Arozena M, Nicolaides KH, Charakida M. Fetal cardiac function at 35-37 weeks’ gestation in pregnancies that subsequently develop pre-eclampsia. Ultrasound Obstet Gynecol. 2021;57(3):417-422. doi: 10.1002/uog.23521 [DOI] [PubMed] [Google Scholar]
- 20.O’Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214(1):103.e1-103.e12. doi: 10.1016/j.ajog.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 21.Wright D, Wright A, Nicolaides KH. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol. 2020;223(1):12-23.e7. doi: 10.1016/j.ajog.2019.11.1247 [DOI] [PubMed] [Google Scholar]
- 22.The R Project . Getting started. Accessed April 7, 2023. https://www.r-project.org/
- 23.Aguilera J, Semmler J, Coronel C, et al. Paired maternal and fetal cardiac functional measurements in women with gestational diabetes mellitus at 35-36 weeks’ gestation. Am J Obstet Gynecol. 2020;223(4):574.e1-574.e15. doi: 10.1016/j.ajog.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 24.Patey O, Carvalho JS, Thilaganathan B. Perinatal changes in fetal cardiac geometry and function in diabetic pregnancy at term. Ultrasound Obstet Gynecol. 2019;54(5):634-642. doi: 10.1002/uog.20187 [DOI] [PubMed] [Google Scholar]
- 25.Yovera L, Zaharia M, Jachymski T, et al. Impact of gestational diabetes mellitus on fetal cardiac morphology and function: cohort comparison of second- and third-trimester fetuses. Ultrasound Obstet Gynecol. 2021;57(4):607-613. doi: 10.1002/uog.22148 [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Liu C, Liu X, Zhang Y, Wang Y. Evaluation of prenatal changes in fetal cardiac morphology and function in maternal diabetes mellitus using a novel fetal speckle-tracking analysis: a prospective cohort study. Cardiovasc Ultrasound. 2021;19(1):25. doi: 10.1186/s12947-021-00256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atiq M, Ikram A, Hussain BM, Saleem B. Assessment of cardiac function in fetuses of gestational diabetic mothers during the second trimester. Pediatr Cardiol. 2017;38(5):941-945. doi: 10.1007/s00246-017-1600-2 [DOI] [PubMed] [Google Scholar]
- 28.Breitweser JA, Meyer RA, Sperling MA, Tsang RC, Kaplan S. Cardiac septal hypertrophy in hyperinsulinemic infants. J Pediatr. 1980;96(3 Pt 2):535-539. doi: 10.1016/S0022-3476(80)80862-6 [DOI] [PubMed] [Google Scholar]
- 29.Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med. 1994;45:245-260. doi: 10.1146/annurev.med.45.1.245 [DOI] [PubMed] [Google Scholar]
- 30.Greco P, Vimercati A, Scioscia M, Rossi AC, Giorgino F, Selvaggi L. Timing of fetal growth acceleration in women with insulin-dependent diabetes. Fetal Diagn Ther. 2003;18(6):437-441. doi: 10.1159/000073139 [DOI] [PubMed] [Google Scholar]
- 31.Aguilera J, Sanchez Sierra A, Abdel Azim S, Georgiopoulos G, Nicolaides KH, Charakida M. Maternal cardiac function in gestational diabetes mellitus at 35-36 weeks’ gestation and 6 months postpartum. Ultrasound Obstet Gynecol. 2020;56(2):247-254. doi: 10.1002/uog.22118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Fitted regression models for each of the studied cardiac indices
Data sharing statement