This comparative effectiveness research study compares autologous hematopoietic stem cell transplant (AHSCT) vs fingolimod, natalizumab, and ocrelizumab for patients with relapsing-remitting multiple sclerosis.
Key Points
Question
What is the comparative effectiveness of autologous hematopoietic stem cell transplant (AHSCT) vs individual most potent disease-modifying therapies for relapsing-remitting multiple sclerosis (MS), such as natalizumab or ocrelizumab?
Findings
In this observational comparative effectiveness study of 4915 individuals using a composite cohort from specialized MS centers and the MSBase international registry, the effectiveness of AHSCT was compared with 1 medium-efficacy and 2 high-efficacy disease-modifying therapies (fingolimod, natalizumab, and ocrelizumab) in patients with relapsing-remitting MS, high frequency of relapses, and moderate disability. Over 5 years, AHSCT was associated with substantially lower relapse rate than fingolimod and marginally lower relapse rate than natalizumab and was also associated with a higher rate of recovery from disability compared with fingolimod and natalizumab, but no evidence of difference in clinical outcomes between AHSCT and ocrelizumab was found at 3-year follow-up.
Meaning
The results indicate that in relapsing-remitting MS, the clinical effectiveness of AHSCT is considerably superior to fingolimod and marginally superior to natalizumab.
Abstract
Importance
Autologous hematopoietic stem cell transplant (AHSCT) is available for treatment of highly active multiple sclerosis (MS).
Objective
To compare the effectiveness of AHSCT vs fingolimod, natalizumab, and ocrelizumab in relapsing-remitting MS by emulating pairwise trials.
Design, Setting, and Participants
This comparative treatment effectiveness study included 6 specialist MS centers with AHSCT programs and international MSBase registry between 2006 and 2021. The study included patients with relapsing-remitting MS treated with AHSCT, fingolimod, natalizumab, or ocrelizumab with 2 or more years study follow-up including 2 or more disability assessments. Patients were matched on a propensity score derived from clinical and demographic characteristics.
Exposure
AHSCT vs fingolimod, natalizumab, or ocrelizumab.
Main outcomes
Pairwise-censored groups were compared on annualized relapse rates (ARR) and freedom from relapses and 6-month confirmed Expanded Disability Status Scale (EDSS) score worsening and improvement.
Results
Of 4915 individuals, 167 were treated with AHSCT; 2558, fingolimod; 1490, natalizumab; and 700, ocrelizumab. The prematch AHSCT cohort was younger and with greater disability than the fingolimod, natalizumab, and ocrelizumab cohorts; the matched groups were closely aligned. The proportion of women ranged from 65% to 70%, and the mean (SD) age ranged from 35.3 (9.4) to 37.1 (10.6) years. The mean (SD) disease duration ranged from 7.9 (5.6) to 8.7 (5.4) years, EDSS score ranged from 3.5 (1.6) to 3.9 (1.9), and frequency of relapses ranged from 0.77 (0.94) to 0.86 (0.89) in the preceding year. Compared with the fingolimod group (769 [30.0%]), AHSCT (144 [86.2%]) was associated with fewer relapses (ARR: mean [SD], 0.09 [0.30] vs 0.20 [0.44]), similar risk of disability worsening (hazard ratio [HR], 1.70; 95% CI, 0.91-3.17), and higher chance of disability improvement (HR, 2.70; 95% CI, 1.71-4.26) over 5 years. Compared with natalizumab (730 [49.0%]), AHSCT (146 [87.4%]) was associated with marginally lower ARR (mean [SD], 0.08 [0.31] vs 0.10 [0.34]), similar risk of disability worsening (HR, 1.06; 95% CI, 0.54-2.09), and higher chance of disability improvement (HR, 2.68; 95% CI, 1.72-4.18) over 5 years. AHSCT (110 [65.9%]) and ocrelizumab (343 [49.0%]) were associated with similar ARR (mean [SD], 0.09 [0.34] vs 0.06 [0.32]), disability worsening (HR, 1.77; 95% CI, 0.61-5.08), and disability improvement (HR, 1.37; 95% CI, 0.66-2.82) over 3 years. AHSCT-related mortality occurred in 1 of 159 patients (0.6%).
Conclusion
In this study, the association of AHSCT with preventing relapses and facilitating recovery from disability was considerably superior to fingolimod and marginally superior to natalizumab. This study did not find evidence for difference in the effectiveness of AHSCT and ocrelizumab over a shorter available follow-up time.
Introduction
Chemotherapy followed by autologous hematopoietic stem cell transplant (AHSCT) is a potent immunosuppressant/immune-reconstitution therapy that is occasionally used to treat highly inflammatory multiple sclerosis (MS) with suboptimal response to conventional disease-modifying therapies (DMTs). As a result of ablation and subsequent reconstitution of the immune system, it is particularly effective in temporarily eliminating neuroinflammation within the central nervous system.1 Single-arm cohort studies reported prolonged freedom from relapses and worsening of disability in aggressive MS post-AHSCT.2,3,4,5,6 Only 1 open-label randomized clinical trial compared the efficacy of AHSCT with a combination of DMT and non-DMT interventions in relapsing-remitting MS.7
AHSCT is associated with significant risks, including early complications of immune ablation and 0.3% to 2% treatment-related mortality.1,8 The risk of death has declined over recent years, mainly as a result of improved patient selection and transplant center experience.9 Therefore, AHSCT represents a higher-risk but potentially higher-yield therapy with long-term benefit. However, to define the role of AHSCT in active MS, we need to understand its comparative effectiveness relative to the most effective available DMTs. High-quality cohorts have helped establish the comparative effectiveness among DMTs.10,11,12,13,14,15 Emulation of clinical trials in existing data sets supports treatment decisions, especially where randomized trials would not be feasible.16,17 A scenario ideally suited to this approach is a comparison of AHSCT with high-efficacy DMTs.18,19
In this study, we emulated a clinical trial that compared clinical effectiveness of AHSCT with 2 high-efficacy DMTs (natalizumab, ocrelizumab) and 1 moderate-efficacy DMT (fingolimod).
Methods
Patients and Data
Data recorded between May 2006 and December 2021 were obtained from 6 cohorts treated with AHSCT at specialized centers (in Ottawa, Canada; Uppsala, Sweden; Sheffield, UK; Bergen, Norway; Sydney, Australia; and Melbourne, Australia) and 94 centers in 27 countries from the MSBase registry. The study was approved by the Melbourne Health Human Research Ethics Committee and the site institutional review boards. Patients provided written informed consent, as required. The data are the property of the individual centers; they can be requested for replication of this study, at the discretion of each principal investigator. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The inclusion criteria were definite relapsing-remitting MS,20,21,22 first exposure to one of the study therapies, no exposure to alemtuzumab or participation in randomized clinical trials within the prior 10 years, minimum recorded follow-up 2 months prior to treatment start and 2 postbaseline disability scores (including ≥1 while undergoing treatment), and persistence on study therapy for 1 month or longer and minimum data set (consisting of sex, age, date of first MS symptom, dates of clinical relapses, clinical MS course, and disability score at treatment commencement (−9 months to +1 month). All consecutive patients treated with AHSCT were included. Data on race and ethnicity were not collected.
Procedures
Patients received AHSCT following protocols specific to the treating centers.2,3,5,23 Autologous hematopoietic stem cells were mobilized using cyclophosphamide 2 to 4.5 g/m2 intravenously with granulocyte colony stimulating factor 5 to 10 μg/kg. In a small number of patients, the mobilization used granulocyte colony stimulating factor only or in combination with methylprednisolone. The cells were then harvested by leukapheresis and cryopreserved. In approximately one-third of patients, the graft was depleted of mature immune cells with CD34 immunomagnetic selection. The transplant conditioning regimens were commenced more than 3 weeks after mobilization and included BEAM (carmustine, 300 mg/m2; etoposide, 200-800 mg/m2; cytarabine, 200 mg/m2; and melphalan, 140 mg/m2); busulfan with cyclophosphamide, 50 mg/kg; or cyclophosphamide, 200 mg, with antithymocyte globulin, 10 mg/kg. Rabbit/horse antithymocyte globulin was used in 84% of patients. Infection prophylaxis was used as per local protocols.
Patients included in the DMT arms were treated either with fingolimod (0.5 mg oral daily), ocrelizumab (600 mg intravenously every 6 months), or natalizumab (300 μg intravenously every 4 weeks). Baseline was defined as the first day of AHSCT conditioning or commencement of the DMT. Patients were censored at discontinuing therapy (with the minimum duration of treatment effect set at 60 days after starting fingolimod or natalizumab, 6 months after ocrelizumab, and 5 years after AHSCT),24 commencing another DMT, or at the last recorded disability score, whichever occurred first.
The analyzed data were recorded as part of routine practice, mostly at tertiary MS services, with real-time data entry. The MSBase study protocol (Supplement 1) stipulates minimum annual acquisition of disability scores, but patients with less frequent visits were not excluded.25 Data from different sources were mapped, combined, and underwent a rigorous quality procedure (eTable 1 in Supplement 2).26
Outcomes
The primary end point was the annualized relapse rate (ARR) during treatment with study therapy, per the standard analytical protocol (Supplement 1). A relapse was defined as new symptoms or exacerbation of existing symptoms persisting for 24 hours or longer, in the absence of concurrent illness/fever, and occurring 30 days or longer after a previous relapse.27 Confirmation of relapses by Expanded Disability Status Scale (EDSS) was not mandated. Individual ARR between baseline and censoring was calculated.
Secondary end points were the cumulative hazards of first postbaseline relapse and the proportions of patients free from disability worsening and with disability improvement. Disability was scored by EDSS scorers (Neurostatus certification was required at each site), excluding scores recorded 30 days or less of a prior relapse. Disability worsening was defined as an increase in EDSS by 1 step (1.5 steps if baseline EDSS score = 0 and 0.5 steps if baseline EDSS score >5.5) confirmed by subsequent EDSS scores over 6 months or longer. Disability improvement was defined as a decrease in EDSS score by 1 step (1.5 step if baseline EDSS score = 1.5 and 0.5 steps if baseline EDSS score >6) confirmed by subsequent EDSS scores over 6 months or longer.28
Safety information was recorded in the AHSCT group and included febrile neutropenia, serum sickness, intensive care unit admission, infectious and other complications after discharge, and mortality.
Statistical Analysis
This study emulated 3 clinical trials comparing AHSCT with fingolimod, natalizumab, and ocrelizumab (eTable 2 in Supplement 2).29 Matching and statistical analyses were conducted using R version 4.1.1 (R Foundation). Individual patients were matched on their propensity of receiving either of the compared therapies in 1:10 variable matching ratio without replacement within a caliper of 0.1 SDs of the propensity score. Individual propensity scores were calculated using a multivariable logistic model of treatment allocation that used demographic and clinical variables available at baseline as independent variables: sex, age, EDSS score, number of relapses 12 and 24 months before baseline, time from first symptom of MS to baseline, the most effective prior DMT, and geographical region.
All subsequent analyses were designed as paired models with weighting to account for the variable matching ratio (cumulative weight per patient ≤1). The pairwise-censored study follow-up was determined in each matched pair as the shorter of the 2 patient follow-up periods, to mitigate attrition bias, informative censoring, and differential treatment persistence.12
ARRs were compared with a weighted negative binomial model with cluster effect for matched pairs. The cumulative hazards of first relapse, disability worsening, and disability improvement were evaluated with weighted conditional proportional hazards models (Cox) adjusted for visit frequency and with robust estimation of variance. Interaction term for treatment and time was introduced in the models where Schoenfeld global test indicated violation of the proportionality of hazards assumption.
Robustness of the statistically significant differences to unidentified confounders was quantified with Hodges-Lehmann Γ.30 Where no evidence of difference between the compared groups was found, the minimum detectable effect at α = 0.05 and 1 − β = 0.80 was estimated with 200 simulations per treatment pair and outcome.
Results
Of 4915 individuals who fulfilled the inclusion criteria, 167 (3.4%) were treated with AHSCT, 2558 (52.0%) with fingolimod, 1490 (30.3%) with natalizumab, and 700 (14.2%) with ocrelizumab (Figure 1 and eTable 3 in Supplement 2). In the groups, the proportion of women ranged from 65% (223 of 343) to 70% (506 of 730), the mean (SD) age ranged from 35.3 (9.4) to 37.1 (10.6) years, the mean (SD) disease duration ranged from 7.9 (5.6) to 8.7 (5.4) years, the mean (SD) EDSS score ranged from 3.5 (1.6) to 3.9 (1.9), and the mean (SD) frequency of relapses ranged from 0.77 (0.94) to 0.86 (0.89) in the preceding year. Among the AHSCT cohort, the conditioning intensity was used as follows: high-intensity in 43 patients (26%), intermediate-intensity myeloablative in 49 patients (29%), intermediate-intensity lymphoablative in 64 patients (38%), and low- to intermediate-intensity in 11 patients (7%).19 As expected, the 4 unmatched groups differed in their baseline characteristics (eTable 4 in Supplement 2). From the logistic models used to derive the propensity scores, it is apparent that patients tended to commence AHSCT at younger age, higher disability, and shorter disease duration compared with the 3 studied DMTs (eTable 5 in Supplement 2).
Figure 1. CONSORT Diagram .
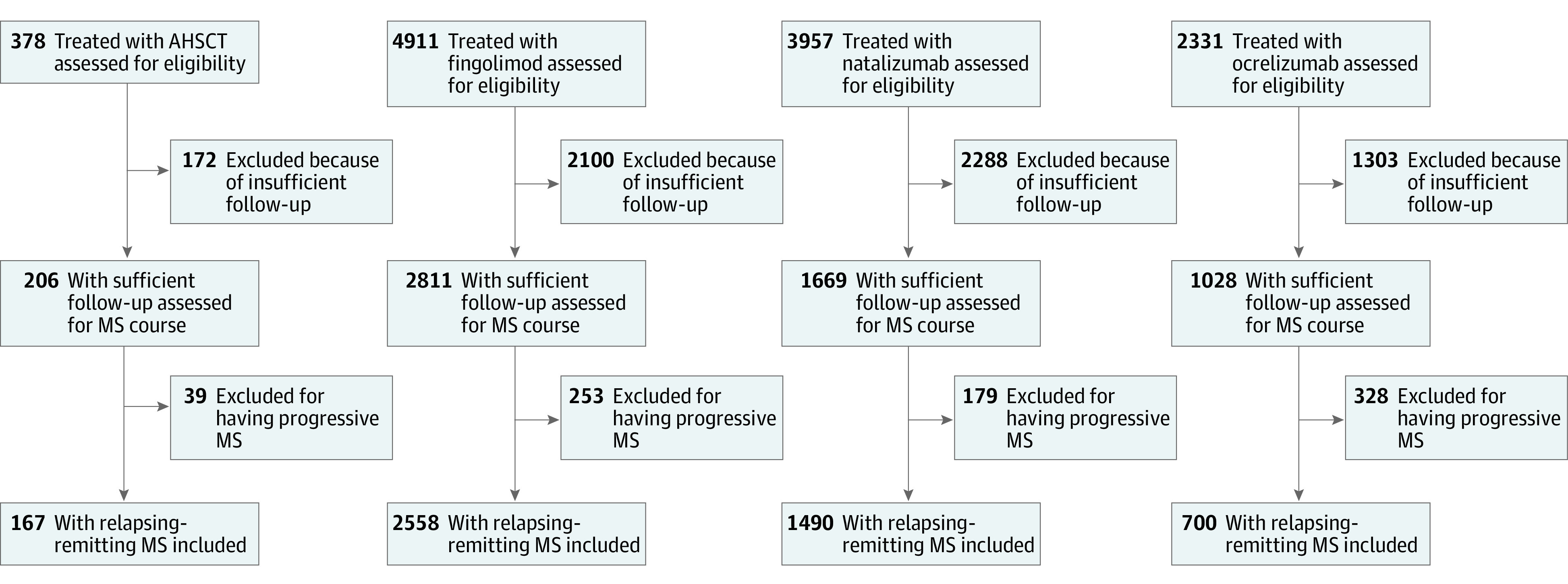
AHSCT indicates autologous hematopoietic stem cell transplant; MS, multiple sclerosis.
The numbers of patients retained in the 3 pairwise matched comparisons are shown in the Table. The matching procedure significantly decreased the differences in propensity scores between the compared groups from 0.35-0.41 to 0.002-0.005, corresponding to a 99.0% to 99.5% improvement in the overall balance. The close match on individual characteristics is demonstrated in the Table (standardized differences ≤10% for all matched characteristics). As a result of pairwise censoring, studied follow-up was identical in the matched groups. The groups were not matched on the between-visit intervals, for which the analyses were then adjusted.
Table. Characteristics of the Matched Patient Groups at Baseline.
| Characteristic | Matched comparison: AHSCT and fingolimod | Matched comparison: AHSCT and natalizumab | Matched comparison: AHSCT and ocrelizumab | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AHSCT | Fingolimod | d | AHSCT | Natalizumab | d | AHSCT | Ocrelizumab | d | |
| Patients matched, No. | 144 | 769 | NA | 146 | 730 | NA | 110 | 343 | NA |
| Male, No. (%) | 44 (30.6) | 224 (29.1) | 0.03 | 45 (30.8) | 224 (30.6) | 0.01 | 36 (32.7) | 120 (35.0) | 0.05 |
| Female, No. (%) | 100 (69.4) | 545 (70.9) | NA | 101 (69.2) | 506 (69.4) | NA | 74 (67.3) | 223 (65.0) | NA |
| Age, mean (SD), y | 35.7 (8.7) | 35.3 (9.4) | 0.04 | 35.5 (8.7) | 36.0 (9.0) | 0.06 | 37.0 (8.6) | 37.1 (10.6) | 0.01 |
| MS duration, mean (SD), y | 8.12 (5.58) | 8.17 (6.07) | 0.01 | 7.92 (5.63) | 8.17 (6.22) | 0.04 | 8.68 (5.42) | 8.48 (7.34) | 0.03 |
| Relapses in prior 12 mo, mean (SD) | 0.80 (0.97) | 0.81 (0.92) | 0.02 | 0.82 (1.01) | 0.86 (0.89) | 0.04 | 0.79 (0.95) | 0.77 (0.94) | 0.03 |
| Relapses in prior 24 mo, mean (SD) | 1.12 (1.27) | 1.17 (1.20) | 0.04 | 1.17 (1.33) | 1.19 (1.14) | 0.02 | 1.15 (1.25) | 1.08 (1.19) | 0.06 |
| Baseline EDSS score, mean (SD) | 3.74 (1.63) | 3.75 (1.82) | 0.00 | 3.86 (1.66) | 3.88 (1.92) | 0.02 | 3.50 (1.60) | 3.58 (1.87) | 0.05 |
| Patients with prebaseline progression, No. (%) | 23 (16.0) | 168 (21.8) | 0.15 | 23 (15.8) | 197 (27.0) | 0.28 | 20 (18.2) | 69 (20.0) | 0.05 |
| Top prebaseline DMT (%) | |||||||||
| Low efficacy | 18 (12.5) | 104 (13.5) | 0.05 | 18 (12.3) | 87 (12.0) | 0.03 | 14 (12.7) | 43 (12.5) | 0.03 |
| Medium efficacy | 9 (6.2) | 46 (5.9) | 12 (8.2) | 55 (7.5) | 10 (9.1) | 30 (8.7) | |||
| High efficacy | 24 (16.7) | 139 (18.2) | 17 (11.6) | 88 (12.1) | 22 (20.0) | 73 (21.3) | |||
| Unknown | 93 (64.6) | 480 (62.4) | 99 (67.8) | 500 (68.5) | 64 (58.2) | 197 (57.5) | |||
| Region, No. (%) | |||||||||
| Asia-Pacific | 46 (31.9) | 236 (30.7) | 0.03 | 46 (31.5) | 230 (31.5) | 0.07 | 45 (40.9) | 148 (43.2) | 0.05 |
| Europe | 73 (50.7) | 392 (51.0) | 73 (50.0) | 346 (47.4) | 50 (45.5) | 148 (43.0) | |||
| North America | 25 (17.4) | 141 (18.3) | 27 (18.5) | 154 (21.1) | 15 (13.6) | 47 (13.8) | |||
| Study follow-up, mean (SD), ya | 4.01 (2.59) | 2.84 (2.43) | 0.46 | 4.08 (2.67) | 2.51 (2.22) | 0.64 | 3.78 (2.43) | 1.52 (0.94) | 1.22 |
| Year of baseline, median (IQR) | 2015 (2013-2017) | 2013 (2012-2015) | 0.17 | 2015 (2013-2016) | 2012 (2010-2015) | 0.44 | 2016 (2014-2017) | 2018 (2018-2019) | 1.40 |
| MRI: T2 lesion, No. (%) | |||||||||
| 0 | 0. (0.0) | 4 (0.5) | 0.76 | 0 (0.0) | 1 (0.1) | 0.84 | 0 (0.0) | 9 (2.5) | 1.04 |
| 1-2 | 3 (2.1) | 27 (3.5) | 3 (2.1) | 35 (4.8) | 3 (2.7) | 9 (2.7) | |||
| 3-8 | 5 (3.5) | 130 (17.0) | 4 (2.7) | 125 (17.2) | 5 (4.5) | 53 (15.6) | |||
| ≥9 | 45 (31.2) | 374 (48.6) | 46 (31.5) | 367 (50.3) | 38 (34.5) | 220 (64.1) | |||
| Unknown | 91 (63.2) | 234 (30.5) | 93 (63.7) | 202 (27.7) | 64 (58.2) | 52 (15.1) | |||
| Visit interval, mean (SD), mo | 8.38 (4.43) | 4.46 (4.02) | 0.93 | 8.39 (4.42) | 3.99 (4.41) | 0.99 | 8.77 (4.70) | 5.48 (3.57) | 0.79 |
Abbreviations: AHSCT, autologous hematopoietic stem cell transplant; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; MS, multiple sclerosis; NA, not applicable.
Study follow-up is shown before pairwise-censoring was applied.
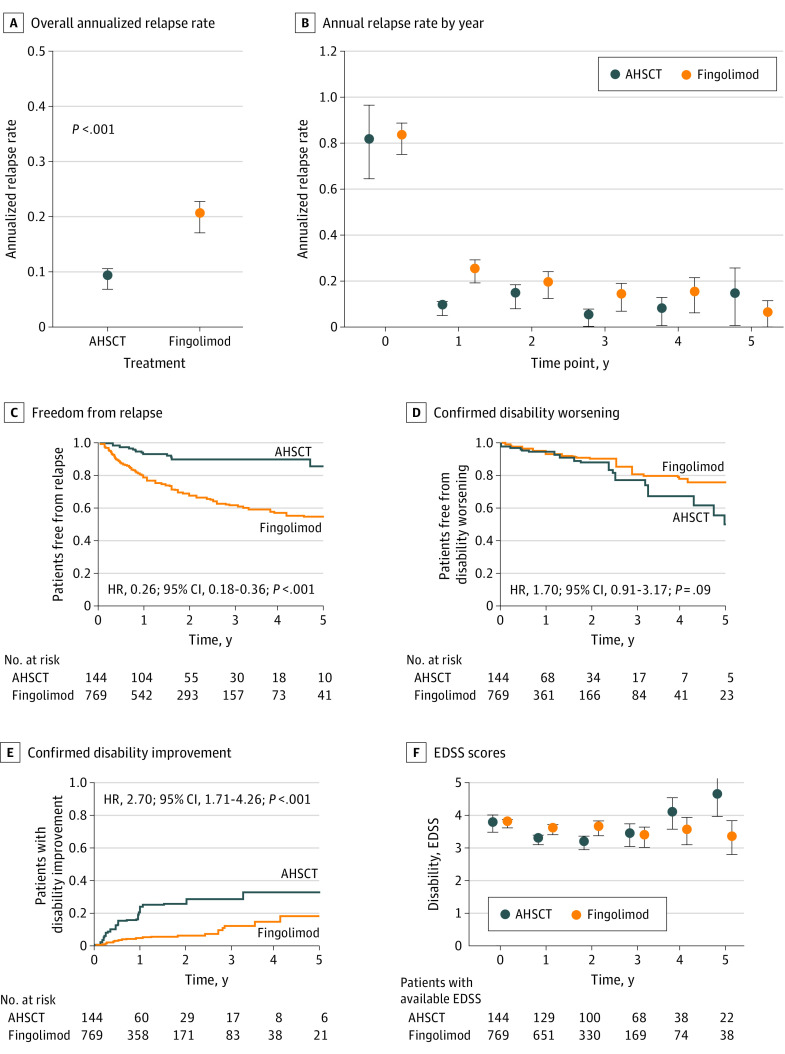
Patients treated with AHSCT experienced fewer relapses than those treated with fingolimod (Figure 2; ARR, mean [SD], 0.09 [0.30] vs 0.20 [0.44], respectively; P < .001). This observation was robust to unmeasured confounding (Γ > 100%) and confirmed by the cumulative hazard of relapse (hazard ratio [HR], 0.26; 95% CI, 0.18-0.36). We did not find evidence for difference in the cumulative hazards of 6-month confirmed disability worsening over up to 5 years (HR, 1.70; 95% CI, 0.91-3.17). The association of AHSCT with facilitating 6-month confirmed improvement of disability was superior to fingolimod (HR, 2.70; 95% CI, 1.71-4.26).
Figure 2. Comparative Association Between Autologous Hematopoietic Stem Cell Transplant (AHSCT) and Fingolimod.
Error bars indicate 95% CI. EDSS indicates Expanded Disability Status Scale; HR, hazard ratio.
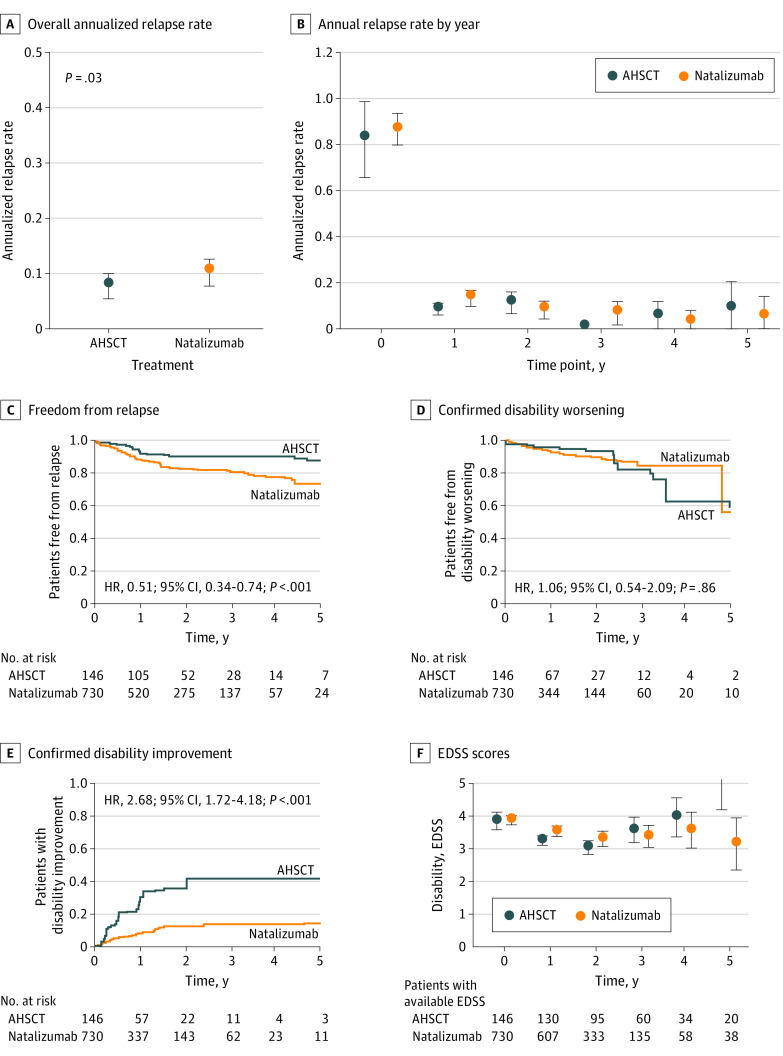
The ARR in the AHSCT group was marginally lower than in the natalizumab group (Figure 3; mean [SD], 0.08 [0.31] vs 0.10 [0.34], respectively; P = .03), as also confirmed by the cumulative hazard of relapses (HR, 0.51; 95% CI, 0.34-0.74). This observation was moderately robust to unmeasured confounding (Γ = 20%). The study did not find evidence for difference in the 6-month confirmed disability worsening between AHSCT and natalizumab (HR, 1.06; 95% CI, 0.54-2.09), with similar proportions of patients who experienced disability worsening by years 2 and 5. The association of AHSCT with 6-month confirmed improvement of disability during the 5-year follow-up was consistently superior to natalizumab (HR, 2.68; 95% CI, 1.72-4.18).
Figure 3. Comparative Association Between Autologous Hematopoietic Stem Cell Transplant (AHSCT) and Natalizumab.
Error bars indicate 95% CI. EDSS indicates Expanded Disability Status Scale; HR, hazard ratio.
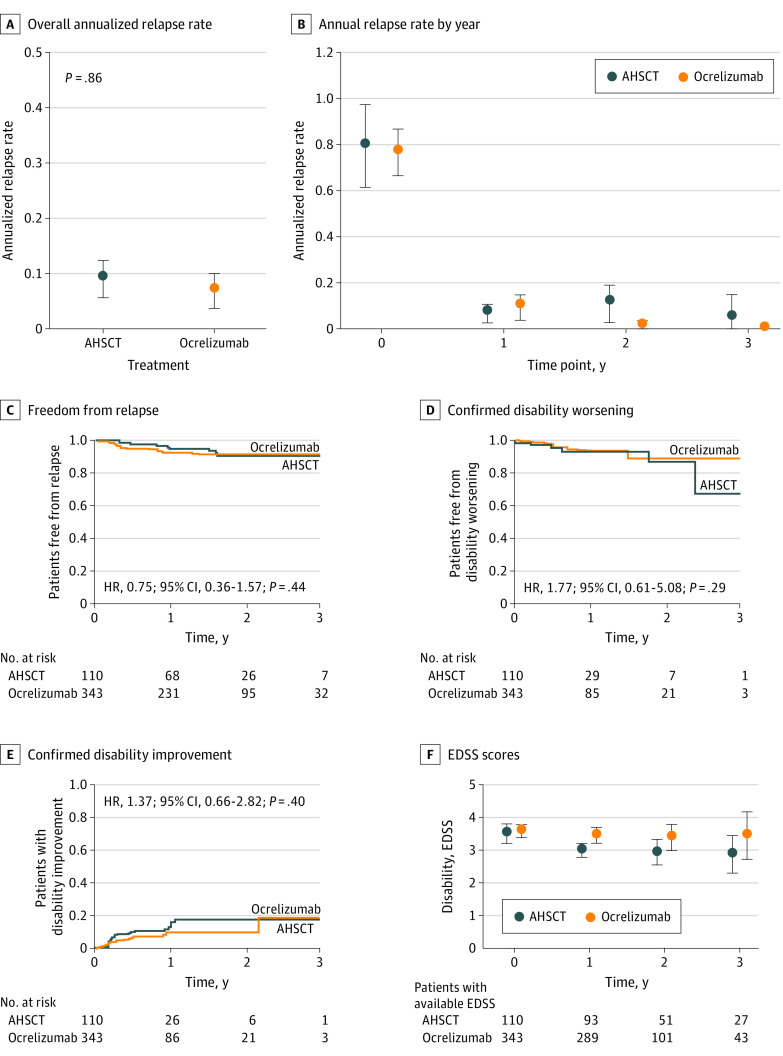
The analyzable follow-up for ocrelizumab was relatively shorter, up to 3 years from commencing study therapy. The risk of relapses was similar in the AHSCT and the ocrelizumab groups, as demonstrated by ARR (Figure 4; mean [SD], 0.09 [0.34] vs 0.06 [0.32], respectively; P = .86) and cumulative hazard of relapses (HR, 0.75; 95% CI, 0.36-1.57). This observation was moderately robust to potential unmeasured confounding (Γ = 40%). The cumulative hazards and the proportions of patients who remained free from 6-month confirmed disability worsening (HR, 1.77; 95% CI, 0.61-5.08) and experienced 6-month confirmed disability improvement (HR, 1.37; 95% CI, 0.66-2.82) were similar.
Figure 4. Comparative Association Between Autologous Hematopoietic Stem Cell Transplant (AHSCT) and Ocrelizumab.
Error bars indicate 95% CI. EDSS indicates Expanded Disability Status Scale; HR, hazard ratio.
According to the power analysis, the emulated trials were sufficiently powered to detect minimum differences of 0.17 relapses per year and 19% to 69% of the cumulative hazards of outcome events (eTable 6 in Supplement 2).
Safety data were available for patients treated with AHSCT. Among 159 patients who were matched in at least one of the pairwise analyses, 37 (23.3%) experienced febrile neutropenia during mobilization, 18 (11.3%) experienced serum sickness, and 14 (8.8%) required intensive care unit admission. Overall, 82 serious adverse events were recorded in 58 patients after discharge post-AHSCT; these consisted mainly of infections (49 [59.8%]), especially of viral etiology (34 [41.5%]; eTable 7 in Supplement 2). Treatment-related death was reported in 1 patient (0.6%; due to veno-occlusive disease of the liver postbusulfan).
Discussion
We have used composite data from 6 AHSCT centers and the international MSBase registry to emulate comparative trials of AHSCT vs 2 high-efficacy and 1 medium-efficacy DMTs for MS. The results support AHSCT as highly efficacious when used to treat highly active relapsing-remitting MS. The association of AHSCT with preventing relapses is substantially superior to fingolimod, marginally superior to natalizumab, and, with a shorter follow-up, appears similar to ocrelizumab. The study did not find evidence for a difference in the probability of disability worsening between AHSCT and the comparator DMTs and in the probability of disability improvement over a shorter available follow-up between AHSCT and ocrelizumab. AHSCT is associated with a higher rate of recovery from disability in comparison with fingolimod and natalizumab, especially during the initial year posttreatment, when it was observed among approximately 30% of the patients treated with AHSCT. This is of particular interest, as natalizumab is associated with a particularly high (25%) probability of confirmed reduction of neurological disability shortly after its commencement.12,31
To date and to our knowledge, only 2 randomized clinical trials of AHSCT have been completed. A phase 2 trial compared a mixed group of 9 patients with relapsing or progressive MS treated with myeloablative AHSCT with 12 patients treated with mitoxantrone. The trial concluded that AHSCT was more effective than mitoxantrone in reducing clinical and radiological episodic inflammatory activity.32 The phase 3 Multiple Sclerosis International Stem Cell Transplant (MIST) trial7 compared 55 patients with relapsing-remitting MS randomized to nonmyeloablative AHSCT with the same number randomized to escalation of DMT. The trial reported superiority of AHSCT in reducing the risk of disability worsening, relapses, and magnetic resonance imaging (MRI) activity. Because the interventions in the DMT escalation group ranged from interferon beta to natalizumab with or without add-on methylprednisolone, rituximab, plasmapheresis, cyclophosphamide, or intravenous immunoglobulins, the study did not generate evidence regarding the effectiveness of AHSCT head to head with the most potent available DMTs.
Presently, 3 randomized clinical trials comparing AHSCT (cyclophosphamide–antithymocyte globulin protocols) to composite comparator groups treated with specific high-efficacy DMTs in highly active MS are underway.8 The RAM-MS trial (phase 3 trial in Scandinavia and the Netherlands; NCT03477500) will compare the efficacy of AHSCT against alemtuzumab, ocrelizumab and cladribine. The STAR-MS trial (phase 3 trial in the UK; NCT03477500) uses a composite comparator group of alemtuzumab, ocrelizumab and cladribine. The COAST trial (phase 2 trial in Germany; NCT03822351) compares AHSCT vs a composite comparator of ocrelizumab or alemtuzumab. In addition, 2 randomized clinical trials are comparing AHSCT with BEAM–antithymocyte globulin conditioning against a range of high-efficacy DMTs representing the best standard care: BEAT-MS (phase 3 trial in the US; NCT04047628) and NET-MS (phase 2 trial in Italy). These trials will generate important evidence to guide the use of AHSCT in the future. Their results are expected to become available over the next decade.
The present study enables us to draw conclusions separately about the effectiveness of AHSCT vs 2 high-efficacy and 1 medium-efficacy DMT among patients with highly active relapsing-remitting MS. The cohort represents typical clinical scenarios in which AHSCT is presently considered: highly inflammatory disease in young patients with prior failures of potent DMTs and mild to moderate disability. With the comparison of AHSCT against fingolimod, we have established discriminative ability of the matched analysis, clearly demonstrating the expected superiority of AHSCT. In comparison with natalizumab, the relapse activity over 5 years was marginally lower during the treatment with AHSCT (absolute difference of 1 relapse per 50 patient-years). In none of the comparisons did the lower relapse rates associated with AHSCT translate into reducing the risk of disability worsening. On the other hand, AHSCT was associated with higher chance of partial recovery from the previously accumulated neurological disability when compared with fingolimod and natalizumab. Interestingly, we did not find evidence of difference between the risk of relapses during treatment with AHSCT and ocrelizumab, studied over a shorter, 3-year follow-up.
The observation that AHSCT showed superiority in clinical outcomes over fingolimod and, to a lesser extent, natalizumab, but not ocrelizumab, is intriguing. While this may be attributed to the shorter study follow-up available in the ocrelizumab cohort, it may also be due to the differences in the mechanisms of action among the therapies. Fingolimod and natalizumab are antitrafficking agents, sequestrating lymphocytes outside of the central nervous system, whereas ocrelizumab acts through depletion of CD20-positive cells, a mechanism that is more similar to the immunosuppressive effect of AHSCT.33
The safety profile of AHSCT is consistent with the previous cohort experience. A considerable number of patients experienced febrile neutropenia during mobilization with cyclophosphamide and 9% required intensive care unit admission. Doses lower than 2 g/m2 are associated with a lower risk of this complication. Whether the lymphodepleting effect of cyclophosphamide is dose-dependent and whether the mononuclear content of the graft impacts on the outcome is unknown. Almost one-third of patients developed infectious complications at later stages, following recovery from the transplant procedures. Only 1 treatment-related death (0.6%) was reported.
Limitations
The main limitation of this study is its lack of true randomization. However, randomization to AHSCT or DMT with appropriate blinding is extremely problematic, given the considerably different intensities of treatment protocols, persistence, and safety profiles.34 Therefore, it has been argued that observational data analyzed with appropriate statistical methodology represent an optimal solution to establishing evidence for comparative effectiveness of AHSCT.35 We have used well-established methods to emulate clinical trials using a large composite database of patients treated with AHSCT or DMTs, and this methodology provides this study with larger power and generalizability than previous randomized trials.17 We have applied matching, pairwise censoring, and model adjustment to mitigate the potential biases, an approach whose validity was demonstrated in our previous studies.12,36 As the result of strict inclusion and matching criteria, we achieved a close alignment of the compared treatment groups on their demographic and clinical characteristics. While the study did not allow direct comparison of the safety of AHSCT and the DMTs, the systematic acquisition of safety information in the AHSCT cohort enabled us to report short- and long-term safety outcomes of AHSCT. Because MRI information was unavailable in more than half of the AHSCT cohort, this study did not include MRI in matching or as one of its outcomes. However, MRI characteristics at baseline were similar between the matched groups where the information was available. Our previous studies did not show any effect of inclusion of MRI in matching on their results.11,12 To account for geographic differences in cohorts and outcomes,37 we have matched patients on their geographic location. Some of the patients in the AHSCT group would be followed up as part of open-label clinical trials. To mitigate this potential source of ascertainment bias, we have accounted for differences in follow-up, we have adjusted models for the frequency of visits with EDSS scores. To explore the specific effectiveness of conditioning regimens on the effectiveness of AHSCT, a dedicated study with specific design will be required.
Conclusions
We show that over 5 years, AHSCT is associated with a lower risk of relapses and a higher chance of recovery from disability in highly active relapsing-remitting MS when compared with fingolimod and natalizumab. Over the limited follow-up 3 years, we did not find its clinical effect superior to that of ocrelizumab. Although AHSCT requires a complex treatment procedure, its one-off nature may offer practical advantages over the continuously administered therapies.8 AHSCT is associated with considerable risks, but the risk of treatment-associated mortality is low.
Study protocol and statistical analysis plan
eTable 1. Data quality procedure
eTable 2. Summary of the study protocol (target trial)
eTable 3. Patient disposition per centre
eTable 4. Characteristics of the included unmatched patients at baseline
eTable 5. Logistic regression models used to estimate the propensity scores
eTable 6. Power analysis
eTable 7. Serious adverse events reported after AHSCT
Nonauthor Collaborators. MSBase Study Group Collaborators
Data Sharing Statement
References
- 1.Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13(7):391-405. doi: 10.1038/nrneurol.2017.81 [DOI] [PubMed] [Google Scholar]
- 2.Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388(10044):576-585. doi: 10.1016/S0140-6736(16)30169-6 [DOI] [PubMed] [Google Scholar]
- 3.Burman J, Iacobaeus E, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85(10):1116-1121. doi: 10.1136/jnnp-2013-307207 [DOI] [PubMed] [Google Scholar]
- 4.Burt RK, Balabanov R, Han X, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313(3):275-284. doi: 10.1001/jama.2014.17986 [DOI] [PubMed] [Google Scholar]
- 5.Krasulová E, Trneny M, Kozák T, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler. 2010;16(6):685-693. doi: 10.1177/1352458510364538 [DOI] [PubMed] [Google Scholar]
- 6.Nash RA, Hutton GJ, Racke MK, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology. 2017;88(9):842-852. doi: 10.1212/WNL.0000000000003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321(2):165-174. doi: 10.1001/jama.2018.18743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharrack B, Petrie J, Coles A, Snowden JA. Is stem cell transplantation safe and effective in multiple sclerosis? BMJ. 2022;377:e061514. doi: 10.1136/bmj-2020-061514 [DOI] [PubMed] [Google Scholar]
- 9.Muraro PA, Pasquini M, Atkins HL, et al. ; Multiple Sclerosis–Autologous Hematopoietic Stem Cell Transplantation (MS-AHSCT) Long-term Outcomes Study Group . Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74(4):459-469. doi: 10.1001/jamaneurol.2016.5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320-327. doi: 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalincik T, Horakova D, Spelman T, et al. ; MSBase Study Group . Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol. 2015;77(3):425-435. doi: 10.1002/ana.24339 [DOI] [PubMed] [Google Scholar]
- 12.Kalincik T, Brown JWL, Robertson N, et al. ; MSBase Study Group . Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16(4):271-281. doi: 10.1016/S1474-4422(17)30007-8 [DOI] [PubMed] [Google Scholar]
- 13.Iaffaldano P, Lucisano G, Pozzilli C, et al. ; Italian iMed-Web database . Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain. 2015;138(pt 11):3275-3286. doi: 10.1093/brain/awv260 [DOI] [PubMed] [Google Scholar]
- 14.Barbin L, Rousseau C, Jousset N, et al. ; CFSEP and OFSEP groups . Comparative efficacy of fingolimod vs natalizumab: a French multicenter observational study. Neurology. 2016;86(8):771-778. doi: 10.1212/WNL.0000000000002395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78(10):1197-1204. doi: 10.1001/jamaneurol.2021.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalincik T, Sormani MP. Comparative effectiveness of rituximab in multiple sclerosis. Nat Rev Neurol. 2021;17(1):3-4. doi: 10.1038/s41582-020-00412-5 [DOI] [PubMed] [Google Scholar]
- 17.Hernán MA. Methods of public health research: strengthening causal inference from observational data. N Engl J Med. 2021;385(15):1345-1348. doi: 10.1056/NEJMp2113319 [DOI] [PubMed] [Google Scholar]
- 18.Tappenden P, Wang Y, Sharrack B, et al. Evaluating the clinical effectiveness of autologous haematopoietic stem cell transplantation versus disease-modifying therapy in multiple sclerosis using a matching-adjusted indirect comparison: an exploratory study from the Autoimmune Diseases Working Party (ADWP) of the European Society of Bone and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2020;55(7):1473-1475.https://pubmed.ncbi.nlm.nih.gov/31745252 doi: 10.1038/s41409-019-0747-2 [DOI] [PubMed] [Google Scholar]
- 19.Sharrack B, Saccardi R, Alexander T, et al. ; European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT) and EBMT (JACIE) . Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. 2020;55(2):283-306. doi: 10.1038/s41409-019-0684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 22.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703 [DOI] [PubMed] [Google Scholar]
- 23.Moore JJ, Massey JC, Ford CD, et al. Prospective phase II clinical trial of autologous haematopoietic stem cell transplant for treatment refractory multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(5):514-521. doi: 10.1136/jnnp-2018-319446 [DOI] [PubMed] [Google Scholar]
- 24.Roos I, Malpas C, Leray E, et al. ; MSBase and OFSEP . Disease reactivation after cessation of disease-modifying therapy in patients with relapsing-remitting multiple sclerosis. Neurology. 2022;99(17):e1926-e1944. doi: 10.1212/WNL.0000000000201029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MSBase study protocol. Accessed July 6, 2022. https://www.msbase.org/about-us/documents-and-resources/
- 26.Kalincik T, Kuhle J, Pucci E, et al. ; MSBase Scientific Leadership Group and MSBase Study Group . Data quality evaluation for observational multiple sclerosis registries. Mult Scler. 2017;23(5):647-655. doi: 10.1177/1352458516662728 [DOI] [PubMed] [Google Scholar]
- 27.Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552-568. doi: 10.1111/j.1749-6632.1965.tb20235.x [DOI] [PubMed] [Google Scholar]
- 28.Kalincik T, Cutter G, Spelman T, et al. Defining reliable disability outcomes in multiple sclerosis. Brain. 2015;138(Pt 11):3287-3298. doi: 10.1093/brain/awv258 [DOI] [PubMed] [Google Scholar]
- 29.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum PR. Observational Studies. 2nd ed. Springer-Verlag; 2002. [Google Scholar]
- 31.Belachew S, Phan-Ba R, Bartholomé E, et al. Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(2):240-245. doi: 10.1111/j.1468-1331.2010.03112.x [DOI] [PubMed] [Google Scholar]
- 32.Mancardi GL, Sormani MP, Gualandi F, et al. ; ASTIMS Haemato-Neurological Collaborative Group, On behalf of the Autoimmune Disease Working Party (ADWP) of the European Group for Blood and Marrow Transplantation (EBMT); ASTIMS Haemato-Neurological Collaborative Group On behalf of the Autoimmune Disease Working Party ADWP of the European Group for Blood and Marrow Transplantation EBMT . Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. 2015;84(10):981-988. doi: 10.1212/WNL.0000000000001329 [DOI] [PubMed] [Google Scholar]
- 33.Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999-e2008. doi: 10.1212/WNL.0000000000010380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasquini MC, Griffith LM, Arnold DL, et al. Hematopoietic stem cell transplantation for multiple sclerosis: collaboration of the CIBMTR and EBMT to facilitate international clinical studies. Biol Blood Marrow Transplant. 2010;16(8):1076-1083. doi: 10.1016/j.bbmt.2010.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sormani MP. Real-world studies provide reliable comparisons of disease modifying therapies in MS: no. Mult Scler. 2020;26(2):161-162. doi: 10.1177/1352458519845837 [DOI] [PubMed] [Google Scholar]
- 36.Kalincik T, Butzkueven H. Observational data: understanding the real MS world. Mult Scler. 2016;22(13):1642-1648. doi: 10.1177/1352458516653667 [DOI] [PubMed] [Google Scholar]
- 37.Bovis F, Signori A, Carmisciano L, et al. Expanded disability status scale progression assessment heterogeneity in multiple sclerosis according to geographical areas. Ann Neurol. 2018;84(4):621-625. doi: 10.1002/ana.25323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study protocol and statistical analysis plan
eTable 1. Data quality procedure
eTable 2. Summary of the study protocol (target trial)
eTable 3. Patient disposition per centre
eTable 4. Characteristics of the included unmatched patients at baseline
eTable 5. Logistic regression models used to estimate the propensity scores
eTable 6. Power analysis
eTable 7. Serious adverse events reported after AHSCT
Nonauthor Collaborators. MSBase Study Group Collaborators
Data Sharing Statement