Abstract
Background and Objective
To investigate the efficacy and safety of IV infusion of tirofiban before endovascular thrombectomy for patients with large vessel occlusion due to intracranial atherosclerotic disease. The secondary objective was to identify potential mediators for the clinical effect of tirofiban.
Methods
Post hoc exploratory analysis of the Endovascular Treatment With versus Without Tirofiban for Patients with Large Vessel Occlusion Stroke (RESCUE BT) trial, which was a randomized, double-blinded, placebo-controlled trial at 55 centers in China from October 2018 to October 2021. Patients with occlusion of the internal carotid artery or middle cerebral artery due to intracranial atherosclerosis were included. The primary efficacy outcome was the proportion of patients achieving functional independence (defined as modified Rankin scale 0–2) at 90 days. Binary logistic regression and causal mediation analyses were used to estimate the treatment effect of tirofiban and the potential mediators.
Results
This study included 435 patients, of whom 71.5% were men. The median age was 65 (interquartile range [IQR] 56–72) years, with a median NIH Stroke Scale of 14 (IQR 10–19). Patients in the tirofiban group had higher rates of functional independence at 90 days than patients in the placebo group (adjusted odds ratio 1.68; 95% CI 1.11–2.56, p = 0.02) without an increased risk of mortality or symptomatic intracranial hemorrhage. Tirofiban was associated with fewer thrombectomy passes (median [IQR] 1 [1–2] vs 1 [1–2], p = 0.004), which was an independent predictor of functional independence. Mediation analysis showed tirofiban-reduced thrombectomy passes explained 20.0% (95% CI 4.1%–76.0%) of the effect of tirofiban on functional independence.
Discussion
In this post hoc analysis of the RESCUE BT trial, tirofiban was an effective and well-tolerated adjuvant medication of endovascular thrombectomy for patients with large vessel occlusion due to intracranial atherosclerosis. These findings need to be confirmed in future trials.
Trial Registration Information
The RESCUE BT trial was registered on the Chinese Clinical Trial Registry: chictr.org.cn, ChiCTR-INR-17014167.
Classification of Evidence
This study provides Class II evidence that tirofiban plus endovascular therapy improves 90-day outcome for patients with large vessel occlusion due to intracranial atherosclerosis.
Intracranial atherosclerotic disease (ICAD) is one of the most common causes of large vessel occlusive (LVO) stroke, especially in the Asian, Black, and Hispanic population.1-3 LVO stroke related to ICAD has a high risk of recanalization failure after thrombectomy and often requires additional angioplasty and/or permanent stent.1 One of the major concerns is thrombectomy, and rescue treatment could aggravate endothelial injury, leading to platelet aggregation and subsequent reocclusion.
Tirofiban, a selective glycoprotein IIb/IIIa receptor inhibitor, could effectively inhibit the bridging of fibrinogen with platelet and prevent arterial thrombosis.4 Given the robust evidence in percutaneous coronary intervention,5,6 tirofiban has been commonly used as an adjuvant medication during endovascular therapy (EVT) in acute ischemic stroke,7,8 though this indication has not been approved by the US Food and Drug Administration. Several studies have indicated that tirofiban may decrease the risk of arterial reocclusion for ICAD-related LVO stroke and improve the clinical outcomes after EVT for stroke patients with atherosclerotic etiology.7,9 However, selection bias and small sample size limited the reliability of their results. The Endovascular Treatment With versus Without Tirofiban for Patients with Large Vessel Occlusion Stroke (RESCUE BT) trial was a randomized trial to test the efficacy and safety of tirofiban plus EVT for patients with proximal intracranial large vessel occlusion, which demonstrated that IV tirofiban vs placebo before EVT did not improve the functional disability at 90 days.10 Nevertheless, it remains unclear as to whether tirofiban could be offered to patients with stroke attributable to ICAD.
In this secondary analysis of the RESCUE BT trial, we sought to estimate the association between tirofiban and outcomes after EVT in patients with LVO stroke due to ICAD. Furthermore, we sought to identify the potential mediators for tirofiban treatment using a causal mediation analysis11,12 and assess the heterogeneity of treatment effect in key subgroups. The primary research question was whether adding tirofiban to EVT could be effective and safe for patients with LVO stroke due to ICAD.
Methods
Study Design and Participants
The RESCUE BT was a prospective, double-blind, randomized clinical trial of IV tirofiban plus EVT vs placebo plus EVT for patients presenting with an occlusion of the internal carotid artery (ICA) or middle cerebral artery (MCA) within 24 hours of symptom onset. The trial protocol and patient eligibility criteria have been previously reported.10 In brief, patient randomization was stratified by age, baseline NIH Stroke Scale (NIHSS), occlusion site, and participating center. Patients who had received dual antiplatelet therapy or IV thrombolysis (IVT) were excluded from trial enrollment. The study drug was initiated before EVT at a dose of 10 μg/kg IV bolus followed by an infusion of 0.15 μg/kg/min for up to 24 hours (eMethod 1, links.lww.com/WNL/C698).
Standard Protocol Approvals, Registrations, and Patient Consents
The RESCUE BT trial was registered on the Chinese Clinical Trial Registry (chictr.org.cn, ChiCTR-INR-17014167). The study was approved by the ethics committee of the Xinqiao Hospital, Army Medical University, and all participating centers. Written informed consent was obtained from all patients or their proxy.
Etiologic Identification of ICAD
The case report forms and source documents (including medical history, clinical features, laboratory test, 24-hour ECG, echocardiography, noninvasive brain imaging, and angiography of DICOM format) were centrally assessed by 2 senior neurologists who were unaware of treatment allocation (Dr. Zi and Dr. Li). Discrepant cases were resolved by consensus. The ICAD-related LVO stroke was defined as acute in situ thrombo-occlusion secondary to underlying ICAD after excluding embolism or other etiologies according to the Trial of Org 10172 in Acute Stroke Treatment criteria.13 If there was an established source of the embolism, and/or primary thrombectomy led to completely recanalized of the occluded vessel, an embolic occlusion was identified. Supporting evidence of ICAD on angiography were remnant stenosis ≥50% or a stenosis with significant distal flow disturbance, transient visualization of eccentric plaque contour, or reocclusion tendency at the target arterial lesion after thrombus removal.13,14 The artery stenosis was free from vasospasm, dissection, vasculitis, or Moyamoya disease.
Variables and Imaging Assessment
Demographic variables, vascular risk factors, baseline NIHSS score, workflow measures, and treatment information were prospectively recorded during enrollment.
All imaging data were evaluated based on central evaluation in the RESCUE BT imaging core laboratory. The location of the target occlusion was assessed based on CT or MR angiography on admission and were classified as ICA, MCA M1 segment (M1 MCA), and MCA M2 segment (M2 MCA). The extent of ischemic injury was assessed using the Alberta Stroke Program Early CT Score (ASPECTS).15 Reperfusion at final angiography was assessed using the expanded Thrombolysis in Cerebral Ischemia score, with grade 2b 50, 2c, or 3 indicating substantial, near-complete, or complete reperfusion, respectively.16 Recurrent occlusion of the reperfused arteries were assessed based on follow-up CT or MR angiography examined within 48 hours after thrombectomy.
Outcomes
The primary efficacy outcome was the rate of functional independence, defined as modified Rankin scale (mRS) score of 0–2, at 90 days. The mRS was adjudicated by 2 neurologists who were blind to treatment allocation through review of the patients' structured video or voice recordings. No patient was lost during follow-up. A secondary efficacy outcome was the level of mRS at 90 days. The safety outcomes included all-cause death within 90 days, any intracranial hemorrhage (ICH), symptomatic ICH (sICH), and fatal ICH within 48 hours. ICH was assessed according to the Heidelberg criteria,17 and symptomatic hemorrhage was defined as ICH associated with clinical deterioration of 4 points or more in the NIHSS score.
Statistical Analysis
Statistical analyses were performed based on the intention-to-treat (ITT) population. Missing data were imputed using the mode (ICH, 2/435 [0.5%]; sICH, 2/435 [0.5%]) or multiple imputation (reocclusion, 76/382 [19.9%]). Demographic factors, medical history, and baseline characteristics between groups were compared using the χ2 or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables.
We first identified the independent predictors of functional independence after EVT in stroke patients due to ICAD. Variables significant at α < 0.10 in multivariable regression served as the confounding factors for subsequent analyses of treatment effect. The clinical outcomes were compared between the 2 treatment arms using binary logistic regression, ordinal logistic regression, Poisson logistic regression, and linear logistic regression, as appropriate. Adjusted odds ratio (aOR), common odds ratio (OR), relative risks, and β coefficient were reported with 95% CI. We also performed the analyses based on the per-protocol population to verify the robustness of our results.
To identify the potential mediator of the effect of tirofiban on functional outcomes, we performed cause mediation analysis (detailed in eMethod 2, links.lww.com/WNL/C698) from the following factors: first pass effect, thrombectomy passes, procedure time, reperfusion at final angiogram, and postprocedural reocclusion of the target artery. These variables were selected based on clinical practice and previous reports.9,18 In subgroup analysis, we established 5 separate models and tested the interaction of treatment with each variable (age, ASPECTS, onset-to-randomization time, occlusion location, or intracranial angioplasty) by bringing their multiplicative interaction terms into the base model. All tests were 2-sided, and statistical analyses were performed using STATA version 15.2 (StataCorp LLC, College Station, TX) and R Studio software (version 1.3.1093) with the mediation package.
Data Availability
Anonymized data will be shared to qualified investigators whose proposal of data use has been approved by the corresponding author and the RESCUE BT investigators.
Results
Patient Characteristics
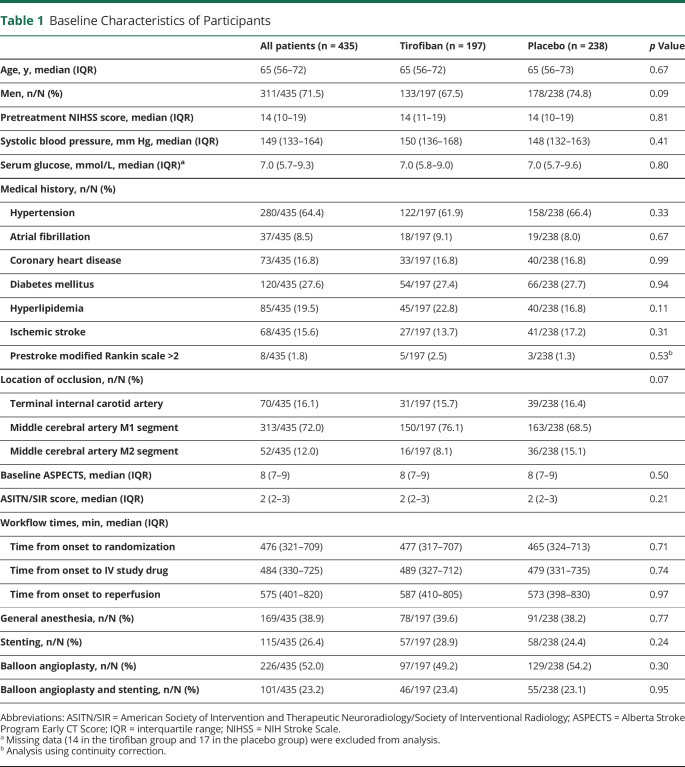
Of the 950 patients randomized in the RESCUE BT trial, 2 patients who withdrew informed consent, 406 patients with cardioembolism, 77 patients with unknown stroke etiology, and 30 patients with other stroke etiology were excluded (eFigure 1, links.lww.com/WNL/C698). A total of 435 patients with acute ischemic stroke due to ICAD were included in this analysis, including 197 patients assigned to the tirofiban group and 238 patients assigned to the placebo group. The median (interquartile range, IQR) age was 65 (56–72) years, the median NIHSS score was 14 (10–19), and 311 (71.5%) patients were men.
Baseline characteristics and treatment information in both treatment arms are summarized in Table 1. The baseline characteristics were well balanced for age, baseline NIHSS score and ASPECTS, but randomized assignment resulted in numerically lower rates of men (67.5% vs 74.8%) and occlusion of the M2 MCA (8.1% vs 15.1%) in the tirofiban group than in the placebo group. There were 57 (28.9%) patients who received stenting in the tirofiban group and 58 (24.4%) patients in the placebo group (p = 0.24). The rates of balloon angioplasty were also similar (49.2% vs 54.2%, p = 0.30) between the 2 groups.
Table 1.
Baseline Characteristics of Participants
Outcomes of Tirofiban vs Placebo
In the cohort comparing patients receiving tirofiban with those receiving placebo, the predictors of functional independence at 90 days included age, pretreatment systolic blood pressure, history of diabetes, baseline NIHSS, baseline ASPECTS, and location of occlusion (eTable 1, links.lww.com/WNL/C698). All subsequent analyses were adjusted for these covariates.
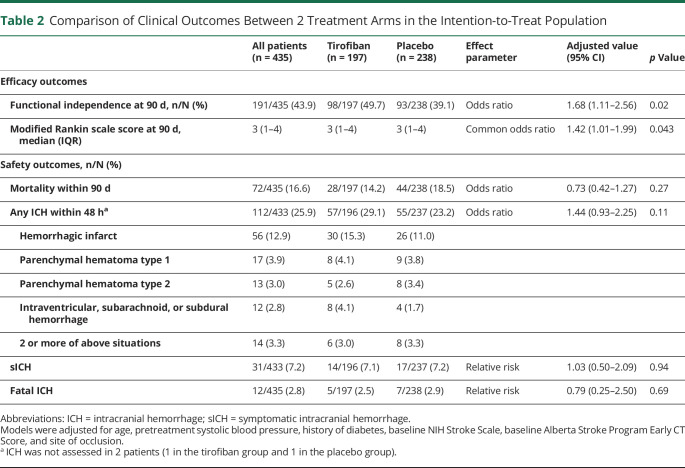
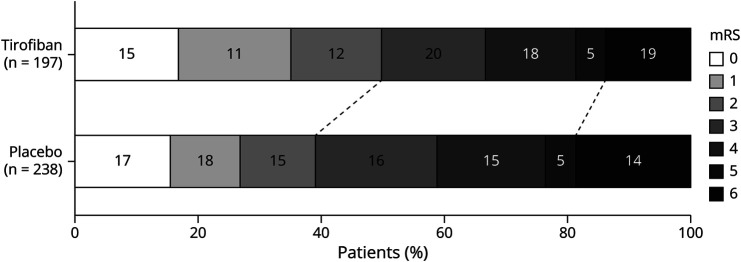
There was a higher rate of functional independence at 90 days in the tirofiban group than in the placebo group (49.7% vs 39.1%, aOR 1.68, 95% CI 1.11–2.56, p = 0.02; Table 2). The adjusted common OR for tirofiban when compared with that for placebo was 1.42 (95% CI 1.01–1.99, p = 0.043; Figure 1). Patients in the tirofiban group had numerically lower mortality (14.2% vs 18.5%) and numerically higher rates of any ICH (29.1% vs 23.2%), but the differences did not reach statistical significance. The rates of sICH were similar, with 14/197 (7.1%) patients in the tirofiban group and 17/238 (7.1%) patients in the placebo group (adjusted relative risk 1.03; 95% CI 0.50–2.09, p = 0.94). Sensitivity analysis in the per-protocol population showed that tirofiban was associated with a higher rate of 90-day functional independence (57.6% vs 38.8%, aOR 2.17, 95% CI 1.31–3.60, p = 0.003) and less severity of disability (adjusted common OR 1.82, 95% CI 1.20–2.76, p = 0.005, eTable 2 and eFigure 2, links.lww.com/WNL/C698).
Table 2.
Comparison of Clinical Outcomes Between 2 Treatment Arms in the Intention-to-Treat Population
Figure 1. Level of Disability Measured by mRS at 90 Days in the Intention-to-Treat Analysis.
mRS = modified Rankin scale.
Mediation Analysis
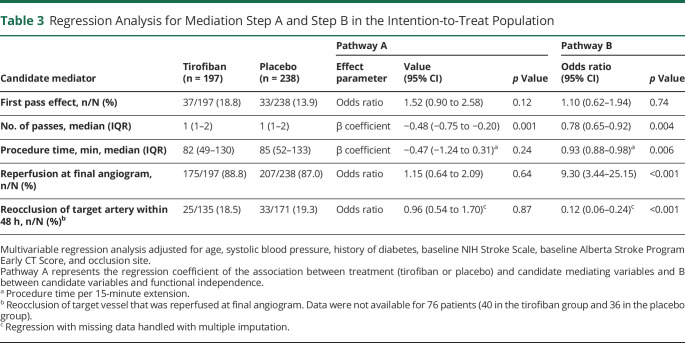
The rate of first pass effect in the tirofiban group was numerically higher than that of the placebo group (18.8% vs 13.9%, p = 0.17). There was no difference in the procedure time, reperfusion at final angiogram, or postprocedural reocclusion of the target artery between both arms, though these 3 variables were independently associated with functional independence at 90 days (Table 3). The number of thrombectomy passes was lower in the tirofiban group than in the placebo group (1 [1–2] vs 1 [1–2], p = 0.004; β = −0.48; 95% CI −0.75 to −0.20; p = 0.001) and was also an independent predictor of functional independence (aOR 0.78; 95% CI 0.65–0.92; p = 0.004). Therefore, thrombectomy passes were analyzed as a potential mediator of the effect of tirofiban on functional independence.
Table 3.
Regression Analysis for Mediation Step A and Step B in the Intention-to-Treat Population
Mediation analyses indicated a partial mediation effect of thrombectomy pass in the ITT population (Figure 2). After adjustment for thrombectomy pass, tirofiban still had a substantial relationship with functional independence (step c′: aOR 1.54; 95% CI 1.00–2.35; p = 0.048). Treatment-reduced passes of thrombectomy accounted for 20.0% (95% CI 4.1%–76.0%) of the beneficial effect of tirofiban on functional independence. This proportion was 10.9% (95% CI 0.6%–43.0%) in the per-protocol population (eTable 3 and eFigure 3, links.lww.com/WNL/C698).
Figure 2. Explained Proportions in the Mediation Analyses of the Effect of Tirofiban on Functional Independence Mediated by the Number of Thrombectomy Passes in the Intention-to-Treat Analysis.
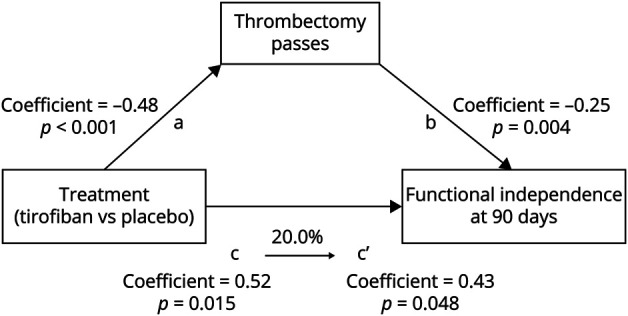
The coefficients of the regression equation of each step (steps a, b, c, and c′) and the percentage of indirect effect mediated by thrombectomy passes are described. Multivariable regressions adjusted for age, pretreatment systolic blood pressure, history of diabetes, baseline NIH Stroke Scale, Alberta Stroke Program Early CT Score, and location of occlusion.
Modifiers of Treatment Effect of Tirofiban
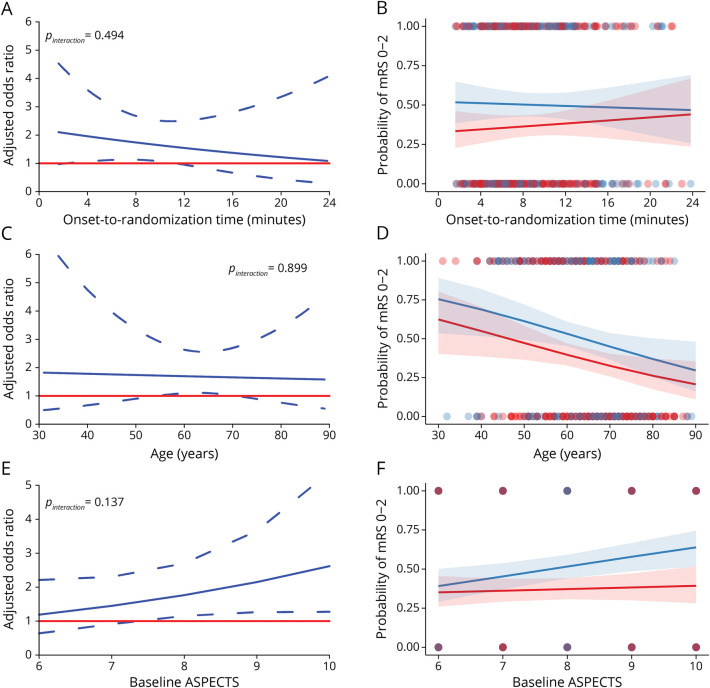
The absolute benefit of tirofiban vs placebo on functional independence showed a directionally downward trend with longer time to treatment, until 24 hours after onset, but the interaction between treatment and onset-to-randomization time was not noted (p interaction = 0.49; Figure 3, A and B). Longer time to treatment showed a crossover pattern of effect on mortality, but the p for interaction was 0.41 (eFigure 4A, links.lww.com/WNL/C698). The difference of sICH between the 2 groups was not visually suggestive nor statistically significant with time delay (p interaction = 0.38, eFigure 4I). Efficacy of tirofiban vs placebo was consistent irrespective of age ranging from 30 to 90 years (p interaction = 0.90 for functional independence) and safety (p interaction = 0.67 for mortality; p interaction = 0.64 for sICH). Similar results on treatment-by-time interactions and treatment-by-age interactions were observed in the per-protocol populations (eFigure 5).
Figure 3. Tirofiban Treatment Effect and Probability of Functional Independence.
The left panels show the treatment effect of tirofiban associated with onset-to-randomization time (A), age (C), and baseline ASPECTS (E). An odds ratio greater than 1 indicates higher odds of functional independence with tirofiban than placebo. The right panels show the probability and 95% CI of functional independence changes with onset-to-randomization (B), age (D), and ASPECTS (F). The blue lines and shading indicate the values in the tirofiban group while the red lines and shading indicate the values in the placebo group. The analyses are based on all patients with atherosclerotic etiology in the RESCUE BT trial. ASPECTS = Alberta Stroke Program Early CT Score.
The interaction between treatment and ASPECTS (range 6–10) on 90-day mRS 0–2 showed directional increase as ASPECTS declined from 10 to 6, but was not significant (p interaction = 0.14). There was also no obvious heterogeneity in the treatment effect of tirofiban by ASPECTS for mortality (p interaction = 0.35), ICH (p interaction = 0.30) and sICH (p interaction = 0.97). After excluding patients who violated the protocol, interactions between ASPECTS and treatment for the efficacy and safety outcomes were also not noted (eFigure 5, links.lww.com/WNL/C698).
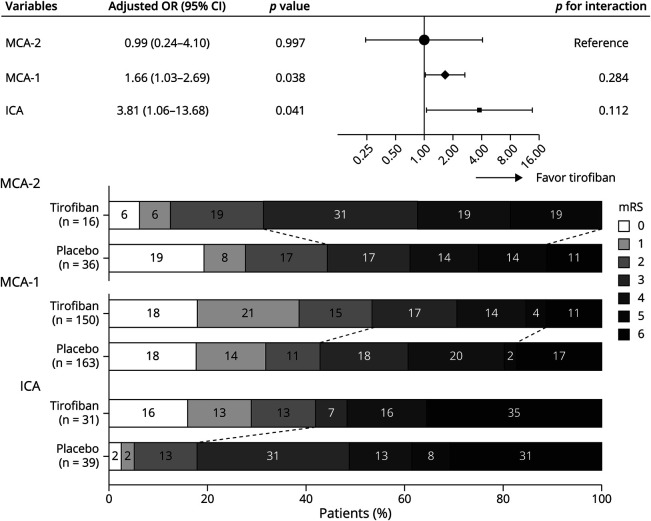
An analysis stratified by site of occlusion in Figure 4 showed a trend toward improved functional independence by tirofiban in occlusion of the ICA (aOR 3.81; 95% CI 1.06–13.68) and the M1 MCA (aOR 1.66; 95% CI 1.03–2.69) but no association between treatment and functional outcomes for M2 MCA occlusion. The interactions between treatment and site of occlusion were not statistically significant (p interaction = 0.11 between ICA and M2 MCA and p interaction = 0.28 between M1 and M2 MCA). Other outcomes according to the location of occlusion are listed in eTable 4 (links.lww.com/WNL/C698). The treatment effect of tirofiban by occlusion site in the per-protocol population was similar to that in the ITT analysis (eFigure 6).
Figure 4. Association Between Treatment With Functional Independence at 90 Days by Location of Occlusion.
Reported ORs are adjusted for age, pretreatment systolic blood pressure, history of diabetes, baseline NIH Stroke Scale, and Alberta Stroke Program Early CT Score. The distribution of the mRS scores in the 2 treatment arms are also shown. ICA = internal carotid artery; MCA = middle cerebral artery; mRS = modified Rankin scale; OR = odds ratio.
Tirofiban was associated with higher rates of functional independence compared with placebo only in patients who did not receive balloon angioplasty or stenting (aOR 2.09; 95% CI 1.09–4.03, eTable 5 and eFigure 7, links.lww.com/WNL/C698). However, there was no significant interaction between treatment and intracranial angioplasty (p interaction = 0.59 between none vs balloon angioplasty only, and p interaction = 0.29 between none vs stenting) in the ITT analyses.
Classification of Evidence
This study provides Class II evidence that tirofiban plus EVT improved 90-day outcome for patients with LVO stroke due to intracranial atherosclerosis. This study is Class II because of the post hoc analysis of a randomized trial.
Discussion
This post hoc analysis of the RESCUE BT randomized trial explored the treatment effect of tirofiban for patients presenting with intracranial atherosclerotic LVO stroke in the 24-hour time window. IV tirofiban before EVT significantly improved the 90-day functional independence, though the risk of any ICH was numerically increased. A lower number of thrombectomy passes could be a mediator in the benefit of tirofiban. We did not find significant heterogeneity of the effects of tirofiban in exploratory subgroup analyses.
Intracranial bleeding is a feared complication for any antithrombotic drug during EVT. Although tirofiban resulted in a numerically higher rate of any ICH, the rates of symptomatic or fatal ICH were similar between the 2 treatment arms. In view of the improved functional outcomes and numerically decreased mortality in the tirofiban group, the tirofiban-related ICH may be an acceptable risk in patients with ICAD-related LVO stroke. Our results are in contrast to a prior study and 1 pooled analysis, showing that tirofiban significantly increased the risk of fatal hemorrhage after thrombectomy.19,20 However, in the study of Kellert et al., 70.0% of patients treated with EVT in the tirofiban group also received IVT, whereas we excluded patients who had received IVT or dual antiplatelet therapy from our trial. Moreover, the underlying stroke etiology and route of medication administration may be important considerations when taking into account tirofiban-related bleeding risk. Prior reports indicated that tirofiban was safer in patients with LVO stroke related to ICAD than in those with cardioembolism.7 Intraarterial (vs IV) administration was associated with higher hemorrhagic and death rates,8 thus supporting our results.
The increased thrombectomy passes was an independent predictor of unfavorable outcomes, as described by our study and others,21 possibly due to delayed recanalization and damage of vascular intima. Of importance, the number of thrombectomy passes was significantly reduced by tirofiban in our study. This finding could be explained from the following 2 aspects. In contrast to the utilization of tirofiban as rescue after thrombectomy in previous studies,22,23 tirofiban was initiated after occlusion confirmation on noninvasive imaging and randomization in our study. In this scenario, tirofiban may help to prevent platelet aggregation, thrombus extension, and even promote thrombolysis24 before thrombectomy, thus facilitating thrombus debulking. This explanation could be reflected in the numerically higher rate of first pass effect in the tirofiban group. Second, it is not uncommon for mural thrombus and/or reocclusion to form after initial thrombus removal by thrombectomy for in situ atherosclerotic occlusion. Tirofiban could inhibit fibrinogen-dependent platelet aggregation and prevent mural thrombus and/or reocclusion,25 which may be helpful for the neurointerventionalist to distinguish atherosclerotic stenosis and directly resort to balloon dilatation or stent implantation, so as to avert additional thrombectomy passes.
The lack of an association between tirofiban and reperfusion at final angiogram is consistent with prior studies.19,26 We also did not find that tirofiban could reduce postprocedural reocclusion of reperfused vessels. However, because not all patients were assessed for the presence of reocclusion within 48 hours, this may have biased our results. Some reports have indicated that there was a lower risk of reocclusion after tirofiban infusion in patients with residual stenosis after EVT.27,28 We speculate that the lack of relationship might also be due to aggressive rescue therapy to reduce residual stenosis in most participating centers, with the proportion of angioplasty or permanent stenting in up to 55.5% of patients. The association between tirofiban and reocclusion still needs to be investigated.
Whether the benefits of tirofiban apply to diverse patient subgroups is critical for individualized treatment in clinical practice. We found the benefit of tirofiban was uniform across patients with a wide age range (30–90 years), onset-to-randomization time (0–24 hours), and extent of ischemic injury (ASPECTS 6–10). These results were encouraging and hint at a great potential of tirofiban as an adjuvant agent to EVT in patients with ICAD-related LVO stroke.29-31 We found tirofiban plus EVT was superior to placebo plus EVT for functional independence in ICA and M1 MCA occlusion, but not in M2 MCA occlusion. The treatment modification was likely underpowered because the false-negative risk prevails in subgroup analyses, particularly when multiple subgroups with unequal sample sizes exist.32 Of interest, the beneficial effect of tirofiban on 3-month functional outcome was attenuated and disappeared in patients who underwent balloon angioplasty only and stenting, respectively. The similar rates of favorable outcome between 2 treatments in the stenting subgroup suggests a ceiling effect of tirofiban in facilitating additional benefit in these patients. It is possible that tirofiban failed to benefit from reducing number of thrombectomy passes, probably because that once there is a commitment to stent, additional passes are often not performed because of reperfusion achieved or risk of additional instrumentation with a deployed stent. Because these subgroups were of small sample size, we interpret our findings with caution.
In this study, we performed the ITT analysis and per-protocol analysis. The ITT analysis included all patients randomized at study entry into the RESCUE BT trial. The ITT analysis attenuates selection bias and includes and reflects potential noncompliance and violations of adherence to the protocol in clinical practice.33 For example, the tirofiban was terminated early if the patient suffered from procedure-related ICH, which was a violation of therapeutic plans but can happen in daily practice. The per-protocol analysis considered the actual effect of tirofiban in patients who completed the trial without significant deviations from the protocol, but exclusion of patients may reduce the balance of risk factors. The 2 complementary strategies strengthen the credibility of the conclusions.
The main limitation of our study was that this was an exploratory analysis in an ICAD subgroup of the RESCUE BT trial and may have been underpowered. Patients were not randomized by stroke subtype (i.e., cardioembolic vs ICAD) because the precise etiology of the patient's LVO stroke can be difficult to decipher based on noninvasive imaging before EVT. Still, our results are to be considered hypothesis generating, and our study provides additional information in selecting patients with probable ICAD-related LVO stroke who may benefit from tirofiban as adjunctive therapy to EVT in future randomized trials. Moreover, tirofiban is often used in current practice when ICAD is considered at the LVO stroke etiology, especially after angioplasty or stenting, reocclusion tendency, or failure of thrombectomy. Our results lend support for the safety of tirofiban in these situations, given the safe profile of tirofiban application before EVT, though the effect size of its benefit is unclear.
In addition, the dosage of tirofiban in this study was administered as in studies of myocardial infarction,34,35 which may have resulted in a numerically higher risk of any ICH. Further dose exploration studies are needed to determine the optimal dosage in patient with ischemic stroke because of the dose-dependent effect of tirofiban on bleeding complications.36 Only candidate mediators measured during the trial were evaluated. The 20.0% effect of tirofiban on functional independence could be attributed to reduced thrombectomy passes, suggesting there may be additional mechanisms to support its benefits. Furthermore, this study represents a Chinese population with particularly high rates of ICAD, which is possibly due to genetic factors, diet, and vascular risk factor profile.1 The generalizability of our finding is uncertain across other ethnic populations.
The secondary analysis of the RESCUE BT trial provided support for the efficacy and safety of tirofiban plus EVT in large vessel occlusion due to intracranial atherosclerosis. In addition, we identified a reduced number of thrombectomy passes as a potential mediator of the beneficial effect of tirofiban. These findings need to be confirmed in further randomized trials.
Glossary
- aOR
adjusted OR
- ASPECTS
Alberta Stroke Program Early CT Score
- EVT
endovascular therapy
- ICA
internal carotid artery
- ICAD
intracranial atherosclerotic disease
- ICH
intracranial hemorrhage
- IQR
interquartile range
- ITT
intention-to-treat
- IVT
IV thrombolysis
- LVO
large vessel occlusive
- M1 MCA
MCA M1 segment
- M2 MCA
MCA M2 segment
- MCA
middle cerebral artery
- mRS
modified Rankin scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- RESCUE BT
Endovascular Treatment With versus Without Tirofiban for Patients with Large Vessel Occlusion Stroke
- sICH
symptomatic ICH
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The RESCUE BT trial was supported by Lunan Pharmaceutical Group Co., Ltd., and Youth Program of National Natural Science Foundation of China (Nos. 81801157 and 81901236).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Toyoda K, Koga M, Hayakawa M, Yamagami H. Acute reperfusion therapy and stroke care in Asia after successful endovascular trials. Stroke. 2015;46(6):1474-1481. [DOI] [PubMed] [Google Scholar]
- 2.Mehndiratta MM, Khan M, Mehndiratta P, Wasay M. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry. 2014;85(12):1308-1312. [DOI] [PubMed] [Google Scholar]
- 3.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12(11):1106-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang M, Huo X, Miao Z, Wang Y. Platelet glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs. 2019;79(5):515-529. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344(25):1879-1887. [DOI] [PubMed] [Google Scholar]
- 6.Montalescot G, Borentain M, Payot L, Collet JP, Thomas D. Early vs late administration of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention of acute ST-segment elevation myocardial infarction: a meta-analysis. JAMA. 2004;292(3):362-366. [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Li X, Zhao Z, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol. 2019;10:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Wu Y, Gao X, et al. Intraarterial versus intravenous tirofiban as an adjunct to endovascular thrombectomy for acute ischemic stroke. Stroke. 2020;51(10):2925-2933. [DOI] [PubMed] [Google Scholar]
- 9.Baek BH, Yoon W, Lee YY, Kim SK, Kim JT, Park MS. Intravenous tirofiban infusion after angioplasty and stenting in intracranial atherosclerotic stenosis-related stroke. Stroke. 2021;52(5):1601-1608. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Z, Li F, Sang H, et al. Effect of intravenous tirofiban vs placebo before endovascular thrombectomy on functional outcomes in large vessel occlusion stroke: the RESCUE BT randomized clinical trial. JAMA. 2022;328(6):543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buse JB, Bain SC, Mann JFE, et al. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43(7):1546-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boers AMM, Jansen IGH, Brown S, et al. Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol. 2019;76(2):194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. [DOI] [PubMed] [Google Scholar]
- 14.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58(5):688-697. [DOI] [PubMed] [Google Scholar]
- 15.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670-1674. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. [DOI] [PubMed] [Google Scholar]
- 17.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Yang Y, Xie Y, Yuan Z, Li X, Li J. Therapeutic effect of pre-operative tirofiban on patients with acute ischemic stroke with mechanical thrombectomy within 6-24 hours. Interv Neuroradiol. 2019;25(6):705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, Guo Y, Shen J, et al. Efficacy and safety of tirofiban therapy in patients receiving endovascular treatment after large vessel ischaemic stroke: a systematic review and meta-analysis. J Clin Neurosci. 2020;80:112-120. [DOI] [PubMed] [Google Scholar]
- 20.Kellert L, Hametner C, Rohde S, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013;44(5):1453-1455. [DOI] [PubMed] [Google Scholar]
- 21.Alawieh A, Vargas J, Fargen KM, et al. Impact of procedure time on outcomes of thrombectomy for stroke. J Am Coll Cardiol. 2019;73(8):879-890. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Huo X, Gao F, et al. Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol. 2020;27(6):1056-1061. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48(12):3289-3294. [DOI] [PubMed] [Google Scholar]
- 24.Gold HK, Garabedian HD, Dinsmore RE, et al. Restoration of coronary flow in myocardial infarction by intravenous chimeric 7E3 antibody without exogenous plasminogen activators. Observations in animals and humans. Circulation. 1997;95(7):1755-1759. [DOI] [PubMed] [Google Scholar]
- 25.Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37(5):350-355. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Guo ZN, Yan X, et al. Safety and efficacy of tirofiban combined with endovascular therapy compared with endovascular therapy alone in acute ischemic stroke: a meta-analysis. Neuroradiology. 2021;63(1):17-25. [DOI] [PubMed] [Google Scholar]
- 27.Yan Z, Shi Z, Wang Y, et al. Efficacy and safety of low-dose tirofiban for acute intracranial atherosclerotic stenosis related occlusion with residual stenosis after endovascular treatment. J Stroke Cerebrovasc Dis. 2020;29(4):104619. [DOI] [PubMed] [Google Scholar]
- 28.Kim YW, Sohn SI, Yoo J, et al. Local tirofiban infusion for remnant stenosis in large vessel occlusion: tirofiban ASSIST study. BMC Neurol. 2020;20(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833-1844. [DOI] [PubMed] [Google Scholar]
- 30.Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. [DOI] [PubMed] [Google Scholar]
- 31.Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325(3):234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses. J Clin Epidemiol. 2004;57(3):229-236. [DOI] [PubMed] [Google Scholar]
- 33.Tripepi G, Chesnaye NC, Dekker FW, Zoccali C, Jager KJ. Intention to treat and per protocol analysis in clinical trials. Nephrology. 2020;25(7):513-517. [DOI] [PubMed] [Google Scholar]
- 34.van 't Hof AW, Ernst N, de Boer MJ, et al. Facilitation of primary coronary angioplasty by early start of a glycoprotein 2b/3a inhibitor: results of the ongoing tirofiban in myocardial infarction evaluation (On-TIME) trial. Eur Heart J. 2004;25(10):837-846. [DOI] [PubMed] [Google Scholar]
- 35.Lee DP, Herity NA, Hiatt BL, et al. Adjunctive platelet glycoprotein IIb/IIIa receptor inhibition with tirofiban before primary angioplasty improves angiographic outcomes: results of the TIrofiban Given in the Emergency Room before Primary Angioplasty (TIGER-PA) pilot trial. Circulation. 2003;107(11):1497-1501. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Yin C, Yang J, Jiang L, Parsons MW, Lin L. Endovascular thrombectomy. Stroke. 2018;49(11):2783-2785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared to qualified investigators whose proposal of data use has been approved by the corresponding author and the RESCUE BT investigators.