Abstract
Background and Objectives
Limbic-predominant age-related TDP-43 encephalopathy (LATE) affects similar neuroanatomical networks as Alzheimer disease (AD) and is often comorbid with AD, though frequently missed in clinical diagnosis. The primary aim of this study was to elucidate the clinical and cognitive differences at baseline between patients with autopsy-confirmed LATE and patients with AD and comorbid LATE + AD.
Methods
Clinical and neuropathologic datasets were requested from the National Alzheimer Coordination Center. Baseline data from individuals older than 75 years during death without neuropathologic indication of frontotemporal lobar degeneration were included in analyses. Pathologically defined groups reflecting LATE, AD, and comorbid LATE + AD were identified. Group differences in clinical characteristics and cognition were explored through analysis of variance and the χ2 using measures from the Uniform Data Set measures.
Results
Pathology groups included 31 individuals with LATE (mean age: 80.6 ± 5.4 years), 393 with AD (mean age: 77.8 ± 6.4 years), and 262 with LATE + AD (mean age: 77.8 ± 6.6 years) without significant differences in sex, education, or race. Compared with participants with AD and LATE + AD pathology, participants with LATE pathology lived significantly longer (mean visits: LATE = 7.3 ± 3.7; AD = 5.8 ± 3.0; and LATE + AD = 5.8 ± 3.0; F(2,683) = 3.7, p < 0.05), reported later onset of cognitive decline (mean onset: LATE = 78.8 ± 5.7; AD = 72.5 ± 7.0; and LATE + AD = 72.9 ± 7.0; F(2,516) = 6.2, p < 0.01), and were more likely to be diagnosed as cognitively normal at baseline (LATE = 41.9%; AD = 25.4%; and LATE + AD = 12%; χ2 = 38.7, p < 0.001). Individuals with LATE (45.2%) also reported fewer memory complaints than those with AD (74.4%) or LATE + AD (66.4%; χ2 = 13.3, p = 0.001) and were less likely to be classified as impaired on the Mini-Mental State Examination (LATE = 6.5%; AD = 24.2%; and LATE + AD = 40.1%; χ2 = 29.20, p < 0.001). Across all neuropsychological measures, participants with LATE + AD pathology performed significantly worse than the AD and LATE groups.
Discussion
Those with LATE pathology were older when cognitive symptoms began and lived longer than participants with AD or LATE + AD pathology. Participants with LATE pathology were also more likely to be classified as “cognitively normal” based on objective screening and self-report measures, and they had higher scores on neuropsychological testing. Consistent with prior literature, comorbid pathologies led to more significant cognitive and functional impairment. Early disease characteristics based on clinical presentation alone were insufficient for differentiating LATE from AD, reiterating the need for a validated biomarker.
Limbic-predominant age-related TDP-43 encephalopathy (LATE) is believed to be the second most common neurodegenerative disease, after Alzheimer disease (AD), in the “oldest old” individuals older than 90 years.1 The neuropathologic changes associated with LATE are accumulation of phosphorylated transactive response DNA binding protein of 43 kDA (TDP-43) primarily located with limbic system, with or without hippocampal sclerosis (HS).1 While there are no published clinical diagnostic criteria for a clinical diagnosis of LATE, individuals with LATE typically present with an amnestic memory profile similar to AD.1 However, patients with LATE typically have prominent unilateral or bilateral hippocampal atrophy, negative amyloid and tau imaging, negative dopamine transporter scan, and normal levels of amyloid and phosphorylated tau in the CSF.2
Although clinical and pathologic changes associated with LATE have been known for several years, its recognition as a possibly discrete pathology process is relatively new. Compared with other neurodegenerative diseases, little is known about the impact of LATE neuropathologic changes (LATE-NC) on clinical symptoms and cognition, despite the fact LATE-NC has been found in 20%–60% of aged brains at autopsy.1,3,4 Cognitively, patients with LATE-NC demonstrate fairly isolated impairments in episodic memory1,5 and working memory.6 There are conflicting reports on the expected disease course of LATE, with some studies suggesting a faster progression and some demonstrating a more gradual decline relative to other neurodegenerative diseases.1,6,7 Some of this variability may be attributable to the extent and location of TDP-43 pathology because lower scores across neuropsychological measures have been associated with the spread of TDP-43 pathology outside the amygdala.6 However, there have otherwise been few studies evaluating LATE-specific cognitive impairments or clinical trajectories.
As people age, differential diagnosis of neurodegenerative diseases based on clinical presentation alone becomes increasingly difficult due to the combined influence of comorbid neuropathologies.8 This is especially true for LATE (and likely why it was only discovered recently) because LATE pathology affects similar neuroanatomical networks as AD and is often comorbid with AD, with an estimated 13%–36% of individuals having both TDP-43 and AD neuropathologic changes (ADNC) on autopsy.1,9 Autopsy data further suggest that a significant proportion, up to 20%, of patients with clinically diagnosed AD were actually misdiagnosed based on neurologic examination and neuroimaging, and patients' cognitive decline was likely more attributable to LATE-NC.10 There is also evidence suggesting that comorbid LATE-NC can exacerbate AD symptoms. Not only has the presence of both LATE-NC and ADNC been associated with lower scores on the Mini-Mental State Examination (MMSE), but there is also evidence of more rapid cognitive decline and more pronounced hippocampal atrophy in those with comorbid pathology compared with those with AD pathology alone.5,11
Without a validated biomarker, it will remain difficult to accurately diagnose LATE, if diagnosis remains solely dependent on the clinical presentation, given its syndromic overlap with AD. Although it is relatively clear that the comorbid presence of LATE-NC worsens cognition in patients with AD, the unique profile of cognitive impairment in patients who only have LATE-NC and how this compares with patients with pure AD and comorbid LATE-NC and ADNC remains unclear, especially early in the disease course. The primary aim of this study was to further investigate the cognitive characteristics of patients with LATE to better understand how these characteristics may differ from patients with pure AD and patients with both LATE-NC and ADNC. We hypothesized that patients with pure LATE would demonstrate less impairment on nonmemory-related neuropsychological measures, including measures of global cognition, semantic knowledge, working memory, executive functioning, and processing speed compared with patients with pure AD due to the isolated nature of LATE-NC in the hippocampus. We also expected that the presence of comorbid disease would exacerbate cognitive impairments, with patients with comorbid LATE + AD pathology performing worse than either LATE-NC or ADNC alone across neuropsychological measures.
Methods
Participants
This study used data from the Alzheimer Disease Research Center (ADRC) network, housed at the National Alzheimer Coordinating Center (NACC), which was collected between 2005 and 2020. A detailed description of NACC data collection and the Uniform Data Set (UDS) is available in the published literature.12,13 Data included in analyses were restricted to deceased participants who (1) had neuropathology data available and (2) were aged 75 years or older during death; this resulted in 19,880 data entries. We further excluded participants with clinical diagnoses or neuropathologic indications of frontotemporal lobar disease (FTLD), multiple system atrophy, Down syndrome, amyotrophic lateral sclerosis, or prion disease or participants with missing data on the presence of TDP-43 in the amygdala, hippocampus, or entorhinal/inferior temporal cortex. We limited analyses to data from baseline visits to investigate how participants initially present to clinics and research centers. With these criteria, the study dataset included 686 participants who were then grouped based on presence of pathology at autopsy: (1) evidence of LATE pathology only, (2) evidence of AD pathology only, and (3) evidence of comorbid LATE + AD pathology.
Demographic variables of interest were age, sex, race, handedness, and years of education. Clinical variables of interest included the number of UDS visits, age at symptom onset, age during death, and disease duration, which was defined as the time between symptom onset and death. Disease characteristics include consensus diagnosis after the first UDS visit (normal cognition, mild cognitive impairment, and dementia), presence of motor symptoms, presence of behavioral symptoms, participants' reports of memory decline, and caregivers' reports of memory decline.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants enrolled in research at ADRCs. There is an additional optional consent process for brain donation on death for all participants.
Neuropathology Data
Autopsy data on participants were collected using the standardized NACC Neuropathology Form.14 Because these autopsies are conducted at ADRCs across the country, there are a minimum number of recommended brain regions to be assessed.15 ADRCs conduct autopsies based on the established criteria for the assessment of AD, Lewy body disease (LBD), FTLD, cerebrovascular disease, and rarer neurologic pathologies. Staining procedures and additional information regarding the neuropathologic examination can be found in NACC Neuropathology Form14 and has been extensively documented.14
Most participants who have NACC Neuropathology Form data have Braak staging for neurofibrillary degeneration (stages 0–VI) and Consortium to Establish a Registry for Alzheimer Disease scores for neurotic plaque density (non, sparse, moderate, and frequent).15,16 Beginning in 2014, Thal phase for amyloid plaques (phases 0–5) is available for participants assessed with version 10 of the NACC Neuropathology Form.17 In participants who have had Thal phase assessed, the National Institute on Aging–Alzheimer Association (NIA-AA) AD score is available (none, low, intermediate, or high AD).18 For purposes of this study, a pathologic diagnosis of ADNC was defined by intermediate or high likelihood based on the NIA-AA criteria.7
Starting with version 10 of the NACC Neuropathology Form, the presence or absence of TDP-43–immunoreactive inclusions have been assessed in the amygdala, hippocampus, entorhinal/inferior temporal cortex, and neocortex. For this study, a pathologic diagnosis of LATE-NC was defined by the presence of TDP-43 inclusions in the amygdala, hippocampus, or entorhinal/inferior temporal cortex.19 To look at comorbid pathologies, the presence of LBD was classified based on a modification to the McKeith criteria to assess brainstem-, limbic-, neocortical-, or amygdala-predominant disease.20 Arteriolosclerosis is quantified as either none-to-mild or moderate-to-severe, and neuropathologists additionally note the presence of lacunar/gross infarcts, microinfarcts, cerebral amyloid angiopathy (CAA), and HS. Participants with the presence of FTLD with tau pathology, FTLD with TDP-43 pathology, and other FTLD subtypes were excluded from the study.
Neuropsychological Data
The neuropsychological test battery from the UDS consists of brief measures of episodic memory, attention, processing speed, language, visual spatial abilities, and executive functioning. There are 2 versions of the neuropsychological battery, C1 and C2. Version C1 contained the following neuropsychological assessments: MMSE, Wechsler Memory Scale-Revised Logical Memory, Benson Complex Figure Copy, Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Span and Digit-Symbol, Category Fluency, Trail Making Test, Phonemic Fluency, and Boston Naming Test–Short Form, and it has been well validated to detect cognitive aging and the early stages of AD.21 In 2015, version C2 was released, which replaced proprietary tests with nonproprietary tests.13 Because most of the participants with autopsy data were administered version C1, the raw scores from primary indices for the MMSE (out of 30 points), Logical Memory (out of 25 points), Category Fluency, Boston Naming Test–Short Form (out of 30 points), Trail Making Test A and B, Digit-Symbol, and Digit Span Forward and Backward from C1 were used in analyses. Data from version C2 were not included in this study, given the change in cognitive measures. Consistent with prior literature, participants were classified as impaired on the MMSE if their total score was ≤ 23.22 Given the presence of mood symptoms in many neurodegenerative diseases, total scores on the Geriatric Depression Scale (GDS) were analyzed. To assess functional decline, the total score from the Functional Assessment Scale was also analyzed.
Analyses
All statistical analyses were completed using IBM SPSS (version 26 or 28). Categorical variables, including sex, race, handedness, APOE status, consensus diagnosis, presence of behavior and motor symptoms, reported memory loss, and comorbid pathologies, were summarized using frequency and percentage. Continuous variables, including age, education, number of UDS visits, age at initial visit, age at initial symptoms, age at death, and disease duration (defined as time between onset of symptoms and death), were summarized using mean value and SD. Differences in patient demographics, clinical characteristics, comorbid pathologies, and impairment classification on the MMSE between the 3 pathology groups were assessed through χ2 tests for categorical variables. Owing to small sample sizes in some tabulated cells, we report the likelihood ratio χ2 test results. If overall significance was found, we used Bonferroni-adjusted post hoc tests to determine which pathology groups differed at α = 0.05. Uninformative responses (e.g., “no coparticipation” or “unknown”) were not retained for analyses; removal did not affect the statistical outcome of the global tests. Univariate analysis of variance (ANOVA) was used for continuous variables.
Differences in cognition between groups were assessed with ANOVA using neuropsychological variables as primary outcomes. Post hoc comparisons using the Bonferroni adjustment to maintain a type I error rate of 0.05 for multiple comparisons were conducted when main effect results were significant.
Data Availability
Qualified researchers may obtain access to all deidentified clinical, cognitive, and autopsy data used for this study by submitting a request to NACC (naccdata.org/requesting-data/data-request-process).
Results
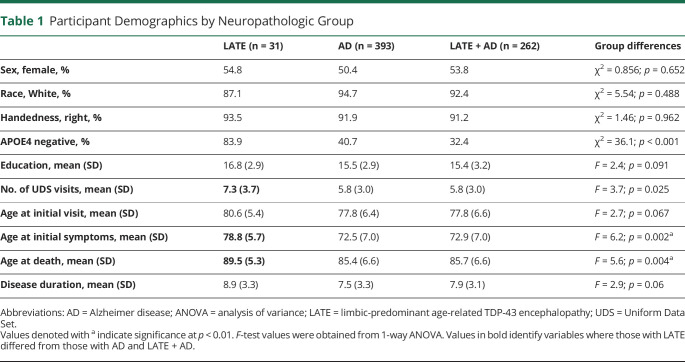
Of the 686 participants included, 31 met criteria for LATE-NC without the presence of ADNC, 393 participants met criteria for ADNC without the presence of LATE-NC, and 262 participants met criteria for comorbid LATE + AD. There were no significant differences between groups on age at first visit, sex, race, handedness, education, or disease duration (Table 1).
Table 1.
Participant Demographics by Neuropathologic Group
Participants with LATE-NC had significantly more UDS visits from the time of enrollment to the time of death (F(2,683) = 3.7, p < 0.05) than ADNC or LATE + AD groups. Participants with LATE-NC were also less likely to have an APOE4 allele and were older when cognitive symptom emerged (F(2,516) = 6.2, p < 0.01) and during death (F(2,685) = 5.6, p < 0.01) than the ADNC and LATE + AD groups (Table 1).
Clinical Characteristics
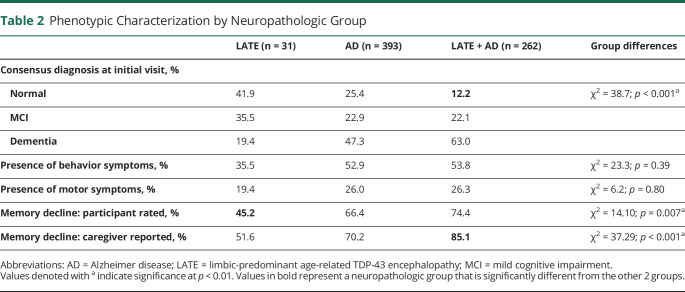
The proportion of patients diagnosed as cognitively normal at their initial visit was higher in the pure LATE (41.9%) and pure AD (25.4%) groups than in the LATE + AD (12%) pathology group (χ2 = 38.7, p < 0.001). There were also differences in participant-reported memory decline between pathology groups, with individuals with LATE-NC reporting significantly fewer memory complaints than those with ADNC or LATE + AD (χ2 = 13.3, p = 0.001). Caregiver-reported memory decline also differed between groups (χ2 = 24.7, p < 0.001), with the highest prevalence reported from those caring for people with LATE + AD; however, caregiver-reported memory concerns did not differ between those with ADNC and LATE-NC alone. There were no differences in the prevalence of behavioral changes or motor symptoms between groups (Table 2).
Table 2.
Phenotypic Characterization by Neuropathologic Group
Comorbid Pathology
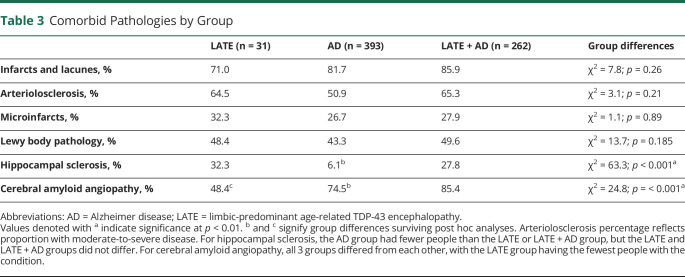
Regarding additional comorbidities, there were no differences in the prevalence of arteriolosclerosis between groups (χ2 = 3.1, p = 0.209). Regarding the presence of unilateral or bilateral HS, the LATE-NC group and comorbid LATE + AD group had significantly more people with HS than the ADNC group (χ2 = 63.3, p < 0.001). Finally, individuals in the LATE + AD group had significantly higher presence of CAA (85.5% of participants), followed by the ADNC group (74.6%), and the LATE-NC group (48.4%; χ2 = 24.8, p < 0.001). There were no significant differences between groups on the presence of infarcts or lacunes, microinfarcts, or Lewy body pathology (Table 3).
Table 3.
Comorbid Pathologies by Group
Neuropsychological Measures
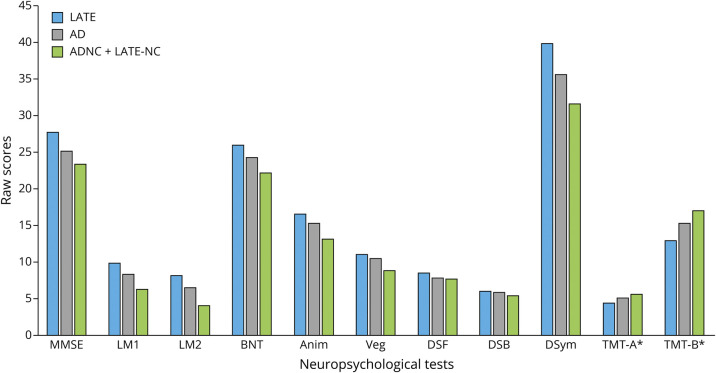
Differences in raw scores on neuropsychological measures between pathology groups are presented in Figure. There were significant differences between groups on total MMSE score (F(2,620) = 15.3, p < 0.001). Post hoc analyses revealed differences between each group, with the LATE-NC performing better than the ADNC group, and both the LATE-NC and ADNC groups performing better than those with comorbid pathology. Using a cutoff score of ≤23, there was a significant difference between pathology groups on the percentage of participants with an impaired score (χ2 = 29.20, p < 0.001). Among those with LATE-NC, 6.5% of participants were classified as impaired at baseline, compared with 24.2% of those with ADNC and 40.1% of those with LATE + AD. Logical Memory learning also differed between groups (F(2,604) = 14.4, p < 0.001), with the LATE + AD group showing the worst performance; there were no significant differences between the LATE-NC and ADNC groups. Logical Memory delayed recall followed a similar pattern, with the LATE + AD group performing worse than both the LATE-NC group and the ADNC group; there were again no significant differences between the LATE-NC and ADNC groups. The Boston Naming Test–Short Form (BNT-SF) also differed between groups (F(2,595) = 11.4, p < 0.001), with the LATE + AD group again performing worse than the LATE-NC group and the ADNC group, but the LATE-NC and ADNC groups did not significantly differ. Category fluency also differed between pathology groups (F(2,636) = 10.0, p < 0.001) and again showed that for animal naming, the LATE-NC group performed the best, followed by the ADNC group, both of which performed significantly better than the LATE + AD group; similar differences were also observed for vegetable naming. Significant differences emerged on the Trail Making Test B (F(2,549) = 4.2, p < 0.05), with the LATE + AD group performing worse than the LATE-NC group. There were no significant differences between the LATE-NC and ADNC groups and the ADNC and LATE + AD groups on post hoc analyses. Finally, significant differences emerged on WAIS-R Digit Symbol measure (F(2,556) = 7.8, p < 0.001) with the LATE + AD group performing worse than the LATE-NC group and the ADNC group. There were no significant differences between the LATE-NC and ADNC groups on post hoc analyses. There were no significant differences between groups on Digit Span Forward or Trail Making Test A, and although Digit Span Backward was significantly different between the 3 groups (F(2,606) = 3.5, p = 0.03), this significant difference did not withstand post hoc comparisons.
Figure. Neuropsychological Test Performance by Pathology Group.
Anim = Animal Naming; BNT = Boston Naming Test-Short Form; DSB = Digit Span Backward; DSF = Digit Span Forward; DSym = Digit Symbol Substitution; LM1 = Logical Memory I; LM2 = Logical Memory 2; MMSE = Mini-Mental State Examination; TMT-A = Trail Making Test, A; TMT-B = Trail Making Test, B; Veg = Vegetable Naming. All values represent raw, unadjusted performance. Values denoted with * have been divided by 10 for ease of visualization.
Regarding functional status, there were significant differences between groups on the level of functional impairment reported (F(2,662) = 12.0, p < 0.001), with the LATE + AD group reporting more functional impairment than the LATE-NC group and the ADNC group. There were no significant differences between the LATE-NC and ADNC group on post hoc analyses. There were no significant differences found between groups on the GDS.
Discussion
In an autopsy-confirmed sample, we found that there are clear differences at initial presentation between those with LATE pathology relative to those with AD pathology and comorbid AD + LATE. Perhaps most notably, patients who are ultimately found to possess LATE pathologic changes on autopsy are more likely to be classified at baseline as cognitively normal based on consensus diagnosis. They also have fewer subjective memory complaints compared with those with ADNC or comorbid disease and are more likely to perform normally on cognitive screenings (e.g., MMSE). Taken together, these findings demonstrate that those with LATE-NC are initially presenting as cognitively healthy individuals with few cognitive complaints. Patients with LATE-NC are also likely to be older during initial symptom onset and older during death, though these participants demonstrated a shorter disease duration than patients with ADNC or LATE + AD. Consistent with the prior literature, participants with comorbid LATE + AD had significantly worse cognitive performance across measures of memory, language, working memory, and processing speed than either pathology alone, reiterating that the presence of comorbid pathologies leads to more cognitive impairment and a faster trajectory of cognitive decline.5-7,23
Currently, there are no established in vivo diagnostic criteria for LATE. Differentiating participants with LATE from AD requires autopsy data or confirmatory rule out with AD biomarkers (e.g., amyloid or tau PET scan or CSF studies), though this remains particularly challenging in the absence of supporting biomarkers, especially early in the disease course (e.g., screening visits). Our findings show that individuals with LATE initially present with fewer cognitive and functional complaints than individuals with AD or comorbid LATE + AD, which is reflected on objective measures of cognition. Across measures of memory, semantic knowledge, working memory, and processing speed, participants with LATE performed better than participants with AD and LATE + AD. However, post hoc analyses indicated that objective cognitive testing is better at differentiating the comorbid disease process from either disease on its own, but differences between the pure, single pathology groups were less clear. Out of all the cognitive measures assessed, the MMSE total score was the only measure to show significant differences among all 3 groups.
Our finding that participants with LATE-NC are more likely to initially present to clinics with cognitive performances within the normal range has a significant impact for research, especially clinical trials and observational studies of aging. While highly sensitive AD biomarkers (e.g., amyloid PET) can be used to determine eligibility and study enrollment in intervention trials, these measures are expensive and labor intensive. Many research studies rely heavily on the clinical presentation, augmented by objective cognitive measures and screenings, such as the MMSE or Montreal Cognitive Assessment. Our findings suggest that individuals with LATE pathology have a significantly higher likelihood of being classified as normal and would thus have been enrolled in the control group. This contamination of participants in a control group with abnormal neuropathology would be a serious threat to the internal validity of the trial and thus undermine the ability to detect the impact of the AD intervention. These findings highlight the importance of including additional advanced biomarkers, not just for AD pathology but for additional pathologies, when screening participants for study inclusion, such as fluorodeoxyglucose-PET imaging, and relying less on cognitive screening measures. Our findings also lend further support for calls to develop a fluid or imaging biomarker specific to LATE-NC, which would improve both trial enrollment and our ability to learn more about LATE, especially early in the disease course.
In our study, participants with LATE were older at the age at symptom onset and older at the age of death, consistent with the prior literature suggesting LATE symptoms typically develop later than AD symptoms.1 Contrary to other studies in the literature, however, our findings suggest that participants with LATE have a similar disease duration defined as time between onset of cognitive symptoms and death, compared with participants with AD and comorbid disease. This may be because many people with LATE-NC are likely to have very subtle (if any) cognitive impairment during the initial stages of disease, only reporting onset of symptoms later in the disease course due to a higher burden of neuropathology. We recognize that this is at odds with prior studies that have found that participants with LATE pathology have a slower trajectory of cognitive decline, while participants with LATE + AD tend to have a more rapid rate of decline.1,6,7 These prior studies tended to use mixed-effect models to determine the rate of cognitive decline over time between participants with and without LATE pathology, while our study exclusively examined the length of time between symptom onset and death. Our findings are similar to those from Boyle et al.23 on the predicted trajectory of cognitive decline, who found that individuals with TDP-43 pathology had a shorter time between symptom onset and death compared with AD, LBD, and cerebrovascular disease. Further longitudinal research is needed to track the progression of individuals with LATE pathology.
When comorbid pathology was investigated, participants with any LATE pathology had significantly more participants with unilateral or bilateral HS than the pure AD, which is consistent with the potential diagnostic criteria for LATE. Not surprisingly, individuals with any AD pathology on autopsy had higher levels of CAA than individuals with pure LATE pathology.
There are several limitations to this study. First, we had a significantly lower number of participants with pure LATE pathology, which stems from the fact that autopsy data on TDP-43 inclusions in the limbic system were not included as a standard until 2014. Because of this, it is possible that individuals in the AD group may have also had LATE pathology, but because it was not evaluated, these participants were diagnosed with AD. Moreover, our sample of people with evidence of LATE-NC was underpowered for subgroup analyses based on clinical disease stages, which should be explored in the future. Second, we chose to examine initial visits, which often used the C1 neuropsychological battery. As such, there are no normative data or age-adjusted standardized scores available for these measures. Because this research investigated differences in the raw scores, it was difficult to investigate clinically meaningful differences between cognitive profiles; however, given that consensus diagnoses were available and likely based on standardized interpretation, it is a reasonable assumption that clinical interpretations of the baseline neuropsychological data would have been normatively intact. Future research should focus on investigating cognitive differences between AD, LATE, and comorbid pathology using normative data to investigate meaningful differences to further investigate whether LATE has a distinct profile of cognitive impairments compared with the normative sample. We also chose to focus on the cognitive differences between groups though there is certainly interest in understanding whether there are any psychiatric and behavioral differences. The present dataset had limited information of behavioral changes beyond clinician ratings, though this is certainly another direction for targeted study.7,24
It is also important to note that while incredibly rich in many ways, the representativeness of the NACC dataset sample is biased toward older adults who are primarily non-Hispanic White, highly educated, and from higher socioeconomic backgrounds. The extent to which these findings generalize to racially and ethnically diverse populations and those from areas of greater neighborhood disadvantage is unclear but is an incredibly important area for further inquiry. Moreover, the NACC dataset is enriched for AD and as such has many APOE+ individuals. This may explain the relatively low rate of LATE on its own in our cohort relative to recently published data from an aggregated community-based cohort.25 Finally, future studies should focus on the diagnosis of LATE in vivo, such as investigating the ratio of hippocampal atrophy to whole brain atrophy when both LATE and AD are possible differential diagnoses.
Glossary
- AD
Alzheimer disease
- ADNC
AD neuropathologic change
- ADRC
Alzheimer Disease Research Center
- ANOVA
analysis of variance
- CAA
cerebral amyloid angiopathy
- FTLD
frontotemporal lobar disease
- GDS
Geriatric Depression Scale
- HS
hippocampal sclerosis
- LATE
limbic-predominant age-related TDP-43 encephalopathy
- LATE-NC
LATE neuropathologic change
- LBD
Lewy body disease
- MMSE
Mini-Mental State Examination
- NACC
National Alzheimer Coordinating Center
- NIA-AA
National Institute on Aging-Alzheimer Association
- TDP-43
phosphorylated transactive response DNA binding protein of 43 kDA
- UDS
Uniform Data Set
- WAIS-R
Wechsler Adult Intelligence Scale-Revised
Appendix. Authors
Study Funding
The NACC database is funded by the NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), and P30 AG072959 (PI James Leverenz, MD).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527. doi: 10.1093/BRAIN/AWZ099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budson AE, Solomon PR. Limbic-predominant age-related TDP-43 encephalopathy. In: Budson AE, Solomon PR, eds. Memory Loss, Alzheimer's Disease, and Dementia: A Practical Guide for Clinicians. 3rd ed. Elsevier; 2022:77-83. [Google Scholar]
- 3.Harrison WT, Lusk JB, Liu B, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol. 2021;142(5):917-919. doi: 10.1007/S00401-021-02360-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeżewski RC, Poljak A, Mather KA, Sachdev PS, Shepherd CE. The prevalence of limbic-predominant age-related TDP-43 encephalopathy in the Sydney brain bank. Alzheimers Dement. 2020;16(S2):e037885. doi: 10.1002/ALZ.037885 [DOI] [Google Scholar]
- 5.Nelson PT. LATE neuropathologic changes with little or no Alzheimer disease is common and is associated with cognitive impairment but not frontotemporal dementia. J Neuropathol Exp Neurol. 2021;80(7):649-651. doi: 10.1093/JNEN/NLAB050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70(11):1418-1424. doi: 10.1001/JAMANEUROL.2013.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapasi A, Yu L, Boyle PA, Barnes LL, Bennett DA, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy, ADNC pathology, and cognitive decline in aging. Neurology. 2020;95(14):e1951-e1962. doi: 10.1212/WNL.0000000000010454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JL, Corrada MM, Kovacs GG, et al. Non-Alzheimer's contributions to dementia and cognitive resilience in the 90+ Study. Acta Neuropathol. 2018;136(3):377-388. doi: 10.1007/S00401-018-1872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DX, Bajaj S, McRae-McKee K, Hadjichrysanthou C, Anderson RM, Collinge J. Association of TDP-43 proteinopathy, cerebral amyloid angiopathy, and Lewy bodies with cognitive impairment in individuals with or without Alzheimer's disease neuropathology. Sci Rep. 2020;10(1):14579. doi: 10.1038/s41598-020-71305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85(1):114-124. doi: 10.1002/ANA.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal S, Yu L, Nag S, et al. Neocortical-type Lewy bodies and limbic-predominant age-related TDP-43 encephalopathy neuropathologic change in community-dwelling older persons. Alzheimers Dement. 2020;16(S3):e047449. doi: 10.1002/ALZ.047449 [DOI] [Google Scholar]
- 12.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249-258. doi: 10.1097/WAD.0B013E318142774E [DOI] [PubMed] [Google Scholar]
- 13.Besser L, Kukull W, Knopman DS, et al. Version 3 of the national Alzheimer's coordinating center's uniform data set. Alzheimer Dis Assoc Disord. 2018;32(4):351-358. doi: 10.1097/WAD.0000000000000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer's Coordinating Center's Neuropathology Form-available data and new analyses. J Neuropathol Exp Neurol. 2018;77(8):717-726. doi: 10.1093/jnen/nly049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/S00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389-404. doi: 10.1007/S00401-006-0127-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791-1800. doi: 10.1212/WNL.58.12.1791 [DOI] [PubMed] [Google Scholar]
- 18.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8(1):1-13. doi: 10.1016/J.JALZ.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besser LM, Teylan MA, Nelson PT. Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological Associations. J Neuropathol Exp Neurol. 2020;79(3):305-313. doi: 10.1093/JNEN/NLZ126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. doi: 10.1212/01.WNL.0000187889.17253.B1 [DOI] [PubMed] [Google Scholar]
- 21.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91-101. doi: 10.1097/WAD.0B013E318191C7DD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Relationship between the Montreal Cognitive Assessment and Mini-Mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15(1):107. doi: 10.1186/S12877-015-0103-3/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74-83. doi: 10.1002/ANA.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017;140(3):804-812. doi: 10.1093/BRAIN/AWW341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol. 2022;144(1):27-44. doi: 10.1007/S00401-022-02444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may obtain access to all deidentified clinical, cognitive, and autopsy data used for this study by submitting a request to NACC (naccdata.org/requesting-data/data-request-process).