Abstract
Background and Objectives
To investigate the frequency of induced EEG burst suppression pattern during continuous IV anesthesia (IVAD) and associated outcomes in adult patients treated for refractory status epilepticus (RSE).
Methods
Patients with RSE treated with anesthetics at a Swiss academic care center from 2011 to 2019 were included. Clinical data and semiquantitative EEG analyses were assessed. Burst suppression was categorized as incomplete burst suppression (with ≥20% and <50% suppression proportion) or complete burst suppression (with ≥50% suppression proportion). The frequency of induced burst suppression and association of burst suppression with outcomes (persistent seizure termination, in-hospital survival, and return to premorbid neurologic function) were the endpoints.
Results
We identified 147 patients with RSE treated with IVAD. Among 102 patients without cerebral anoxia, incomplete burst suppression was achieved in 14 (14%) with a median of 23 hours (interquartile range [IQR] 1–29) and complete burst suppression was achieved in 21 (21%) with a median of 51 hours (IQR 16–104). Age, Charlson comorbidity index, RSE with motor symptoms, the Status Epilepticus Severity Score and arterial hypotension requiring vasopressors were identified as potential confounders in univariable comparisons between patients with and without any burst suppression. Multivariable analyses revealed no associations between any burst suppression and the predefined endpoints. However, among 45 patients with cerebral anoxia, induced burst suppression was associated with persistent seizure termination (72% without vs 29% with burst suppression, p = 0.004) and survival (50% vs 14% p = 0.005).
Discussion
In adult patients with RSE treated with IVAD, burst suppression with ≥50% suppression proportion was achieved in every fifth patient and not associated with persistent seizure termination, in-hospital survival, or return to premorbid neurologic function.
Status epilepticus (SE) is a neurologic emergency with ongoing epileptic seizures1 that comes along with a high morbidity and mortality.2-4 When first-line and second-line antiseizure treatments fail to terminate seizure activity, SE becomes refractory (RSE), often representing a life-threatening condition requiring a transfer to the intensive care unit (ICU) to stop seizures. In such a situation, international guidelines recommend the induction of an artificial deep coma by continuous IV administration of anesthetic drugs (IVADs) for 24–48 hours with the aim to end RSE, monitored by EEG.5,6 EEG surrogates for RSE termination are either cessation of electrographic seizures, the emergence of a therapeutic burst suppression/attenuation pattern, or an isoelectric curve, but recommendations for the establishment of one of these specific EEG targets in certain clinical situations are not provided. To achieve these surrogate aims, an EEG-guided titration of IVADs is frequently necessary. A multinational survey identified burst suppression as the preferred titration target for IVADs in RSE,7 and an interburst interval of approximately 10 seconds is suggested according to experts' opinion.8
However, maintaining a continuous burst suppression is difficult despite constant administration of IVADs.9 Furthermore, there is little evidence for burst suppression as being an adequate surrogate target, which is limited by methodological issues as acknowledged by international guidelines.5,6 A current definition of burst suppression specifies a suppression/attenuation proportion of ≥50% of the recording.10 However, data supporting the adequacy of this threshold are lacking. In the context of the ongoing debate whether high-dose and prolonged IVADs in patients with RSE cause harm that may outweigh presumed benefits,11-16 there is an utter need for further studies. Hence, we aimed to investigate the frequency of induced burst suppression during anesthesia and whether burst suppression was associated with specific outcomes in patients treated with IVADs for RSE.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This observational cohort study was performed at the ICU of the University Hospital of Basel, a Swiss tertiary academic medical care center. We followed the Strengthening the Reporting of Observational Studies in Epidemiology-guidelines.17 Patients' consent was waived after approval of the study by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz) in compliance with the 1964 Declaration of Helsinki and its later amendments.
Data Collection
Clinical data in this study are part of the STatus EPilepticus Unicenter Population study (ClinicalTrials.gov ID: NCT04204863) and the REfractory Status Epilepticus Treatment: Quality and Efficacy of Coma Induction study (NCT04333082), performed at the University Hospital of Basel, collecting data of adult (18 years or older) patients with SE. From January 1, 2011, to December 31, 2019, all clinical, laboratory and epileptologic data of all consecutive patients with RSE were collected. All data were extracted from electronic medical records and managed in the password-encrypted online digital browser-based, metadata-driven database organizer Research Electronic Data Capture.18 The following clinical data were collected from individual patients' charts: age, sex, diseases, Glasgow Coma Score (GCS) at SE onset, and etiology of RSE (categorized as potential nonfatal and fatal etiologies, as defined elsewhere19,20). Patients with anoxic brain injury (ABI) included those with acute brain damage after cardiac arrest with resuscitation. Duration of mechanical ventilation, continuous administration of anesthetics and vasopressors, the use of nonsedating antiseizure drugs, complications during RSE including organ failure, infections, and arterial hypotension requiring the use of continuously administered vasopressors were noted. The length of ICU stay and hospital stay, return to premorbid neurologic function, and death at hospital discharge were assessed.
Illness severity was assessed by the Status Epilepticus Severity Score (STESS; range 0–6),21,22 the Charlson comorbidity index (range 0–37),23 the Simplified Acute Physiology Score II (range 0–163),24 and the Acute Physiology and Chronic Health Evaluation II (range 0–71).25 For patients with ABI, additional information regarding the time from cardiorespiratory arrest to start of resuscitation, duration of cardiopulmonary resuscitation, and initial rhythms were assessed.
EEG Recording and Semiquantitative Analysis
Burst suppression describes an EEG pattern consisting of alternating epochs of high-voltage broad-spectrum oscillations (bursts) and electrical suppression. Burst suppression was defined according to the current American Clinical Neurophysiology Society's (ACNS) Standardized Critical Care EEG Terminology with bursts that must average ≥0.5 seconds, have at least 4 phases, and may last up to 30 seconds, whereas suppression/attenuation is either <10 μV or ≥10 μV and <50% of the higher voltage background activity. The proportion of suppression/attenuation must be between 50% and 99% of the recording,10 which is based on an experts' consensus.
As part of routine clinical practice, all video-EEGs were recorded at a sampling rate of 256 Hz using 21 superficial scalp electrodes placed according to the international 10-20 system. All continuous EEGs (cEEGs; defined as records ≥1 hour) and spot-EEGs were first qualitatively analyzed by 2 EEG readers, noting the presence of burst suppression or attenuation/suppression epochs in clinical practice.
Computational algorithms to quantify burst suppression need an extensive preprocessing before analysis, and commercially available software is prone to over-reading or under-reading and needs human proofreading for quality assessment.26 For this study, we chose an adaptation of a previously published pragmatic approach27 to quantitatively assess burst suppression, which represents also a feasible approach in clinical practice directly at the patient's bedside: all EEGs during the period of IVADs were visually reanalyzed to quantify burst suppression by 2 trained EEG readers (U.F. and A.L.J.) with the aim to reach full agreement regarding the predefined characteristics regarding burst suppression. In rare cases of missing consensus, the recordings were assessed with a third EEG reader (R.S.) to reach agreement. For every hour of EEG recording, the first 2-minute epoch was continuously assessed for segments with suppression/attenuation fulfilling the ACNS criteria that were rounded to whole seconds (Figure 1). In case of severe artefacts on cEEG, a representative 2-minute epoch within ±15 minutes was analyzed.
Figure 1. Semiquantification of Burst Suppression.
Within 2-minute epochs, suppression/attenuation segments were visually identified and rounded to whole seconds (bold lines): Absence of any burst suppression pattern (A, ≤20% cumulative suppression/attenuation segments of an exemplary 15-second EEG epoch); incomplete burst suppression (B, ≥20% and <50% suppression/attenuation proportion); complete burst suppression (C, ≥50% and <100% suppression/attenuation). All EEGs shown in anterior-posterior bipolar montage using the international 10-20 system for the placement of superficial scalp electrodes, low-pass filter 0.5 Hz, high-pass filter 70 Hz; scale bar: horizontal 1 second, vertical 70 µV.
The following 3 burst suppression categories were noted: presence of (1) a complete burst suppression with ≥50% suppression/attenuation (cumulative ≥60 seconds of a 2-minute epoch) in line with the ACNS definition10; (2) an incomplete burst suppression that morphologically resembles a burst suppression, fulfilling all ACNS definitions except the duration of suppression/attenuation with proportions of suppression/attenuation episodes between 20% and 49% (cumulative between 24 and 59 seconds of a 2-minute epoch); (3) absence of any burst suppression (cumulative <24 seconds of a 2-minute epoch; Figure 1). Each case with RSE was assigned to none, incomplete, or complete burst suppression according to the maximal level of suppression reached during the entire EEG monitoring.
Definition, Duration, and Severity of RSE
RSE and SE were defined according to the International League Against Epilepsy task force on the classification of SE.1 As previously described,28,29 RSE duration was defined as the length between clinical and/or EEG evidence of seizure onset and termination, as verified by EEG. For video-EEG monitoring, 2 strategies were used during the study period, including cEEG and spot-EEGs at least every 12 hours. Thus, SE duration represents an approximation with a maximal inaccuracy of 12 hours. Video-EEG monitoring was used in patients with persistent altered consciousness after SE termination to exclude recurrent SE.
Antiseizure Treatment
Diagnostic and therapeutic procedures followed the guidelines of the American Epilepsy Society and the Neurocritical Care Society and were guided by the same neurologists and neurointensivists during the entire study period.6,30 First-line treatment was IV benzodiazepine bolus that was repeated in case of seizure persistency. For SE refractory to benzodiazepines, second-line treatment, including levetiracetam, lacosamide, valproic acid, or phenytoin, was started. In patients with SE refractory to first-line and second-line antiseizure treatments (i.e., RSE), nonsedating antiseizure drugs, such as pregabalin, sultiame, zonisamide, topiramate, oxcarbazepine or perampanel, were added, and continuously administered anesthetics including propofol and midazolam were started as third-line treatment. As a routine clinical practice, anesthetics were titrated on the discretion of the treating physicians in consultation with a board-certified neurologist with the aim to achieve an electrographic proof of either seizure cessation or a burst suppression as defined earlier and depending on multiple factors including the patients' hemodynamic stability during and their reaction on the administration of anesthetics for at least 24 hours. If SE reoccurred after third-line anesthetic treatment, barbiturates were started and titrated to induce a maximal burst suppression.31
Outcomes
The primary outcome was the frequency of induced burst suppression during deep anesthesia. Secondary outcomes were the association of the presence and the duration of burst suppression with predefined endpoints, including persistent seizure termination (i.e., no recurrent seizures after weaning of IVAD until hospital discharge or death), in-hospital survival, or return to premorbid neurologic function at hospital discharge.
Statistics
Patients were first categorized according to the presence or absence of any burst suppression. Second, burst suppression was categorized into complete or incomplete, as defined earlier. The χ2 or Fisher exact test, where appropriate, was used for univariable comparisons of proportions. For continuous variables, the Mann-Whitney U test or the Kruskal-Wallis test was applied. Discrete variables were expressed as counts (percentages), and continuous variables were expressed as medians and interquartile ranges (IQRs). All clinical, treatment-related, and EEG variables with significant differences between the burst suppression groups were included in univariable and multivariable logistic regression models for all secondary endpoints. For multivariable logistic regression models, Hosmer-Lemeshow chi-square goodness-of-fit tests were performed. These tests provide summary measures of calibration based on a comparison of observed and estimated outcomes.32 Patients with RSE after ABI were excluded for the main analyses because this etiology of RSE is known to be independently associated with a high mortality.33 For this patient subgroup, multivariable analyses were not performed because of the limited sample size. Two-sided p values ≤0.05 were considered significant. Statistical analysis was performed with STATA software, version 16.1 (Stata Corp., College Station, TX).
Data Availability
The corresponding author has full access to all data of the study. Due to data protection reasons, data cannot be made publicly available. However, anonymized grouped data will be made available on reasonable request to qualifed investigators.
Results
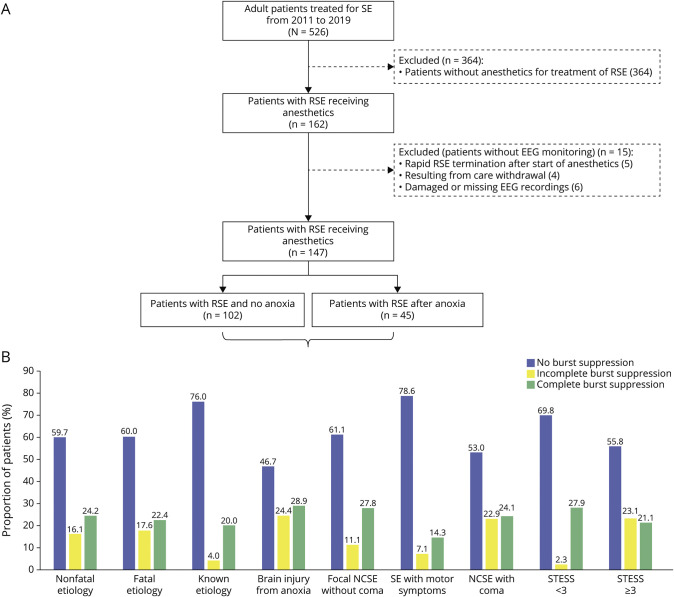
Of 526 adult patients with SE, 162 patients with RSE were treated with IVADs, of whom EEG recording was available in 147 patients (Figure 2A). Of these 147 patients, 45 had ABI (none with spontaneous burst suppression). Demographics and clinical and treatment characteristics are summarized in eTable 1 (links.lww.com/WNL/C684). Among 102 patients without ABI, burst suppression was established in 35 (34.3%) with incomplete burst suppression in 14 (13.7%) with a median of 22.8 hours (IQR 1.0–28.9) and complete burst suppression in 21 (20.5%) with a median of 50.9 hours (IQR 16.1–103.7) (Figure 2B). A complete background suppression was not achieved in any patient.
Figure 2. Flowchart and EEG Characteristics Among the Subgroups of the Study Population.
(A) Proportion of patients with and without achieved burst suppression during the treatment of refractory status epilepticus. (B) Proportion of patients with and without complete or incomplete burst suppression among specific subgroups. NCSE = nonconvulsive status epilepticus; RSE = refractory status epilepticus; SE = status epilepticus; STESS = Status Epilepticus Severity Score.
Univariable Comparisons
Univariable comparison of demographics and clinical and treatment characteristics of patients without ABI with or without burst suppression are outlined in Tables 1 and 2. The Charlson comorbidity index, RSE with motor symptoms, the choice of IVADs, and arterial hypotension requiring vasopressors differed significantly between patients achieving any and no burst suppression (Table 1).
Table 1.
Univariable Comparisons Between 102 Patients With RSE Without ABI With or Without Any Induced Burst Suppression
Table 2.
Univariable Comparisons Between 102 Patients With RSE Without ABI With Incomplete, Complete, or No Burst Suppression
In addition, patients with any burst suppression when compared with patients without burst suppression had a longer median ICU (19 vs 8 days, p = 0.031) and hospital stay (25 vs 20 days, p < 0.001), a longer median duration of mechanical ventilation (13 vs 3 hours, p < 0.001), were monitored on cEEG more often (97% vs 66%, p = 0.005), and had a longer cumulative median duration of EEG monitoring (29.2 vs 4.3 hours, p = 0.007). All patients received benzodiazepines as first-line and second-line antiseizure drugs. Patients achieving any burst suppression did not have more nonanesthetic antiseizure drugs than those without burst suppression (median 4 vs 3, p = 0.075).
When discriminating any burst suppression into incomplete and complete burst suppression, age and STESS showed additional significant differences. Patients with complete burst suppression needed more often vasopressors due to arterial hypotension (Table 2).
Multivariable Logistic Regression Analyses
Multivariable analyses in patients with RSE without ABI adjusted for the potential confounders age, Charlson comorbidity index, RSE with motor symptoms, STESS, and arterial hypotension requiring vasopressors are summarized in Table 3. After adjustment, the multivariable models showed no associations between the presence of any burst suppression or duration of burst suppression and persistent seizure termination, in-hospital survival, or return to premorbid neurologic function in survivors.
Table 3.
Multivariable Logistic Regression Analyses Regarding the Associations Between Burst Suppression and Outcomes in Patients With RSE Without ABI
Patients With RSE After ABI
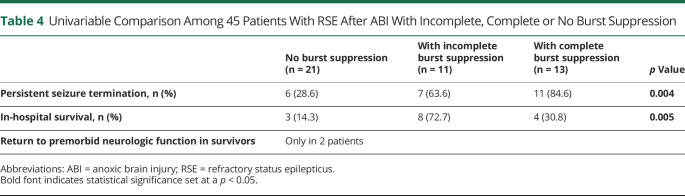
Subsequent univariable comparisons among 45 patients with RSE after ABI are summarized in Table 4. In our cohort, all patients with ABI had brain injury from cardiorespiratory arrest. In comparison with the absence of burst suppression, induction of any burst suppression was significantly associated with persistent seizure termination (29% vs 72%) and in-hospital survival (14% vs 50%). Two of 15 surviving patients (4% of 45 patients with anoxia treated with IVADs) had full recovery with return to premorbid neurologic status. Of the 12 surviving patients with any burst suppression induced by IVADs (1 with STESS 2 and 11 with STESS ≥3), none had a Glasgow Outcome Scale (GOS) more than 3 (vegetative state to severe disability). Thereof, 5 were transferred to neurorehabilitation and 8 were discharged to a hospice. No patient with ABI had spontaneous burst suppression due to ABI per se before IVAD administration. We refrained from performing multivariable analyses due to the limited sample size. Except higher median GCS at SE onset in the 2 patients with ABI and return to premorbid neurologic function, a detailed comparison of demographics and clinical characteristics did not show any marked difference between patients with ABI with or without return to premorbid neurologic function (eTable 2, links.lww.com/WNL/C684).
Table 4.
Univariable Comparison Among 45 Patients With RSE After ABI With Incomplete, Complete or No Burst Suppression
Discussion
We analyzed the frequency and duration of induced burst suppression and effects on outcomes in a single-center 9-year cohort of adult patients with RSE. Our main findings indicated that burst suppression was achieved in up to a third of patients in whom deep anesthesia reflected by burst suppression or persistent seizure suppression was targeted and that in only every fifth patient burst suppression fulfilled the current ACNS criteria.9 These findings are reflected by previous studies that demonstrated the difficulties to establish a continuous burst suppression. A retrospective study demonstrated a remarkable interpatient and intrapatient variability of suppression proportions despite a continuous rate of IVADs with most patients not meeting the goal of a suppression rate of 80% and some also not fulfilling the ACNS burst suppression criteria despite cEEG monitoring.9 Even in a prospective randomized study, burst suppression was not possible to be established in some patients.34 As a point of consideration in our cohort, the titration goal has not always specified to be a burst suppression. At our institution, physicians followed the international guidelines that recommend either achieving seizure cessation or burst suppression with no further advice whichever is preferred in which clinical situation.5,6 Thus, the fact that merely 60% of our patients did not reach burst suppression is not necessarily equivalent to a treatment failure in all cases, but may rather indicate the clinicians' balance between deepening of anesthesia to achieve burst suppression and the associated risks that may come along with artificially induced deep coma.11,14,15
Our secondary findings showed that while burst suppression was associated with prolonged mechanical ventilation, severe arterial hypotension requiring the administration of vasopressors, and prolonged ICU stay and hospital stay as expected from a more intense anesthetic treatment, the achievement and duration of burst suppression was not associated with clinical outcomes, including persistent seizure termination, in-hospital survival, and return to premorbid neurologic function after adjusting for potential confounders. Previous retrospective studies showed heterogeneous results on burst suppression and outcome. One study showed improved survival if the titration goal of an isoelectric EEG or absence of seizures was achieved, but not burst suppression, although adjustment for confounders regarding mortality was not conducted.35 Contrariwise, another study, where almost 90% of patients qualified for a super-RSE, found worse outcomes in patients with burst suppression or isoelectric EEG. Conversely, seizure suppression alone was associated with good outcome.36 Other studies could not demonstrate an impact of burst suppression on outcome.26,37-40 However, most of these studies were also limited by the lack of multivariable models and burst suppression presence based on EEG reports rather than EEG evaluation itself. Furthermore, burst suppression was not uniformly defined.
The evidence provided from this study and previous research is insufficient to suggest that using burst suppression with a suppression/attenuation proportion ≥50% is more effective than using burst suppression with a smaller suppression/attenuation proportion or not using it at all. This conclusion applies specifically to patients with RSE not following ABI. Our multivariable analyses regarding associations between complete burst suppression and outcomes might have been underpowered (i.e., type II error), as reflected by the large confidence intervals. However, the fact that several other studies yielded similar findings strengthen our results.26,37-40 Nevertheless, studies on larger cohorts are needed to confirm these results.
Our analyses of the subgroup of patients with RSE after ABI revealed a significant increase of persistent seizure termination and survival in patients in whom any burst suppression was achieved. Further analyses adjusting for potential confounders were not feasible due to the limited sample size. Despite obvious differences in the study design and data collection, our results contrast the conclusions of a recent multicenter trial, which could not identify a benefit of suppressing rhythmic and periodic EEG activity by antiseizure drugs in comatose survivors of cardiac arrest.41 As a marked difference, our subgroup consisted of patients with RSE after ABI, whereas in the recent multicenter trial, this was not specified.42 While at first glance, these results were promising, a closer look showed that no surviving patient with any burst suppression had a GOS of 4–5 and the bias of “self-fulfilling prophecy” cannot be excluded. Likewise, there remains the possibility that the responsible clinicians treated more aggressively when they believed that individual patients may benefit. Nevertheless, these findings merit further investigation. Larger studies are needed in this field to identify possible subgroups, such as patients with RSE, who could benefit from aggressive antiseizure treatment.
Our study strengths include a large and representative cohort of adult patients with RSE. In contrast to previous studies,36,38 this investigation performed semiquantitative visual analyses of all EEGs during IVAD treatment for the assessment of burst suppression, which was based on the 2021 ACNS Standardized Critical Care EEG Terminology. We investigated whether burst suppression that did not completely fulfill this definition yielded similar results.
Besides the limitations discussed earlier, we report some additional ones: the single-center design limits the generalizability of the results, although our cohort demonstrates similar characteristics to other adult SE studies regarding age,43-47 outcome,44,48,49 etiologies,44-46,48 complications,47,49 SE severity,44,45 and SE types.43,48 Because the treatment was not randomized, we cannot exclude that patients were selected for deeper anesthesia based on etiology and severity of SE. Due to the retrospective nature of our study, no further information regarding the reasons for aiming at seizure cessation, burst suppression, or isoelectric EEG was available. There may be a potential underestimation of RSE duration, especially with nonconvulsive SE.3 Furthermore, breakthrough seizures were not assessed. However, analysis regarding RSE duration and breakthrough seizures was not the aim of this study. We lacked cEEG recordings from the onset until the end of IVADs, and short periods of burst suppression may have been missed with our semiquantitative approach. Hence, the cumulative duration of burst suppression should be cautiously interpreted. However, most patients had several hours of EEG recordings, and thus, the risk that burst suppression was systematically missed is low.
We report a low number of adult patients in RSE with achieved burst suppression, which may reflect a combination of the known difficulties in maintaining burst suppression and the current guidelines' simultaneously stating different treatment targets. While achievement and duration of burst suppression was associated with prolonged hospital stay and ICU stay, mechanical ventilation, and the use of vasopressors, it was not associated with any of the examined outcomes including persistent seizure termination, in-hospital survival, and return to premorbid neurologic function. Furthermore, not achieving burst suppression was not associated with adverse outcomes. Further and larger randomized studies are needed to validate our findings in adult patients with RSE and to explore whether certain subgroups benefit from burst suppression in comparison with seizure cessation as the titration goal for IVADs.
Acknowledgment
The authors thank Sarah Tschudin-Sutter, MD, MSc (University Hospital Basel), for her statistical assistance.
Glossary
- ABI
anoxic brain injury
- ACNS
American Clinical Neurophysiology Society
- cEEG
continuous EEG
- GCS
Glasgow Coma Score
- GOS
Glasgow Outcome Scale
- ICU
intensive care unit
- IQR
interquartile range
- IVAD
IV administration of anesthetic drug
- RSE
refractory status epilepticus
- SE
status epilepticus
- STESS
Status Epilepticus Severity Score
Appendix. Authors

Footnotes
See page 889
Study Funding
This study was funded by the University Hospital Basel.
Disclosure
U. Fisch, A.L. Jünger, S.M. Baumann, and S. Semmlack report no disclosures. G.M. De Marchis has been receiving support from the Swiss National Science Foundation (numbers 32003B_200573 and PBBEP3_139388); Spezialprogramm Nachwuchsförderung Klinische Forschung, University of Basel; Science Funds (Wissenschaftspool) of the University Hospital Basel; Swiss Heart Foundation; ProPatient Foundation Basel; Bangerter-Rhyner-Stiftung; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; Fondazione Dr Ettore Balli; De Quervain research grant; Thermo Fisher GmbH; and Novartis grant; travel honoraria by Bayer and BMS/Pfizer; speaker honoraria by Bayer and Medtronic; and consultant honoraria by Bayer and Novartis. He is a member of the Steering Committee of PACIFIC Stroke (NCT04304508). Industry payments are made to the research fund of the University Hospital Basel. S. Hunziker is supported by the Swiss National Foundation (SNF) (Ref 10001C_192850/1 and 10531C_182422), the Gottfried Julia Bangerter-Rhyner Foundation (8472/HEG-DSV), and the Swiss Society of General Internal Medicine (SSGIM). S. Rüegg received unconditional research grants from UCB-pharma. He received honoraria from serving on the scientific advisory boards of Angellini/Arvelle, Bial, Eisai, GW, and UCB-pharma and from serving as a consultant for Angellini/Arvelle, Eisai, Pfizer, Novartis, Sandoz, and UCB-pharma. He does not hold any stocks of any pharmaceutical industries or manufacturers of medical devices. He received funding from UCB-pharma and Swiss National Science Foundation Grants: grant number 320030_169379/1 and coapplicant for grants numbers 33CM30_125115/1 and 33CM30_140338/1; he disclosed that he is the past president of the Swiss League Against Epilepsy (no payments), Editor of Zeitschrift für Epileptologie (Journal of the German, Austrian, and Swiss League Against Epilepsy) (no payments). S. Marsch reports no disclosures. R. Sutter received research grants from the Swiss National Foundation (number 320030_169379), the Research Fund of the University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He received personal grants from UCB-pharma and holds stocks from Novartis, Roche, Alcon, and Johnson & Johnson. Go to Neurology.org/N for full disclosures.
References
- 1.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515-1523. [DOI] [PubMed] [Google Scholar]
- 2.Sutter R, Kaplan PW, Rüegg S. Outcome predictors for status epilepticus: what really counts. Nat Rev Neurol. 2013;9:525-534. [DOI] [PubMed] [Google Scholar]
- 3.Sutter R, Semmlack S, Kaplan PW. Nonconvulsive status epilepticus in adults: insights into the invisible. Nat Rev Neurol. 2016;12(5):281-293. [DOI] [PubMed] [Google Scholar]
- 4.Sutter R, Marsch S, Fuhr P, Rüegg S. Mortality and recovery from refractory status epilepticus in the ICU: a 7-year observational study. Epilepsia. 2013;54(3):502-511. [DOI] [PubMed] [Google Scholar]
- 5.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348-355. [DOI] [PubMed] [Google Scholar]
- 6.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3-23. [DOI] [PubMed] [Google Scholar]
- 7.Holtkamp M, Masuhr F, Harms L, Einhäupl KM, Meierkord H, Buchheim K. The management of refractory generalised convulsive and complex partial status epilepticus in three European countries: a survey among epileptologists and critical care neurologists. J Neurol Neurosurg Psychiatry. 2003;74(8):1095-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol. 2011;10:922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An J, Jonnalagadda D, Moura V, Purdon PL, Brown EN, Westover MB. Variability in pharmacologically-induced coma for treatment of refractory status epilepticus. PLoS One. 2018;13(10):e0205789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38:1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutter R, Marsch S, Fuhr P, Kaplan PW, Rüegg S. Anesthetic drugs in status epilepticus: risk or rescue? A six-year cohort study. Neurology. 2014;82(8):656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutter R, De Marchis GM, Semmlack S, et al. Anesthetics and outcome in status epilepticus: a matched two-center cohort study. CNS Drugs. 2017;31(1):65-74. [DOI] [PubMed] [Google Scholar]
- 13.De Stefano P, Baumann SM, Semmlack S, et al. Safety and efficacy of coma induction following first-line treatment in status epilepticus: a 2-center study. Neurology. 2021;97(6):e564-e576. [DOI] [PubMed] [Google Scholar]
- 14.Kowalski RG, Ziai WC, Rees RN, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med. 2012;40(9):2677-2684. [DOI] [PubMed] [Google Scholar]
- 15.Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med. 2015;43(5):1003-1009. [DOI] [PubMed] [Google Scholar]
- 16.Hocker S. Anesthetic drugs for the treatment of status epilepticus. Epilepsia. 2018;59(suppl 2):188-192. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77(5):611-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann SM, Semmlack S, Rybitschka A, et al. Prolonged mechanical ventilation in patients with terminated status epilepticus and outcome: an observational cohort study. Epilepsia. 2021;62(12):3042-3057. [DOI] [PubMed] [Google Scholar]
- 21.Rossetti AO, Logroscino G, Bromfield EB. A clinical score for prognosis of status epilepticus in adults. Neurology. 2006;66(11):1736-1738. [DOI] [PubMed] [Google Scholar]
- 22.Sutter R, Kaplan PW, Rüegg S. Independent external validation of the Status Epilepticus Severity Score. Crit Care Med. 2013;41(12):e475-e479. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 24.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957-2963. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591-597. [DOI] [PubMed] [Google Scholar]
- 26.Peedicail J, Mehdiratta N, Zhu S, Nedjadrasul P, Ng MC. Quantitative burst suppression on serial intermittent EEG in refractory status epilepticus. Clin Neurophysiol Pract. 2021;6:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furbass F, Herta J, Koren J, et al. Monitoring burst suppression in critically ill patients: multi-centric evaluation of a novel method. Clin Neurophysiol. 2016;127(4):2038-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutter R, Semmlack S, Kaplan PW, Opic P, Marsch S, Ruegg S. Prolonged status epilepticus: early recognition and prediction of full recovery in a 12-year cohort. Epilepsia. 2019;60(1):42-52. [DOI] [PubMed] [Google Scholar]
- 29.Baumann SM, Semmlack S, De Marchis GM, et al. Frequency and implications of complications in the ICU after status epilepticus. No calm after the storm. Crit Care Med. 2020;48(12):1779-1789. [DOI] [PubMed] [Google Scholar]
- 30.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroeger D, Amzica F. Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci. 2007;27(39):10597-10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosmer DWL S . A goodness of-fit test for the multiple logistic regression model. Commun Stat. 1980;10:1043-1069. [Google Scholar]
- 33.Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69(3):255-260. [DOI] [PubMed] [Google Scholar]
- 34.Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14(1):4-10. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamurthy KB, Drislane FW. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia. 1999;40(6):759-762. [DOI] [PubMed] [Google Scholar]
- 36.Hocker SE, Britton JW, Mandrekar JN, Wijdicks EFM, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol. 2013;70(1):72. [DOI] [PubMed] [Google Scholar]
- 37.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43(2):146-153. [DOI] [PubMed] [Google Scholar]
- 38.Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62(11):1698. [DOI] [PubMed] [Google Scholar]
- 39.Kang BS, Jung KH, Shin JW, et al. Induction of burst suppression or coma using intravenous anesthetics in refractory status epilepticus. J Clin Neurosci. 2015;22(5):854-858. [DOI] [PubMed] [Google Scholar]
- 40.Phabphal K, Chisurajinda S, Somboon T, Unwongse K, Geater A. Does burst suppression achieve seizure control in refractory status epilepticus? BMC Neurol. 2018;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruijter BJ, Keijzer HM, Tjepkema-Cloostermans MC, et al. Treating rhythmic and periodic EEG patterns in comatose survivors of cardiac arrest. N Engl J Med. 2022;386(8):724-734. [DOI] [PubMed] [Google Scholar]
- 42.De Stefano P, Kaplan PW, Sutter R. Not all rhythmicities and periodicities in coma EEG are fatal: when simplification becomes dangerous. Epilepsia. 2022;63(8):2164-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51(2):251-256. [DOI] [PubMed] [Google Scholar]
- 44.Fatuzzo D, Novy J, Rossetti AO. Use of newer antiepileptic drugs and prognosis in adults with status epilepticus: comparison between 2009 and 2017. Epilepsia. 2018;59(7):e98-e102. [DOI] [PubMed] [Google Scholar]
- 45.Beuchat I, Novy J, Rossetti AO. Newer antiepileptic drugs in status epilepticus: prescription trends and outcomes in comparison with traditional agents. CNS Drugs. 2017;31(4):327-334. [DOI] [PubMed] [Google Scholar]
- 46.Strzelczyk A, Ansorge S, Hapfelmeier J, Bonthapally V, Erder MH, Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. 2017;58(9):1533-1541. [DOI] [PubMed] [Google Scholar]
- 47.Belluzzo M, Furlanis G, Stragapede L, Monti F. Role of comorbidities and in-hospital complications in short-term status epilepticus outcome. Clin Neurol Neurosurg. 2017;154:13-18. [DOI] [PubMed] [Google Scholar]
- 48.Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42(6):714-718. [DOI] [PubMed] [Google Scholar]
- 49.Zelano J, Moller F, Dobesberger J, Trinka E, Kumlien E. Infections in status epilepticus: a retrospective 5-year cohort study. Seizure. 2014;23(8):603-606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author has full access to all data of the study. Due to data protection reasons, data cannot be made publicly available. However, anonymized grouped data will be made available on reasonable request to qualifed investigators.