Pericytes are fundamental components of the neurovascular unit (NVU). They interact with endothelial cells, basal lamina (basement membrane), and glial cells and have a critical role in maintenance of blood-brain barrier (BBB) stability, local control of capillary blood flow, angiogenesis, and immune responses. Pericytes may also function as stem cells with potential to differentiate to into smooth muscle, glial cells, or neurons. A wide range on neurologic disorders, including vascular disorders, neurodegenerative disorders such as Alzheimer disease (AD), traumatic injury, and multiple sclerosis (MS) are associated with changes in pericyte structure and function affecting the NVU. Dysfunctional pericyte signaling may be a potential biomarker of NVU pathology and provides therapeutic targets for neuroprotection. The functions of pericytes and their role in neurologic disorders have been the subject of several comprehensive reviews.1-13
Development, Heterogeneity, and Dynamics of Pericytes
The NVU is a fundamental structural and functional unit in the CNS and includes endothelial cells, pericytes, vascular smooth muscle cells, basal lamina, astrocytes, microglia, and neurons (Figure).14 The CNS has the highest density of pericytes compared with other tissues; about 70%–80% of microvessels are covered by pericytes.15 Pericytes are contractile cells that are located between endothelial cells and other cells of the NVU and are almost entirely embedded within the basal lamina. Pericytes constitute a heterogeneous population from the standpoint of their origin, location, morphology, protein expression, and function.16 Forebrain pericytes originate from neural crest cells, whereas those in the brainstem and spinal cord originate from mesenchymal stem cells.4 During development, the newly formed capillaries attract pericytes by secreting platelet-derived growth factor (PDGF)-B, which binds to the PDGF receptor β (PDGFRβ).17 The expression of PGDRβ in pericytes depends on their integrin-mediated adhesion to laminin in the basal lamina.4 Pericytes situated at different locations along capillaries have different morphology. Precapillary pericytes have many circumferential processes that wrap around blood vessels; midcapillary pericytes are long, spindle-shaped cells that extend processes parallel to the length of microvessels, and postcapillary pericytes are short stellate-shaped cells that cover the abluminal surface of postcapillaries and postcapillary venules.18 Pericytes express several molecular markers that vary across different tissues, locations within the vascular tree, developmental state, and pathologic setting.4,16 Common markers include PGDRβ, α-smooth muscle actin, and potassium channel Kir6.1. Adhesion between pericytes and endothelial cells is promoted by transforming growth factor-β (TGF-β) secreted from both cell types in a paracrine and autocrine manner.19 TGF-β also upregulates expression of α-smooth muscle actin, which is the primary contractile protein in pericytes.19 Pericytes may also express vascular cell adhesion molecule (V-CAM) and immunologic markers such as Fc receptor, CD4, CD11b, and major histocompatibility complex (MHC) Class I and II molecules among other markers.5,20,21 In mature adult brains, pericytes residing in a stable microvessel network are relatively quiescent with a low rate of turnover. Both physiologic conditions that require expansion of the vasculature, such as increased metabolic activity, or pathologic conditions such as hypoxia and inflammation trigger pericyte activation, proliferation, self-renewal, and differentiation. Pathologic insults can both increase the proliferation of pericytes in preexisting pools around brain vessels and recruit pericyte progenitor cells from the bone marrow.6 In culture, pericytes can behave as multipotential stem cells that can differentiate into multiple lineages, including smooth muscle cells, oligodendrocyte precursor cells, microglia, and neurons.5,22-25 Maintenance of the self-renewal and multipotent state of pericytes depends on their interactions with laminin secreted by astrocytes.26 However, the differentiation potential of pericytes varies according to the specific tissue27 and is epigenetically regulated, for example, by tissue-specific histone modification patterns.28
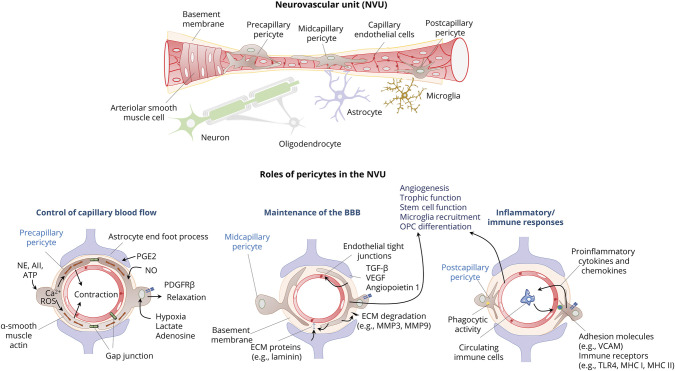
Figure. Components of the NVU and Roles of Pericytes.
The neurovascular unit (NVU) includes endothelial cells, pericytes, vascular smooth muscle cells, basal lamina (or basement membrane), astrocytes, microglia, and neurons. Pericytes are located between endothelial cells and other cells of the NVU and are embedded within the basal lamina. Newly formed capillaries attract pericytes by secreting platelet-derived growth factor (PDGF)-B, which binds to the PDGF receptor β (PDGFRβ) in pericytes. Precapillary pericytes wrap around blood vessels and express high levels of α-smooth muscle actin, which allows them to bidirectionally regulate blood flow in response to signals that elicit changes in intracellular calcium (Ca2+). Gap junctions between pericytes with each other and with endothelial allow propagation of vasomotor responses. Glutamatergic synaptic activity promotes pericyte relaxation and capillary vasodilation by activating production of nitric oxide (NO) and prostaglandin E2 (PgE2) in synaptic terminal and astrocytes, respectively. Hypoxia, lactate, and adenosine can also relax pericytes. In contrast, adenosine triphosphate (ATP), norepinephrine (NE) angiotensin II (AII), and accumulation of intracellular reactive oxygen species (ROS) promote pericyte contraction. Midcapillary pericytes have a major role in maintenance of the blood-brain barrier (BBB). They secrete transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and angiopoietin 1, which induces tight junction formation and promote angiogenesis. Pericytes participate in the formation and degradation of extracellular matrix (ECM) proteins of the basement membrane. They synthesize ECM proteins such as laminin and also produce metalloproteinase (MMP)-2 and MMP-9, which enhance ECM degradation. Pericytes express vascular cell adhesion molecule (V-CAM) and immune receptors such as Toll-like receptor 4 (TLR4), and major histocompatibility complex (MHC) molecules I and II have phagocytic activity, secrete proinflammatory cytokines and chemokines, and attract immune cells from the bloodstream. In response to microenvironmental changes, pericytes secrete different molecules that function as paracrine signals including growth factors that regulate differentiation of various progenitors in the neurovascular unit. Pericytes may also behave as multipotential stem cells that can differentiate into multiple lineages, including smooth muscle cells, oligodendrocyte precursor cells, microglia, and neurons.
Functions of Pericytes in the NVU
Pericytes have several fundamental roles in the NVU, including maintenance of the BBB, regulation of capillary flow, paracrine interactions with other cells regulating angiogenesis, and survival, phagocytosis, and inflammatory responses.3,6,7,13,29-32 Pericytes extend processes that preferentially wrap around tight junctions between neighboring endothelial cells forming the BBB33-35 and the blood-retina barrier.36 The basement membrane separating pericytes and endothelial cells has hole-like structures that allow endothelial cell–pericyte communication and interactions via gap and adhesion junctions. These close interactions enable pericytes to provide structural and nutritional support to endothelial cells to enhance the barrier function of the BBB.37-39 For example, pericytes secrete angiopoietin 1, which induces tight junction formation between endothelial cells.40,41 Pericytes participate in the formation and degradation of extracellular matrix (ECM) proteins of the basement membrane. This special form of matrisome surrounds the cerebral microvessels at the interface between endothelial cells, contractile cells (smooth muscle cells and pericytes), and astrocyte endfeet.42,43 Pericytes, like endothelial cells and astrocytes, synthesize laminin, collagen IV, nidogen, and perlecan, which are the main ECM proteins of the basement membrane.41,42 The production of ECM proteins by pericytes is stimulated by TGF-β, which leads to upregulation of cadherin-2 and tight junction stabilization in cerebral endothelial cells.3 Pericytes also produce metalloproteinase (MMP)-2 and MMP-9, which enhance ECM degradation during early stages of angiogenesis. However, they also produce tissue inhibitor of MMP-3 (TIMP-3), which facilitates vessel maturation and stabilization.44,45 Pericytes function synergically with astrocytes to maintain the normal function of the BBB.46-48 For example, astrocytes release laminin, which binds to integrin α2 on pericytes and maintains pericytes in a BBB stabilizing status preventing them from switching to a contractile status.26 Pericytes regulate the polarization of aquaporin-4 (AQP-4) to the perivascular astrocytic end foot membrane.49
In addition to their fundamental structural functions, pericytes have a major role in the regulation of local cerebral blood flow. Ensheathing pericytes at the arteriole-capillary transitional zone, like arteriolar smooth muscle cells, control large-scale, rapid changes in blood flow, whereas capillary pericytes act on slower and smaller scales and control resting capillary tone and flow heterogeneity according to local demands.31,50,51 Pericytes contain contractile proteins such as α-smooth muscle actin, tropomyosin, and desmin.51 This allows pericytes to bidirectionally regulate blood flow depending on neuronal activity, metabolic state, and neurotransmitter signals resulting in changes in intracellular calcium (Ca2+).52,53 Ensheathing pericytes at the arteriole-capillary transition zone express the highest levels of α-smooth muscle actin and exhibit highly regular oscillatory Ca2+ fluctuations compared with capillary and perivenular pericytes and thus have the primary responsible for regulation of microvascular blood flow.54 Adjoining membranes of neighboring pericytes are interconnected via gap junctions, which allow them to function as a syncytium along the microvascular wall.32,52 Propagation of vasomotor responses in response to local changes of neural activity also depends on connexin-43 gap junctions between pericytes and endothelial cells.55,56
In physiologic conditions, pericytes contribute to neurovascular coupling by contracting or relaxing according to the energy demands of nervous tissue. Pericyte-mediated capillary dilation occurs before arteriolar dilation in response to a focal increase in energy demand from a small group of nearby neural cells.32 In contrast, regional blood flow is primarily controlled by arteriolar smooth muscle contractility.57 Several mediators, including neurotransmitters, PDGF-β, adenosine triphosphate (ATP), adenosine, and nitric oxide (NO), control pericyte contractility. For example, glutamate released during excitatory synaptic activity promotes pericyte relaxation and capillary vasodilation via a mechanism that involves NO and prostaglandin E2.58 PDGF-β can activate nonspecific cation channels, chloride channels, and ATP-sensitive potassium channels to regulate contractility of pericytes according to the metabolic status. Signals of increased energy consumption, such as hypoxia, lactate, adenosine, low pH, and transient elevation of reactive oxygen species (ROS), can also relax pericytes.31,59 In contrast, increased levels of ATP induce pericyte contraction via P2X7 receptors.60 Pericytes also respond to vasoactive substances such as norepinephrine61 and angiotensin II.62 They express angiotensin-converting enzyme 2 (ACE2), which converts vasoconstricting angiotensin II into vasodilating angiotensin-(1-7).62 The proportion of microvascular pericytes that are able to contract varies depending on the tissue, species, developmental stage, and localization along the arteriovenous length.63
In response to microenvironmental changes, pericytes may also secrete different molecules that function as paracrine signals. The pericyte secretome is both tissue and stimulus specific and includes growth factors, ECM proteins, and pro- and anti-inflammatory cytokines.64 Some pericyte signals regulate cell survival and differentiation within the NVU.65 For example, pericytes release vascular endothelial growth factor (VEGF), angiopoietin 1, and other signals that promote angiogenesis.8 Angiopoietin 1 and glial-derived neurotrophic factor enhance tight junction formation,66 whereas VEGF and TGF-β1 protect endothelial cells.19,67 Pericytes also provide trophic factors for glial cells and neurons.38,68 For example, pericytes contact oligodendrocyte precursor cells, and both cell populations reciprocally regulate their proliferation and survival.65,69,70 Pericytes induce the differentiation of new oligodendrocytes from precursor cells by secreting laminin alpha2 chain in the microvascular basal lamina, thus promoting myelin development.68,70-72
Pericytes are involved in inflammatory and immune responses at the NVU.73 They express pattern recognition receptors such as Toll-like receptor 4 and Fc receptors, may develop macrophage-like phagocytotic ability, and contain cytoplasmic lysosome-like granules; this indicates that they recognize abnormal antigens and serve as scavenger cells.74 Pericytes also express adhesion molecules that promote leukocyte recruitment, may secrete proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-gamma, and produce ROS, NO, and other inflammatory mediators during immune reactions.5,29,38,75-77 Perivenular pericytes may have major role in regulating peripheral immune cells infiltration in response to insults affecting the NVU.7 However, in some conditions, pericytes may inhibit proliferation and cytokine production of activated T cells, thus protecting endothelial cells from inflammation-mediated apoptosis.78 This indicates that in physiologic conditions, pericytes could prevent unnecessary immune reactions, while in response to injury cells, they can readily initiate inflammatory responses.
Clinical Correlations
Animal models show that pericyte loss or dysfunction is associated with BBB impairment, microaneurysms, and endothelial cell hyperplasia.2,35,61,79 Pericytes may be affected by aging and in a wide variety of neurologic disorders.8,10,13,80 Studies on the effects of aging on pericyte number and BBB function have provided contradictory results.2,81,82 However, with aging, pericytes may accumulate vacuolar inclusions and show increased expression of α-smooth muscle actin, altered length and orientation of desmin filaments, and reduced contacts with endothelial cells.83,84 Whereas accumulation of vacuolar inclusions may reflect phagocytotic activity as a defense against potential BBB leakage,83 changes desmin filaments can reflect pericyte sclerosis with resultant impairment of their capacity to regulate local blood flow.2 Several factors, including oxidative stress, may contribute to pericyte dysfunction during aging.85 Hyperlipidemia,67 diabetes mellitus,86 and hypertension87 may all affect pericyte function. The role of pericytes in neurologic disorders has been extensively reviewed,8-13,78,88-95 and only few concepts will be emphasized here.
Cerebral Ischemia
Pericytes are among the first cell types that react to brain hypoxia-ischemia and participate in various compensatory, pathologic, and repair processes during ischemic stroke.8 These changes may have either beneficial or deleterious effects according to the timing and severity of the insult. For example, studies in experimental models show that during short-duration ischemia, pericytes relax in response to increased levels of PDGF-β, adenosine, and NO.59,88 However, during sustained ischemia, pericyte reverse their phenotype to produce constriction in response to ROS and peroxynitrite generated during ischemia and reperfusion87; this contributes to the non-reflow phenomenon after ischemic stroke.53 In this setting, pericytes constrict capillaries through a Ca2+-induced α-smooth muscle actin contraction and subsequently die due to an uncontrolled overload of intracellular Ca2+ potentiated by ROS generated by pericyte mitochondria, endothelial cells, and astrocyte endfeet.96,97
Pericytes may also have a protective or deleterious effect on the maintenance of BBB integrity in the setting of ischemia. In response to PDGF signaling, pericytes change from a quiescent flat into an ameboid morphology, separate from the basal lamina, and migrate toward the hypoperfused area. This migration may be protective as pericytes can release trophic factors such as VEGF, angiopoietin 1, glial-derived neurotrophic factor, and TGF-β1, which promote angiogenesis and maintain BBB integrity, as well as other neuroprotective factors for other cellular components of the NVU.8,19,38,66,68,89,98 Pericytes may also exert neuroprotective effects by adopting phagocytic activity and functioning as multipotential stem cells that generate microglia, thus contributing to clearance of tissue debris thereby alleviating local inflammation and reducing secondary tissue damage.90,99 Accumulation of oxygen free radicals promotes nuclear factor erythroid-2–related factor 2 signaling in pericytes, allowing them to acquire stem-like characteristics.100 However, pericytes may not be able to reconstruct a functional NVU and rather contribute to formation of a glial scar under pathologic conditions.101
Pericytes may also participate in deleterious responses in the setting of ischemia. They express nicotinamide adenine dinucleotide phosphate oxidase, which is upregulated in the peri-infarct region and generates superoxide, promoting MMP-9 release resulting in disruption of the BBB.30,102-104 Furthermore, MMP-9, bone morphogenetic protein (BMP)-4, and endostatin-1 secreted by pericytes may inhibit angiogenesis.105,106 Ischemic injury can be aggravated by inflammatory responses due to recruitment and transmigration of immune cells to ischemic or peri-ischemic tissue.107 Pericytes express intercellular adhesion molecule 1,108 which interacts with integrins on leukocytes to guide leukocyte migration.109
Small Vessel Disease
Pericytes may have a role in small vessel disease associated with disorders of the matrisome.43 A common mechanism in several of these disorders is disturbed TGF-β signaling.110 As mentioned earlier in this review, TGF-β mediates numerous cellular processes including pericyte and endothelial cell proliferation, differentiation, and vascular remodeling. This cytokine is secreted by both pericytes and endothelial cells as a proform that needs activation by proteases, thrombospondin, or integrins.111 In basal conditions, mature TGF-β is kept in a functionally inactive state by forming a complex with latent TGF-β–binding protein 1 that is anchored to the ECM.112 Latent TGF-β–binding protein 1 is a substrate of the high temperature requirement A1 (Htra1) serine protease/peptidase encoded by the HTRA1 gene. Activated TGF-β acts via 2 types of receptors, both of which are expressed in pericytes and elicit distinct intracellular transcription factor cascades that promote vessel maturation, inhibit pericyte proliferation, and induce upregulation of expression of contractile proteins.113 The recruitment and proper attachment of pericytes to endothelial cells leads to activation of secreted TGF-β signaling, promoting VGEF receptor expression and differentiation of endothelial cells, inhibition of their proliferation, and formation and stabilization of the basement membrane and BBB.113 Knockouts of many of the proteins involved in this signaling pathway lead to severe vascular abnormalities.3 Pathogenic HTRA1 variants leading to reduced enzymatic activity are associated with cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy.91 These variants reduce TGF-β release from its complex with TGF–β binding protein 1 but also increase levels of TGF-β, as it is a substrate of the serine protease encoded by HTRA1.114 Thus, the precise mechanisms by which HTRA1 variants affect TGF-β signaling in the microvasculature are still incompletely defined.92 A postmortem study in brains of patients with acquired small vessel disease showed high expression of BMP-4, a member of the TGFB superfamily in white matter pericytes; this was associated with increased angiogenesis and astrogliogenesis at the expense of oligodendrocyte precursor cell proliferation and maturation, thereby aggravating white matter damage.115 Pericytes may also be involved in the vascular complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.116 SARS-CoV-2 binding removes ACE2 from the cell surface membrane and thus impairs conversion of angiotensin II to angiotensin-(1-7)62 leading pericyte-mediated angiotensin II evoked cerebral capillary constriction.116
Familial Cerebral Cavernous Malformations
Familial cerebral cavernous malformation (CCM) is caused by loss-of-function variants in CCM1, CCM2, or CCM3 genes in endothelial cells and consists of multiple, dilated capillary channels formed by a single layer of endothelium and lacking parenchymal cells. Given the ubiquitous expression of CCM proteins, it is not known why CCM lesions are confined to the brain.93 Whereas the primary defect in CCMs is thought to be intrinsic to endothelial cells, recent evidence indicates a contribution of pericyte dysfunction. For example, studies in experimental models indicate that Ccm3 deletion in pericytes also induces CCM lesions by destabilizing pericyte–endothelial cell interactions.93 Pericytes in Ccm1 knockout mice have increased expression of PDGFRβ and ECM genes, particularly that encoding fibronectin-1; human CCM lesions had abundant fibronectin deposition in pericytes.117
Alzheimer Disease
Disturbances of the NVU, including structural alterations of the brain capillaries, impaired control of microcirculation, and disruption of the BBB are well-recognized features of AD.14,94,95,118-122 Involvement of pericytes is particularly relevant given their key role in microvascular stability, capillary density, and angiogenesis.80,118,123,124 For example, β-amyloid targets pericytes, eliciting vasoconstriction and disrupting capillary blood flow.11,12 Cerebrovascular pericytes acting together with perivascular astroglial cells have also been implicated in the production of lipidated, reactive forms of ApoE, which in turn suppresses pericyte motility and adhesion to endothelial cells.125 Dysfunctional microvascular pericytes release soluble PDGFRβ, which has been proposed as a biomarker of BBB integrity and predictor of neurodegeneration.126
Multiple Sclerosis
Studies in experimental models and in postmortem brain tissues of patients with MS show that pericytes and other perivascular cell populations have different behavior depending on the lesion type and clinical course of the disease.127 Active lesions contain higher numbers of proliferative perivascular cells than inactive lesions, whereas chronic lesions have lower numbers of proliferative perivascular cells than normal-appearing white matter.127 In experimental allergic encephalomyelitis, oligodendrocyte precursor cells make more contacts with pericytes leading to tight junction impairment and BBB leakage.71,128,129 Activation of P2X7 receptors by ATP released during inflammation leads to downregulation of both PDGFRβ in pericytes and claudin-5 in endothelial cells also leading to BBB disruption.130 Pericytes may also contribute to neuroinflammation in MS by their ability to secrete adhesion molecules, chemokines, and cytokines that assist in the recruitment and migration of immune cells.75-77,131 As shown in traumatic injury models, microglial-released TNF-α initiates a nuclear factor kappa B pathway–inducible NO synthase cascade that results in pericyte damage.132
Perspective
As a fundamental component of the NVU, pericytes have a major role in preserving the neural microenvironment. Their dysfunction leads to BBB disruption, disturbed local blood flow, and neuroinflammation. Pericytes thus provide a major potential target for neuroprotection. Treatments targeting hyperlipidemia, diabetes, or hypertension may be beneficial in ischemic injury or neurodegeneration in part by protecting pericytes. In preclinical studies, some drugs affecting pericytes may be neuroprotective against ischemic injury. For example, in spontaneously hypertensive rats, the antiplatelet drug cilostazol prevented the detachment of pericytes from microvessels and enhanced pericyte proliferation while inhibiting production of MMP-9.133 A study showed that short-term treatment with cilostazol and isosorbide mononitrate, alone or in combination, improved MRI-measured cerebrovascular function in patients with lacunar stroke.134 In experimental models, the free radical scavenger edaravone also promoted pericyte proliferation, increased pericyte coverage of endothelial cells, and reduced production of MMP-9, thus attenuating BBB destruction during reperfusion injury.135 However, these drugs also affect other cells of the NVU besides pericytes. Furthermore, these promising results may not be reproducible in clinical conditions, given the functional heterogeneity of pericytes and their varying and sometimes opposite responses depending on the environmental context, timing of injury, and other variables. Identification of specific molecular biomarkers may allow more precise targeting of pericytes as a major tool to protect NVU integrity in pathologic conditions.
Acknowledgment
The author thanks Dr. Kelly Flemming, MD, Consultant of the Division of Cerebrovascular Disease at the Mayo Clinic, Rochester, MN, for her careful review and helpful suggestions on this paper.
Glossary
- ACE2
angiotensin-converting enzyme 2
- AD
Alzheimer disease
- AQP-4
aquaporin-4
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- BMP
bone morphogenetic protein
- Ca2+
calcium
- CCM
cerebral cavernous malformation
- ECM
extracellular matrix
- Htra1
high temperature requirement A1
- MHC
major histocompatibility complex
- MMP
metalloproteinase
- MS
multiple sclerosis
- NO
nitric oxide
- NVU
neurovascular unit
- PDGF
platelet-derived growth factor
- PDGFRβ
PDGF receptor β
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TGF-β
transforming growth factor-β
- TIMP-3
tissue inhibitor of MMP-3
- TNF-α
tumor necrosis factor-α
- V-CAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
Study Funding
No targeted funding reported.
Disclosure
The author reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1-11. [DOI] [PubMed] [Google Scholar]
- 2.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193-215. [DOI] [PubMed] [Google Scholar]
- 5.Lange S, Trost A, Tempfer H, et al. Brain pericyte plasticity as a potential drug target in CNS repair. Drug Discov Today. 2013;18(9-10):456-463. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu R, Yamashita T. Pericyte function in the physiological central nervous system. Neurosci Res. 2014;81-82:38-41. [DOI] [PubMed] [Google Scholar]
- 7.Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai W, Liu H, Zhao J, et al. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8(2):107-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Korte N, Nortley R, Sethi H, Tang Y, Attwell D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018;136(4):507-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirunpattarasilp C, Attwell D, Freitas F. The role of pericytes in brain disorders: from the periphery to the brain. J Neurochem. 2019;150(6):648-665. [DOI] [PubMed] [Google Scholar]
- 11.Nortley R, Korte N, Izquierdo P, et al. Amyloid beta oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science. 2019;365(6450):eaav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korte N, Nortley R, Attwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer's disease. Acta Neuropathol. 2020;140(6):793-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girolamo F, Errede M, Bizzoca A, Virgintino D, Ribatti D. Central nervous system pericytes contribute to health and disease. Cells. 2022;11(10):1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24(9):1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci. 2020;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, D'Souza SS, Moskvin OV, et al. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 2017;19(9):1902-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nwadozi E, Rudnicki M, Haas TL. Metabolic coordination of pericyte phenotypes: therapeutic implications. Front Cell Dev Biol. 2020;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2(4):041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor β1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci USA. 2003;100(26):15859-15864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurtado-Alvarado G, Cabanas-Morales AM, Gomez-Gonzalez B. Pericytes: brain-immune interface modulators. Front Integr Neurosci. 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina-Flores F, Hurtado-Alvarado G, Deli MA, Gomez-Gonzalez B. The active role of pericytes during neuroinflammation in the adult brain. Cell Mol Neurobiol. 2022;43(2):525-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14(16):1581-1593. [DOI] [PubMed] [Google Scholar]
- 23.Paul G, Ozen I, Christophersen NS, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7(4):e35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakata M, Nakagomi T, Maeda M, Nakano-Doi A, Momota Y, Matsuyama T. Induction of perivascular neural stem cells and possible contribution to neurogenesis following transient brain ischemia/reperfusion injury. Transl Stroke Res. 2017;8(2):131-143. [DOI] [PubMed] [Google Scholar]
- 25.Farahani RM, Rezaei-Lotfi S, Simonian M, Xaymardan M, Hunter N. Neural microvascular pericytes contribute to human adult neurogenesis. J Comp Neurol. 2019;527(4):780-796. [DOI] [PubMed] [Google Scholar]
- 26.Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5(1):3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vezzani B, Pierantozzi E, Sorrentino V. Not all pericytes are born equal: pericytes from human adult tissues present different differentiation properties. Stem Cells Dev. 2016;25(20):1549-1558. [DOI] [PubMed] [Google Scholar]
- 28.Yianni V, Sharpe PT. Molecular programming of perivascular stem cell precursors. Stem Cells. 2018;36(12):1890-1904. [DOI] [PubMed] [Google Scholar]
- 29.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40(3 suppl):S13-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Agalliu D, Yu C, Fisher M. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18(25):3653-3662. [DOI] [PubMed] [Google Scholar]
- 31.Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol. 2014;9(5):591-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99(1):21-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Profaci CP, Munji RN, Pulido RS, Daneman R. The blood-brain barrier in health and disease: important unanswered questions. J Exp Med. 2020;217:e20190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quaegebeur A, Segura I, Carmeliet P. Pericytes: blood-brain barrier safeguards against neurodegeneration? Neuron. 2010;68(3):321-323. [DOI] [PubMed] [Google Scholar]
- 36.Park DY, Lee J, Kim J, et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017;8(1):15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab. 2012;32(10):1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ElAli A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15(4):6453-6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557-561. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89(2):503-513. [DOI] [PubMed] [Google Scholar]
- 41.Huang H. Pericyte-endothelial interactions in the retinal microvasculature. Int J Mol Sci. 2020;21(19):7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol. 2019;4(2):78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joutel A, Haddad I, Ratelade J, Nelson MT. Perturbations of the cerebrovascular matrisome: a convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab. 2016;36(1):143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badaut J, Bix GJ. Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Transl Stroke Res. 2014;5(3):394-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders WB, Bohnsack BL, Faske JB, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175(1):179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009;87(3):653-659. [DOI] [PubMed] [Google Scholar]
- 47.Itoh Y, Toriumi H, Yamada S, Hoshino H, Suzuki N. Astrocytes and pericytes cooperatively maintain a capillary-like structure composed of endothelial cells on gel matrix. Brain Res. 2011;1406:74-83. [DOI] [PubMed] [Google Scholar]
- 48.Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31(2):693-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gundersen GA, Vindedal GF, Skare O, Nagelhus EA. Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct Funct. 2014;219(6):2181-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartmann DA, Coelho-Santos V, Shih AY. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol. 2022;84(1):331-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30(1):35-44. [DOI] [PubMed] [Google Scholar]
- 52.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15(9):1031-1037. [DOI] [PubMed] [Google Scholar]
- 54.Gluck C, Ferrari KD, Binini N, et al. Distinct signatures of calcium activity in brain mural cells. Elife. 2021;10:e70591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanova E, Kovacs-Oller T, Sagdullaev BT. Vascular pericyte impairment and connexin43 gap junction deficit contribute to vasomotor decline in diabetic retinopathy. J Neurosci. 2017;37(32):7580-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovacs-Oller T, Ivanova E, Bianchimano P, Sagdullaev BT. The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov. 2020;6(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87(1):95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q, Chen Y, Li B, et al. Hemoglobin induced NO/cGMP suppression deteriorate microcirculation via pericyte phenotype transformation after subarachnoid hemorrhage in rats. Sci Rep. 2016;6(1):22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Q, Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 2001;907(1-2):93-99. [DOI] [PubMed] [Google Scholar]
- 60.Kawamura H, Sugiyama T, Wu DM, et al. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551(3):787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkler EA, Rutledge WC, Kalani MYS, Rolston JD. Pericytes regulate cerebral blood flow and neuronal health at a capillary level. Neurosurgery. 2017;81(5):N37-N38. [DOI] [PubMed] [Google Scholar]
- 62.Hirunpattarasilp C, James G, Kwanthongdee J, et al. SARS-CoV-2 triggers pericyte-mediated cerebral capillary constriction. Brain. 2022;146(2):727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Klett F, Priller J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab. 2015;35(6):883-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaceb A, Paul G. Pericyte secretome. Adv Exp Med Biol. 2018;1109:139-163. [DOI] [PubMed] [Google Scholar]
- 65.Shibahara T, Ago T, Nakamura K, et al. Pericyte-mediated tissue repair through PDGFRβ promotes peri-infarct astrogliosis, oligodendrogenesis, and functional recovery after acute ischemic stroke. eNeuro. 2020;7(2):ENEURO.0474-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimizu F, Sano Y, Saito K, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37(2):401-409. [DOI] [PubMed] [Google Scholar]
- 67.Zechariah A, ElAli A, Hagemann N, et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1561-1567. [DOI] [PubMed] [Google Scholar]
- 68.Ishitsuka K, Ago T, Arimura K, et al. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc Res. 2012;83(3):352-359. [DOI] [PubMed] [Google Scholar]
- 69.Maki T, Maeda M, Uemura M, et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett. 2015;597:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva ME, Lange S, Hinrichsen B, et al. Pericytes favor oligodendrocyte fate choice in adult neural stem cells. Front Cell Neurosci. 2019;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girolamo F, Errede M, Longo G, et al. Defining the role of NG2-expressing cells in experimental models of multiple sclerosis. A biofunctional analysis of the neurovascular unit in wild type and NG2 null mice. PLoS One. 2019;14(3):e0213508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24(3):326-337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Torok O, Schreiner B, Schaffenrath J, et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc Natl Acad Sci USA. 2021;118(10):e2016587118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014;1550:1-8. [DOI] [PubMed] [Google Scholar]
- 75.Rustenhoven J, Jansson D, Smyth LC, Dragunow M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci. 2017;38(3):291-304. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharya A, Kaushik DK, Lozinski BM, Yong VW. Beyond barrier functions: roles of pericytes in homeostasis and regulation of neuroinflammation. J Neurosci Res. 2020;98(12):2390-2405. [DOI] [PubMed] [Google Scholar]
- 77.Kaushik DK, Bhattacharya A, Lozinski BM, Wee Yong V. Pericytes as mediators of infiltration of macrophages in multiple sclerosis. J Neuroinflammation. 2021;18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tu Z, Li Y, Smith DS, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52(12):9005-9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55(3):261-268. [DOI] [PubMed] [Google Scholar]
- 80.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol. 2014;24(4):371-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters A, Sethares C. Age-related changes in the morphology of cerebral capillaries do not correlate with cognitive decline. J Comp Neurol. 2012;520(6):1339-1347. [DOI] [PubMed] [Google Scholar]
- 82.Peinado MA, Quesada A, Pedrosa JA, et al. Quantitative and ultrastructural changes in glia and pericytes in the parietal cortex of the aging rat. Microsc Res Tech. 1998;43(1):34-42. [DOI] [PubMed] [Google Scholar]
- 83.Alba C, Vidal L, Diaz F, Villena A, de Vargas IP. Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Res Bull. 2004;64(2):145-153. [DOI] [PubMed] [Google Scholar]
- 84.Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27(12):1838-1847. [DOI] [PubMed] [Google Scholar]
- 85.van Leeuwen E, Hampton MB, Smyth LCD. Redox signalling and regulation of the blood-brain barrier. Int J Biochem Cell Biol. 2020;125:105794. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Zhang H, Wang S, et al. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am J Physiol Heart Circ Physiol. 2021;320(2):H549-H562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Presa JL, Saravia F, Bagi Z, Filosa JA. Vasculo-neuronal coupling and neurovascular coupling at the neurovascular unit: impact of hypertension. Front Physiol. 2020;11:584135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arimura K, Ago T, Kamouchi M, et al. PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9:1-9. [DOI] [PubMed] [Google Scholar]
- 89.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6(3):241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakuma R, Kawahara M, Nakano-Doi A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation. 2016;13(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beaufort N, Scharrer E, Kremmer E, et al. Cerebral small vessel disease-related protease HtrA1 processes latent TGF-beta binding protein 1 and facilitates TGF-beta signaling. Proc Natl Acad Sci USA. 2014;111(46):16496-16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Dong F, Hoh J. Loss of HtrA1-induced attenuation of TGF-beta signaling in fibroblasts might not be the main mechanism of CARASIL pathogenesis. Proc Natl Acad Sci USA. 2015;112(14):E1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Min W, Zhou JH. Endothelial cell-pericyte interactions in the pathogenesis of cerebral cavernous malformations (CCMs). Cold Spring Harb Perspect Med. 2022;13(3):a041188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64(6):575-611. [DOI] [PubMed] [Google Scholar]
- 95.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol. 2013;23(3):303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalkara T, Alarcon-Martinez L, Yemisci M. Pericytes in ischemic stroke. Adv Exp Med Biol. 2019;1147:189-213. [DOI] [PubMed] [Google Scholar]
- 97.Gursoy-Ozdemir Y, Yemisci M, Dalkara T. Microvascular protection is essential for successful neuroprotection in stroke. J Neurochem. 2012;123(suppl 2):2-11. [DOI] [PubMed] [Google Scholar]
- 98.Wang L, Xiong X, Zhang L, Shen J. Neurovascular unit: a critical role in ischemic stroke. CNS Neurosci Ther. 2021;27(1):7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ozen I, Deierborg T, Miharada K, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128(3):381-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakuma R, Kobayashi M, Kobashi R, et al. Brain pericytes acquire stemness via the Nrf2-dependent antioxidant system. Stem Cells. 2022;40(7):641-654. [DOI] [PubMed] [Google Scholar]
- 101.Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12(1):5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35(4):998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takata F, Dohgu S, Matsumoto J, et al. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflammation. 2011;8(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Machida T, Dohgu S, Takata F, et al. Role of thrombin-PAR1-PKCθ/δ axis in brain pericytes in thrombin-induced MMP-9 production and blood-brain barrier dysfunction in vitro. Neuroscience. 2017;350:146-157. [DOI] [PubMed] [Google Scholar]
- 105.Kane R, Godson C, O'Brien C. Chordin-like 1, a bone morphogenetic protein-4 antagonist, is upregulated by hypoxia in human retinal pericytes and plays a role in regulating angiogenesis. Mol Vis. 2008;14:1138-1148. [PMC free article] [PubMed] [Google Scholar]
- 106.Wu P, Yonekura H, Li H, et al. Hypoxia down-regulates endostatin production by human microvascular endothelial cells and pericytes. Biochem Biophys Res Commun. 2001;288(5):1149-1154. [DOI] [PubMed] [Google Scholar]
- 107.Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res. 2014;5:543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stark K, Eckart A, Haidari S, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol. 2013;14(1):41-51. [DOI] [PubMed] [Google Scholar]
- 109.Proebstl D, Voisin MB, Woodfin A, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209(6):1219-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haffner C, Malik R, Dichgans M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J Cereb Blood Flow Metab. 2016;36(1):158-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630-638. [DOI] [PubMed] [Google Scholar]
- 112.Muller K, Courtois G, Ursini MV, Schwaninger M. New insight into the pathogenesis of cerebral small-vessel diseases. Stroke. 2017;48(2):520-527. [DOI] [PubMed] [Google Scholar]
- 113.Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO. Differential TGF-beta signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci. 2010;51(4):1857-1865. [DOI] [PubMed] [Google Scholar]
- 114.Hara K, Shiga A, Fukutake T, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360(17):1729-1739. [DOI] [PubMed] [Google Scholar]
- 115.Uemura MT, Ihara M, Maki T, et al. Pericyte-derived bone morphogenetic protein 4 underlies white matter damage after chronic hypoperfusion. Brain Pathol. 2018;28:521-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khaddaj-Mallat R, Aldib N, Bernard M, et al. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol Dis. 2021;161:105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai Z, Li J, Li Y, et al. Role of pericytes in the development of cerebral cavernous malformations. iScience. 2022;25(12):105642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baloyannis SJ. Brain capillaries in Alzheimer's disease. Hell J Nucl Med. 2015;18(suppl 1):152. [PubMed] [Google Scholar]
- 119.Halliday MR, Rege SV, Ma Q, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab. 2016;36(1):216-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miners JS, Schulz I, Love S. Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer's disease. J Cereb Blood Flow Metab. 2018;38(1):103-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Damodarasamy M, Vernon RB, Pathan JL, et al. The microvascular extracellular matrix in brains with Alzheimer's disease neuropathologic change (ADNC) and cerebral amyloid angiopathy (CAA). Fluids Barriers CNS. 2020;17(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20(3):406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Procter TV, Williams A, Montagne A. Interplay between brain pericytes and endothelial cells in dementia. Am J Pathol. 2021;191(11):1917-1931. [DOI] [PubMed] [Google Scholar]
- 125.Casey CS, Atagi Y, Yamazaki Y, et al. Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA protein-mediated pathway. J Biol Chem. 2015;290(22):14208-14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iacobaeus E, Sugars RV, Tornqvist Andren A, et al. Dynamic changes in brain mesenchymal perivascular cells associate with multiple sclerosis disease duration, active inflammation, and demyelination. Stem Cells Transl Med. 2017;6(10):1840-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De La Fuente AG, Lange S, Silva ME, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017;20(8):1755-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferrara G, Errede M, Girolamo F, et al. NG2, a common denominator for neuroinflammation, blood-brain barrier alteration, and oligodendrocyte precursor response in EAE, plays a role in dendritic cell activation. Acta Neuropathol. 2016;132(1):23-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grygorowicz T, Dabrowska-Bouta B, Struzynska L. Administration of an antagonist of P2X7 receptor to EAE rats prevents a decrease of expression of claudin-5 in cerebral capillaries. Purinergic Signal. 2018;14(4):385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keough MB, Rogers JA, Zhang P, et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun. 2016;7(1):11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng S, Wang C, Lin L, et al. TNF-α impairs pericyte-mediated cerebral microcirculation via the NF-κB/iNOS axis after experimental traumatic brain injury. J Neurotrauma. 2023;40(3-4):349-364. [DOI] [PubMed] [Google Scholar]
- 133.Omote Y, Deguchi K, Kono S, et al. Neurovascular protection of cilostazol in stroke-prone spontaneous hypertensive rats associated with angiogenesis and pericyte proliferation. J Neurosci Res. 2014;92(3):369-374. [DOI] [PubMed] [Google Scholar]
- 134.Blair GW, Janssen E, Stringer MS, et al. Effects of cilostazol and isosorbide mononitrate on cerebral hemodynamics in the LACI-1 randomized controlled trial. Stroke. 2022;53(1):29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deguchi K, Liu N, Liu W, et al. Pericyte protection by edaravone after tissue plasminogen activator treatment in rat cerebral ischemia. J Neurosci Res. 2014;92(11):1509-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]