Abstract
Background and Objectives
To evaluate brain volume changes caused by different subclasses of anti–β-amyloid (Aβ) drugs trailed in patients with Alzheimer disease.
Methods
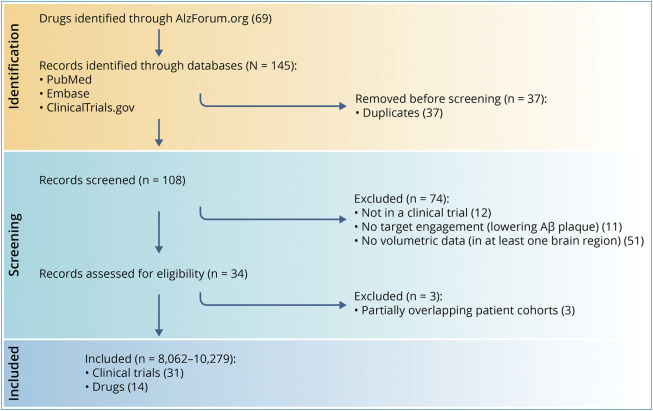
PubMed, Embase, and ClinicalTrials.gov databases were searched for clinical trials of anti-Aβ drugs. This systematic review and meta-analysis included adults enrolled in randomized controlled trials of anti-Aβ drugs (n = 8,062–10,279). The inclusion criteria were as follows: (1) randomized controlled trials of patients treated with anti-Aβ drugs that have demonstrated to favorably change at least one biomarker of pathologic Aβ and (2) detailed MRI data sufficient to assess the volumetric changes in at least one brain region. MRI brain volumes were used as the primary outcome measure; brain regions commonly reported include hippocampus, lateral ventricle, and whole brain. Amyloid-related imaging abnormalities (ARIAs) were investigated when reported in clinical trials. Of the 145 trials reviewed, 31 were included in the final analyses.
Results
A meta-analysis on the highest dose of each trial on hippocampus, ventricle, and whole brain revealed drug-induced acceleration of volume changes that varied by anti-Aβ drug class. Secretase inhibitors accelerated atrophy to the hippocampus (Δ placebo - Δ drug: −37.1 µL [19.6% more than placebo]; 95% CI −47.0 to −27.1) and whole brain (Δ placebo - Δ drug: −3.3 mL [21.8% more than placebo]; 95% CI −4.1 to 2.5). Conversely, ARIA-inducing monoclonal antibodies accelerated ventricular enlargement (Δ placebo - Δ drug: +2.1 mL [38.7% more than placebo]; 95% CI 1.5–2.8) where a striking correlation between ventricular volume and ARIA frequency was observed (r = 0.86, p = 6.22 × 10−7). Mild cognitively impaired participants treated with anti-Aβ drugs were projected to have a material regression toward brain volumes typical of Alzheimer dementia ∼8 months earlier than if they were untreated.
Discussion
These findings reveal the potential for anti-Aβ therapies to compromise long-term brain health by accelerating brain atrophy and provide new insight into the adverse impact of ARIA. Six recommendations emerge from these findings.
The potential benefit of anti–β-amyloid (Aβ) drugs to cognition in Alzheimer disease remains under active debate. A recent meta-analysis concluded that anti-Aβ therapies do not slow cognitive decline in Alzheimer disease.1 Yet, supporters of anti-Aβ therapies suggest that drugs that substantially reduce plaque report more promising effects to cognition.2 Despite Food and Drug Administration (FDA) approval of the monoclonal antibody therapy, aducanumab (marketed as Aduhelm), there has been limited use of this drug in the community. Another monoclonal antibody, lecanemab (Eisai/Biogen), was recently reported to slow clinical decline by 27%3 and has likewise received accelerated approval by the FDA, whereas gantenerumab (Roche) failed to provide a clinical benefit. The field also retains hope in another monoclonal antibody, donanemab (Eli Lilly), which will report phase 3 findings in 2023. All these drugs under active development cause MRI-detectable amyloid-related imaging abnormalities (ARIAs). These side effects are often clinically silent or are associated with non–life-threating symptoms such as migraine that resolve over 3–4 months of treatment suspension.4 In severe cases, ARIA may require hospitalization. The long-term consequence of ARIA to brain health has not been investigated.
Anti-Aβ drugs also frequently cause accelerated changes to brain volume.5-12 This is concerning because loss of brain tissue is the proximate cause of cognitive dysfunction in Alzheimer disease and volume changes are supportive and objective evidence of disease progression. These volume changes have been rationalized as being small or due to factors other than neuron loss.5,6,11 An often-cited alternative explanation is that the drug-induced volume change may be attributable to loss of plaque that occupies volume within the brain. However, Aβ is a low abundant peptide, even in Alzheimer disease.13 We previously calculated that the loss of plaque volume accounted for less than a thousandth of the change in brain volume caused by donanemab.14 The long-term consequence of drug-induced volume loss to brain health has not been investigated. One article that investigated volume changes more extensively found that the β-secretase inhibitor, verubecestat, caused nonprogressive changes to brain structures (hippocampus, whole brain, ventricles, and cortex).10 Verubecestat caused loss of brain volume particularly in amyloid-rich regions, but this was inversely related to change in plaque load. The change in brain volume was moderately (0.44 < r < 0.55) associated with cognitive change. Other anti-Aβ drugs are reported to cause progressive volumetric changes, but there has been little exploration into the impact of these adverse findings.3,5-9
With the approval of aducanumab and lecanemab by the FDA and donanemab in an advanced clinical trial, we may now be emerging into a new therapeutic era for Alzheimer disease. Yet, potentially adverse volumetric changes caused by anti-Aβ drugs have hardly been investigated (publicly) by the pharmaceutical companies that control the data. We have been unsuccessful in our requests to access these data. There is also no publicly available evidence that the FDA reviewed volumetric data for aducanumab when they approved the drug. The clinical review of aducanumab by the FDA's Centre for Drug Evaluation and Research15 lists changes in volume to brain structures as a key pharmacodynamic end point (safety and efficacy), but they do not comment on the result in an otherwise exhaustive analysis of the trial data. Simple questions such as which brain regions are affected by anti-Aβ drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and APOE ε4 genotype can and should be addressed. Answers to these questions will be informative of the nature of drug-induced volume changes, the consequence of those changes, and which individuals are at risk. To gain further insight into the adverse effects of anti-Aβ drugs, we performed a systematic review and meta-analysis of brain volume changes in clinical trials of anti-Aβ drugs.
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16,17 A prespecified protocol was not published before conducting this study.
Search Methods and Study Selection
On August 8, 2022 (and updated on December 10, 2022), we searched the Alzheimer Research Forum (alzforum.org) for “Amyloid-Related” drugs for “Alzheimer Disease” and “Mild Cognitive Impairment,” which yielded a comprehensive list of 69 drugs identified in eTable 1 (links.lww.com/WNL/C712). We then searched for trials of these drugs in PubMed, Embase, and ClinicalTrials.gov databases. The search terms combined synonyms using the preidentified drugs as keywords, “Alzheimer Disease,” “Mild Cognitive Impairment,” and “brain volume.” Details and specific search queries are presented in eTables 2–4.
The inclusion criteria were as follows: (1) randomized controlled trials of patients treated with anti-Aβ drugs that have demonstrated to favorably change at least one biomarker of pathologic Aβ and (2) detailed MRI data sufficient to assess the volumetric changes in at least one brain region. The exclusion criteria were as follows: (1) studies that were not clinical trials and (2) data that were reported in a conference abstract, review article, letter, editorial, comment, note, short survey, or chapter or a summary of other studies. The search and study selection were conducted by 2 reviewers (F.A.) and (S.A.), and disagreements were resolved by consensus.
Volumetric Data Extraction
We obtained data from ClinicalTrials.gov, peer-reviewed publications, and company presentations (for the lecanemab, “CLARITY” trial only). When available, mean and 95% CIs were obtained for volumetric changes from baseline; however, several sources reported data as mean (SE), mean (SD), or percentage changes from baseline. Percentage changes were used to calculate mean volume changes using baseline volumes of whole brain, hippocampus, and lateral ventricle when these were reported in the publication or on ClinicalTrials.gov. If not, we imputed a baseline from a representative study.10 Five studies did not report baseline values: lecanemab (NCT03887455), IV immunoglobulin (IVIg: NCT00818662 and NCT01300728), and crenezumab (NCT01397578 and NCT02670083)—except for lecanemab, none of these studies reported a negative impact of drug on volume changes to any structure. The semagacestat studies (NCT00762411 and NCT00594568) reported left and right hippocampus volumes separately, so we combined both the left and right using (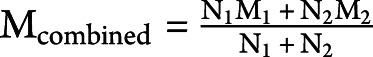 ) to determine a single mean value for the hippocampus and a single value for the SD (
) to determine a single mean value for the hippocampus and a single value for the SD (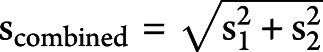 ). When exact values were not available, data were approximated from graphs. When volumetric data were measured but not reported (gantenerumab; NCT01224106), we contacted pharmaceutical companies directly in person and through subsequent e-mails. We additionally approached pharmaceutical companies for access to more extensive data (described in the eAppendix 1, links.lww.com/WNL/C712). The summary of volumetric data sources is presented in eTable 5. This analysis is based on all data available to us on August 23, 2022, and reviewed on December 10, 2022. We assumed that studies with no data available were missing completely at random. The results for a drug with notable volumetric benefits would almost certainly be publicly reported if it were measured, so if the missing completely at random assumption was violated, any bias would likely be in a direction favorable to anti-Aβ drugs.
). When exact values were not available, data were approximated from graphs. When volumetric data were measured but not reported (gantenerumab; NCT01224106), we contacted pharmaceutical companies directly in person and through subsequent e-mails. We additionally approached pharmaceutical companies for access to more extensive data (described in the eAppendix 1, links.lww.com/WNL/C712). The summary of volumetric data sources is presented in eTable 5. This analysis is based on all data available to us on August 23, 2022, and reviewed on December 10, 2022. We assumed that studies with no data available were missing completely at random. The results for a drug with notable volumetric benefits would almost certainly be publicly reported if it were measured, so if the missing completely at random assumption was violated, any bias would likely be in a direction favorable to anti-Aβ drugs.
Aβ Plaque and ARIA Data Extraction
When available, we obtained data from ClinicalTrials.gov and peer-reviewed publications. For most studies that measured Aβ plaque using PET, data available were the mean change from baseline (standardized uptake value ratio [SUVR]). Owing to a range of radioisotopes (i.e., [18F] flutemetamol, 18F-florbetapir, and Pittsburgh compound-B [PIB-PET]), we converted values to a % change from baseline (using in study baseline mean values). For studies that did not provide baseline PET values (solanezumab: NCT01900665 and IVIg: NCT00818662 and NCT01300728), the average baseline from other studies using the same tracer was used (PIB-PET: 2.17, florbetapir: 1.43; eTable 7, links.lww.com/WNL/C712). These drugs caused less than 5% difference in SUVR, so the imputed baseline values are unlikely to have a material impact on the analysis. Frequency of ARIA was obtained when available. Studies that verbally reported “no ARIA” ceased ARIA measurements part way through the trial as “deemed unnecessary based on Data Monitoring Committee and regulatory feedback” or did not report ARIA were assumed to be 0%. We focused on ARIA-E (edema) not ARIA-H (hemorrhagic) because the latter was infrequently reported in the clinical trials that were included in the analysis and is known to be correlated with ARIA-E.4
Statistical Analyses
A random effects meta-analysis for mean differences was performed using the metafor package18 in R version 4.2.0 (2022-04-22 ucrt) for each brain region. For each trial, the arm with the highest dose was included for the overall analysis. The analysis was run on the raw mean difference19 between the treatment and the control groups for each trial without assuming homoscedasticity. For one trial (NCT01760005), a shared control group (n = 28) was used to compare 2 drugs (solanezumab and gantenerumab); in this occasion, we split the control group into 2 groups of size (n = 14) for the meta-analyses. This method can bias the summary statistics of a meta-analysis; however, a sensitivity analysis on these drugs showed negligible effect on results and no impact on interpretation or inference.
Data from all trials of anti-Aβ drugs and doses were used to investigate the relationship between brain volumetric changes and ARIA frequency or Aβ reduction measured by PET (SUVR % change relative to baseline) using a series of Pearson regressions. For the analysis focused on plaque, we excluded trials where no acceleration of volume changes was observed and where decreased plaque was also observed because it is not possible that decreased plaque could decrease volume changes. For the analysis focused on ARIA, we excluded trials where no acceleration of volume changes was observed and increased ARIA was also neither reported nor detected because it is not possible that ARIA could decrease volume changes. Furthermore, a subgroup analysis was performed to measure the effect of monoclonal antibodies, secretase inhibitors, and studies that reported ARIA and no ARIA effects.
We modeled the projected influence of anti-Aβ drugs on brain volumes of patients with mild cognitive impairment (MCI). We used previously measured volumes of hippocampus, whole brain and lateral ventricle in MCI and patients with Alzheimer dementia, and the calculated rate of volume change in MCI.20 We assigned these attributes to a modeled placebo group. Assuming that the annualized percentage increased the rate of volume change induced by anti-Aβ drugs would be constant over the natural history of Alzheimer disease, we estimated the time it would take patients with MCI to develop Alzheimer dementia-sized brain structures if they were administered an anti-Aβ drug by using our calculations of weighted percentage change from our meta-analyses of pooled monoclonal antibodies with ARIA, monoclonal antibodies with no ARIA, secretase inhibitors, and all drugs.
Standard Protocol Approvals, Registrations, and Patient Consents
This analysis was not preregistered. We followed the PRISMA statement for transparent reporting of systematic reviews16,17 and produced a detailed PRISMA flowchart (Figure 1) and checklist. This work was based on publicly available data only, so it did not require review by an institutional research board. No individual-level data are included in this manuscript. All data are aggregated data from clinical trials that are publicly available.
Figure 1. Flowchart of Trial Exclusions and Inclusions.
Aβ = β-amyloid. Created with Biorender.com.
Data Availability
This work was based on publicly available data only. On reasonable request, statistical code and the data set are available from the corresponding author.
Results
Anti-Aβ Drugs That Report Brain Volume
Overall, 145 studies were identified that potentially met the inclusion criteria (Figure 1). Two authors reviewed these trials, and 31 met the inclusion criteria (51 treatment arms, 8,062–10,279 participants; Figure 1; eTable 5, links.lww.com/WNL/C712). The following drugs were represented in this analysis: aducanumab (Biogen, Cambridge, MA), bapineuzumab (Janssen Biotech, Philadelphia, PA; Pfizer, New York, NY), crenezumab (Hoffmann-La Roche/Genetech, Basel, Switzerland), donanemab (Eli Lilly, Indianapolis, IN), BAN2401 (lecanemab; Biogen, Cambridge, MA; Eisai, Tokyo, Japan), solanezumab (LY2062430; Eli Lilly), LY450139 (semagacestat; Eli Lilly), gantenerumab (Chugai Pharmaceutical, Tokyo, Japan; Hoffmann-La Roche), tramiprosate (Neurochem, Inc., Laval, QC, Canada), AAC-001 (Pfizer, New York, NY), IVIg (Takeda, Tokyo, Japan), and verubecestat (MK-8931; Merck Sharp & Dohme, Kenilworth, NJ). Of 31 clinical trials that measured brain volume loss, one study (combined of 2 trials) investigated the association with ARIA, one study investigated the association with cognitive outcomes, and 4 studies stratified by APOE ε4 (eTable 6). The brain regions commonly investigated include hippocampus, ventricles, and whole brain. The average number of brain regions measured was 2.5, with donanemab (NCT03367403) standing out from the rest of the drug trials with 14 brain regions measured.
Effects of Anti-Aβ Drugs on Brain Volume Changes
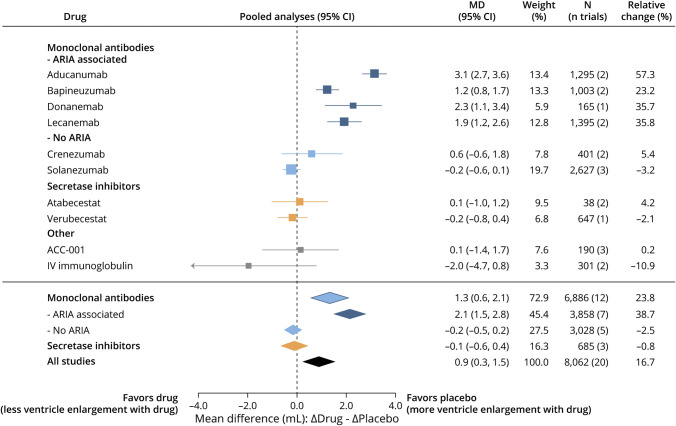
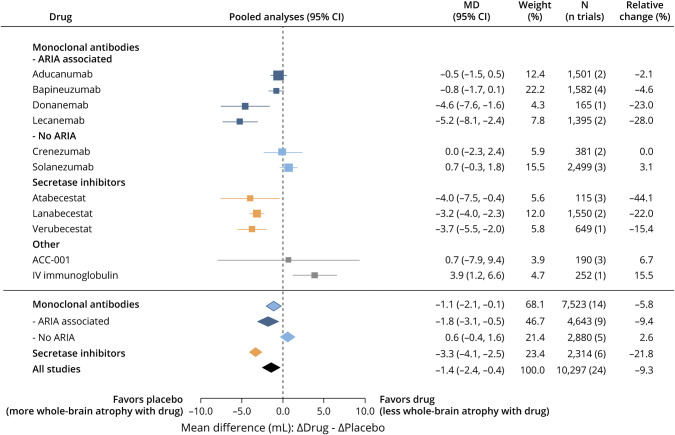
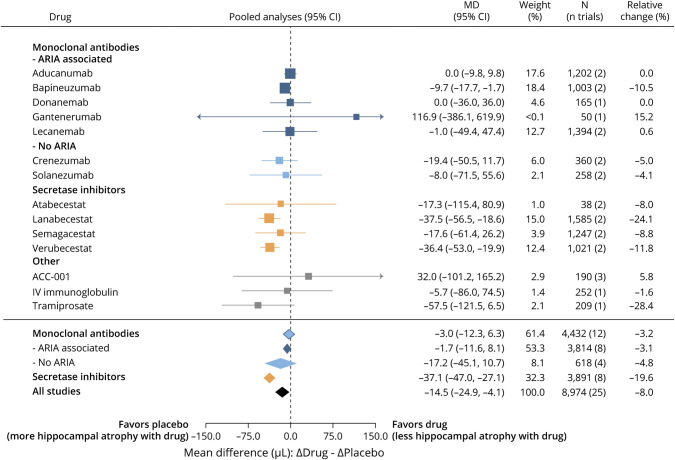
Pooled random effects meta-analyses of the highest drug dose used in all trials demonstrated that anti-Aβ drugs significantly accelerated volumetric changes to the ventricles (Δ placebo - Δ drug: +0.9 mL [16.7% more than placebo]; 95% CI 0.3–1.5; Figure 2 for summary, eFigure 1 for individual studies, links.lww.com/WNL/C712), whole brain (Δ placebo - Δ drug: −1.4 mL [9.3% more than placebo]; 95% CI −2.4 to −0.4; Figure 3, eFigure 2), and hippocampus (Δ placebo - Δ drug: −14.5 μL [8% more than placebo]; 95% CI −24.9 to −4.1; Figure 4, eFigure 3). Funnel plots were used to investigate publication bias for each brain region. Most studies fell within the inverse variance funnel, showing little publication bias in the hippocampus. There was evidence for possible publication bias across all brain regions. However, investigating this bias by subgroup showed that the distance outside the funnel was due to the effects explored by the subgroups investigated rather than publication bias. This is supported also by the primary variable investigated by these studies which was not brain atrophy/ventricular enlargement. Sensitivity analyses (Cook distance, DFBETA, and hat value analyses) showed that no particular study had a strong influence on the results.
Figure 2. Effect of Anti-Aβ Drugs on Lateral Ventricular Enlargement.
Summary data from the highest dose of all drug trials for each drug. N includes placebo and high-dose groups from each study. Aβ = β-amyloid; ARIA = amyloid-related imaging abnormality; CI: 95% confidence interval; MD = mean difference; Relative change (%): the mean difference between volume changes in drug and placebo as a ratio to the change in placebo.
Figure 3. Effect of Anti-Aβ Drugs on Whole-Brain Atrophy.
Summary data from the highest dose of all drug trials for each drug. N includes placebo and high-dose groups from each study. Aβ = β-amyloid; ARIA = amyloid-related imaging abnormality; CI: 95% confidence interval; MD = mean difference; Relative change (%): the mean difference between volume changes in drug and placebo as a ratio to the change in placebo.
Figure 4. Effect of Anti-Aβ Drugs on Hippocampal Atrophy.
Summary data from the highest dose of all drug trials for each drug. N includes placebo and high-dose groups from each study. Aβ = β-amyloid; ARIA = amyloid-related imaging abnormality; CI: 95% confidence interval; MD = mean difference; Relative change (%): the mean difference between volume changes in drug and placebo as a ratio to the change in placebo.
To assess the presence and extent of between-study variation, we observed the I2 for each region. The plots revealed considerable heterogeneity between trials, and I2 values indicate moderate heterogeneity for the hippocampal regions (I2 = 64.01%) and substantial heterogeneity across the ventricles (I2 = 87.64%) and whole-brain (I2 = 76.88%) regions. These results supported conducting subgroup analyses by drug mechanism and by the presence of ARIA. Subgroup analysis was performed for each of the following mechanisms: secretase inhibitors, monoclonal antibodies, monoclonal antibodies that cause increased ARIA, and monoclonal antibodies that do not increase ARIA (<1% increase).
Secretase inhibitors accelerated atrophy of the hippocampus (Δ placebo - Δ drug: −37.1 µL [19.6% more than placebo]; 95% CI −47.0 to −27.1) and whole brain (Δ placebo - Δ drug: −3.3 mL [21.8% more than placebo]; 95% CI −4.1 to −2.5), but not ventricles. Conversely, monoclonal antibodies caused accelerated ventricular enlargement (Δ placebo - Δ drug: +1.3 mL [23.8% more than placebo]; 95% CI 0.6–2.1), which was driven by the subset of monoclonal antibodies that cause increased ARIA (Δ placebo - Δ drug: +2.1 mL [38.7% more than placebo]; 95% CI 1.5–2.8; Figure 2). ARIA-inducing monoclonal antibodies had a variable effect on whole-brain volume, with accelerated whole-brain volume loss caused by donanemab (Δ placebo - Δ drug: −4.6 mL [23% more than placebo]; 95% CI −7.6 to −1.6) and lecanemab (Δ placebo - Δ drug: −5.2 mL [36.4% more than placebo]; 95% CI −8.1 to −2.4), which was not observed with aducanumab and bapineuzumab. Monoclonal antibodies did not materially cause accelerated volume loss to the hippocampus regardless of whether they caused increased ARIA.
ARIA Frequency and Brain Volume Changes
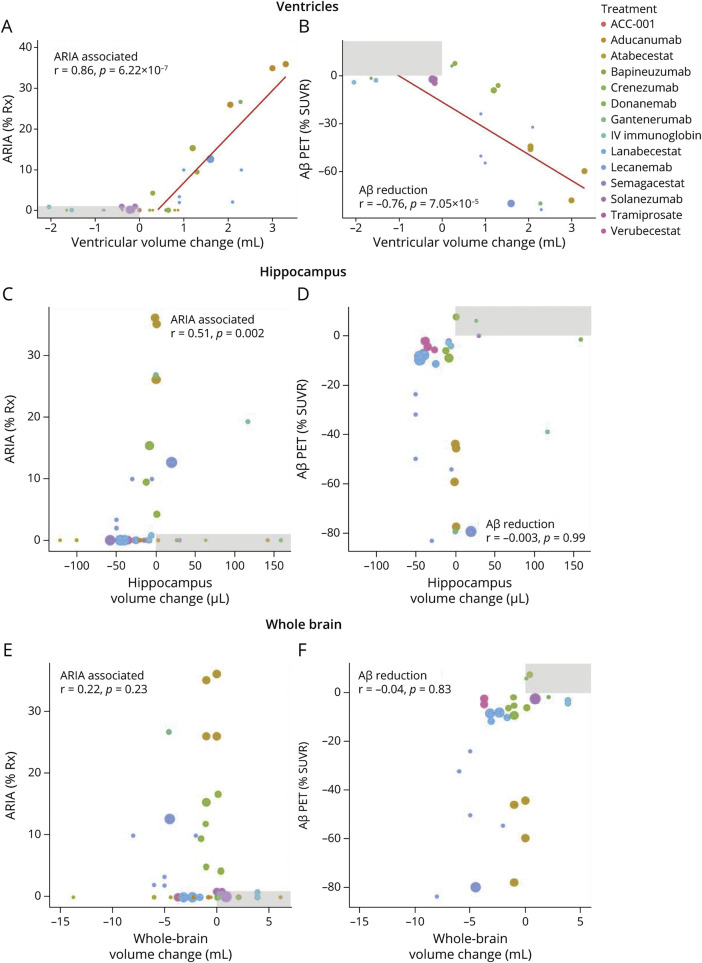
Given that ARIA-associated monoclonal antibodies caused markedly accelerated enlargement to the lateral ventricle compared with other anti-Aβ drugs included in this analysis, we next investigated the association between the frequency of ARIA reported in the trials and the extent of volume changes to the different structures induced by drugs at different doses. Increased frequency of ARIA induced by anti-Aβ therapies was dependent on the drug type and dose. After excluding trials where no acceleration of volume changes was observed and increased ARIA was also neither reported or detected (since it is not possible that ARIA could decrease volume changes), the enlargement of ventricles was strongly correlated with % ARIA (r = 0.86, p = 6.22 × 10−7; Figure 5A). The frequency of ARIA was also associated with hippocampus volume change (r = 0.51; p = 0.002), but not the whole brain when explored in a similar analyses (Figure 5, C and E).
Figure 5. Association of Volume Change With ARIA and Amyloid Reduction.
Data from all trials of anti-Aβ drugs and doses were used to investigate correlations between brain volumetric changes and ARIA frequency or Aβ reduction measured by PET (SUVR % change relative to baseline). For analysis focused on ARIA, we excluded trials (shaded in gray) where no acceleration of volume changes was observed and increased ARIA was also neither reported nor detected (since it is not possible that ARIA could decrease volume changes). For analysis focused on plaque, we excluded trials (shaded in gray) where no acceleration of volume changes was observed and decreased plaque was also observed (since it is not possible that decreased plaque could decrease volume changes). A series of Pearson regressions were performed between ventricular enlargement and ARIA (A), ventricular enlargement and Aβ plaque reduction (B), hippocampus atrophy and ARIA (C), hippocampus atrophy and Aβ plaque reduction (D), whole-brain atrophy and ARIA (E), and whole-brain atrophy and Aβ plaque reduction (F). Bubble size represents sample size of drug-treated arm. Aβ = β-amyloid; ARIA = amyloid-related imaging abnormality; SUVR = standardized uptake value ratio.
Reduction in PET-Determined Aβ Plaque and Brain Volume Changes
Brain volume loss caused by anti-Aβ drugs has previously been attributed to loss of plaque volume in response to treatment.5,6,11 Hence, we explored whether change in brain volume was associated with the reduction in Aβ plaque measured by PET. The mean reduction in SUVR was 23.6% (eFigure 4, links.lww.com/WNL/C712). We found that the extent of SUVR reduction was correlated with the degree of enlargement to the lateral ventricle (r = −0.76; p = 7.5 × 10−5) (Figure 5B) but not volume changes to hippocampus (Figure 5C) or whole brain (Figure 5F). However, we found that the frequency of ARIA was also correlated with the extent of SUVR reduction by anti-Aβ drugs (r = −0.682; p = 1.70 × 10−5; eFigure 4), which may confound associations between SUVR reduction and volume changes.
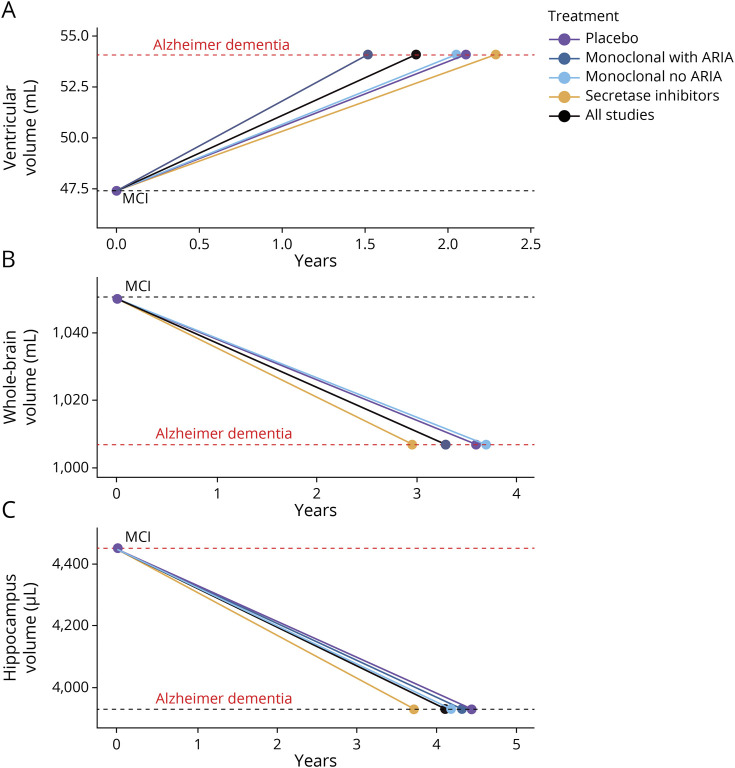
Modeling the Effect of Aβ Drugs on Brain Volume Changes
To contextualize the clinical relevance of these findings, we modeled the projected influence of anti-Aβ drugs on the volume changes of lateral ventricles (Figure 6A), hippocampus (Figure 6B), and whole-brain atrophy (Figure 6C) that occurs between MCI and Alzheimer dementia. Using prior measured volumes of these structures in patients with MCI and Alzheimer dementia and calculated rates of change20 as an imputed placebo reference, we applied our estimates of percentage increased rate of volume change above placebo that is induced by the different drug classes from our meta-analyses. This analysis predicted that patients with MCI taking secretase inhibitors will develop whole-brain volume (treated: 3.0 years; nontreated: 3.6 years; difference 7.7 months) and hippocampal volume (treated: 3.7 years; nontreated: 4.4 years; difference 8.7 months) typical of someone with Alzheimer dementia in a materially earlier time point. The effect of monoclonal antibodies with and without ARIA had a negligible effect on time to develop an Alzheimer dementia-sized hippocampus. Rather, monoclonal antibodies that induced ARIA accelerated the timeframe for patients with MCI to develop enlargement of the lateral ventricle typical of Alzheimer dementia (treated: 1.5 years, nontreated: 2.1 years; difference 7.0 months), which was not observed with monoclonal antibodies that did not induce ARIA (2.1 years) or secretase inhibitors (2.3 years).
Figure 6. Modeling of Volume Changes.
The projected influence of anti-Aβ drug classes on the volume changes of (A) ventricle, (B) hippocampus, and (C) whole-brain atrophy that occur between MCI and Alzheimer dementia (obtained from reference 20). The placebo (purple) is an average rate of volumetric change in participants with MCI.20 The projected rate of volume change induced by pooled monoclonal antibodies with ARIA (dark blue), monoclonal antibodies with no ARIA (light blue), secretase inhibitors (orange), and all drugs (black) used the percentage difference on placebo derived from forest plots (Figures 2–4). Aβ = β-amyloid; ARIA = amyloid-related imaging abnormality; MCI = mild cognitive impairment.
Discussion
We combined all available brain volumetric results from 31 clinical trials of anti-Aβ drugs to conduct meta-analyses of drug-related changes to hippocampus (n = 8,974), lateral ventricles (n = 8,062), and whole brain (n = 10,279). Anti-Aβ drugs significantly accelerated volumetric changes to each region, but the effect on region was dependent on drug class. Secretase inhibitors had a prominent impact compared with monoclonal antibodies on volume changes to hippocampus and whole brain. Conversely, monoclonal antibodies but not secretases inhibitors caused accelerated ventricular enlargement. Monoclonal antibodies that cause ARIA had a greater impact on the ventricles but not the other structures, and we found an arresting correlation between the frequencies of ARIA with the degree of ventricular enlargement. These data reveal the potential for anti-Aβ therapies to compromise long-term brain health by materially accelerating brain atrophy and provide new insight into the adverse impact of ARIA.
These meta-analyses permit robust conclusions regarding the effect of anti-Aβ drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings. We do not have data from individual cases to determine whether people who develop ARIA exhibit accelerated changes to the ventricles. In the only example that we could find, a clinical trial of bapineuzumab showed that patients who acquired ARIA had accelerated volume changes.21 The statistical analysis plan for the phase 3 clinical trials of aducanumab prespecifies analyzing volume changes after ARIA stratification,5 but the data have not been presented.
ARIA is unlikely to account for the accelerated change to hippocampus and whole brain because ARIA frequency was not associated with volume changes to these structures. These were instead affected by secretase inhibitors, which report no/minor increases to ARIA frequency. How do secretase inhibitors cause reductions to hippocampus and whole brain if not by ARIA? It is not possible to rule out off-target toxic effects of these drugs, but it is also conceivable that lowering Aβ levels damages the brain by the restriction of a vital function of Aβ. The sequence of Aβ is highly conserved among vertebrates,22 and all healthy brains contain Aβ. Indeed, the production of Aβ requires complex mechanisms that suggest an important reason for its presence. Soluble levels of Aβ decrease in Alzheimer disease CSF,23 and most familial mutations in presenilin lower Aβ levels.24 Physiologic concentrations (picomolar and low nanomolar) of Aβ are neurotrophic and promote long-term potentiation (LTP) in mice.25 Conversely, decreasing Aβ in mice using antibodies or in APP knockout mice inhibits LTP and cognition.26,27 Hence, it is possible that lowering functional Aβ using secretase inhibitors could be the cause of accelerated cognitive decline and brain atrophy of whole brain and hippocampus reported in clinical trials.28-30
Although loss of functional Aβ may contribute to brain damage, it is unlikely that reduction in Aβ plaque volume could account for the degree of brain volume change reported in clinical trials of anti-Aβ drugs. The amount of Aβ in the Alzheimer disease brain is too little,14 and in the clinical trial of verubecestat (the only study that explored this association), there was an inverse association between plaque loss and hippocampal volume change.10 Furthermore, the donanemab trial demonstrates a spatial and temporal dissociation between plaque loss and brain volume changes.6 Donanemab caused a major (75%) reduction in plaque load by 24 weeks, but the brain volume changes emerged from 52 weeks to the following regions: bilateral cortex, lateral parietal lobe, precuneus, ventricles, whole brain, whole temporal lobe, and white matter. It would be difficult to envision how an early loss of plaque predominantly in gray matter could account for the subsequent marked white matter loss caused by donanemab. It has also recently been shown that the whole-brain atrophy induced by donanemab was associated with plasma neurofilament light chain protein, which is positive evidence that the volume changes indeed reflect neurodegeneration.31 In the current analysis, monoclonal antibodies caused a greater decrease in plaque than did the secretase inhibitors, but the latter had a much larger effect on whole-brain and hippocampal volume. The degree of volume loss to these structures was not associated with the degree of plaque removal.
While these findings argue against plaque loss as an explanation for volume changes, we indeed observed that ventricular volume loss was associated with the extent of plaque removal; an association that was not as strong as that between %ARIA and ventricular volume. Since we also observed that ARIA frequency was correlated with the extent of plaque removal, it is possible that either of these variables (plaque or ARIA) confounds the association of the other with ventricular volume. We noted that in every trial where ARIA was reported, a corresponding and proportional increase in ventricular enlargement was observed. By contrast, in trials where SUVR was reduced less than the mean of the group (−23.6%), there were only 4 of 10 occasions where the ventricle was enlarged, suggesting that loss of plaque volume cannot account for ventricular enlargement, at least when plaque reduction was modest (<mean). It is conceivable that ventricular enlargement could be contributed by reduction in plaque volume where it is more extensive (>mean SUVR reduction), but donanemab and lecanemab which caused the greatest reduction in SUVR compared with all other drugs did not cause the greatest acceleration to ventricular enlargement. These findings imply that the extent of SUVR change is related to the frequency of ARIA, with the latter being the more likely driver of ventricular volume enlargement. In support of this hypothesis, the one study that we identified that examined volume changes after stratification by ARIA reported that bapineuzumab-treated patients who developed ARIA-E had significantly more ventricular enlargement (approximately 30%) compared with patients who did not develop ARIA-E.21
It is not clear why ARIA or the different drug classes cause distinct changes to hippocampus, lateral ventricle, and whole brain, and these findings call for further research into a range of brain structures to examine regional trends. It is possible that the symptoms that result from drug-induced brain volume loss to different structures are not related to cognition—especially for monoclonal antibodies that tend not to affect the hippocampus. Therefore, the clinical impact of drug-induced brain damage may not have been measured using the clinical instruments used in the trials of these drugs. It is possible that volume changes do not reflect neurodegeneration, for example, these drugs may cause CSF malabsorption (e.g., by blocking glymphatics) leading to a temporary and/or nondeleterious outcome, but when dealing with the possibility of brain damage, we should be cautious in our interpretation and gather more data before dismissing this finding and its usual interpretation. Our analysis calls for urgent revaluation of prior trials and renewed procedures to monitor patients in current trials and in the community, which we enumerate in 6 recommendations:
Clinicians who prescribe ARIA-inducing anti-Aβ monoclonal antibodies should inform current and new patients that these drugs have been shown to accelerate markers of neurodegeneration (for example, ventricular enlargement).
Clinicians should review the volumetric data from the clinical trials of ARIA-inducing anti-Aβ monoclonal antibodies when assessing the risk/benefit profile of these therapies.
Clinicians should monitor brain volume changes of individual patients who receive ARIA-inducing anti-Aβ monoclonal antibodies to determine whether continued treatment is appropriate.
The data safety monitoring boards serving current clinical trials of anti-Aβ drugs should review volumetric data to determine whether patient safety is at risk, particularly in patients who develop ARIA.
Ethics boards that approve trials for anti-Aβ drugs should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine whether brain atrophy is progressive, particularly in patients who develop ARIA.
Pharmaceutical companies that have conducted trials of anti-Aβ drugs should interrogate prior data on brain volume (e.g., stratifications by ARIA and analysis of additional brain structures), report the findings, and release the data for researchers to investigate.
Glossary
- Aβ
β-amyloid
- ARIA
amyloid-related imaging abnormality
- ARIA-E
ARIA edema
- ARIA-H
ARIA hemorrhagic
- FDA
Food and Drug Administration
- IVIg
IV immunoglobulin
- LTP
long-term potentiation
- MCI
mild cognitive impairment
- PIB
Pittsburgh compound B
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SUVR
standardized uptake value ratio
Appendix. Authors

Footnotes
Editorial, page 941
Study Funding
Analysis was supported by funds from the Australian National Health & Medical Research Council (NHMRC: GNT2008359). The Florey Institute of Neuroscience and Mental Health acknowledges support from the Victorian Government, particularly funding from the Operational Infrastructure Support Grant.
Disclosure
F. Alves and P. Kalinowski declare support from the NIH National Institute on Aging for the submitted work. S. Ayton declares support from the NIH National Institute on Aging for the submitted work and reports acting as a consultant for Eisai in the past 3 years; no other relationships or activities that could appear to have influenced the submitted work. Go to Neurology.org/N for full disclosures.
References
- 1.Ackley SF, Zimmerman SC, Brenowitz WD, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ. 2021;372:n156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haass C, Selkoe D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. 2022;20(7):e3001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9-21. [DOI] [PubMed] [Google Scholar]
- 4.Salloway S, Chalkias S, Barkhof F, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. [DOI] [PubMed] [Google Scholar]
- 6.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691-1704. [DOI] [PubMed] [Google Scholar]
- 7.Novak G, Streffer JR, Timmers M, et al. Long-term safety and tolerability of atabecestat (JNJ-54861911), an oral BACE1 inhibitor, in early Alzheimer's disease spectrum patients: a randomized, double-blind, placebo-controlled study and a two-period extension study. Alzheimers Res Ther. 2020;12(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling R, Henley D, Aisen PS, et al. Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: a truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 2021;78(3):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sur C, Kost J, Scott D, et al. BACE inhibition causes rapid, regional, and non-progressive volume reduction in Alzheimer's disease brain. Brain. 2020;143(12):3816-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak G, Fox N, Clegg S, et al. Changes in brain volume with bapineuzumab in mild to moderate Alzheimer's disease. J Alzheimers Dis. 2016;49(4):1123-1134. [DOI] [PubMed] [Google Scholar]
- 12.Wessels AM, Tariot PN, Zimmer JA, et al. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 2020;77(2):199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts BR, Lind M, Wagen AZ, et al. Biochemically-defined pools of amyloid-β in sporadic Alzheimer's disease: correlation with amyloid PET. Brain. 2017;140(5):1486-1498. [DOI] [PubMed] [Google Scholar]
- 14.Ayton S. Brain volume loss due to donanemab. Eur J Neurol. 2021;28(9):e67-e68. [DOI] [PubMed] [Google Scholar]
- 15.Krudys K. BLA761178: Aduhelm (Aducanumab) Clincal Review(s). FDA Center for Drug Evaluation and Research; 2021. [Google Scholar]
- 16.Park HY, Suh CH, Woo S, Kim PH, Kim KW. Quality reporting of systematic review and meta-analysis according to PRISMA 2020 guidelines: results from recently published papers in the Korean Journal of Radiology. Korean J Radiol. 2022;23(3):355-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. [Google Scholar]
- 19.Borenstein M, ed. Effect Sizes for Continuous Data. Russell Sage Foundation; 2009. [Google Scholar]
- 20.Schott JM, Bartlett JW, Barnes J, Leung KK, Ourselin S, Fox NC. Reduced sample sizes for atrophy outcomes in Alzheimer's disease trials: baseline adjustment. Neurobiol Aging. 2010;31(8):1452-1462.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu E, Wang D, Sperling R, et al. Biomarker pattern of ARIA-E participants in phase 3 randomized clinical trials with bapineuzumab. Neurology. 2018;90(10):e877-e886. [DOI] [PubMed] [Google Scholar]
- 22.Moir RD, Tanzi RE. Low evolutionary selection pressure in senescence does not explain the persistence of Aβ in the vertebrate genome. Front Aging Neurosci. 2019;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Agboola F, Grant E, et al. Accelerated longitudinal changes and ordering of Alzheimer disease biomarkers across the adult lifespan. Brain. 2022;145(12):4459-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Zhou R, Yang G, Shi Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc Natl Acad Sci USA. 2017;114(4):E476-E485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulisano W, Melone M, Ripoli C, et al. Neuromodulatory action of picomolar extracellular Aβ42 oligomers on presynaptic and postsynaptic mechanisms underlying synaptic function and memory. J Neurosci. 2019;39(30):5986-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmeri A, Ricciarelli R, Gulisano W, et al. Amyloid-beta peptide is needed for cGMP-induced long-term potentiation and memory. J Neurosci. 2017;37(29):6926-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayton S, Lei P, Hare DJ, et al. Parkinson's disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci. 2015;35(8):3591-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer's disease. N Engl J Med. 2018;378(18):1691-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary results of a trial of atabecestat in preclinical Alzheimer's disease. N Engl J Med. 2019;380(15):1483-1485. [DOI] [PubMed] [Google Scholar]
- 30.Doody RS, Raman R, Farlow M, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369(4):341-350. [DOI] [PubMed] [Google Scholar]
- 31.Pontecorvo MJ, Lu M, Burnham SC, et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(12):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This work was based on publicly available data only. On reasonable request, statistical code and the data set are available from the corresponding author.