Abstract
Background and Objectives
People with epilepsy (PWE) are at risk of premature death with considerable variability according to the study population. We aimed to estimate the risk and causes of death in PWE according to age, disease severity, disease course, comorbidities, and socioeconomic status in Korea.
Methods
We conducted a nationwide population-based retrospective cohort study using the National Health Insurance database linked with the national death register. Newly treated PWE from 2008 to 2016 who were identified by antiseizure medication (ASM) prescriptions and diagnostic codes for epilepsy/seizure were included and observed until 2017. We assessed all-cause and cause-specific crude mortality rates and standardized mortality ratios (SMRs).
Results
Among 138,998 PWE, 20,095 deaths were identified, and the mean follow-up period was 4.79 years. The SMR was 2.25 in the overall group of PWE, with a higher value in the younger age group at diagnosis and a shorter time interval after diagnosis. The SMR in the monotherapy group was 1.56, while that in the group with 4 or more ASMs was 4.93. PWE without any comorbidities had an SMR of 1.61. PWE who were rural residents had a higher SMR than those who were urban residents (2.47 vs 2.03, respectively). The causes of death among PWE were cerebrovascular disease (18.9%, SMR 4.50), malignant neoplasms outside the CNS (15.7%, SMR 1.37), malignant neoplasms of the CNS (6.7%, SMR 46.95), pneumonia (6.0%, SMR 2.08), and external causes (7.2%, SMR 2.17), including suicide (2.6%, SMR 2.07). Epilepsy itself and status epilepticus accounted for 1.9% of the overall death. The excess mortality associated with pneumonia and external causes was persistently high, whereas the excess mortality associated with malignancy and cerebrovascular diseases tended to decrease with increasing time since diagnosis.
Discussion
This study showed excess mortality in PWE, even in those without comorbidities and those receiving monotherapy. Regional disparities and sustained risks of deaths from external causes over 10 years imply potential points of intervention. In addition to active control of seizures, education about injury prevention, monitoring for suicidal ideation, and efforts to improve accessibility to epilepsy care are all required to reduce mortality.
People with epilepsy (PWE) have a 2- to 3-fold higher risk of premature death than the general population.1,2 According to a recent systematic review, the mortality of PWE has changed little since the 1950s.3 Premature mortality in PWE is related to the etiology of epilepsy, comorbidities and direct/indirect effects of seizures, and antiseizure therapies. External causes such as accidents and suicide are important preventable causes of death in PWE,4,5 and excess mortality from external causes differs, particularly between low-middle–income and high-income countries.6 The accurate estimation of epilepsy-related mortality and identification of preventable causes of death are important in reducing mortality among PWE. In Asia, mortality among PWE in population-based studies has been reported in only India7,8 and China9,10 a decade ago, mostly in resource-limited settings.11
In Korea, public attitudes toward epilepsy are negative compared with those in Western societies, and the social stigmatization of epilepsy persists,12,13 although the advanced technologies required for the diagnosis and treatment of epilepsy have been available since the early 2000s. Korea had the highest general population suicide rate among 34 Organization for Economic Cooperation and Development countries from the late 1990s to 2017.14 In addition, the Korean population has the fastest rate of aging in the world,15 and the number of elderly epilepsy patients is also rapidly increasing.16 Therefore, we expect that a unique trend of epilepsy-associated mortality might be observed in Korean PWE.
In this study, we aimed to investigate the mortality rate and causes of death among PWE in Korea using the nationwide healthcare database linked to the national death register. We estimated all-cause mortality and stratified the results by demographic and clinical factors, economic status, and residential area. Furthermore, we assessed cause-specific mortality in PWE.
Methods
Design and Data Sources
We conducted a nationwide population-based retrospective cohort study using the National Health Insurance Service (NHIS) database in Korea from January 2006 to December 2017 and vital statistics from Statistics Korea from January 2008 to December 2017.
Korea provides healthcare coverage to all citizens with mandatory social health insurance and medical aid. The NHIS database has comprehensive data on healthcare utilization, covering the entire population and all medical facilities, including inpatient and outpatient visits; laboratory examinations; prescription records; related diagnoses coded according to the International Classification of Diseases, Tenth Revision (ICD-10); demographic variables; and income-based insurance contributions.17
Mortality and causes of death statistics were acquired from Statistics Korea, a central organization for statistics under the Ministry of Economy and Finance. Causes of death were classified into 236 categories according to ICD-10 codes, as recommended by the World Health Organization (WHO), based on the underlying cause of death described in the death certificate issued by physicians and complemented by 22 types of administrative data, including data from the NHIS, cancer registry of the National Cancer Center, criminal investigation records and transport accident investigations from the National Police Agency, autopsy records from the National Forensic Service, and emergency records from the National Emergency Medical Center.18 Statistics Korea provides the mortality rate of the mid-year Korean population for each age, sex, residential region, and year of death stratum. This national death register was linked with the NHIS database of PWE using anonymized identification numbers.
This study was approved by the institutional review boards of Sungkyunkwan University (approval number: 2018-06-006) and the NHIS for Bioethics Policy (NHIS-2018-1-342). The requirement for informed consent was waived because of the deidentification of the data.
Study Participants and Stratification Variables
We included PWE who were newly treated between January 2008 and December 2016. PWE were identified by both diagnostic codes for epilepsy/seizure and an antiseizure medication (ASM) prescription as recommended by the International League Against Epilepsy.19–21 The ICD-10 diagnostic codes for epilepsy/seizure were G40 (epilepsy), G41 (status epilepticus), F803 (Landau-Kleffner syndrome), and R56 (convulsion), excluding R56.0 (febrile convulsion). ASMs included carbamazepine, clobazam, ethosuximide, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, perampanel, phenobarbital, phenytoin, pregabalin, primidone, stiripentol, topiramate, vigabatrin, valproate, zonisamide, lacosamide, and rufinamide.
The operational definition of PWE was individuals who had at least 2 documented visits with epilepsy/seizure-related codes and who were prescribed ASMs for more than 180 days. One or more ASM prescriptions under a diagnostic code for epilepsy/seizure (G40, G41, F803, or R56) showed a positive predictive value of 81.0% for epilepsy in a previous study using the Korean NHIS database.20 To improve diagnostic validity excluding acute symptomatic seizures and prophylactic usage, we added criteria for the number of medical encounters and duration of treatment in this study.
Newly treated PWE were defined as individuals who had neither epilepsy-related codes nor an ASM prescription within the 2 years before diagnosis. Newly treated PWE were followed from the day when the inclusion criteria were met (180 days after the first visit) to December 31, 2017, or the date of death to avoid immortal time bias.
We assessed mortality depending on the number of ASMs at censoring, the occurrence of status epilepticus, and the number of hospitalizations during follow-up and comorbidities at baseline. Status epilepticus was ascertained by hospitalization or emergency department visits under the diagnostic code G41. Comorbidities were classified using ICD-10 codes from the year before the epilepsy case definition was met, and the Charlson Comorbidity Index (CCI)22 was used to quantify patients’ comorbidities (eTable 1, links.lww.com/WNL/C697).
Mortality was stratified according to economic status and residential area at censoring. Economic status was classified according to the type of health security service (medical aid vs national health insurance) and income level. Medical aid provides support for approximately 3%23 of the Korean population who are unable to pay the health insurance premium due to low income and reduced work ability or the persons who are devoted to the nation, while national health insurance provides coverage to the remaining people. NHIS premiums are charged according to the monthly household income, which enabled us to divide the national health insurance group into 5 subgroups (the fifth quintile for the highest income). Among people with disability, 18% benefited from medical aid in 2016.24 Residential areas were classified as capital, noncapital urban, and noncapital rural areas. The capital area included Seoul, Incheon, and Gyeonggi Provinces, which are mostly urban areas. The noncapital urban areas included the Busan, Daegu, Gwangju, Daejeon, and Ulsan metropolitan cities and the Sejong special self-governing city. The noncapital rural areas included Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju Provinces.
Outcome Assessment and Statistical Analysis
Data on dates and causes of death were retrieved for all individuals who died between 2008 and 2017. Causes of death were categorized as epilepsy, status epilepticus, malignant neoplasm, cerebrovascular disease, pneumonia, ischemic heart disease, diabetes mellitus, external causes, including suicide, transportation accidents, falls, accidental drowning, and other causes (eTable 1, links.lww.com/WNL/C697).
The crude mortality rate (CMR), which is the number of deaths divided by person-years at risk, was calculated for internal comparison. Excess mortality was estimated by standardized mortality ratios (SMRs) and compared with that of the general population. All-cause and cause-specific SMRs were calculated by dividing the number of observed deaths in the epilepsy cohort by the expected number of deaths, which were calculated by applying the mortality rate of the mid-year Korean population for each age, sex, and year of death stratum to the number of patient-years at risk. SMR of PWE depending on demographic and clinical characteristics was calculated by applying the mortality rate of the overall general population because detailed mortality data depending on each stratum of the general population were not available. We additionally conducted internal standardization, calculating the SMR of PWE in each residential area with the mortality data of the regional population. The CIs of the SMRs assumed that the observed number of deaths followed a Poisson distribution. All statistical analyses were performed using SAS Enterprise Guide 7.1 for Windows (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
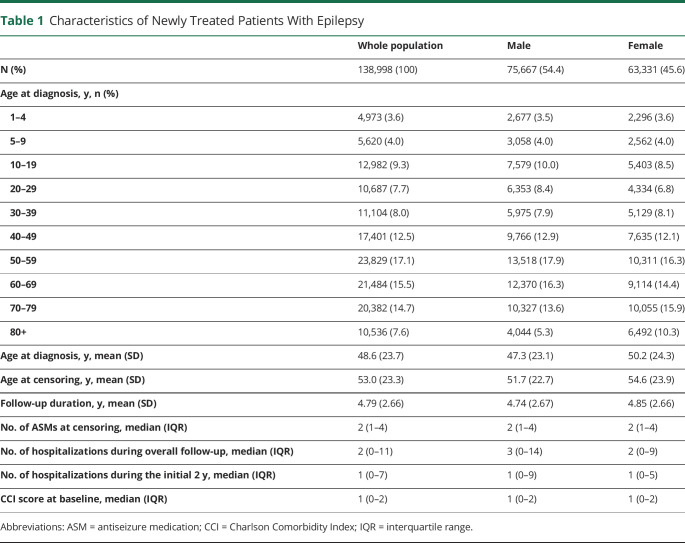
The baseline characteristics of the study population are presented in Table 1. Overall, 138,998 newly treated PWE (54.4% male) were identified during the study period. Their mean age at diagnosis was 48.6 years, and the mean follow-up duration was 4.79 years. The median number of ASMs prescribed at the last follow-up was 2 (interquartile range 1–4): 1 ASM in 35.2% of the sample, 2–3 ASMs in 51.8%, and 4 or more ASMs in 13.0%. A total of 2.2% of the patients had status epilepticus during the follow-up period.
Table 1.
Characteristics of Newly Treated Patients With Epilepsy
All-Cause Mortality
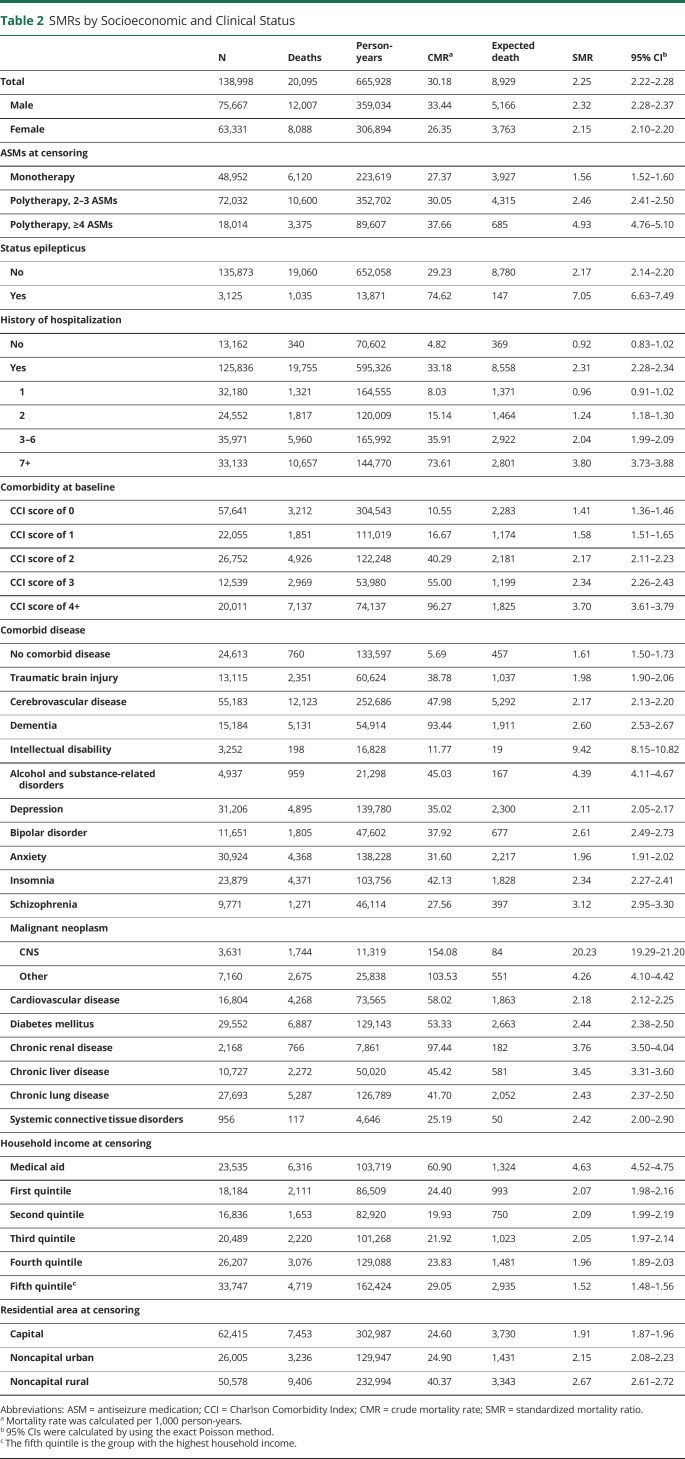
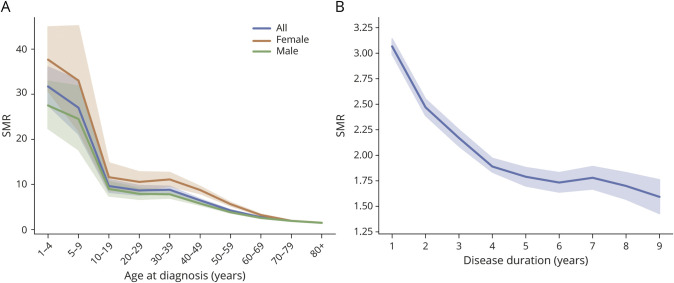
Over 665,928 person-years of follow-up, 20,095 PWE died, with a CMR of 30.18 per 1,000 person-years; the CMRs were 33.44 and 26.35 per 1,000 person-years in men and women, respectively (Table 2). The SMR was 2.25 (95% CI 2.22–2.28) overall, 2.32 (95% CI 2.28–2.37) in men, and 2.15 (95% CI 2.10–2.20) in women. The age-specific SMR was higher in women than in men across all age groups. The SMR was the highest in children aged 4 years and younger, at 31.57 (95% CI 27.55–36.02); it then decreased dramatically to 9.58 (95% CI 8.24–11.08) in teens and decreased gradually with age, reaching 1.48 (95% CI 1.44–1.52) in those aged 80 years or older (Figure 1A and eTable 2, links.lww.com/WNL/C697).
Table 2.
SMRs by Socioeconomic and Clinical Status
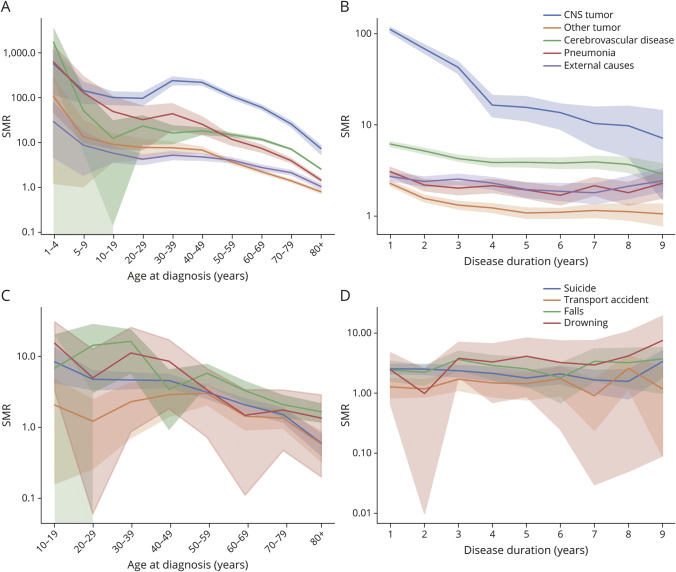
Figure 1. SMRs by Age at Diagnosis (A) and Disease Duration (B).
SMR = standardized mortality ratio.
Regarding disease duration, the SMR was highest in the first year after diagnosis, at 3.07 (95% CI 2.99–3.14), with a decreasing tendency until the fourth year (1.89, 95% CI 1.81–1.97); it was relatively stable thereafter (Figure 1B and eTable 3, links.lww.com/WNL/C697).
The SMR, depending on the number of ASMs, was 1.56 (95% CI 1.53–1.60) in the monotherapy group, 2.46 (2.41–2.50) in the group with 2–3 ASMs, and 4.93 (4.76–5.10) in the group with 4 or more ASMs. The SMR in the group with status epilepticus was 7.05 (95% CI 6.63–7.49). The mortality rate of PWE without a history of hospitalization (SMR 0.92, 95% CI 0.83–1.02) or with 1 hospitalization (SMR 0.96, 95% CI 0.91–1.02) during the follow-up period did not differ from that of the general population. However, in those with 2 or more hospitalizations, the SMR increased in proportion to the number of hospitalizations (Table 2).
PWE with malignant CNS neoplasms (SMR 20.23, 95% CI 19.29–21.20) and intellectual disability (SMR 9.42, 95% CI 8.15–10.82) had particularly high SMRs. Psychiatric comorbidities, such as alcohol/substance-related disorders (SMR 4.39, 95% CI 4.11–4.67), schizophrenia (SMR 3.12, 95% CI 2.95–3.30), and bipolar disorder (SMR 2.61, 95% CI 2.49–2.73), had relatively high SMRs. PWE without CNS illnesses or major systemic comorbidities also had a higher mortality rate than the general population, with an SMR of 1.61 (95% CI 1.50–1.72). PWE with the higher CCI score had the higher SMR, but PWE with a CCI score = 0 also had excess mortality (SMR 1.41, 95% CI 1.36–1.46) (Table 2).
According to economic status, the SMR was the highest in the medical aid group (4.63, 95% CI 4.52–4.75) and lowest in the group with a household income in the fifth quintile (the highest income) (1.52, 95% CI 1.48–1.56). However, there was no significant difference among the first to fourth quintile groups. The SMR was higher in PWE in the following order: those living in noncapital rural areas (2.67, 95% CI 2.61–2.72), those living in noncapital urban areas (2.15, 95% CI 2.08–2.23), and those living in capital areas (1.91, 95% CI 1.87–1.96) (Table 2). When the SMR of PWE was assessed in comparison with the general population living in the same areas, the SMR in the noncapital rural area group was still higher (2.47, 95% CI 2.41–2.53) than that in the noncapital urban area (2.03, 95% CI 1.98–2.09) and capital area groups (2.00, 95% CI 1.92–2.09).
Causes of Death and Cause-Specific Mortality Rates
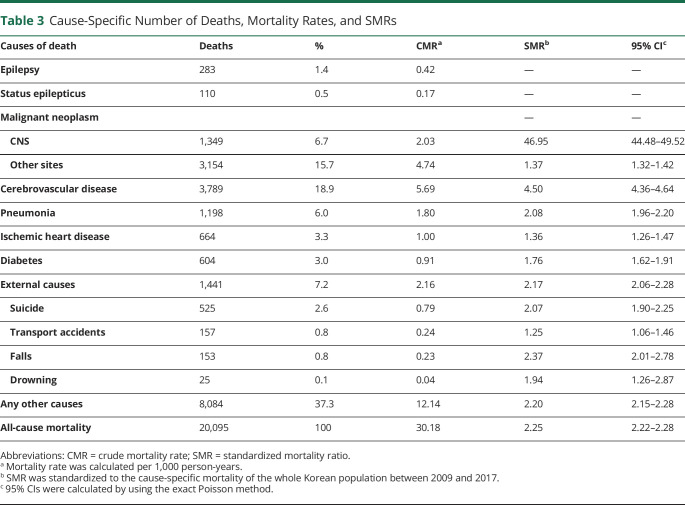
The causes of death are presented in Table 3. The top 5 causes of death were as follows: cerebrovascular disease (18.9%), malignant tumors at sites other than the CNS (15.7%), external causes (7.2%), malignant CNS neoplasms (6.7%), and pneumonia (6.0%). Among the external causes, suicide was the most common cause of death (525 patients, 2.6%). There were 157 (0.8%) deaths due to transport accidents, 153 (0.8%) due to falls, and 25 (0.1%) due to drowning. Epilepsy and status epilepticus accounted for 1.9% of all deaths (eFigure 1, links.lww.com/WNL/C696).
Table 3.
Cause-Specific Number of Deaths, Mortality Rates, and SMRs
The SMRs for malignant CNS neoplasms (46.95, 95% CI 44.48–49.52), cerebrovascular disease (4.50, 95% CI 4.36–4.64), pneumonia (2.08, 95% CI 1.96–2.0) and external causes (2.17, 95% CI 2.06–2.28) were higher than 2. Among the external causes, the risk of death due to falls (2.37, 95% CI 2.01–2.78) and suicide (2.07, 95% CI 1.90–2.25) was elevated by 2 or more times. Mortality rates associated with drowning, transport accidents, diabetes, ischemic heart disease, and malignant neoplasms of sites other than the CNS were also higher in PWE than in the general population (Table 3).
The cause-specific SMRs for the top 5 causes tended to decrease with age after 30 years. The SMR for suicide was highest in young people and then gradually decreased. The SMRs for transport accidents were highest for individuals aged 40–50 years (Figure 2, A and C, eTables 4 and 5, links.lww.com/WNL/C697).
Figure 2. Cause-Specific SMRs by Age at Diagnosis and Disease Duration.
Cause-specific SMRs by age at diagnosis (A) and disease duration (B) of 5 common causes of death. The SMRs of 4 common external causes of death by age at diagnosis (C) and disease duration (D). Due to the small number of individuals younger than 10 years, we deliberately omitted the number in Figure 2C to prevent breaches of personal information. SMR = standardized mortality ratio.
The SMRs for tumors and cerebrovascular disease tended to decrease with time, while the SMR for pneumonia was relatively static during follow-up. The SMRs for external causes, including transport accidents, falls and drowning, showed increasing tendencies over time. The SMR for suicide showed a tendency to decrease up to the eighth year, followed by an increase in the ninth year (Figure 2, B and D, eTables 6 and 7, links.lww.com/WNL/C697).
Discussion
This nationwide population-based 10-year cohort study of newly treated PWE showed a 2.3-fold higher risk of premature death in PWE than in the general population. Excess mortality was observed even in the groups without comorbidities and with monotherapy but not in the groups who had been admitted to the hospital only once or never. The risk of mortality due to external causes was 2 times higher in PWE than that in the general population and accounted for 7% of the overall deaths. The most common cause of unnatural death was suicide, and the greatest excess risk of suicide was observed in young PWE.
The all-cause SMR among PWE in urban (2.0) and rural (2.5) Korea was consistent with the results of previous population-based studies representing incidence cohorts including people of all ages (weighted median SMR of 2.3 in developed countries and 2.6 in developing countries).25,26 In Asia, the SMRs were reported to range from 2.6 in urban India7 to 4.9 in rural China,10 which were all assessed from field surveys. Longer follow-up of the same rural Chinese cohort resulted in a drop in the SMR from 3.9 to 2.9 due to increased acquisition of outcome information.9,27 The SMR of rural residents in this study is close to that of the previous studies conducted in India and China, although they are not exactly comparable because those studies included prevalent cases, while this study included only newly treated cases. In addition, previous Chinese studies targeted people with convulsive epilepsy who were not taking standard antiseizure treatment before enrollment and were treated with phenobarbital by the WHO project, while this study included participants who had any type of epilepsy and received various ASMs. Mortality in patients with newly treated epilepsy was assessed in Hong Kong using the hospitalization database, showing an SMR of 5.09.28 The higher SMR in the Hong Kong study might be because of the inclusion of only individuals who required hospitalization and had a shorter follow-up duration.
Excess mortality was the highest in the medical aid group and the lowest in the highest income (fifth quintile) group, without a significant difference among the first to fourth quintile income groups. Medical costs associated with epilepsy are fully covered by medical aid funds for medical aid beneficiaries but are partly paid out of pocket by national health insurance subscribers. Therefore, the high SMR in medical aid recipients could be attributable to disease severity, disability, or unmet care needs that are not covered by medical aid.
Higher mortality of the general population in rural areas and its association with relatively fewer medical resources have been reported in Korea.29 This study demonstrated even higher excess mortality in PWE than in the general population in rural areas. Regional disparities in the mortality of PWE suggest that accessibility to specialty care, which affects adherence and proper management, is still insufficient in Korea, which has a universal health security system.
The SMR was higher in those with a younger age at diagnosis, consistent with previous studies that showed that the mortality risk tended to be higher in children (median SMR 7.5, range 3.1–22.4) than in adults (median SMR 2.6, range 1.3–8.7).3,25,26 This may be due to the higher incidence of a congenital symptomatic etiology in children and relatively lower mortality rates in the general population at younger ages.
We showed a gradual decrease in the SMR over time after diagnosis, in line with previous population-based studies.30–32 This high mortality risk shortly after diagnosis can be explained in part by underlying etiologies with poor prognoses, such as malignant neoplasms and cerebrovascular disease, and by the time required for seizure control after diagnosis.
Mortality depending on disease severity was assessed in a few studies,33,34 and no excess mortality was shown in seizure-free patients.35,36 In this study, PWE who were hospitalized once or never had no significant excess mortality, but PWE who received monotherapy and who did not have status epilepticus had excess mortality.
Excess mortality from epilepsy itself, excluding the effect of underlying causes or comorbidities, has rarely been estimated. This can be extrapolated from the SMR of PWE without comorbid disease in this study (1.61), which was close to the SMR of people with idiopathic or cryptogenic epilepsy in previous studies showing modest SMR increases.26,34
The most frequent causes of death were malignant neoplasm and cerebrovascular disease, which are mostly presumed to be underlying etiologies of epilepsy.
Mortality directly due to epilepsy accounted for a smaller portion of deaths in this study than in previous studies (seizure or status epilepticus, drowning, proportionated mortality 2% vs 12.1%–14.8%).3 The excess risk of mortality caused by transport accidents (SMR or hazard ratio [HR] 1.3–6.0) and falls (SMR or HR 8.5–9.8), which are presumed to be associated with seizures, was also lower than that reported in previous studies.3 This could be attributed to adequate seizure control but could also be explained by the relatively high proportion of elderly individuals with comorbidities in Korea compared with other study populations.
Despite the SMR for suicide (2.07) being in the low range of previous studies (3.3, 95% CI 2.8–3.7 in meta-analysis), the CMR (0.79) was in the high range (0.48 in meta-analysis),37 close to that in China (0.99)27 and Sweden (0.81),38 due to the high suicide rate in the general population in Korea. In this study, the SMR seemed to decrease over time but increased again in the ninth year. The high suicide risk in the early period may be due to inadequate seizure control and sudden changes in daily living activities, such as job loss. A possible explanation for the re-elevation of the suicide rate could be depression from long-standing epilepsy. Further studies are needed to confirm these trends in the suicide mortality rate and to elucidate the cause of suicide during long-term follow-up.
This is a population-based study on mortality associated with epilepsy in an Asian country with a universal healthcare system in which standard treatment for epilepsy, including most ASMs, is available. The major strength of this study is that we examined the entire unselected population of a country using the healthcare database. This ensured greater generalizability and enabled a stratified risk assessment of death according to age, disease course, disease severity, comorbidities, economic status, and residential area as well as cause-specific risk. Long-term follow-up and accurate identification of outcomes were efficiently performed by reconstructing the epilepsy cohort using NHIS data linked with the death register. This method will be useful for the surveillance of mortality in PWE. To our knowledge, this is the only study that has assessed the cause-specific mortality risk among PWE according to age and disease course. Understanding cause-specific mortality risk, particularly the risk of external causes, is important because they are mostly preventable. These results could be helpful in guiding whether to limit daily activities such as driving and timely and targeted interventions to prevent mortality. Another strength is that we included only newly treated patients, which allowed the complete observation of the clinical phase of the disease from the diagnosis of epilepsy to death.21,39
The major limitation of this study is that we identified epilepsy cases based on diagnostic codes registered for administrative purposes. We prioritized the high-positive predictive value of the operational definition of epilepsy for the study of mortality. However, by restricting the participants to those who were treated with ASMs for 180 days or more, we could not assess deaths that occurred during the first 180 days after the initiation of treatment. In addition, the 2-year disease-free period before epilepsy diagnosis used in this study could be insufficient to identify newly developed cases. Third, the comparison group for the analyses depending on comorbidities and income level was the overall general population, which was not stratified. Therefore, the excess mortality of PWE includes the risk from each comorbidity or the reason for which these individuals could benefit from medical aid, such as severe disability, and from epilepsy itself.
This study demonstrated a wide range of mortality risks in PWE depending on age at diagnosis, disease duration, disease severity, and comorbidities. We identified regional disparities within Korea, and this result urges a public health approach to improve accessibility to epilepsy care. The high rate of suicide and increasing rate of deaths caused by accidents over time are also notable findings and are potentially avoidable. To reduce mortality in PWE, comprehensive efforts including a national policy against stigma of epilepsy and clinicians' total management, such as risk stratification, education about injury prevention, and monitoring for suicidal ideation with psychological intervention, as well as active control of seizures, are needed.
Glossary
- ASM
antiseizure medication
- CCI
Charlson Comorbidity Index
- CMR
crude mortality rate
- HR
hazard ratio
- ICD-10
International Classification of Diseases, Tenth Revision
- NHIS
National Health Insurance Service
- PWE
people with epilepsy
- SMR
standardized mortality ratio
- WHO
World Health Organization
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
This work was supported by the Soonchunhyang University Research Fund and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI19C0481 and HC19C0180).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(2):185-191. doi: 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Epilepsy: A Public Health Imperative. WHO; 2019. [Google Scholar]
- 3.Mbizvo GK, Bennett K, Simpson CR, Duncan SE, Chin RFM. Epilepsy-related and other causes of mortality in people with epilepsy: a systematic review of systematic reviews. Epilepsy Res. 2019;157:106192. doi: 10.1016/j.eplepsyres.2019.106192. [DOI] [PubMed] [Google Scholar]
- 4.Mahler B, Carlsson S, Andersson T, Tomson T. Risk for injuries and accidents in epilepsy: a prospective population-based cohort study. Neurology. 2018;90(9):e779-e789. doi: 10.1212/wnl.0000000000005035. [DOI] [PubMed] [Google Scholar]
- 5.Gorton HC, Webb RT, Carr MJ, DelPozo-Banos M, John A, Ashcroft DM. Risk of unnatural mortality in people with epilepsy. JAMA Neurol. 2018;75(8):929-938. doi: 10.1001/jamaneurol.2018.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watila MM, Balarabe SA, Ojo O, Keezer MR, Sander JW. Overall and cause-specific premature mortality in epilepsy: a systematic review. Epilepsy Behav. 2018;87:213-225. doi: 10.1016/j.yebeh.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee TK, Ray BK, Das SK, et al. A longitudinal study of epilepsy in Kolkata, India. Epilepsia. 2010;51(12):2384-2391. doi: 10.1111/j.1528-1167.2010.02740.x. [DOI] [PubMed] [Google Scholar]
- 8.Carpio A, Bharucha NE, Jallon P, et al. Mortality of epilepsy in developing countries. Epilepsia. 2005;46(suppl 11):28-32. doi: 10.1111/j.1528-1167.2005.00404.x. [DOI] [PubMed] [Google Scholar]
- 9.Ding D, Wang W, Wu J, et al. Premature mortality risk in people with convulsive epilepsy: long follow-up of a cohort in rural China. Epilepsia. 2013;54(3):512-517. doi: 10.1111/epi.12048. [DOI] [PubMed] [Google Scholar]
- 10.Mu J, Liu L, Zhang Q, et al. Causes of death among people with convulsive epilepsy in rural West China. A prospective study. Neurology. 2011;77(2):132-137. doi: 10.1212/wnl.0b013e318223c784. [DOI] [PubMed] [Google Scholar]
- 11.Trinka E, Kwan P, Lee B, Dash A. Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia. 2019;60(suppl 1):7-21. doi: 10.1111/epi.14458. [DOI] [PubMed] [Google Scholar]
- 12.Lee SA, Yoo HJ, Lee BI. Factors contributing to the stigma of epilepsy. Seizure. 2005;14(3):157-163. doi: 10.1016/j.seizure.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JK, Jung KY, Park KW, et al. Familiarity with, understanding of, and attitudes toward epilepsy among people with epilepsy and healthy controls in South Korea. Epilepsy Behav. 2009;16(2):260-267. doi: 10.1016/j.yebeh.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Organization for Economic Cooperation and Development. Education at a Glance 2019. OECD; 2019. [Google Scholar]
- 15.Yang S, Khang Y-H, Harper S, Davey Smith G, Leon DA, Lynch J. Understanding the rapid increase in life expectancy in South Korea. Am J Public Health. 2010;100(5):896-903. doi: 10.2105/ajph.2009.160341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon JY, Lee H, Shin JY, Moon HJ, Lee SY, Kim JM. Increasing trends in the incidence and prevalence of epilepsy in Korea. J Clin Neurol. 2021;17(3):393-399. doi: 10.3988/jcn.2021.17.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheol Seong S, Kim YY, Khang YH, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799-800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin HY, Lee JY, Kim JE, et al. Cause-of-death statistics in 2016 in the Republic of Korea. J Korean Med Assoc. 2018;61(9):573-584. doi: 10.5124/jkma.2018.61.9.573. [DOI] [Google Scholar]
- 19.Mbizvo GK, Bennett KH, Schnier C, Simpson CR, Duncan SE, Chin RF. The accuracy of using administrative healthcare data to identify epilepsy cases: a systematic review of validation studies. Epilepsia. 2020;61(7):1319-1335. doi: 10.1111/epi.16547. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Chung SE, Kim DW, et al. Estimating the prevalence of treated epilepsy using administrative health data and its validity: ESSENCE study. J Clin Neurol. 2016;12(4):434-440. doi: 10.3988/jcn.2016.12.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(suppl 7):2-26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 22.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288-1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.KOSIS. Status of receiving medical aids [online]. Accessed July 21, 2021. https://www.index.go.kr/unity/potal/main/EachDtlPageDetail.do?idx_cd=1406
- 24.Hong MJ, Lee C, Lee C, et al. Are high medical costs incurred by people with disabilities excessive?: an empirical analysis of Korean National Health Insurance Data. PLoS One. 2022;17(1):e0262653. doi: 10.1371/journal.pone.0262653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levira F, Thurman DJ, Sander JW, et al. Premature mortality of epilepsy in low- and middle-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58(1):6-16. doi: 10.1111/epi.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurman DJ, Logroscino G, Beghi E, et al. The burden of premature mortality of epilepsy in high-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58(1):17-26. doi: 10.1111/epi.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding D, Wang W, Wu J, et al. Premature mortality in people with epilepsy in rural China: a prospective study. Lancet Neurol. 2006;5(10):823-827. doi: 10.1016/s1474-4422(06)70528-2. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Liew D, Kwan P. Excess mortality and hospitalized morbidity in newly treated epilepsy patients. Neurology. 2016;87(7):718-725. doi: 10.1212/wnl.0000000000002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang I, Kim BHS. Regional disparity of medical resources and its effect on age-standardized mortality rates in Korea. Ann Reg Sci. 2019;62(2):305-325. doi: 10.1007/s00168-019-00897-z. [DOI] [Google Scholar]
- 30.Ngugi AK, Bottomley C, Fegan G, et al. Premature mortality in active convulsive epilepsy in rural Kenya: causes and associated factors. Neurology. 2014;82(7):582-589. doi: 10.1212/wnl.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiboriboon K, Schiltz NK, Bakaki PM, Lhatoo SD, Koroukian SM. Premature mortality in poor health and low income adults with epilepsy. Epilepsia. 2014;55(11):1781-1788. doi: 10.1111/epi.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lhatoo SD, Johnson AL, Goodridge DM, MacDonald BK, Sander JWAS, Shorvon SD. Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol. 2001;49(3):336-344. doi: 10.1002/ana.70. [DOI] [PubMed] [Google Scholar]
- 33.Mohanraj R, Norrie J, Stephen LJ, Kelly K, Hitiris N, Brodie MJ. Mortality in adults with newly diagnosed and chronic epilepsy: a retrospective comparative study. Lancet Neurol. 2006;5(6):481-487. doi: 10.1016/s1474-4422(06)70448-3. [DOI] [PubMed] [Google Scholar]
- 34.Nevalainen O, Ansakorpi H, Simola M, et al. Epilepsy-related clinical characteristics and mortality: a systematic review and meta-analysis. Neurology. 2014;83(21):1968-1977. doi: 10.1212/wnl.0000000000001005. [DOI] [PubMed] [Google Scholar]
- 35.Trinka E, Bauer G, Oberaigner W, Ndayisaba JP, Seppi K, Granbichler CA. Cause-specific mortality among patients with epilepsy: results from a 30-year cohort study. Epilepsia. 2013;54(3):495-501. doi: 10.1111/epi.12014. [DOI] [PubMed] [Google Scholar]
- 36.Sillanpää M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363(26):2522-2529. doi: 10.1056/nejmoa0911610. [DOI] [PubMed] [Google Scholar]
- 37.Bell GS, Gaitatzis A, Bell CL, Johnson AL, Sander JW. Suicide in people with epilepsy: how great is the risk? Epilepsia. 2009;50(8):1933-1942. doi: 10.1111/j.1528-1167.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- 38.Fazel S, Wolf A, Långström N, Newton CR, Lichtenstein P. Premature mortality in epilepsy and the role of psychiatric comorbidity: a total population study. Lancet. 2013;382(9905):1646-1654. doi: 10.1016/s0140-6736(13)60899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34(8):592-596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]