Abstract
Introduction:
Pre-procedural fasting (nil per os, NPO) is a commonly implemented protocol to prevent aspiration during certain diagnostic and therapeutic procedures. However, evidence suggests aspiration risk is quite low. Current guidelines support a reduced fasting duration before procedures necessitating anesthesia or sedation, but many health systems persist in the use of NPO past midnight.
Methods:
An interprofessional team at our academic health system initiated a quality improvement project to decrease use of NPO past midnight before inpatient diagnostic and therapeutic procedures. Diagnostic imaging protocols previously requiring NPO were amended to reflect evidence based fasting requirements. A pre-procedure clear liquid diet was also implemented. Implementation steps, feasibility (process measures), and safety (balancing measures) are reported.
Results:
NPO requirements were removed from 70% of existing diagnostic imaging and therapeutic orders. After these amended protocols and implementation of a pre-procedure clear liquid diet, we displayed an immediate 50% reduction in NPO past midnight usage. Further stakeholder engagement/education and interventions reduced NPO past midnight usage to only 33% of peri-procedural diet orders. Surgery remains the most common indication for continued use of NPO. Aspiration events and procedural delays were rare.
Conclusion:
Through implementation of a pre-procedural clear liquid diet and amending diagnostic imaging protocols, we significantly and sustainably reduced use of NPO before inpatient diagnostic and therapeutic procedures. Risk of aspiration and procedural delay were indeed rare suggesting protocols to reduce the inpatient fasting duration can be implemented safely.
Keywords: fasting, peri-procedural, quality improvement
Introduction:
Pre-procedural fasting (nil per os, NPO) is common before diagnostic and therapeutic procedures to reduce aspiration risk. Patients frequently remain nil per os (NPO) for durations exceeding 16 hours, resulting in adverse effects such as hypotension during anesthesia, postoperative delirium, pain and anxiety, and extended lengths of stay2. This is at least partially due to physiologic changes caused by prolonged fasting including induction of a catabolic state and enhanced insulin resistance1. Conversely, the prevalence of peri-procedural aspiration is low, occurring in less than 0.02% of adults3 and less than 0.1% of children4.
Current research suggests shorter fasting times may be preferred. Historically, it was thought that prolonged NPO would lead to lower gastric volumes, higher gastric pH, and lower risks for aspiration during anesthesia. More recent research suggests that increasing gastric volume (such as during po intake of liquids) stimulates gastric mucosal stretch receptors, leading to faster emptying and lower residual volume5. Using gastric ultrasound, Cho et al demonstrated similar gastric volumes between patients fasting since midnight and patients free to consume a carbohydrate drink up to two hours before anesthesia6. In a systematic review assessing aspiration risk by duration of NPO before colonoscopy, shorter fasting duration did not lead to increased aspiration risk7.
American Society of Anesthesiologists (ASA) guidelines8 allow clear liquid ingestion up to two hours before most elective procedures requiring general anesthesia, regional anesthesia, or procedural sedation and analgesia. Yet many health systems maintain NPO past midnight (NPO p MN) orders, especially for inpatient procedures. In fact, inpatient NPO orders are often implemented for procedures not necessitating prolonged fasting durations9. Black et al encouraged liberalization of fluid intake up to two hours before anesthesia even for inpatients with difficult care coordination10. In this report we describe a quality improvement project at a single academic health system seeking to reduce fasting duration before inpatient diagnostic and therapeutic procedures. We aimed to reduce the use of NPO p MN for hospitalized children and adults by 50% within six months of implementation.
Methods:
Setting/Study Population
The University of Texas Medical Branch (UTMB) is an academic health system in Southeast Texas encompassing five hospitals and over 1000 inpatient beds. This includes 1 main academic hospital, 3 community satellite hospitals, and 1 hospital for incarcerated individuals. Interventions bridged across all campuses and all ages. As prisoners are a protected vulnerable population, data from the correctional care hospital is excluded from the analysis. The UTMB institutional review board approved this work through an expedited review. Standards for Quality Improvement Reporting Excellence 2.0 were used in constructing this report11.
Change Management Strategy
Adapting the institutional peri-procedural diet protocol was viewed as a radical shift necessitating a significant culture change. As such, we sought to adopt Kurt Lewin’s Change Management Model of unfreezing, transition and freezing12. Specifically, we recognized that implementation would require many frontline change agents and would likely be met with a period of confusion and uncertainty. We further adopted Kotter’s 8 steps for leading change to guide our implementation strategy12. The basic interventions and timeline to implementation are displayed in Figure 1.
Figure 1:
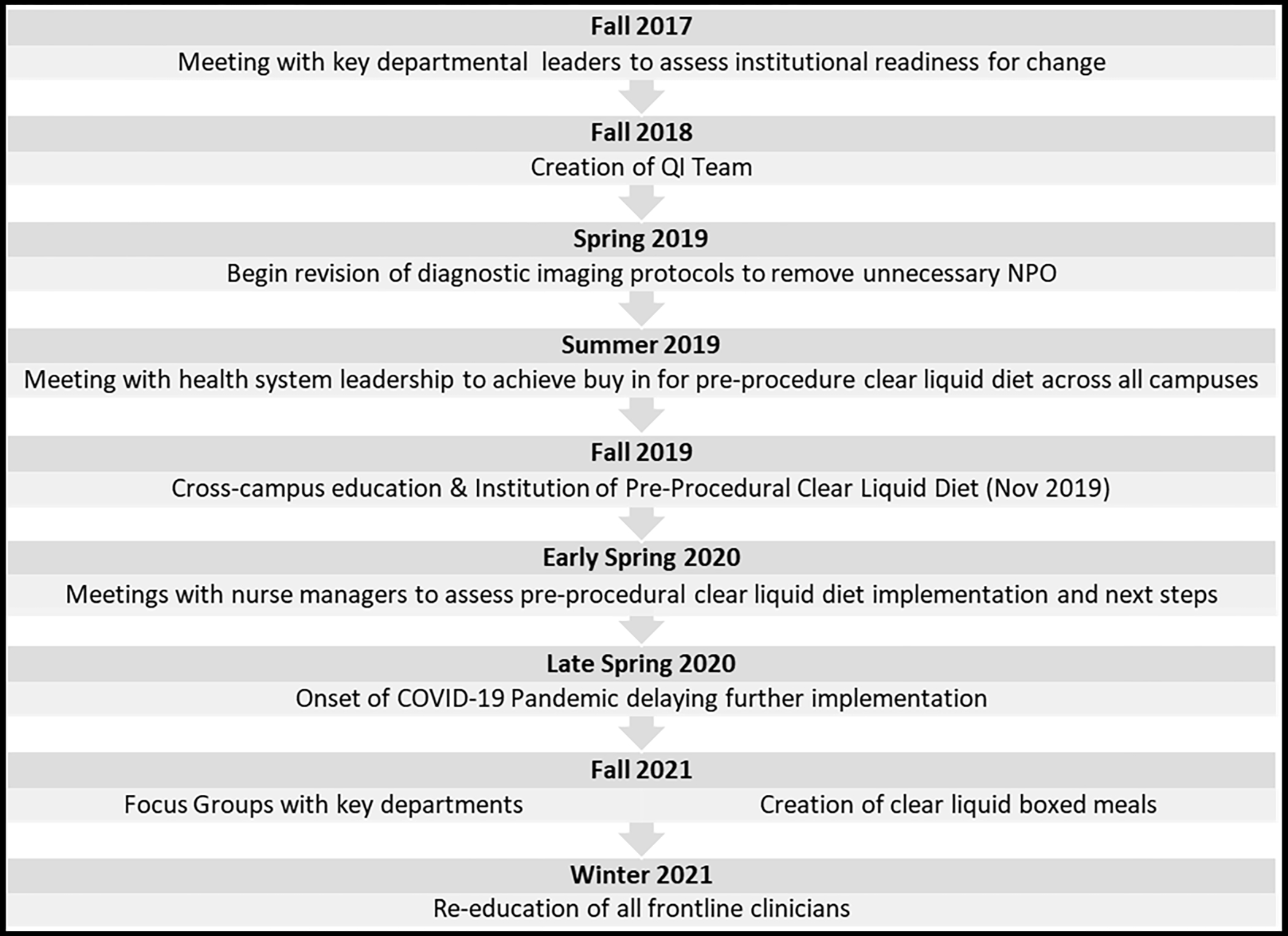
Timeline and Steps to Implementation
Interventions
The first step towards project implementation was meeting with key departmental chairpersons to assess buy in and establish a sense of urgency. After receiving unanimous support to move forward, an interprofessional quality improvement team was created consisting of nurses, physicians, dietitians, clinical data management specialists, and electronic medical record (EMR) builders. Each team member was strategically chosen to represent the diagnostic and therapeutic procedural areas across the health system. Specifically, we sought representation for the operating suites, diagnostic and interventional radiology, cardiovascular services, the endoscopy suites, and the bronchoscopy suites.
The team rapidly identified a barrier in our diagnostic imaging protocols. Several diagnostic studies required NPO despite a lack of evidence to necessitate fasting. A sub-group of general internal medicine and radiology physicians convened to review the existing diagnostic imaging protocols (e.g., computed tomography, ultrasound, magnetic resonance imaging, nuclear medicine, fluoroscopy). These subject matter experts revised all written protocols and directed necessary EMR updates. Frontline staff were educated about the updated protocols via the EMR, e-mails, institutional newsletters, departmental postings, and face-to-face in-services.
Following this, the team convened with health system leadership to secure institutional support for a pre-procedural clear liquid diet. Though ASA guidelines allow intake of solids within 6–8 hours of procedural sedation, we focused on pre-procedure clear liquids to minimize risk of procedural delays and aspiration. We feared that the unpredictable scheduling of inpatient procedures would limit interpretation of longer fasting windows for solid substances.
A pre-procedure clear liquid diet was built into the existing EMR to replace NPO p MN in all existing procedural order sets. If an NPO p MN order was written independently, the entering provider was prompted to convert to a clear liquid diet but could continue with NPO p MN if the patient’s condition required. Our orders did not affect other NPO orders (e.g., for dysphagia or active vomiting). We educated nurses to convert patients to strict NPO two hours before their procedures. This was implemented at the bedside; we did not enter a separate NPO order at the onset of this two-hour window. For procedures with unpredictable start times (i.e., add-ons), we instructed nurses to initiate NPO at 5am for expected am starts or 10am for expected pm starts. Frontline staff were educated about the new diet orders via the EMR, e-mails, institutional newsletters, departmental postings, and face-to-face in-services.
To monitor potential adverse events, frontline staff were instructed to report peri-procedural aspiration events or procedural delays through the health system’s online patient safety monitoring program. We also reviewed cases of aspiration as reported through the anesthesia department’s internal event reporting system.
After the initial implementation, we held focus groups with key stakeholders to assess process barriers and safety concerns. These stakeholders included the physicians from departments of anesthesiology, general and orthopedic surgery, and obstetrics/gynecology, as well as nurse managers from key inpatient units and procedural areas.
Stakeholder feedback guided us to implement additional important interventions. Some staff voiced a lack of clarity as to the purpose of the diet changes. Thus, all staff were re-educated about the importance of reducing NPO duration for highest quality patient care. Anesthesiologists voiced uncertainty as to the safety of some clear liquid diet components so they worked with dietitians to re-affirm each approved item. Nursing staff reported inconsistent access to clear liquid diet floor stock. In response, kitchen staff created clear liquid “boxed meals” with an educational sticker outlining approved clear liquid diet contents. This also reinforced the allowable timing of intake before procedures. Boxes were distributed to nursing units which permitted use after hours. Finally, all staff raised concerns for the unpredictable nature of inpatient procedures and the inconsistent communication about timing from procedural areas. We assimilated contact information for all procedural areas and dispersed this to inpatient nursing units to ease communication about procedural start times.
Timeline
We began revising the diagnostic imaging protocols in spring 2019. This was complete by fall 2019. We instituted the pre-procedure clear liquid diet also in fall 2019 (November). For baseline comparison, we used the 12-month period from November 2018 to October 2019. The post-intervention reporting period includes November 2019 to April 2022.
Analysis
Our analysis is limited to process measures only. First, we report the number of diagnostic imaging protocols which underwent review and were eligible for revision. We provide the percent of protocols for which removal of NPO was approved. Second, we report the percent of inpatient peri-procedural diet orders which used NPO p MN. For this, we monitored total counts of inpatient orders for NPO p MN plus the pre-procedure clear liquid diet. We assumed that these diet types were ordered in preparation for diagnostic and therapeutic procedures necessitating anesthesia or sedation. We defined persistent use of NPO p MN as the nonconforming unit and constructed a p chart to reflect process variation over time. Finally, to understand where there was persistent use of NPO p MN, we constructed a pareto chart of the indication for NPO p MN orders between February 2022 and April 2022. As a balancing measure, we assessed the safety of our intervention by reporting raw counts of aspiration events and procedural delays throughout the post-implementation period.
Since the length of the fasting window is a critical contributor to patient outcomes, we desired to define the time patients were held NPO. This is typically measured as the elapsed time between pre-procedure and post-procedure diet order entry. However, for the pre-procedure clear liquid diet protocol, nurses enforce the strict 2-hour NPO window at the patient’s bedside without a new EMR diet order. This prohibited us from distinguishing the precise duration of NPO between the two diet types. Instead, we provide best estimates using the elapsed time between the pre-procedure and post-procedure diet orders and the institutional protocol instructions.
Results:
Figure 2 displays the diagnostic imaging protocols amended by imaging type. We identified 164 total diagnostic imaging protocols. Of those, 47 protocols had existing NPO requirements. After subject matter expert review, this was reduced to 14 protocols (a 70% reduction).
Figure 2:
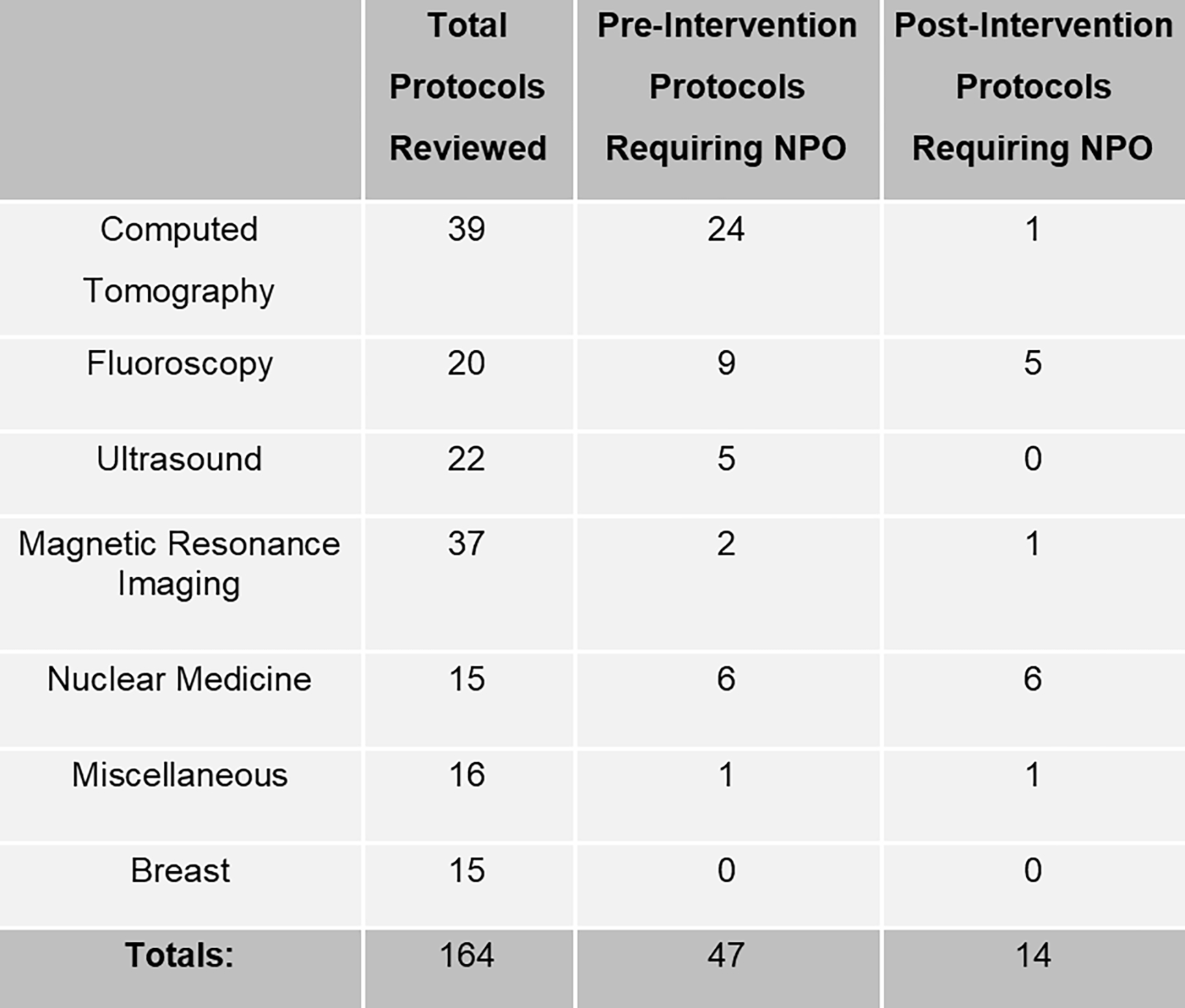
Diagnostic Imaging Protocols Amended
Changes in NPO p MN use throughout the duration of the project are displayed in Figure 3. During the baseline period (November 2018 through October 2019), there was 100% utilization of the NPO p MN diet. Immediately upon implementation of the pre-procedure clear liquid diet (November 2019), NPO p MN use declined to approximately 50% of peri-procedural diet orders. This improvement persisted until December 2021. The second cycle of interventions were implemented fully by winter 2021. After this, NPO p MN use declined to approximately 33% of peri-procedural diet orders. Results from January 2022 through April 2022 all fell below the lower control limit defined following implementation of the pre-procedure clear liquid diet.
Figure 3:
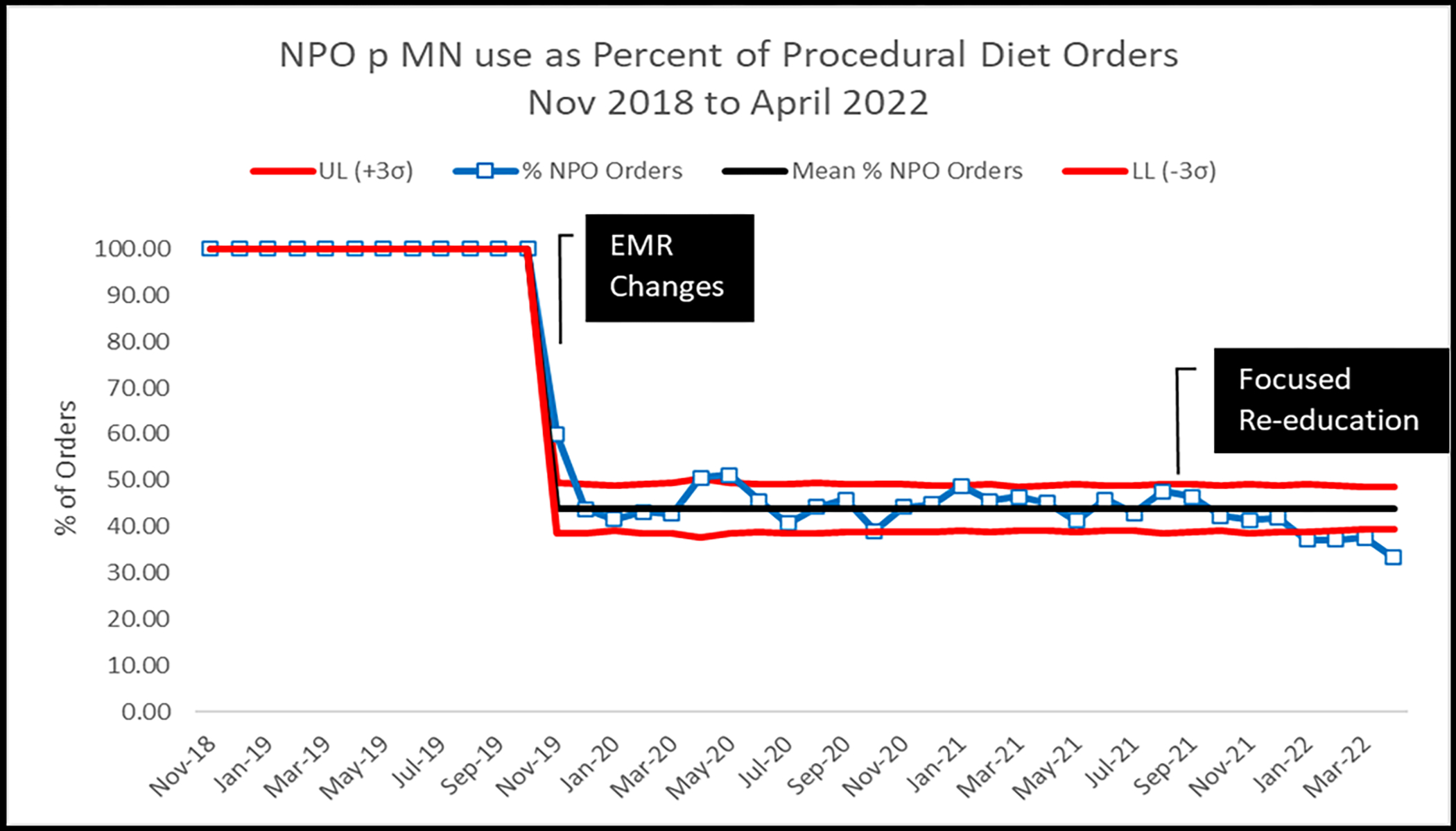
NPO p MN use as Percent of Procedural Diet Orders, Nov 2018 to April 2022
We examined indications for continued use of the NPO p MN order between February 2022 and April 2022. In total, 1085 NPO p MN orders were written (Figure 4). Surgery was the most common indication, comprising 53.5% of NPO p MN orders. Cardiovascular procedures (e.g., cardiac catheterizations, electrophysiology procedures, transesophageal echocardiograms) comprised an additional 15.3%. Gastrointestinal endoscopic procedures (e.g., upper endoscopy, colonoscopy, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound) comprised 12.4%. Together these three indications exceeded 80% of persisting NPO p MN diet orders.
Figure 4:
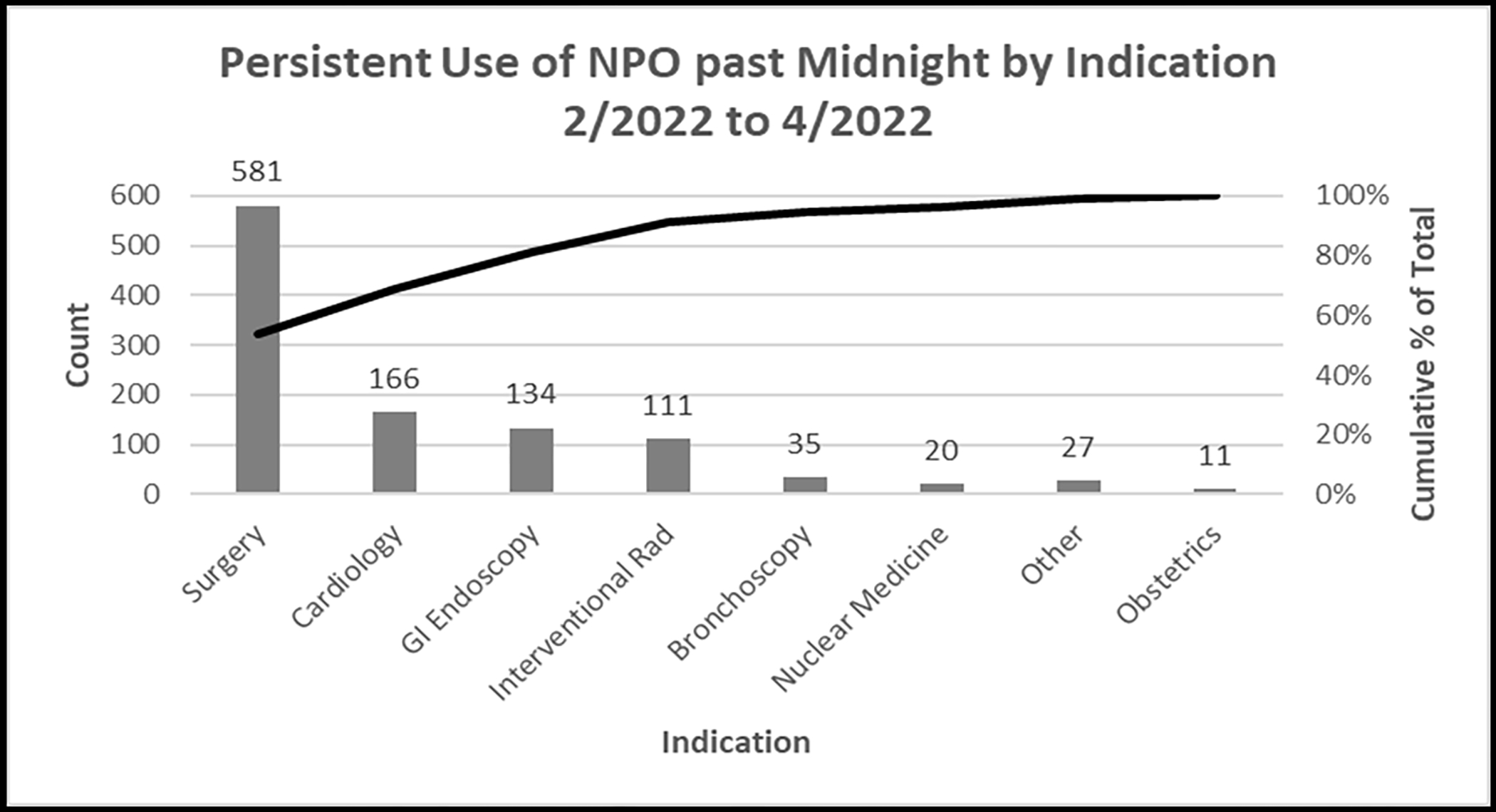
Indication for Persistent NPO p MN Diet Use, February to April 2022
Health system patient safety event reports identified four delayed procedures related to peri-procedural diet. Two patients received full trays despite active NPO p MN orders. One patient with a clear liquid diet had an insufficient bowel prep necessitating procedural delay. Another patient with a clear liquid diet received am meds with pudding for dysphagia concerns. No reports of aspiration were identified in the health system patient safety event reports. Query of the anesthesia internal event reporting system identified four cases of aspiration throughout the post-implementation period. A total of 25,435 peri-procedural diet orders were placed, suggesting an aspiration event rate of only 0.0001 (1 per 10,000 cases).
The duration of the fasting window, defined as elapsed time between pre-procedure and post-procedure diet orders, exceeded 18 hours during the pre-implementation period. This declined to 16 hours for NPO p MN orders during the post-implementation period. While we are not able to extract precise estimates of the duration of the fasting window for the pre-procedure clear liquid diet, we saw similar durations of approximately 16 hours between the clear liquid diet onset at midnight and the post-procedural diet order entry. Conservatively, we assume liquid diet consumption was allowed until at least 5 am for all patients, reducing the duration of NPO to at least 11 hours and likely considerably shorter given many patients were permitted intake hours later into the day.
Discussion:
After removing unnecessary NPO from diagnostic imaging protocols and implementing a pre-procedural clear liquid diet, our institution achieved and sustained over 50% reduction in inpatient pre-procedural NPO p MN orders. This required strong institutional support, a carefully constructed inter-professional team, and persistent re-education of frontline staff. To our knowledge, this is one of the first reports of a quality improvement project implementing an alternative peri-procedural diet protocol across an inpatient setting.
During the planning phase, health system and key departmental leaders provided unanimous support for this initiative. However, subsequent focus groups with frontline staff uncovered barriers to adoption. Many physicians cited lack of knowledge of the new protocols or a belief that the protocols didn’t apply to their patients. We also discovered mistrust of the inpatient scheduling system, difficulty predicting the time for add-on procedures, and difficult cross-departmental communications when schedules changed. Panjier et al reported similar barriers amongst anesthesiologists surveyed in 201613. Both large scale health system and targeted departmental educational campaigns are necessary to establish full buy-in. For procedures with uncertain start times, we instructed nurses to initiate NPO at 5am for expected am starts or 10am for expected pm starts. Clinicians had increased confidence the protocols would not result in procedural delays when these cut points were clearly defined.
Nurses cited different concerns including uncertainty of the approved clear liquid diet contents, limited clear liquid diet components on inpatient units, and poor patient compliance with diet parameters. To overcome this, we developed a “box meal” with approved clear liquid diet contents which included an educational sticker reinforcing the pre-procedural diet allowable items and appropriate timing of intake. This innovation not only permitted education of nurses and patients but provided floor stock to allow access to clear liquid diet components after hours. Reports of similar innovations are rare in the literature. However, Malone et al report an initiative to provide boxed meals for geriatric patients in the emergency department14. Several of their tips apply here, including engagement of nursing in all nutrition focused quality improvement programs, establishing a strategy for access to food after hours, and standardizing protocols across all hospitals within a health system.
For this initiative, we applied a very conservative peri-procedural diet protocol where only clear liquids are permitted on the day of anesthesia or sedation. Most international anesthesia guidelines (including the American Society for Anesthesiologists, the European Society of Anesthesiology and Intensive Care, and the Canadian Anesthesiologists Society) permit intake of non-clear liquids up to 4 hours before anesthesia and the intake of solids up to 6–8 hours before anesthesia8, 15, 16. We were concerned implementing a more liberal diet protocol within the inpatient setting would lead to a greater risk of delayed procedures or aspiration. Depending on the practice setting, solids or non-clear liquids prior to procedures may be permissible.
Additionally, recent literature supports clear liquid diets up to 1 hour before procedures in children17. The European Society of Anesthesiology and Intensive Care and the Canadian Anesthesiologists Society have adapted their guidelines in accordance, though the ASA has not18. Quality improvement initiatives targeting reduced fasting time in the pediatric population are more rapidly gaining ground19, 20.
Use of a clear liquid diet up to 2 hours before testing and treatment requiring anesthesia may not be appropriate for all patients21. Patients with known delays in gastric emptying may require longer duration of NPO to ensure passage of food and liquid. In other cases, procedural guidelines may recommend longer fasting windows, e.g., American Society of Echocardiography guidelines recommend patients undergoing transesophageal echocardiogram be NPO for three hours before procedure22. We maintain an option for NPO p MN for these circumstances. It is possible that these concerns contributed to the residual NPO p MN use we saw in departments such as surgery and cardiology. Adapting a risk adjusted approach to peri-procedural diet orders, as was proposed by the International Committee for the Advancement of Procedural Sedation in 2020, may assist clinicians in striking the right balance of risk and benefit for each patient23. To help us further understand persistent use of NPO p MN, clinicians will be required in the future to enter the patient or procedural circumstances necessitating an NPO p MN order.
Limitations
Our study has several important limitations. We report on a single US academic center’s experience implementing a clear liquid diet before inpatient diagnostic and therapeutic procedures. Results may not be easily generalizable to community health systems, specialty health centers, or non-US practice sites.
Our metrics are also limited to process and balancing measures. Future studies should aim to report on pertinent outcome measures such as peri-procedural complications and the patient experience. Throughout our implementation period, reports of aspiration and procedural delay were quite rare. We relied on clinician reporting of patient safety events through health system and departmental event reporting systems. Under-reporting through these systems is a well-known phenomenon but may be overcome through enhanced teamwork and through providing feedback on reported events24.
A final important limitation of our report is the inability to precisely calculate the difference in fasting duration between diet orders. This would have required patient tracking which was infeasible with the available resources. Future studies should aim to better estimate NPO time saved and peri-procedural nutrient intake to better assess the impact of a more liberal peri-procedural diet on nutritional balance and patient outcomes.
Conclusion
The UTMB community has grown more receptive to reduced fasting duration since implementing the updated diagnostic imaging protocols and pre-procedural clear liquid diet. We have reduced NPO p MN orders to about one-third of remaining inpatient peri-procedural diets with minimal associated risks. Despite demonstrated benefits of reduced fasting duration, many clinicians remain unaware of the option of a reduced fast, with some preferring to keep to outdated practices. Continuous education is necessary, particularly at an academic institution with student and resident trainees. Reinforcing this as a quality-of-care priority requires the engagement of organizational leaders. Auditing and feedback must be maintained. Next steps include investigation into NPO hours saved and peri-procedural nutrient consumption as well as outcome measures such as peri-procedural complications and impact on the patient experience.
Acknowledgements:
We acknowledge the assistance of Amber McIlwain with manuscript preparation.
Footnotes
Conflicts of Interest: None of the authors have any relevant conflicts of interest to disclose.
Financial Disclosures: Dr. Erin Hommel is supported by the Claude D. Pepper Older Americans Independence Center Award [#P30-AG024832], which is funded by the National Institute on Aging (NIA), part of the United States Department of Health and Human Services.
References:
- 1.Friedrich S, Meybohm P, Kranke P. Nulla Per Os (NPO) guidelines: time to revisit? Curr Opin Anaesthesiol. Dec 2020;33(6):740–745. [DOI] [PubMed] [Google Scholar]
- 2.Dorrance M, Copp M. Perioperative fasting: A review. J Perioper Pract. Jul 2020;30(7–8):204–209. [DOI] [PubMed] [Google Scholar]
- 3.Green SM, Mason KP, Krauss BS. Pulmonary aspiration during procedural sedation: a comprehensive systematic review. Br J Anaesth. Mar 1 2017;118(3):344–354. [DOI] [PubMed] [Google Scholar]
- 4.Habre W, Disma N, Virag K, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. May 2017;5(5):412–425. [DOI] [PubMed] [Google Scholar]
- 5.Leiper JB. Fate of ingested fluids: factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev. Sep 2015;73 Suppl 2:57–72. [DOI] [PubMed] [Google Scholar]
- 6.Cho EA, Huh J, Lee SH, et al. Gastric Ultrasound Assessing Gastric Emptying of Preoperative Carbohydrate Drinks: A Randomized Controlled Noninferiority Study. Anesth Analg. Sep 1 2021;133(3):690–697. [DOI] [PubMed] [Google Scholar]
- 7.Shaukat A, Malhotra A, Greer N, MacDonald R, Wels J, Wilt TJ. Systematic Review: Outcomes by Duration of NPO Status prior to Colonoscopy. Gastroenterol Res Pract. 2017;2017:3914942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. Mar 2017;126(3):376–393. [DOI] [PubMed] [Google Scholar]
- 9.Sorita A, Thongprayoon C, Ratelle JT, et al. Characteristics and Outcomes of Fasting Orders Among Medical Inpatients. J Hosp Med. Jan 2017;12(1):36–39. [DOI] [PubMed] [Google Scholar]
- 10.Black MK, Lupa MC, Lemley LW, Dreesen EB, Deaton AM, Wardrop RM 3rd. Things We Do for No Reason: NPO After Midnight. J Hosp Med. Jun 2021;16(6):368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. Dec 2016;25(12):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrow JM, Annamaraju P, Toney-Butler TJ. Change Management. StatPearls. Treasure Island (FL) 2022. [PubMed] [Google Scholar]
- 13.Panjiar P, Kochhar A, Vajifdar H, Bhat K. A prospective survey on knowledge, attitude and current practices of pre-operative fasting amongst anaesthesiologists: A nationwide survey. Indian J Anaesth. May 2019;63(5):350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone M PA, Gallo A, Simonis S. Clinical aspects of providing a meal for an older patient in the emergency department. Journal of Geriatric Emergency Medicine 2020;2(1):1–3. [Google Scholar]
- 15.Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. Aug 2011;28(8):556–569. [DOI] [PubMed] [Google Scholar]
- 16.Dobson G, Chow L, Filteau L, et al. Guidelines to the Practice of Anesthesia - Revised Edition 2020. Can J Anaesth. Jan 2020;67(1):64–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson H, Schmitz A, Frykholm P. Preoperative fasting guidelines in pediatric anesthesia: are we ready for a change? Curr Opin Anaesthesiol. Jun 2018;31(3):342–348. [DOI] [PubMed] [Google Scholar]
- 18.Frykholm P, Disma N, Andersson H, et al. Pre-operative fasting in children: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol. Jan 1 2022;39(1):4–25. [DOI] [PubMed] [Google Scholar]
- 19.Isserman R, Elliott E, Subramanyam R, et al. Quality improvement project to reduce pediatric clear liquid fasting times prior to anesthesia. Paediatr Anaesth. Jul 2019;29(7):698–704. [DOI] [PubMed] [Google Scholar]
- 20.Newton RJG, Stuart GM, Willdridge DJ, Thomas M. Using quality improvement methods to reduce clear fluid fasting times in children on a preoperative ward. Paediatr Anaesth. Aug 2017;27(8):793–800. [DOI] [PubMed] [Google Scholar]
- 21.Presta MV, Bhavani SS, Abdelmalak BB. Nil per os guidelines: what is changing, what is not, and what should? Minerva Anestesiol. Dec 2018;84(12):1413–1419. [DOI] [PubMed] [Google Scholar]
- 22.Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. Sep 2013;26(9):921–964. [DOI] [PubMed] [Google Scholar]
- 23.Green SM, Leroy PL, Roback MG, et al. An international multidisciplinary consensus statement on fasting before procedural sedation in adults and children. Anaesthesia. Mar 2020;75(3):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burlison JD, Quillivan RR, Kath LM, et al. A Multilevel Analysis of U.S. Hospital Patient Safety Culture Relationships With Perceptions of Voluntary Event Reporting. J Patient Saf. Sep 2020;16(3):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]


