Abstract
In this paper, we review and analyze the commonly available wound healing models reported in the literature and discuss their advantages and issues, considering their relevance and translational potential to humans. Our analysis includes different in vitro and in silico as well as in vivo models and experimental techniques. We further explore the new technologies in the study of wound healing to provide an all encompassing review of the most efficient ways to proceed with wound healing experiments. We revealed that there is not one model of wound healing that is superior and can give translatable results to human research. Rather, there are many different models that have specific uses for studying certain processes or stages of wound healing. Our analysis suggests that when performing an experiment to assess stages of wound healing or different therapies to enhance healing, one must consider not only the species that will be used but also the type of model and how this can best replicate the physiology or pathophysiology in humans.
Keywords: wound healing, chronic wounds, animal wound model, pathophysiology, in vivo wound model, in vitro wound model
Wound healing is a complex natural process that occurs as a result of disintegration or damage to skin tissue or organ structures. It is dynamic, involving a variety of cells that must act in a coordinated fashion. Although many wounds heal naturally, some do not heal without intervention resulting in chronic or nonhealing wounds. The development of chronic or nonhealing wounds can result if there is dysfunction in the wound healing process due to prolonged disease states, decreased tissue perfusion, or unregulated inflammatory reactions.
Research of the pathophysiology behind wound healing mechanisms has been an ever-evolving study in medicine as it is necessary to discover more ideal methods to treat chronic wounds that often result in poor outcomes in patient quality of life and even mortality. Wound care has led to excessive costs within healthcare and unnecessary procedures. As of now, the most common approach to chronic wound treatment is regular wound care including cleansing and covering using different wound dressings. Some wounds may benefit from negative pressure wound therapy (NPWT), also referred to as vacuu-assisted wound closure therapy. The device entails placement of an open cell foam or gauze dressing that is sealed with an occlusive drape and connected to a pump that provides negative pressure evenly across the wound bed. NPWT reduces edema and promotes growth of granulation tissue through a combination of macro and micro-deformation. In a similar mechanism, hyperbaric oxygen tanks are used to increase oxygen delivery to the patient’s wound for more optimal healing conditions.1 Despite interventions, many wounds remain nonhealed and can lead to a sequela of complications that may result in amputation. Therefore, studies have targeted more successful ways to regenerate tissue and vascularization in diseased states.
Many efforts have been made to study and model the physiology of the wound healing process using different animals (e.g., rats, rabbits, and pigs) combined with the use of a variety of techniques. Currently, there is no ideal model available that effectively recapitulates the physiological or pathological conditions in humans.
This paper will provide a comprehensive review of the different species and models used to study wound healing. The focus will be on translatability to humans by examining the pros and cons of the specific uses of each model.
Wound Healing Pathophysiology
Chronic wounds are due to prolonged or incomplete healing processes and can arise from a variety of disease states including diabetes mellitus, vascular insufficiency, and inflammatory disorders.2 In the presence of these conditions, an injury to the skin can cause a prolonged inflammatory response with subsequent chronic, nonhealing wound development. Although inflammation is necessary for wound healing, prolonged or excessive states of inflammation can lead to an imbalance of reactive oxygen species (ROS) so that their production exceeds the rate at which they are broken down. The resulting tissue damage may perpetuate wound chronicity or result in exaggerated scar tissue.3
A persistent inflammatory response or prolonged proliferation phase may result in the formation of robust scar tissue.4 Hypertrophic scars are seen when there is injury to the deep dermis followed by excessive collagen deposition. A hypertrophic scar appears as an elevated, red, and tense defective tissue often leading to cosmetic dissatisfaction as well as functional abnormality.5 Histological analysis of such scars shows a flatted dermal−epidermal junction (DEJ) and scar tissue throughout the dermis. The scars are also void of epidermal appendages such as hair follicles, sebaceous glands, and apocrine and eccrine glands sweat glands.6 Growth factor expression suggests that there is an increased expression of TGF-beta in hypertrophic skin tissue.7
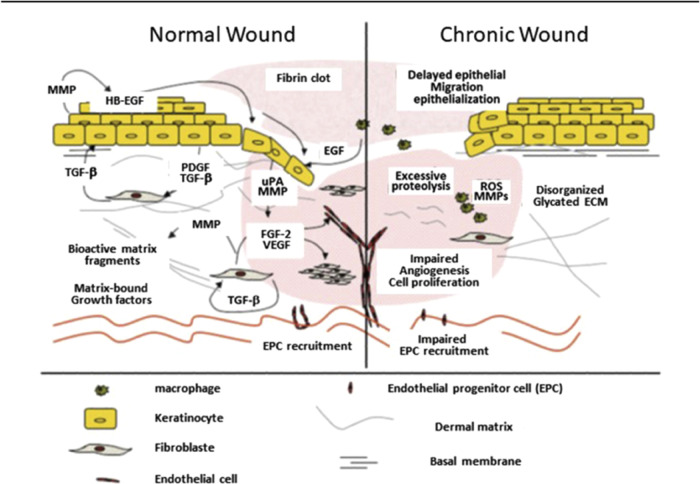
In contrast, failure to re-epithelialize is another factor in the development of chronic, nonhealing wounds. To obtain a new, functional epidermis post injury, two things must happen: the dermal–epidermal junction must reestablish itself to anchor the epidermis to the dermis, and the keratinocytes must terminally differentiate to provide the protective layer of anucleate keratinized squamous cells.8 The major differences between the normal and chronic wound healing processes are summarized in Figure 1.
Figure 1.
Normal wound healing vs. chronic wound healing. In the chronic wound, there is a delayed keratinocyte migration causing delayed re-epithelization, excessive protein cleavage, and reactive oxygen species production resulting in tissue damage. There is also impaired EPC recruitment leading to poor blood vessel formation as well as cell proliferation for the regenerative phase. Reprinted in part with permission from ref (9). Copyright 2012 Wolters Kluwer Health, Inc.
Human Skin Architecture
Although the phases of wound healing across species have many similarities, there are important differences including the rates of wound healing, scar tissue formation, and the types of cells recruited to the sites of injury.10 Many of these differences are related to the distinct architecture of skin and how the underlying structures facilitate the healing processes.
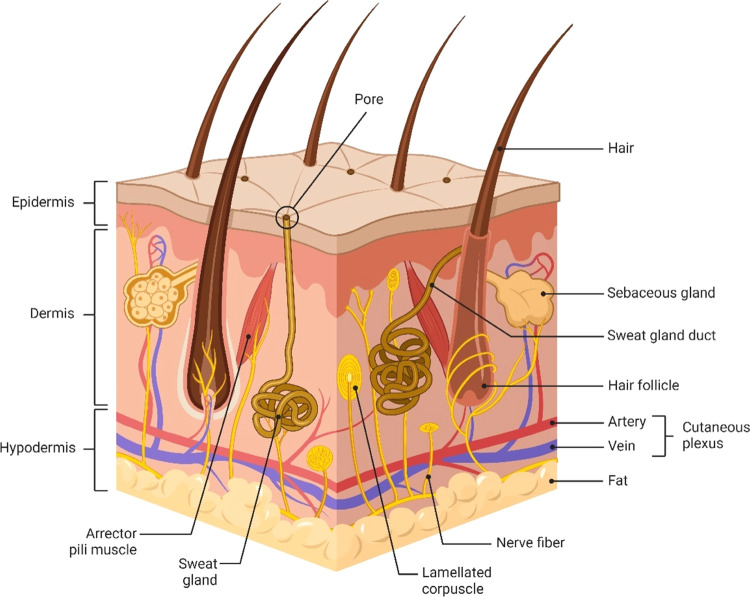
On the outmost surface of human skin is the epidermis which is divided into five layers (Figure 2). From the base of the epidermis to the most external portion, the layers are as follows: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. Details of each layer are:
-
1.
The stratum basale contains the epidermal stem cells that mitotically divide and terminally differentiate into keratinocytes as they migrate upward, a process known as keratinization. This differentiation is important for the production of keratin.11,12
-
2.
The stratum spinosum contains keratinocytes with cytoplasmic processes that look like spines and work to attach adjacent cells by desmosomes. This layer also contains Langerhan’s cells which phagocytose foreign material and act as antigen-presenting cells to activate an immune response.12,13
-
3.
The stratum granulosum is composed of keratinocytes with keratohyalin granules. Keratohyalin granules contain keratin precursors which are released into the cytoplasm to encourage the aggregation of keratin tonofilaments into tonofibrils. The granules also release lipids and other enzymes that create a lipophilic barrier between the stratum granulosum and stratum corneum.11 This serves to prevent fluid loss from the body.12
-
4.
The stratum lucidum is found only in thick skin such as the palm of the hand or the sole of the foot. This translucent layer consists of eleidin which is a derivative of keratohyalin.12
-
5.
Lastly, the stratum corneum, the most external layer contains terminally differentiated keratinocytes without any organelles; known as anucleate keratinized squamous cells. These cells are important for their role in secreting defensins to protect against pathogen entry.14 In this layer, cholesterol sulfate is also lost so that the keratinized epithelium can slough off as dead tissue.12
Figure 2.
Anatomical landmarks of the skin including the different layers, the appendages, and the capillary loops. BioRender is used for drawing this scheme.
Damage to the epidermis will stimulate the proliferation of stem cells in the stratum basale.15,16 These stem cells will then migrate upward and eventually terminally differentiate into anucleate keratinized squamous cells, providing a barrier to pathogen and water loss.17 Failure to re-epithelialize results in loss of the barrier function of the skin which can then lead to dehydration and serious infection.18
Just below the epidermis lies the dermal layer which is sectioned into a superficial and deep layer known as the papillary dermis and the reticular dermis, respectively (Figure 2).19 The papillary dermis is composed mainly of type 3 collagen which is thin with lower tensile strength while the reticular dermis predominantly contains type 1 collagen and is more dense with increased tensile strength.20 Since the stratum basale is an avascular layer,21 the papillary dermis contains many blood vessels to provide nutrients to the epidermis for continued mitotic processes. The reticular dermis also houses cells such as macrophages for stimulating immune responses, and fibroblasts for collagen production.18 In addition, epidermal appendages including sweat glands, hair follicles, and sebaceous glands are located in the reticular dermis.11 These components are protective to the skin and important for sensation and thermoregulatory processes.18
Together, the epidermis and the dermis function as a protective barrier to the inner tissues and organs of the human body. In order for the two layers to remain strongly connected, the DEJ is established.22 The DEJ consists of hemidesmosomes with keratin extensions that anchor the epidermis to the dermis. Another way the DEJ is strengthened is via rete ridges and dermal papillae. Rete ridges are downward epidermal projections into the papillary dermis while dermal papillae are portions of the papillary dermis extending up into the epidermis. The formation of these ridges increases the surface area of the DEJ.8 Each dermal papillae contains at least one capillary loop to provide nutrients and blood to the epidermis. After full-thickness injury, extending beyond the epidermis and dermis, rete ridges fail to reform resulting in flattening of the dermal–epidermal junction.8,22
Below the reticular dermis is the hypodermis (a.k.a subcutaneous fat layer), composed mainly of fat cells.23 This layer separates the skin from the underlying muscle and provides insulation to the body.24,25 In wound healing, the hypodermis is critical for pressure redistribution. For example, those with thin subcutaneous tissue layers who become bedridden are at higher risk of pressure ulcer development because there is no fat pad to redistribute the weight of the body. Conversely, those who are morbidly obese are also at high risk of pressure ulcer development due to the weight of the excess adipose tissue causing increased pressure between the skin and bed.11
Wound Healing Physiology in Humans
The physiology of wound healing is an intricate process with four separate but overlapping phases.11,19,26,27 The first phase is hemostasis which begins immediately after injury and lasts about 1–2 days. The inflammatory phase also begins after injury, peaks 3–5 days post injury, and arrests around day 10. Third, the proliferation or regeneration phase begins at day 1 post injury and completes at days 21–30. The last phase is maturation and remodeling of the tissue which begins simultaneously with the proliferative phase and can last up to 2 years.11,19,26
In the hemostasis phase, vessel constriction allows the activation of platelets via the binding of certain glycoproteins.28 Activated platelets release granules composed of a variety of growth factors, cytokines, hormones, and other cells. One function of the released cells is to induce vasodilation and increase vascular permeability, allowing inflammatory cells to enter the wound site.28−30 Additionally, activated platelets are stimulated to aggregate and form a temporary “platelet plug”.27,31 The clotting cascade concludes with the formation of a more stable “fibrin plug”.27 The final clot releases growth factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) to aid in the proliferation of new cells for reparation of the vascular endothelium and smooth muscle.14,32
Once the bleeding is controlled, the cytokines and growth factors encourage chemotaxis of inflammatory cells such as neutrophils and monocytes. The neutrophils predominate in the initial inflammatory phase to remove any bacteria within the wound via a respiratory burst mechanism. Meanwhile, the monocytes fully differentiate into macrophages which will then phagocytose the dead cells and bacterial particles left over from the respiratory burst.27 Macrophages are also important in releasing additional cytokines and growth factors important for the third phase of the wound healing cycle; proliferation and tissue regeneration. Defective macrophages are often seen in patients with uncontrolled diabetes and thus interfere with the progression of the inflammatory phase to the proliferative and regenerative phase.11,27,33
As the cycle progresses to the proliferative and regenerative phase of wound healing, the fibroblast becomes the dominant cell type. Cytokines and growth factors chemotactically recruit fibroblasts in the dermal layer of uninjured tissue.34 Specifically, PDGF, IL-1B, and TNF-α mediate fibroblast localization to the wound and direct their orientation with correct positioning around the wound.11 Upon activation, fibroblasts synthesize collagen which is the predominant component within the extracellular matrix of the dermis. In addition to collagen synthesis, VEGF from the activated platelets and endothelial progenitor cells (EPCs) from the bone marrow promote angiogenesis so that new blood vessels can become established as part of the regenerative process.34,35 It is suspected that in disease processes such as diabetes, EPC recruitment is hindered because of deficiencies in substances such as nitric oxide, VEGF, matrix metalloproteases (MMPs), and insulin-like growth factor.11,36 Lastly, myofibroblasts have an essential role in this phase by inducing contraction of the wound edges to decrease the surface area of the defect.37
The wound healing process finalizes with the tissue maturation and remodeling phase, mediated by the breakdown of type 3 collagen and replacement with type 1 collagen.38 This change in collagen type enhances the tensile strength of the tissue. It is impossible for the wound to regain 100% normal strength since much of the soft tissue has been replaced with scar tissue. For this reason, the wound is typically noted to have minimal tensile strength at about 21 days post injury because there is still a predominance of type 3 collagen, and 60–80% tensile strength 2–3 months post injury when more type 1 collagen has replaced the type 3 collagen.11 If there is a problem in the breakdown of type 3 collagen or an overproduction of type 1 collagen, a dysfunctional keloid scar can result.39 On the other hand, if there are insufficient amounts of type 1 collagen proliferation secondary to chemotherapy, steroids, or malnutrition, then recurrent wound breakdown can occur.11,40
Wound Healing Physiology in Rodents
Rodents have a much different tissue architecture than humans. They have a panniculus carnosus which is a thin layer of muscle that is only found in the platysma of the neck in humans.41−43 This thin layer of muscle allows for the skin to move independently of the deeper tissues, hence the term “loose skin”.44,45 In contrast, human skin is attached to a much thicker underlying hypodermis or subcutaneous fat layer that lies above the muscle.25 The panniculus carnosus in rodents allows for rapid wound contraction so the edges of the tissue are brought back together for closure, whereas in humans, the wound heals via re-epithelialization by keratinocyte differentiation with a less significant amount of wound contraction.46 This difference can obscure the data collected on wound healing and make certain data poorly translatable to humans. On the other hand, if the rodents are being used to study certain therapeutics in the skin, they can act as sufficient models due to their similar dermal and epidermal layers.
Another limitation to using rodents is seen by the amount of time that the wound healing process takes which is affected by their different structural components. Rodents have remarkably thicker concentrations of epidermal appendages including hair follicles.43,47 The hair follicle cycles between three different phases known as anagen, catagen, and telogen.48 Ansell and colleagues performed a study showing the relationship between the phase of the hair cycle and wound healing progression in mice. The data suggested that wound healing was much more prompt during the anagen or growth phase of the hair cycle. Because of this finding, there must be a component of the hair follicle that assists the process of wound healing which could explain the faster healing processes seen in rodents.49 The thickness of the epidermal layer may also play a role in the speed of wound healing. Mice tend to have a thinner epidermis and as a result have less keratinocyte layers explaining their faster healing rate in comparison to rats.42 Mice can complete the wound healing cycle within 7 days, whereas it takes about 12–14 days in rats and can take up to 2 years in humans.42 Furthermore, gender of the rodents contributes to wound healing properties. Male rodents tend to have a thicker dermis which explains why their skin is about 40% stronger than their female counterparts who have a thicker epidermis and hypodermis.50
There is controversy regarding whether the immune, inflammatory, and genomic systems during wound healing are similar in rodents compared to humans. A study by Seok and colleagues, measured the responses of certain genes, inflammation markers, and immune cells in mice compared to human subjects post trauma, burns, and endotoxemia; the results showed significant differences in all groups, concluding that wound healing studies in rodents are not translatable to humans.51 This information has been cited in many papers. Although, a repeat study done by Takao and colleagues obtained completely opposite results, proving that murine models and human responses are in fact similar. This conclusion was reached by correcting for variables and using the proper statistical analysis between species.52
All in all, rodents are the most used species due to the availability, low cost, easy handling, and maintenance, as well as the extensive knowledge on wound healing from abundant past experiments. Even though they are the most widely used species, we must be careful when translating data to humans. Rodents have quite a different wound healing mechanism than humans primarily due to the difference in skin architecture. Thus, despite the many advantages of using rodents, they should be carefully considered when the goal is to translate wound closure research from rodent animal studies to humans.
Wound Healing Physiology in Pigs
Pigs have been the superior models of wound healing processes due to their anatomical and physiological similarities to humans. Their skin structure allows for partial wound healing by re-epithelialization, although, full-thickness wounds that penetrate the dermis predominantly heal by contraction.44,47 In a similar way, human wounds that breach the dermis also heal with a greater degree of contraction leading to scar tissue formation. The thickness of pig’s epidermis and dermis is similar to humans as well, and they have less epidermal appendages than the other animal models.42 Despite being slightly coarser, the hair follicles of pigs are sparse and extend into the lower portion of the dermis and the upper portion of the hypodermal fat matching the architecture of human hair follicles.53 Additionally, collagen, elastin, immune cells, and growth factors respond in a similar fashion to human tissue.44
Unlike other animal models, the epidermal layer in pigs contains ridges with an alkaline phosphatase cell population in the epithelial cells, similar to rete ridges in human epidermal tissue.47,54 This feature has been used to study the regeneration of the DEJ after full- and partial-thickness wounds.8 A study using Lanyu pigs, showed that full-thickness wounds resulted in scar tissue while partial-thickness wounds allowed for restricted rete ridge and papillary dermis regeneration. In this study, alkaline phosphatase was completely absent upon full-thickness wound healing yet present during partial-thickness wound healing.55 The presence of alkaline phosphatase may have clinical significance in wound healing thus, the anatomically similar ridges in the pig model could provide even more knowledge in future studies particularly explaining the increased wound healing in partial-thickness wounds.
Despite the abundant similarities between pigs and human tissue architecture and physiology, there are limiting differences. Porcine skin contains only apocrine sweat glands, with the presence of eccrine sweat glands restricted to the snout, lips, and carpal organs.53 In contrast, humans have an abundance of eccrine sweat glands all over the body56 and apocrine glands are found mainly in the armpits and groin region, where they open into hair follicles.57 This difference impacts the wound healing model because the eccrine sweat glands appear to be an important source of epidermal stem cells.58 Furthermore, pigs are not the species of choice in many experiments due to their high cost, maintenance, and large housing facilities that are not widely available.
Wound Healing Physiology in Rabbits
Rabbits have been used as models for wound healing, specifically punch biopsies in their ears due to the ability to create multiple biopsies in one ear. The rabbit ear has an epidermis and dermis, but the dermis is attached to a cartilaginous layer below which prevents wound contraction by splinting.44 This rather promotes wound healing by re-epithelialization and granulation tissue formation, similar to humans.42,59 This same idea of splinting to reduce contraction has been employed in the rodent model by placing a tightly adhered ring to the skin around the wound.60,61 However, the rabbit ear having the architecture already present serves as an advantage by decreasing the occurrence of infection from a foreign body implantation.
The cartilaginous properties in the rabbit ear have also been used to study the growth factors and proteoglycan contents upon injury to the cartilage. These studies have been important to learn more about human conditions such as osteoarthritis in which cartilage degeneration occurs with limited restorative properties.62 Another advantage to this model is in the study of hypertrophic scar formation, which tends to occur post injury to the deep dermis. Most animal models including pigs and rodents do not tend to form hypertrophic scars and this is thought to be due to the fibromuscular layer found under the dermis of these species.6 Yet, models to create hypertrophic scars in the ears of rabbits have been established allowing for the study of pathogenesis and therapeutics.63,64
Most importantly, the rabbit ear model is well known for studying the impact of ischemia. This species has significant, large vasculature in their ears, amenable to manipulation by ligating the vessels.65 Moreover, it has been found that the vasculature in the ears of rabbits also tends to form collateral circulation, making the induced ischemia reversible.44 This is notable since humans also have this quality.
Despite their value in these specific examples, the use of rabbits as a wound healing model is restricted due to their limited genetic traceability and species-specific reagents.44
Wound Healing Physiology in Other Animals
Other animal species that have been used for wound healing models include guinea pigs and zebrafish. Guinea pigs are not commonly used, but they are small and inexpensive.47 They have a similar mechanism of wound healing to rodents in which they heal mostly by tissue contraction; limiting data translation to humans.44,66 A major use of Guinea pigs in wound healing has been for the study of collagen synthesis.67 Collagen requires vitamin C as a cofactor for its synthesis.68 Many animal models are able to synthesize their own vitamin C, making it challenging to study collagen synthesis in a vitamin-deficient state. Guinea pigs, like humans, require dietary vitamin C since they are incapable of making endogenous vitamin C.44 This characteristic makes Guinea pigs purposeful for a small subset of studies.
Zebrafish are unique in that they undergo each phase of wound healing in a sequential, nonoverlapping process, whereas in humans and many other species, the phases overlap. The stepwise progression of hemostasis, inflammation, proliferation, and remodeling allows for specific isolation and research on each of these phases.69 For this reason, many studies on the chemical and genetic effects have been carried out in relation to the progression of each phase. Zebrafish heal by re-epithelialization similar to humans, although, they do so extremely rapidly.44 This species has not been used much in studies, therefore is less established than other animal species.
Overall, there is not one specific animal species that provides completely translatable research to human wound healing in any type of model. Each animal species provides different components that make it more advantageous than another for studying the effects of a certain type of wound (Table 1).
Table 1. Similarities and Differences in Wound Healing between Various Animal Species.
| species | advantages | similarities to humans | differences from humans |
|---|---|---|---|
| rodents (rats and mice) | • inexpensive | • epidermal and dermal layers | • “loose skin” with a thin layer of muscle known as the panniculus carnosus underneath |
| • common, therefore many studies have used this species before leading to a wide-ranging knowledge base | • genomic, immune, and inflammatory cells expressed during certain injuries | ||
| • small with easy maintenance and handling | |||
| ○ rats being slightly larger can allow for larger or more numerous wounds. They also have thicker skin which provides benefits in certain studies | • natural wound healing occurs primarily via contraction | ||
| • thick concentrations of epidermal appendages, especially hair follicles | |||
| pigs | • large size for larger and more numerous wounds | • partial-thickness wounds heal by re-epithelialization | • full-thickness wounds heal predominantly by contraction |
| • dermal thickness | • abundant apocrine sweat glands with eccrine sweat glands only at the snout, lips, and carpal organs | ||
| • hair follicle architecture and sparsity | |||
| • collagen, elastin, immune cells, and growth factors respond in similar ways | |||
| • ridges in epidermal layer | • greater risk of infection | ||
| • alkaline phosphatase presence in ridges | |||
| • capillaries in the upper dermis to supply the epidermis | |||
| rabbits | • inexpensive | • ear model heals by re-epithelialization | • large amounts of hair appendages |
| • model to induce a hypertrophic scar formation | |||
| • large vessels in the ear allow for the production of ischemic wounds which form irreversible ischemia via generation of collateral circulation | • tissue aside from ear heals by contraction | ||
| guinea pigs | • small | • require dietary vitamin C | • wound healing by contraction |
| • inexpensive | |||
| zebrafish | • small | • wounds heal by re-epithelialization | • no skin |
| • inexpensive | • rapid re-epithelialization | ||
| • sequential phases of wound healing |
In Vivo Wound Healing in Rodents
Aside from the study of wound healing in healthy tissue, it is possible to create experimental models that are directed at stimulating healing in impaired wound models such as those in patients with diabetes, ischemia, or obesity. In these models, the goal is to simulate similar pathophysiology in the animals to that of human patients with chronic diseases. Then, the models can be used to study the process of wound healing under these certain conditions as well as the effects of therapeutics on complicated wounds. This is a research field of high interest right now due to the increasing burden on the healthcare system of patients with chronic, nonhealing wounds.70
Diabetic wounds are a complicated type of wound that can lead to serious infection71 and subsequent limb amputation72 if not cared for properly. For this reason, it is important that we continue to study the healing process in the diabetic state. Rodents have been used in many experiments to mimic type I and type II diabetic patients.73,74 Type I Diabetes Mellitus (DM) is an autoimmune process against the pancreatic β cells leading to decreased production of insulin.75 In rodent models, there are many ways to simulate this, but the most common are chemical induction by high doses of streptozotocin or alloxan or by obtaining genetically modified rodents.63,76,77 Type II DM is more commonly seen in the human population and is due to a multifactorial process, including genetics and environmental conditions.78 The exact pathophysiology of Type II DM is not completely understood, although there is a strong connection between poor diet leading to chronic hyperglycemic states which results in pancreatic β cell dysfunction and insulin resistance over time.78 A common way to induce Type II DM in rodents is with the genotype db/db, which causes progressively increased insulin resistance as the rodent ages.79
Different induction methods have their advantages and disadvantages. For example, chemical induction creates immune destruction of pancreatic β islets cells, characteristic of the pathophysiology of Type I DM. Although, there is evidence that it is not completely representative of the process that occurs in humans due to the adverse effects of the chemicals. The induction of DM in rodents can cause them to die prematurely, and for this reason, many experiments will create injury to the animals only 1–2 weeks after a hyperglycemic state has been established. This time frame does not allow for the long-term effects of diabetes to manifest.76 The long-term effects, such as cardiovascular disease, decreased vasculature, neuropathy, etc all can affect wound healing. Thus, this model may not be the most appropriate when trying to study complicated wounds in patients with a long-standing history of diabetes mellitus. In contrast to chemical induction, genetically modified rodents such as those with the genotype db/db represent a more natural progression of the disease with prolonged hyperglycemic states and avoid some of the unwanted side effects due to chemical induction.42
DM can lead to ischemic wounds secondary to long-term small vessel disease. None of the diabetic rodent models accurately simulate the long-term effects of diabetic ischemia. Rather, surgically created ischemic models have been applied in rodents to specifically study ischemic wound healing that results from disease states such as diabetes, cardiovascular dysfunction, or pressure ulcers.76 Mcfarlane and colleagues developed the ischemic model in the rodent by making a skin flap on the dorsal side of the rodent. This completely dissociated the skin from the deeper tissues. After dissociation, the flap was sutured back in place allowing revascularization to occur. The process allowed specifically for the study of necrosis and its prevention.80 In more recent years, this model has been modified by adding a silicone sheet under the flap to prevent revascularization and reduce contraction.81 By preventing revascularization, the disease state is more closely mimicked. Additionally, the silicone sheet prevents wound healing by contraction which also allows for more translatable wound healing physiology to humans.
Rat models have been used to mimic the state of obesity. Obesity, which is typically due to a high-calorie diet, is one of the five factors that makes up a condition known as metabolic syndrome.82 Metabolic syndrome has become an overwhelming problem in our societies and has severe consequences including cardiac disease, stroke, and diabetes.83,84 The effect of obesity on wound healing is through a variety of mechanisms and pathways known to increase the concentration of circulating reactive oxygen species (ROS) in the body, resulting in oxidative stress. Normally, ROS at physiological concentrations are extremely important throughout many phases of the wound healing cycle by directly or indirectly promoting hemostasis, fibroblast proliferation, angiogenesis, epithelialization, and more. However, when ROS concentrations are significantly elevated, there is no longer a manageable balance between ROS production and detoxification. Therefore, wound healing becomes dysregulated with increased cell apoptosis and prolonged inflammation.85 To study this process in animals, rats are fed high-fat diets for 1–2 months. This diet results in the elevation of nutritional markers including blood glucose, triglycerides, and cholesterol, as well as increased oxidative stress indicated by elevated malondialdehyde (MDA) and decreased glutathione (GSH).42,86 The nutritional markers suggest that the high-fat diet in rats not only simulates the nutritional effects of obesity but also the oxidative stress that results from obesity in humans. For that reason, the model is practical for studying the wound healing effects in an obese state. The only limitation is that increased ROS has also been known to delay re-epithelialization in human wounds and since rodents heal mainly by contraction, this aspect of the healing would not be translatable.87
Lastly, rodents have known use in the inoculation of bacteria into a wound in order to replicate an infected model.76 The infection can be from direct inoculation of organisms or biofilm production added to the wound. For example, a study by Brandenburg and colleagues added Pseudomonas aeruginosa biofilms via a skin dressing to full-thickness wounds in rodents and compared the wound healing time and properties to a group without inoculation. They found that wounds that harbored the infection had longer rates of healing, prolonged inflammation, decreased re-epithelialization, and decreased collagen formation.88 Other studies have used varieties of bacteria or other organisms to infect the wound and this is extremely beneficial in the study of different therapies against those organisms. Since biofilms have complicated virulence factors that make organisms difficult to treat, this research has been increasingly important. In rodents, this model can be useful to investigate therapies targeted at killing the organism or disrupting its virulence factors but may have poor translatability to humans when comparing healing times and tissue regeneration properties.
Rat Tail Model
One of the major limitations of rodent models is wound contraction resulting in rapid healing which is not in line with the physiological process in humans. Therefore, a novel rat tail wound has been introduced, which exhibits minor wound contraction and significant similarities with human wound healing and hypertrophic scar.89
Typically, the wound is created as a rectangular excision on the dorsal part of the tail. Interestingly, tail wounds usually require 21 days for complete repair and healing compared to conventional back dorsal wounds, which heal within a few days. Consequently, the tail wound is suitable to test the healing potential of proposed drugs and therapy with an extended time course. Moreover, wounds on rat tails can be followed and measured easily without killing the affected animals.44
In Vivo Wound Healing in Other Animals
Since rodents do have their own limitations, it is important to consider what exactly the goal of the experiment is and what animal species will reflect the most accurate and transferrable information to a human patient.
One limitation to using rodents for studying wound healing in a diabetic population was that the results were limited to the early stages of diabetes.76 In order to overcome this, a study by Wang and colleagues created a model in rabbits to more appropriately show the effects of chronic hyperglycemia. DM in the rabbits was chemically induced using alloxan, then treated with insulin. The rabbits survived for up to a year and showed the long-term effects of diabetes such as delayed wound healing, kidney disease, and fatty liver disease.90 On the other hand, diabetic models in pigs have been developed as well since they have similar anatomy and pathophysiology to humans. A study by Velander and colleagues showed that diabetic pigs chemically induced with streptozotocin had delayed re-epithelialization, little to no change in PDGF levels, and decreased levels of TGF-beta as well as IGF-1; similar to humans. The limitation of this study was that the pigs were only kept alive for 18 days so the long-term effects of diabetes could not be determined. The study did note that the pigs tolerated the chemical induction well and other studies showed low mortality and morbidity rates, so it seems as though the pigs could have survived longer to better represent the adverse effects of chronic diabetes.91
The flap method used in rodents to produce an ischemic wound model has also been applied to pigs.76 This model is beneficial to show the effects of ischemia on wound healing by preventing revascularization, but there is still a chance for infection or increased inflammation due to the foreign body reaction that can result from the placement of the silicone sheet between the flap and underlying muscle. Alternatively, rabbits serve as a well-known species for the ischemic wound model. Chien and colleagues designed a simple technique to establish ischemia in the ears of the rabbits. The benefits of this model are that they were able to create four ischemic wounds and four nonischemic wounds in one rabbit by ligating vessels in both ears. They also report that after using this technique on 80 rabbits, none of them suffered complications such as bleeding, infection, or rupture in the skin incisions.92
Rabbits and pigs have both been used to simulate the infected model. A study by Gurjala and colleagues used rabbit ears to induce a punch wound and then subsequently inoculated the wound with Staphylococcus aureus. Their findings showed that S. aureus was able to form a mature biofilm within just 24 h and this biofilm formation did in fact impair wound healing.93 This type of study is useful in that it spotlights the pathophysiology of an infected, chronic wound and can help direct therapeutics in the future. Likewise, similar studies have been done with pigs.94,95 For example, Hirsch and colleagues induced diabetes in pigs and then compared infected diabetic wounds to noninfected diabetic wounds. Results showed that diabetic wounds developed a higher inoculum count of S. aureus and had delayed healing. In addition, this study looked at some of the systemic effects of bacterial infection and found that the diabetic wounds caused increased co-infection with endogenous bacteria, suggesting that diabetic wounds are more prone to infection.96 A drawback to this study was that DM was induced only 14 days prior to creating the wound so the long-term effects of DM could not be established which may have altered the wound healing processes.
Overall, there are many ways to model the various disease states that affect in vivo wound healing. This paper only touched on some of the most common techniques although there is an abundance of others that have been used. In general, it is important to consider the animal species and how the induced disease state will mimic the pathophysiology seen in humans for the most translatable results.
In Vivo Wound Healing Models in Humans
Wound healing models using humans have their own limitations in that it may be hard to find willing participants to obtain enough information or data. Although, it is essential since animal models do not always provide the most translatable results. Each of the methods below has been used in different clinical settings and serves a great purpose to study the different steps of wound healing.
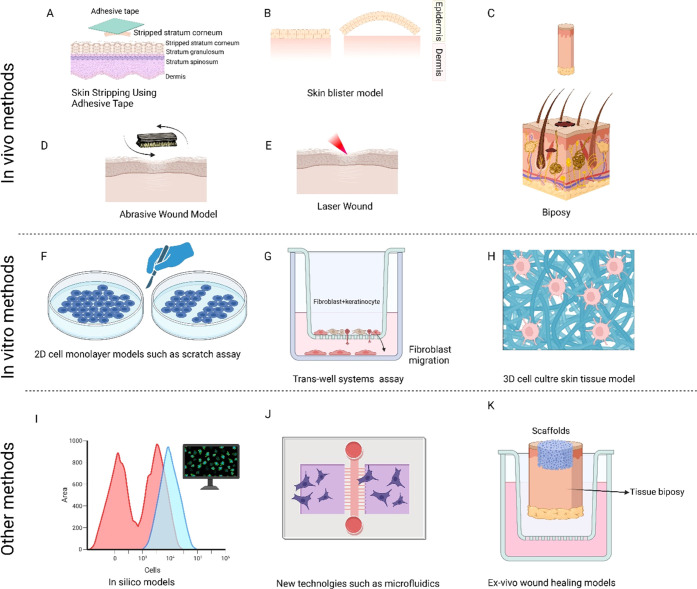
Various wound healing methods and techniques are available in the literature. These methods can be classified mainly into in vivo, in vitro, and other methods such as in silico or new technologies. Figure 3 shows a summary of these methods. The detailed explanation of each method is described in the following paragraphs.
Figure 3.
Example of various models used to study wound healing process. Models based on in vivo methods are (A) skin stripping model; (B) skin blister model; (C) skin biopsy; (D) skin abrasive model; and (E) wound created by laser. Some models based on in vitro models are (F) a variety of methods which includes disrupting the confluency of monolayer cells such as fibroblast using mechanical scratches; (G) trans-well assay for evaluating the migration potential of cells; and (H) three-dimensional (3D) cell cultures using scaffolds. Other methods include (I) in silico methods; (J) new technologies such as microfluidics; and (K) ex vivo wound healing models. BioRender is used for drawing this scheme.
Skin Stripping Using Adhesive Tape
This method works by applying adhesive tape to the skin of a patient. Upon removal of the adhesive tape, the stratum corneum, the most superficial layer of the epidermis, is removed while the remainder of the epidermis remains intact.59,97 The stratum corneum contains the keratinocytes that have completely lost all organelles and desmosome detachment, allowing for sloughing off of the dead tissue layers. This layer is most protective against the entry of foreign material and excessive water loss. Loss of this layer can cause the tissue to become more susceptible to pathogen entry and can cause dehydration which explains the use of this model to collect data on the skin barrier function.98,99 Even though this wound is very minimal, it still stimulates the proliferation of keratinocytes from the stratum basale and promotes their differentiation to anucleate keratinized squamous cells, replicating a wound healing process.97
A study done by Muizzuddin and colleagues used the tape stripping method to study the effects of marital stress on skin barrier strength and healing by measuring the trans-epidermal water loss (TEWL). They used as many tape strippings as needed to reach a TEWL of 18 or higher which indicated that the skin barrier was in fact disrupted. Then, recovery was determined by the TEWL after 3 and 24 h. Their study found no correlation between stress and the skin barrier strength.100
Suction Blister Model
The blister method is based on the idea of creating a clean separation between the epidermis and the dermis. To do so, a constant negative pressure of about 300 mmHg for a period of 1–4 h is applied to tissue resulting in a separation of the skin without any chemical or thermal damage.97 The blister is filled with fluid and cells which can be extracted via fine needle aspiration. This is helpful in studying immune cells that can be difficult to pick up on blood sampling. Holm and colleagues performed a study in which patients were inoculated with Mycobacterium tuberculosis, which caused T cells to leave the lymph tissue, migrate to the site of antigen, and proliferate. Then, using the suction blister method, antigen-specific T cells were readily removed from the aspirate. This project provided great insight as to how we can better study the adaptive immune system with avoiding more invasive methods such as lymphoid tissue biopsy.101 Another study used similar techniques to look at the factors of psychological stress on wound healing and measured the cytokine levels present at the site of the blister during the course of healing.102
After employing the suction blister method, the top layer of the blister can be removed, and the overlying skin will heal without scarring. For this reason, the method has been used to study the changes in wound closure over a series of days to compare wound healing effects of different topical agents or environmental conditions.59,97 Furthermore, the trans-epidermal water loss can be measured to quantify the skin barrier recovery.102
Overall, the suction blister method has many clinical uses and is a great tool in the discovery of wound healing pharmaceutical agents, extraction of inflammatory factors, studying the adaptive immune system, and more.
Abrasive Wound Model
A more invasive approach to studying the effects of wound healing can be done with the abrasive wound model. This procedure requires removal of the epidermis by continued abrading of the skin with a surgical brush. The idea is that the epidermis contains loose cell attachments making their removal by abrasion possible while keeping the basement membrane intact.97 Due to the preserved basement membrane, the patient will heal completely without scar tissue formation. This method and the suction blister method both result in injuries of the same depth, but, the advantage to the abrasive wound model is that it is more representative of a wound that a patient may present with. In other words, the epidermal layer is not removed in a perfect circular fashion, but it is rather rigid as if someone scraped their arm on cement. Accordingly, this method makes a useful resource for the study of different types of wound dressings and their effects on re-epithelialization.
For example, a study done by Kuhlmann and colleagues used the abrasive wound method and then observed healing when an ointment was applied with a standard first aid dressing, ointment with gauze covering, standard first aid dressing alone, and an untreated area covered with gauze. Their results showed that the use of the ointment allowed for significantly faster re-epithelialization as well as more favorable cosmetic outcomes.103 A different study by Wigger-Alberti and colleagues used the same technique to study the differences in wound healing when using a variety of products that are commonly applied to wounded areas. Their results suggested that hydrocolloid and polyurethane products had the best re-epithelialization and cosmetic effects.104
Laser Wounds
The use of lasers to induce and subsequently study the wound healing process is limited. One study done by Ferraq and colleagues used laser and suction blister methods to induce wounds and then compared the different depths of the wound produced versus healing time. Results showed that Er:YAG lasers created shallower wounds with a more uniform depth than the suction blister method, but both methods resulted in similar wound healing times.105
More frequently, lasers as well as microdermabrasions are used therapeutically to promote resurfacing of skin tissue.97 By using these technologies as a treatment, studies have been done to compare laser-induced healing versus other methods. For example, Hopkins and colleagues, compared natural healing to the use of low-level laser therapy (LLLT) on abrasions to the anterior forearm. Results suggested that the laser group healed at faster rates via wound contraction. In addition, untreated wounds on the patients that received LLLT also healed with more contraction suggesting a potential indirect effect of the laser therapy.106
Due to the cost of laser technology, this method is not frequently used to study the effects of wound healing but is more commonly used as a therapy for tissue regeneration and skin resurfacing.107 Recent advances include resurfacing photoaged skin or skin with scar tissue.108 Nevertheless, the cost of equipment is also prohibitive for most research laboratories.
Split-Thickness and Full-Thickness Excisional Wounds
The most invasive method to study the in vivo effects of wound healing is by creating either split-thickness or full-thickness excisions. A split-thickness excisional wound requires the use of a sharp blade to cut parallel to the skin surface until the full epidermis and only a portion of the dermis are removed. To differentiate, a full-thickness excisional wound can be created using the same technique as a split-thickness wound, or with tools such as a punch biopsy or laser to remove the entire epidermal and dermal layers.97 The split-thickness model has been used in wound healing experiments to determine re-epithelialization and different effects on this process. A study performed by Holt and colleagues looked at the effect of age on re-epithelization of post-split-thickness wounds. The findings suggested that older individuals have delayed re-epithelialization.109 In addition to studying re-epithelialization, the model can be used to study different topical agents and their effects on the timeliness of wound healing. For example, Burusapat and colleagues studied the effects of topical aloe vera gel in the healing of a split-thickness biopsy. Data concluded that the aloe vera gel accelerated healing although there was no significant pain relief.110
Since full-thickness wounds excise skin tissue down to the subcutaneous fat, the dermal blood vessels and appendages are disrupted. This type of model has been very useful in studying the overall process of wound healing. After the excision is performed and during the healing process, samples of the injured area can be obtained for the histological determination of tissue regrowth, cells involved, angiogenesis, appendage regrowth or lack thereof, and much more.97 Like split-thickness models, full-thickness models can be used to study the effects of different topical agents on wound healing. Furthermore, this model is more advantageous in that it can be used to study the effects of all aspects of the wound healing process. Many studies have used this model for researching the process of angiogenesis and therapeutics to promote angiogenesis post tissue injury.111,112
In summary, each method involves different tissue layers allowing for the study of the phases of the wound healing cycle and the study of different types of injuries as well as their treatments. For general effects of wound healing and to look at the entire process, a full-thickness biopsy would be best. In contrast, for a model attempting to mimic a real-life abrasion injury, the abrasive wound model would be best. All of these methods may be limited by human participation leading to insufficient data for broader generalizations.
In Vitro Wound Healing Models
In vitro, in silico, and ex vivo models have been developed to tease out the key mechanisms of wound healing. These models provide the advantage of using human cells or tissue and can be used in high-throughput screening of potential therapeutic agents. On the other hand, they lack the true physiological complexity of skin organization, inflammatory components, and surrounding cells that would be attracted by chemotaxis and therefore may not correlate with the in vivo models and results.113
Basically, in vitro models for wound healing are categorized into two-dimensional (2D) cell monolayer models and 3D models, which are more complex and realistic.
2D Cell Monolayer Models
In vitro wound healing assays are usually conducted using the conventional two-dimensional (2D) cell monolayer or single-cell model. Basically, in the 2D in vitro wound healing model, cells in a confluent cell monolayer are disrupted by removing cells via several techniques, creating a region of “wounded” cells.114 The wound cells’ healing potential in terms of the rate at which cells divide and migrate into the wound area is then monitored and evaluated. During the healing phase, the distance between edges is measured and data are acquired using an optical microscope, taking time-lapse images and videos of the destructive area or assessing the expression of specific indicators and molecules by fluorescence spectroscopy. Moreover, evaluating the behavior of cells by nuclear staining and predicting the cellular viability are further used to assess the healing phase of the wounded area.114,115
Interestingly, the regrowth of the destructed cells can be predicted in real-time by measuring the electrical impulses of the cells instead of using the conventional microscopy method to evaluate the healing potential. However, the attachment of cells to the electrodes and the need for a very precise protocol are expected downsides.116
The 2D cell monolayer model provides an effective means to analyze numerous physiological processes involved in the wound repair process, including cellular proliferation, differentiation and viability,117 cell migration,118 angiogenesis,119 and gene expression and protein production.120 The 2D cell monolayer models may use dermal fibroblasts which are involved in extracellular matrix production.121,122
Introducing a wound to the confluent cell monolayer can be performed using several techniques; creating the wound mechanically by scratching devices like a pipette tip is the most common method.113
Although the scratch test is a relatively simple protocol, significant downsides such as irregular scratches, accumulation of cells on the edges of the gap, and interfering with the proliferation and cell migration following the scratch lessen its practical application. Recommendations such as washing and pre-treatment with Mitomycin C have been introduced to improve the scratch assay’s reproducibility.123 Other limitations of the scratch assay include its long duration and the many cells and materials needed to conduct such an assay.124
Another crucial downside of this method and similar methods is that the protocol relies upon creating a linear gap in a monolayer cell. It is challenging to recapture the same wounded area many times since the scratch is longer than the field of view. Generating circular wounds has improved the accuracy and reproducibility of evaluating the wound healing process.125
Other mechanical in vitro wounding methods include stamping,126 thermo-mechanical,127 electrical,128 and optical wounding.129 In the stamping technique, a wound is generated by forcing a stamp, automatically or manually, to mold against a confluent cell layer. Like the scratch method, stamping usually results in an irregular wound area and, consequently, an inaccurate evaluation of the healing potential of the affected cells.130 The thermo-mechanical method is commonly employed as in vitro wound healing assay; however, the transfer of heat into the adjacent cells and its impact on the reproducibility of the assay could not be excluded.
The electrical wounding method applies an elevated current pulse leading to electroporation and cell wounding. This method is fully automated and provides a crucial advantage of monitoring wounding and regrowth of the confluent cell layer and ultimately provides highly reproducible and quantitative data.130
On the other hand, wound generation in a confluent cell monolayer could also be obtained optically by laser-beam-induced cell ablation, where cells will be removed in a defined circular area. Although optical wounding usually produces reproducible results, acquiring a specific instrument for conducting this technique is mandatory.114
Abundant scientific reports support using 2D cell culture models since it is simple and relatively inexpensive. Furthermore, it is considered the first step to understanding several aspects of wound healing and evaluating the healing potential of several compounds before in vivo testing and clinical trials. However, numerous results from in vitro 2D models have not translated into successful in vivo studies since 2D models do not represent the whole natural skin structure and do not mimic the ECM remodeling phase and the complex crosstalk interactions between different cells, which are key in the wound repair process. Consequently, such methods lack the ability to investigate cell signaling mechanisms, the role of immunity, and metabolism.131 Therefore, other advanced models have been introduced to better mimic the physiological complexity and native organization and microenvironment of skin structure.
Trans-Well Systems and Co-Cultured Cell Cultures
The trans-well migration assay or Boyden chamber assay is utilized to analyze the migratory potential of cell mass toward chemotactic responses. It allows analysis of the keratinocyte-fibroblast crosstalk and interaction better than monolayer cultures.76 A variety of cell types can be used in this assay, including epithelial, mesenchymal, cancer cell lines, and primary cells.
Trans-well migration assays use two medium-containing chambers separated by a porous membrane that allows communication between the two cell types. After incubation, the permeable membrane is removed, fixed, and stained. The retained cells on the membrane are then counted to predict the migration potential of cells.132 Incorporating collagen in this assay will create a more complex and native physiological environment to study the migratory behavior of fibroblasts.133
On the other hand, co-cultures usually provide more insightful data on cell-cell interaction than standard monolayer cultures. In particular, crosstalk between keratinocytes and fibroblasts is utmost in wound healing and scarring. Nevertheless, because they are still a 2-D model, co-cultures lack the native structural characteristics of skin where multiple cells interact together. Immune or endothelial cells are not usually incorporated into co-culture models.76
3D Wound Healing Assays
3D wound healing models include histocultures, skin equivalents, and bioengineered and constructed skin to provide a complex biological environment that features the true skin structure and to investigate the expected influence of potential agents or compounds on the different phases of wound healing.131
Histocultures (i.e., three-dimensional tissues that are put into growth medium either with a collagen gel support or simply free floating)134 are developed as cultures of intact skin cultures grown in growth media with or without the support of ECM matrices such as collagen for a better presentation of skin structure and responses and longer preservation of the functions and physiology of the skin structures such as epidermis, dermis and hair follicles.135
3D skin equivalent models have been created from skin cells and ECM matrices that mimic the skin structure and produce functional and structural skin substitutes as dermal equivalent, epidermal equivalent, or constructed from both compartments.136 Skin equivalent models are time and cost-effective alternatives to the in vivo models for wound healing studies. An example of such alternatives is a model for angiogenesis studies where keratinocytes and other cells are combined with ECM matrices, such as Matrigel and collagen, and endothelial cells, which are responsible for creating capillaries in the skin.137 Skin equivalents can also be engineered by specific genetic modifications to the epidermis or dermis structures and predict the impact of such alterations on skin cell responses.138
Artificial and natural polymers are usually included in the skin equivalents to create scaffolds such as collagen, hyaluronic acid, chitosan, fibrin, alginic acid, elastin poly (ethylene glycol), polycaprolactone, poly(vinyl alcohol), or polylactic acid.139
The constructed full-skin model generally comprises a scaffold of fibroblast layer representing the dermal structure enclosed by another layer of keratinocytes to constitute the epidermis-like layer and epidermal bilayer structure.140 It is crucial to indicate here that the constructed skin could be exogenous or endogenous in terms of its origin; the former includes an exogenous scaffold to accommodate the skin cells, while in the endogenous model, skin cells are induced to synthesize their own ECM matrices.140 Wounding of 3D models is commonly conducted using typical techniques or punch biopsies.
3D models very closely mimic the physiological and compositional cellular environment and contain stratum corneum to better understand the diffusion and interactions of several compounds through it. Likewise, 3D skin culture models provide more extended use of cell culture. Nevertheless, 3D skin models are often strenuous and require high-level technical skills.
Although constructed skin mimics the actual skin organization and allows us to understand the crosstalk interactions between these two cells in 3D, it lacks other crucial systems, such as immune and vascular systems and hair follicles.141 Additional types of cells, such as immune or cancer cells, could be co-cultured with this system depending on the study aims and complexity proposed. In this context, several immunocompetent skin models have been developed for specific studies; for example, melanocytes, Langerhans cells, and dendritic cells have been incorporated with skin alternatives for skin sensitization and allergic studies.142−144
For wound healing studies, incorporating macrophages and T cells into the skin 3D models would remarkably improve the relevance of the skin model to investigate the wound healing phases and explore new potential therapeutic candidates.140 Implementing an immunocompetent skin model has been conducted recently by incorporating T cells into in vitro skin models. The infiltration of T cells into these models has been validated by anti-CD3 immunofluorescence staining.145,146
Ex Vivo Wound Healing Models
Ex vivo models are based on the organ culture of skin and reflect the use of full or partial excised skin or biopsies that resemble normal skin. Ex vivo models have been used numerously to understand the pathophysiology and inflammation aspects of skin and to evaluate the healing potential of various compounds and novel materials. For example, excised human or animal skin has been utilized as models to understand the interaction of nanoparticles and nanomaterials with skin and their potential influence on the healing process and potential modulation of numerous inflammatory and anti-inflammatory factors.147−150 In addition, ex vivo models have been used to investigate the wound repair potential of antioxidants151 and photodynamic therapy.152 Ex vivo skin models include normal or pathological skin biopsies, and they provide a 3D representation of skin including cell-to-cell crosstalk and the native microenvironment.
Several ex vivo human skin models have been developed to study skin tissue repair and re-epithelization studies. For example, incisional wounds in the skin or partial-thickness biopsies are commonly used.153 Separation of skin layers (dermal and epidermal) can be achieved by distinct methods based on the aim of the investigation. For instance, the separation of the dermis from the epidermis could be performed by heat, chemical, or enzymatic separation to disturb the electrolyte equilibrium or deactivate critical molecules and compounds, mechanical force separation method by stretching and suction techniques.154 The mechanical separation method preserves the cellular structure of the dermis and epidermis compartments.
Despite the attractiveness of this model to represent the real complexity and microenvironment of skin structure, it lacks innervation, migratory immune cells, and blood supply76 and the standardization and reproducibility of these models are uncertain. Other challenges are considerable variations in methodologies, culture conditions, and limited availability due to the need for fresh tissue. Consequently, its implementation is limited to specific applications.
In Silico Wound Healing Models
Computer modeling is a powerful tool for better understanding biochemical and biological processes. In silico models are computational models derived from known and hypothesized kinetics of the skin structure and provide opportunities to understand cellular responses and phases of wound repair in theory. Besides, they help explore and predict the possible impact and outcomes of inflammation, hypoxia, and other pathological conditions on the rate and success of wound repair155 and are commonly employed to provide a multifactorial approach to potential compound screening. For example, In silico screening has been utilized to select a combination of phyto-constitutes that modulate several targets like inflammatory markers in the wound healing process.156 Furthermore, in silico models have enabled the understanding and modulating of angiogenesis as one of the key phases of wound repair and the effect of therapeutic interventions and vascular modulating agents on the angiogenesis process.157,158
For simulating angiogenesis in wound healing, continuum models and cell-based models are utilized; besides, hybrid models can be used to describe the cellular and tissue levels in studies related to wound healing and angiogenesis analysis.159,160 Moreover, in silico models provide an effective tool for designing tissue substitutes for wound regeneration and investigating the role of growth factors, fibroblasts, and stem cells in epithelium regeneration.161
In silico models lack the physiological characteristics of human skin and are usually confirmed by or combined with in vivo or in vitro models.162 In silico mathematical models for biological and biochemical studies also need close collaboration between scientists and mathematicians, which could be considered a challenge for such models.
New Technologies in the Study of Wound Healing
The in vitro conventional wound healing models and approaches feature several limitations regarding reproducibility, automation, matrix coatings damages, live cell imaging, and variation. Therefore, advanced technologies have been introduced as next-generation wound healing technologies to improve the features of the current models, such as 3D models, in response to the global trends toward miniaturization and automation, and to lessen challenges related to variation, consistency, and reproducibility.
Vascularized 3D Skin Equivalents
3D organotypic skin models and skin equivalents are commonly used in in vitro wound healing studies; however, they lack the native dynamic, complexity, and vascularization of real skin structure. Therefore, various advanced dynamic models have been developed that integrate skin equivalents with vasculature systems to understand angiogenesis, wound healing studies, and/or cancer research.163
Different vascularization techniques have been introduced recently; for example, vascularization was induced by seeding stem cells or endothelial cells into fibrin or collagen hydrogel and then exposing it to vascular endothelial growth factor (VEGF).164,165 Another research group mimicked a microvasculature system into the skin equivalents by casting a simulated vasculature in the collagen matrix using 3D-printed molds.166 Mori et al. have created collagen matrix microvasculature by using removable nylon threads that form channels.163,167 Furthermore, 3D bioprinting has been utilized to generate different skin layers into a perfusable 3D culture system.168
Microfluidics and Skin-on-a-Chip Technologies
The traditional 2D and 3D models cannot simulate in vivo blood circulation due to lacking a vascular network to provide cells with nutrients. Consequently, microfabrication technologies have been introduced in response to the current downsides of traditional 2D cultures and 3D organ models and led to the development of organs-on-chips. These technologies are micrometer-sized microfluidic devices that provide high-throughput capacity and dynamicity of the system that mimics with natural physiology of organs or tissues.169 In microfluidic technology, small amounts of fluids are processed in hollow channels, which exhibit laminar flow as specific physical characteristics due to the channel’s small size. Therefore, a small volume of fluids and the investigated materials or drugs can be used, and fine control of the cellular environment in terms of dynamic fluid flow and external factors can be achieved.133,170
The merits offered by organ-on-chip technologies have been exploited to develop skin-on-a-chip, a more dynamic and reliable skin model cultured under control factors related to chemical ingredients and biochemical parameters.
Skin-on-a-chip approaches are classified based on the approach used to generate skin in the chip; transferred skin-on-a-chip, which is based on the direct introduction of a skin biopsy or a human skin equivalent in the chip of a microfluidic system that is exposed to a direct flow of culture medium, such system results in significant improvement in the life span of the biopsies of skin equivalent for studying. The second one is called in situ skin-on-a-chip, based on the in situ generations of skin tissue directly inside the microfluidic system chip.133 The first method consists of introducing a skin tissue/biopsy in a microfluidic system equipped with a dynamic flow of cell culture medium. This method substantially enhances the life span of the skin tissue/biopsy for testing purposes. The second method lies in generating the skin model directly inside a microfluidic system.133 Another model of on-chip skin culture with a vascular channel that is closely mimicking in vivo conditions to study the vascular absorption of compounds is developed by Mori et al.167
Furthermore, different skin components, such as immune cells, vasculature, and hair follicles, were integrated into the microfluidic system to improve skin-on-chip models’ reliability. For example, vascular networks were built using 3D bioprinting technology and incorporated into human skin equivalent stem cells-derived endothelial cells.171 Ramadan and Ting also developed the immune-competent skin-on-a-chip model. It comprises a microfluidic channel platform of the epidermis and immune systems represented by keratinocytes and monocytes, respectively.172
A model representing the inflammation phase of a wound just after the hemostasis phase has been developed by Biglari et al. The model is composed of three compartments containing three different cells: fibroblasts, endothelial cells, and macrophages. The different types of cells in the compartments communicate with each other, where cytokines are released from M1 macrophages and enhance the migration of fibroblasts and endothelial cells. Then, key cytokines such as TNF-α were produced upon transition of macrophages from M1 to M2 and induced production of ECM, angiogenesis, and differentiation of fibroblasts into myofibroblasts.173
3D Skin Bioprinting Technology
Bioprinting skin tissue is one of the most promising approaches to developing models that mimic tissues and organs and facilitate dermal drug development, drug screening, skin physiology studies, wound healing, and dermal toxicological research. Additionally, 3D bioprinting technology is relatively affordable, conducted at high speed, and scalable. Numerous 3D skin bioprinting models have been developed, and several approaches introduced to mimic the skin physiology and cellular environment. for example, dermis bioprinting, full-skin bioprinting, and bioprinting of blood vessel-containing skin models based on the aim of the experiment and expected data.174,175 Furthermore, the laser-assisted bioprinting technique has been utilized for the bioprinting of fibroblasts and keratinocytes on a stabilizing matrix where neovascularization has been achieved.176
Other 3D skin bioprinting models have been introduced based on Extrusion and Inkjet. A wide range of available cell lines can be suitable for 3D bioprinting, such as fibroblasts, keratinocytes, melanocytes, and hair follicles. Skin biopsies could also be utilized; however, keratinocytes are the primary cell types used for the bioprinting of skin cells.177 Apart from the advantages of 3D bioprinting, greater accuracy in placement of cells and extracellular matrix, as well as having the potential of embedding vasculature in the skin construct as models for wound healing research, the system is quite complex and requires high production costs and specialized staff to conduct the production processes.178
Compared to traditional methods to construct skin tissues, 3D bioprinting offers many advantages including robustness, flexibility, reproducibility, high resolution, and high-throughput culture. These engineered skin models have a capacity to be used in transdermal and topical formulation discovery and dermal toxicity studies.
Conclusions
Each animal species has its own set of unique physiologic wound healing characteristics that make it more advantageous than others for human comparison. For example, pigs have a very similar architecture to human skin making them great models for studying tissue regeneration and re-epithelialization while rabbits are well suited to study tissue ischemia as well as hypertrophic scarring processes. Despite the similarities, there are still differences that should be accounted for such as the shorter duration of wound healing and tissue appendage qualities and distribution. Although there is not a single species that exactly replicates the physiology of human wound healing, with careful consideration, there are certain species that are more suitable than others for studying independent elements of the wound healing process. Not only is it important to choose the correct species to align with a physiologic process but also to account for the best animal to induce a diseased state similar to that in humans. Manuscripts should identify the rationale for the choice of the animal model, the similarities and differences from the human condition that is being modeled, and sufficient detail for other researchers to replicate the model.
The need to mimic the complexity of the human organism for wound healing studies continues to evade researchers. Model development requires close collaboration and communication between basic researchers and the clinicians who treat human wounds. Skin is the largest organ of the body and is exposed to a wide variety of potential impediments to healing. As humans age, they accumulate a multitude of diseases that impact wound healing. While the basic mechanisms of wound healing can be identified and manipulated in cell culture, translation to human wound healing remains limited. The majority of animal models can only simulate acute wounds and the attempts to recapitulate the human co-morbidities that lead to chronic nonhealing wounds have had limited success. As researchers strive to develop novel treatments for human wounds, they should consider a progression of models, from simple to complex. The simple models will verify that the treatment impacts the targeted response, whether that be cell migration, proliferation, angiogenesis or a specific molecular pathway. Once that is verified, progression to a model of increased complexity that mimics the targeted human condition should be performed. For example, if studying diabetic wound healing, the model animal should at least be representative of “middle age,” with verification of long-term elevated blood glucose, metabolic changes, and biomarkers that demonstrate a correlation between the human disease state and recapitulate the chronicity associated with human nonhealing diabetic wounds.179 Although further development of preclinical animal models will improve translatable innovations in wound therapeutics, recent advances in computer modeling and 3D models may provide better insight with the opportunity to manipulate the environment to more closely simulate the complex interactions between human skin cells, the immune response and the critical orchestration of the cytokine response. These models also allow for very high-throughput screening of potential therapeutic molecules which may then be tested directly in humans, bypassing the need for preclinical animal models. This review highlights the need for researchers to identify the most appropriate model for specific therapeutic goals; however, in the end, the most clinically relevant wound healing model remains the human, an elusive and highly variable animal.
Acknowledgments
L.G. and M.M. gratefully acknowledge financial support from the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (grant DK131417).
Author Contributions
⊥ K.F. and N.N.M. equally contributed to this study.
The authors declare the following competing financial interest(s): Morteza Mahmoudi discloses that (i) he is a co-founder and director of the Academic Parity Movement (www.paritymovement.org), a non-profit organization dedicated to addressing academic discrimination, violence and incivility; (ii) he is an advisor for and shareholder in Partners in Global Wound Care (PGWC), and a co-founder of and shareholder in NanoServ Corp. and Targets’ Tip Corp.; and (iii) he receives royalties/honoraria for his published books, plenary lectures, and licensed patent.
References
- Frykberg R. G.; Banks J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwomeh B. C.; Yager D. R.; Cohen I. K. Physiology of the chronic wound. Clin. Plast. Surg. 1998, 25, 341–356. 10.1016/S0094-1298(20)32468-8. [DOI] [PubMed] [Google Scholar]
- James G. A.; Ge Zhao A.; Usui M.; Underwood R. A.; Nguyen H.; Beyenal H.; deLancey Pulcini E.; Agostinho Hunt A.; Bernstein H. C.; Fleckman P.; et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regener. 2016, 24, 373–383. 10.1111/wrr.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg H.; Tilkorn D. J.; Hager S.; Hauser J.; Mirastschijski U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- Zhu Z.; Ding J.; Tredget E. E. The molecular basis of hypertrophic scars. Burns Trauma 2016, 4, 2 10.1186/s41038-015-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidhal Ghazy D.; Rahmah Abu-Raghif A. Effects of Apremilast on Induced Hypertrophic Scar of Rabbits. Arch. Razi Inst. 2021, 76, 1803–1813. 10.22092/ari.2021.356195.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Wang X.-F.; Wang Z.-C.; Lou D.; Fang Q.-Q.; Hu Y.-Y.; Zhao W.-Y.; Zhang L.-Y.; Wu L.-H.; Tan W.-Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020, 129, 110287 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- Roig-Rosello E.; Rousselle P. The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters. Biomolecules 2020, 10, 1607. 10.3390/biom10121607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidova-Rice T. N.; Hamblin M. R.; Herman I. M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1: Normal and Chronic Wounds: Biology, Causes, and Approaches to Care. Adv. Skin Wound Care 2012, 25, 304–314. 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux C. N. Wound healing in animals: a review of physiology and clinical evaluation. Vet. Dermatol. 2022, 33, 91–e27. 10.1111/vde.13032. [DOI] [PubMed] [Google Scholar]
- Doughty D.; McNichol L.. Wound, Ostomy and Continence Nurses Society Core Curriculum; Wolters Kluwer Health, 2014. [Google Scholar]
- Ross M. H.; Pawlina W.; Lippincott W.; Wilkins. Histology: A Text and Atlas; Wolters Kluwer/Lippincott Williams & Wilkins Health, 2015. [Google Scholar]
- Urmacher C. Histology of Normal Skin. Am. J. Surg. Pathol. 1990, 14, 671–686. 10.1097/00000478-199007000-00008. [DOI] [PubMed] [Google Scholar]
- Yousef H.; Alhajj M.; Sharma S.. Anatomy, Skin (Integument), Epidermis StatPearls 2022https://www.statpearls.com/point-of-care/21212 (date accessed, 04/18/2023). [PubMed]
- McLafferty E.; Hendry C.; Farley A. The integumentary system: anatomy, physiology and function of skin. Nurs. Stand. 2012, 27, 35. 10.7748/ns2012.09.27.3.35.c9299. [DOI] [PubMed] [Google Scholar]
- Khavkin J.; Ellis D. A. Aging skin: histology, physiology, and pathology. Facial Plast. Surg. Clin. 2011, 19, 229–234. 10.1016/j.fsc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Lai-Cheong J. E.; McGrath J. A. Structure and function of skin, hair and nails. Medicine 2013, 41, 317–320. 10.1016/j.mpmed.2013.04.017. [DOI] [Google Scholar]
- Takeo M.; Lee W.; Ito M. Wound healing and skin regeneration. Cold Spring Harbor Perspect. Med. 2015, 5, a023267 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantwerker E. A.; Hom D. B. Skin: histology and physiology of wound healing. Clin. Plast. Surg. 2012, 39, 85–97. 10.1016/j.cps.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Watt F. M.; Fujiwara H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harbor Perspect. Biol. 2011, 3, a005124 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker-McGraw M. K.; Jones T. R.; Baer D. G. Soft tissue wounds and principles of healing. Emerg. Med. Clin. North Am. 2007, 25, 1–22. 10.1016/j.emc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Aleemardani M.; Trikić M. Z.; Green N. H.; Claeyssens F. The importance of mimicking dermal-epidermal junction for skin tissue engineering: a review. Bioengineering 2021, 8, 148. 10.3390/bioengineering8110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T.; Zmijewski M. A.; Semak I.; Kim T.-K.; Janjetovic Z.; Slominski R. M.; Zmijewski J. W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. 10.1007/s00018-017-2617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Bai X.; Dai X.; Li Y. The biological processes during wound healing. Regener. Med. 2021, 16, 373–390. 10.2217/rme-2020-0066. [DOI] [PubMed] [Google Scholar]
- Wong R.; Geyer S.; Weninger W.; Guimberteau J. C.; Wong J. K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. 10.1111/exd.12832. [DOI] [PubMed] [Google Scholar]
- Qing C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. 10.1016/j.cjtee.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S.; Lin E. J.; Tartar D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. 10.1007/s13671-018-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S. H.; Sim E. H.; Goh R. Y.; Park J. I.; Han J. Y. Platelet Activation: The Mechanisms and Potential Biomarkers. BioMed Res. Int. 2016, 2016, 9060143 10.1155/2016/9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM Medline.
- Gryglewski R. J.; Dembinska-Kiec A.; Korbut R. A possible role of thromboxane A2 (TXA2) and prostacyclin (PGI2) in circulation. Acta Biol. Med. Ger. 1978, 37, 715–723. [PubMed] [Google Scholar]
- Seaton M.; Hocking A.; Gibran N. S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. 10.1093/ilar/ilv016. [DOI] [PubMed] [Google Scholar]
- Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. 10.1007/s10555-017-9677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancerotto L.; Orgill D. P. Mechanoregulation of Angiogenesis in Wound Healing. Adv. Wound Care 2014, 3, 626–634. 10.1089/wound.2013.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM.
- He L.; Marneros A. G. Macrophages are essential for the early wound healing response and the formation of a fibrovascular scar. Am. J. Pathol. 2013, 182, 2407–2417. 10.1016/j.ajpath.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM.
- Bainbridge P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–411. 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- King A.; Balaji S.; Keswani S. G.; Crombleholme T. M. The Role of Stem Cells in Wound Angiogenesis. Adv. Wound Care 2014, 3, 614–625. 10.1089/wound.2013.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM.
- Demidova-Rice T. N.; Hamblin M. R.; Herman I. M. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. 10.1097/01.ASW.0000418541.31366.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. 10.1016/j.retram.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Bielefeld K. A.; Amini-Nik S.; Alman B. A. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 70, 2059–2081. 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F. L.; Sun Z. L.; Feng Y.; Liu S. Y.; Du Y.; Yu S.; Yang M. L.; Lv G. Z. Epithelial-mesenchymal transition in the formation of hypertrophic scars and keloids. J. Cell. Physiol. 2019, 234, 21662–21669. 10.1002/jcp.28830. [DOI] [PubMed] [Google Scholar]
- Goldberg S. R.; Diegelmann R. F. What makes wounds chronic. Surg. Clin. 2020, 100, 681–693. 10.1016/j.suc.2020.05.001. [DOI] [PubMed] [Google Scholar]
- Naldaiz-Gastesi N.; Bahri O. A.; Lopez de Munain A.; McCullagh K. J.; Izeta A. The panniculus carnosus muscle: an evolutionary enigma at the intersection of distinct research fields. J. Anat. 2018, 233, 275–288. 10.1111/joa.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson-Meyers D. S.; Andrade T. A. M.; Caetano G. F.; Guimaraes F. R.; Leite M. N.; Leite S. N.; Frade M. A. C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM.
- Zomer H. D.; Trentin A. G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. 10.1016/j.jdermsci.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Grada A.; Mervis J.; Falanga V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Invest. Dermatol. 2018, 138, 2095–2105.e2091. 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Volk S. W.; Bohling M. W. Comparative wound healing—are the small animal veterinarian’s clinical patients an improved translational model for human wound healing research?. Wound Repair Regener. 2013, 21, 372–381. 10.1111/wrr.12049. [DOI] [PubMed] [Google Scholar]
- Galiano R. D.; Michaels Jt.; Dobryansky M.; Levine J. P.; Gurtner G. C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regener. 2004, 12, 485–492. 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- Seaton M.; Hocking A.; Gibran N. S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. 10.1093/ilar/ilv016. [DOI] [PubMed] [Google Scholar]
- Baker R. E.; Murray P. J. Understanding hair follicle cycling: a systems approach. Curr. Opin. Genet. Dev. 2012, 22, 607–612. 10.1016/j.gde.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Ansell D. M.; Kloepper J. E.; Thomason H. A.; Paus R.; Hardman M. J. Exploring the ″hair growth-wound healing connection″: anagen phase promotes wound re-epithelialization. J. Invest. Dermatol. 2011, 131, 518–528. 10.1038/jid.2010.291. [DOI] [PubMed] [Google Scholar]
- Rai V.; Moellmer R.; Agrawal D. K. Clinically relevant experimental rodent models of diabetic foot ulcer. Mol. Cell. Biochem. 2022, 477, 1239–1247. 10.1007/s11010-022-04372-w. [DOI] [PubMed] [Google Scholar]
- Seok J.; Warren H. S.; Cuenca A. G.; Mindrinos M. N.; Baker H. V.; Xu W.; Richards D. R.; McDonald-Smith G. P.; Gao H.; Hennessy L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 3507–3512. 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao K.; Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 1167–1172. 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna M.; Yun J. S. Skin of the domestic pig. J. Invest. Dermatol. 1964, 43, 11–21. [PubMed] [Google Scholar]
- Fisher I.; Glick D. Histochemistry XIX. Localization of Alkaline Phosphatase in Normal and Pathological Human Skin. Proc. Soc. Exp. Biol. Med. 1947, 66, 14–18. 10.3181/00379727-66-15967. [DOI] [PubMed] [Google Scholar]
- Lin C. H.; Chiu P. Y.; Hsueh Y. Y.; Shieh S. J.; Wu C. C.; Wong T. W.; Chuong C. M.; Hughes M. W. Regeneration of rete ridges in Lanyu pig (Sus scrofa): Insights for human skin wound healing. Exp. Dermatol. 2019, 28, 472–479. 10.1111/exd.13875. [DOI] [PubMed] [Google Scholar]
- Baker L. B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. 10.1080/23328940.2019.1632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge B. D.; Sanvictores T.; Brodell R. T.. Anatomy, skin sweat glands 2022https://www.statpearls.com/point-of-care/36636 (date accessed, 04/18/2023). [PubMed]
- Biedermann T.; Pontiggia L.; Böttcher-Haberzeth S.; Tharakan S.; Braziulis E.; Schiestl C.; Meuli M.; Reichmann E. Human eccrine sweat gland cells can reconstitute a stratified epidermis. J. Invest. Dermatol. 2010, 130, 1996–2009. 10.1038/jid.2010.83. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Khan A. A.; Nagarajan K. Animal models for the evaluation of wound healing activity. Int. Bull. Drug Res. 2013, 3, 93–107. [Google Scholar]
- Davidson J. M.; Yu F.; Opalenik S. R. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv. Wound Care 2013, 2, 142–148. 10.1089/wound.2012.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Ge J.; Tredget E. E.; Wu Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat. Protoc. 2013, 8, 302–309. 10.1038/nprot.2013.002. [DOI] [PubMed] [Google Scholar]
- Bos P.; Van Osch G.; Frenz D.; Verhaar J.; Verwoerd-Verhoef H. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage 2001, 9, 382–389. 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- Kloeters O.; Tandara A.; Mustoe T. A. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regener. 2007, 15, S40–S45. 10.1111/j.1524-475X.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang J.; Wang Z.; Xia Y.; Zhou M.; Zhong A.; Sun J. Experimental models for cutaneous hypertrophic scar research. Wound Repair Regener. 2020, 28, 126–144. 10.1111/wrr.12760. [DOI] [PubMed] [Google Scholar]
- Lovasova V.; Bem R.; Chlupac J.; Dubsky M.; Husakova J.; Nemcova A.; Fronek J. Animal experimental models of ischemic wounds–A review of literature. Wound Repair Regener. 2022, 30, 268–281. 10.1111/wrr.12996. [DOI] [PubMed] [Google Scholar]
- Yannas I. V.; Tzeranis D. S. Mammals fail to regenerate organs when wound contraction drives scar formation. NPJ Regener. Med. 2021, 6, 39 10.1038/s41536-021-00149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringsdorf W. Jr; Cheraskin E. Vitamin C and human wound healing. Oral Surg., Oral Med., Oral Pathol. 1982, 53, 231–236. 10.1016/0030-4220(82)90295-X. [DOI] [PubMed] [Google Scholar]
- Boo Y. C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. 10.3390/antiox11091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naomi R.; Bahari H.; Yazid M. D.; Embong H.; Othman F. Zebrafish as a model system to study the mechanism of cutaneous wound healing and drug discovery: advantages and challenges. Pharmaceuticals 2021, 14, 1058. 10.3390/ph14101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbrink K.; Ni G.; Sönnergren H.; Schmidtchen A.; Pang C.; Bajpai R.; Car J. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst. Rev. 2017, 6, 15 10.1186/s13643-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C. S. Wound healing in the patient with diabetes mellitus. Nurs. Clin. North Am. 1990, 25, 247–261. 10.1016/S0029-6465(22)00238-9. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- Wu J.; Yan L.-J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2015, 8, 181. 10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A.; Alcolado J. Animal models of diabetes mellitus. Diabetic Med. 2005, 22, 359–370. 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A.; Eisenbarth G. S.; Michels A. W. Type 1 diabetes. Lancet 2014, 383, 69–82. 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami D. G.; Heiba H. H.; Abdellatif A. Wound healing models: A systematic review of animal and non-animal models. Wound Med. 2019, 24, 8–17. 10.1016/j.wndm.2018.12.001. [DOI] [Google Scholar]
- Masson-Meyers D. S.; Andrade T. A.; Caetano G. F.; Guimaraes F. R.; Leite M. N.; Leite S. N.; Frade M. A. C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. 10.1111/iep.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S.; Khunti K.; Davies M. J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- Chen D.; Wang M. W. Development and application of rodent models for type 2 diabetes. Diabetes, Obes. Metab. 2005, 7, 307–317. 10.1111/j.1463-1326.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- McFARLANE R. M.; Deyoung G.; Henry R.; McFarlane R. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast. Reconstr. Surg. 1965, 35, 177–182. 10.1097/00006534-196502000-00007. [DOI] [PubMed] [Google Scholar]
- Trujillo A. N.; Kesl S. L.; Sherwood J.; Wu M.; Gould L. J. Demonstration of the rat ischemic skin wound model. J. Vis. Exp. 2015, 98, e52637 10.3791/52637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després J.-P.; Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Tune J. D.; Goodwill A. G.; Sassoon D. J.; Mather K. J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks F. M. Metabolic syndrome: epidemiology and consequences. J. Clin. Psychiatry 2004, 65, 3–12. [PubMed] [Google Scholar]
- Cano Sanchez M.; Lancel S.; Boulanger E.; Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants 2018, 7, 98. 10.3390/antiox7080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari U.; Kumar V.; Khanna N.; Panda B. P. The effect of high-fat diet-induced obesity on cardiovascular toxicity in Wistar albino rats. Hum. Exp. Toxicol. 2011, 30, 1313–1321. 10.1177/0960327110389499. [DOI] [PubMed] [Google Scholar]
- O’toole E. A.; Goel M.; Woodley D. T. Hydrogen peroxide inhibits human keratinocyte migration. Dermatol. Surg. 1996, 22, 525–529. 10.1016/1076-0512(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Brandenburg K. S.; Calderon D. F.; Kierski P. R.; Czuprynski C. J.; McAnulty J. F. Novel murine model for delayed wound healing using a biological wound dressing with Pseudomonas aeruginosa biofilms. Microb. Pathog. 2018, 122, 30–38. 10.1016/j.micpath.2018.05.043. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Wang W.; Zhou S.; Zhang G.; He J.; Li Q. A Novel Model for Cutaneous Wound Healing and Scarring in the Rat. Plast. Reconstr. Surg. 2019, 143, 468–477. 10.1097/PRS.0000000000005274. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wan R.; Mo Y.; Zhang Q.; Sherwood L. C.; Chien S. Creating a long-term diabetic rabbit model. Exp. Diabetes Res. 2010, 2010, 289614 10.1155/2010/289614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velander P.; Theopold C.; Hirsch T.; Bleiziffer O.; Zuhaili B.; Fossum M.; Hoeller D.; Gheerardyn R.; Chen M.; Visovatti S.; et al. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regener. 2008, 16, 288–293. 10.1111/j.1524-475X.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- Chien S.; Wilhelmi B. J. A simplified technique for producing an ischemic wound model. J. Vis. Exp. 2012, 63, e3341 10.3791/3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurjala A. N.; Geringer M. R.; Seth A. K.; Hong S. J.; Smeltzer M. S.; Galiano R. D.; Leung K. P.; Mustoe T. A. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regener. 2011, 19, 400–410. 10.1111/j.1524-475X.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- Svedman P.; Ljungh A.; Rausing A.; Banck G.; Sandén G.; Miedzobrodzki J.; Wadström T. Staphylococcal wound infection in the pig: Part I. Course. Ann. Plast. Surg. 1989, 23, 212–218. 10.1097/00000637-198909000-00004. [DOI] [PubMed] [Google Scholar]
- Sandén G.; Ljungh Å.; Wadström T.; Banck G.; Miedzobrodski J.; Svedman P. Staphylococcal wound infection in the pig: Part II. Inoculation, quantification of bacteria, and reproducibility. Ann. Plast. Surg. 1989, 23, 219–223. 10.1097/00000637-198909000-00005. [DOI] [PubMed] [Google Scholar]
- Hirsch T.; Spielmann M.; Zuhaili B.; Koehler T.; Fossum M.; Steinau H.-U.; Yao F.; Steinstraesser L.; Onderdonk A. B.; Eriksson E. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 2008, 8, 5 10.1186/1471-2482-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm K. P.; Wilhelm D.; Bielfeldt S. Models of wound healing: an emphasis on clinical studies. Skin Res. Technol. 2017, 23, 3–12. 10.1111/srt.12317. [DOI] [PubMed] [Google Scholar]
- Menon G. K.; Cleary G. W.; Lane M. E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. 10.1016/j.ijpharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Takeo M.; Lee W.; Ito M. Wound healing and skin regeneration. Cold Spring Harbor Perspect. Med. 2015, 5, a023267 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muizzuddin N.; Matsui M. S.; Marenus K. D.; Maes D. H. Impact of stress of marital dissolution on skin barrier recovery: tape stripping and measurement of trans-epidermal water loss (TEWL). Skin Res. Technol. 2003, 9, 34–38. 10.1034/j.1600-0846.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- Holm L. L.; Vukmanovic-Stejic M.; Blauenfeldt T.; Benfield T.; Andersen P.; Akbar A. N.; Ruhwald M. A Suction Blister Protocol to Study Human T-cell Recall Responses In Vivo. J. Vis. Exp. 2018, 57554 10.3791/57554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J.-P.; Kiecolt-Glaser J. K. The impact of psychological stress on wound healing: methods and mechanisms. Immunol. Allergy Clin. North Am. 2011, 31, 81–93. 10.1016/j.iac.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann M.; Wigger-Alberti W.; Mackensen Y.; Ebbinghaus M.; Williams R.; Krause-Kyora F.; Wolber R. Wound healing characteristics of a novel wound healing ointment in an abrasive wound model: A randomised, intra-individual clinical investigation. Wound Med. 2019, 24, 24–32. 10.1016/j.wndm.2019.02.002. [DOI] [Google Scholar]
- Wigger-Alberti W.; Kuhlmann M.; Ekanayake S.; Wilhelm D.; Buettner H.; Callaghan T.; Wilhelm K. Using a novel wound model to investigate the healing properties of products for superficial wounds. J. Wound Care 2009, 18, 123–131. 10.12968/jowc.2009.18.3.39813. [DOI] [PubMed] [Google Scholar]
- Ferraq Y.; Black D. R.; Theunis J.; Mordon S. Superficial wounding model for epidermal barrier repair studies: comparison of Erbium: YAG laser and the suction blister method. Lasers Surg. Med. 2012, 44, 525–532. 10.1002/lsm.22054. [DOI] [PubMed] [Google Scholar]
- Hopkins J. T.; McLoda T. A.; Seegmiller J. G.; Baxter G. D. Low-level. laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled study. J. Athletic Training 2004, 39, 223. [PMC free article] [PubMed] [Google Scholar]
- Janik J. P.; Markus J. L.; Markus R. F.; Al-Dujaili Z.. Laser Resurfacing. In Seminars in Plastic Surgery; Thieme Medical Publishers, 2007; Vol. 21, pp 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaker B. D.; Fuchs C.; Tam J. When wounds are good for you: The regenerative capacity of fractional resurfacing and potential utility in chronic wound prevention. Adv. Wound Care 2019, 8, 679–691. 10.1089/wound.2019.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt D. R.; Kirk S. J.; Regan M. C.; Hurson M.; Lindblad W. J.; Barbul A. Effect of age on wound healing in healthy human beings. Surgery 1992, 112, 293–298. [PubMed] [Google Scholar]
- Burusapat C.; Supawan M.; Pruksapong C.; Pitiseree A.; Suwantemee C. Topical Aloe vera gel for accelerated wound healing of split-thickness skin graft donor sites: A double-blind, randomized, controlled trial and systematic review. Plast. Reconstr. Surg. 2018, 142, 217–226. 10.1097/PRS.0000000000004515. [DOI] [PubMed] [Google Scholar]
- Galeano M.; Altavilla D.; Bitto A.; Minutoli L.; Calò M.; Cascio P. L.; Polito F.; Giugliano G.; Squadrito G.; Mioni C.; et al. Recombinant human erythropoietin improves angiogenesis and wound healing in experimental burn wounds. Crit. Care Med. 2006, 34, 1139–1146. 10.1097/01.CCM.0000206468.18653.EC. [DOI] [PubMed] [Google Scholar]
- Brown N. J.; Smyth E. A.; Cross S. S.; Reed M. W. Angiogenesis induction and regression in human surgical wounds. Wound Repair Regener. 2002, 10, 245–251. 10.1046/j.1524-475x.2002.10408.x. [DOI] [PubMed] [Google Scholar]
- Ud-Din S.; Bayat A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regener. 2017, 25, 164–176. 10.1111/wrr.12513. [DOI] [PubMed] [Google Scholar]
- Stamm A.; Reimers K.; Strauß S.; Vogt P.; Scheper T.; Pepelanova I. In vitro wound healing assays – state of the art. BioNanoMaterials 2016, 17, 79–87. 10.1515/bnm-2016-0002. [DOI] [Google Scholar]
- Jonkman J. E. N.; Cathcart J. A.; Xu F.; Bartolini M. E.; Amon J. E.; Stevens K. M.; Colarusso P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urciuolo F.; Passariello R.; Imparato G.; Casale C.; Netti P. A. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering 2022, 9, 233. 10.3390/bioengineering9060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-H.; Kim J.-Y.; Kim D. J.; Kim M.; Chang M.; Chuck R. S.; Park C. Y. Effect of Nitric Oxide on Human Corneal Epithelial Cell Viability and Corneal Wound Healing. Sci. Rep. 2017, 7, 8093 10.1038/s41598-017-08576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varankar S. S.; Bapat S. A. Migratory Metrics of Wound Healing: A Quantification Approach for in vitro Scratch Assays. Front. Oncol. 2018, 8, 633 10.3389/fonc.2018.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Nogués C.; O’Brien T. In vitro models for assessing therapeutic angiogenesis. Drug Discovery Today 2016, 21, 1495–1503. 10.1016/j.drudis.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Arranz-Valsero I.; Soriano-Romaní L.; García-Posadas L.; López-García A.; Diebold Y. IL-6 as a corneal wound healing mediator in an in vitro scratch assay. Exp. Eye Res. 2014, 125, 183–192. 10.1016/j.exer.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Gaboriau H. P.; Murakami C. S. Skin anatomy and flap physiology. Otolaryngol. Clin. North Am. 2001, 34, 555–569. 10.1016/s0030-6665(05)70005-0. [DOI] [PubMed] [Google Scholar]
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- Vang Mouritzen M.; Jenssen H. Optimized Scratch Assay for In Vitro Testing of Cell Migration with an Automated Optical Camera. J. Vis. Exp. 2018, 57691 10.3791/57691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.-C.; Park A. Y.; Guan J.-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- De Ieso M. L.; Pei J. V. An accurate and cost-effective alternative method for measuring cell migration with the circular wound closure assay. Biosci. Rep. 2018, 38, BSR20180698 10.1042/BSR20180698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Wang Y. L.; Ren F.; Lele T. P. Stamp wound assay for studying coupled cell migration and cell debris clearance. Langmuir 2010, 26, 16672–16676. 10.1021/la103542y. [DOI] [PubMed] [Google Scholar]; From NLM.
- Hettler A.; Werner S.; Eick S.; Laufer S.; Weise F. A new in vitro model to study cellular responses after thermomechanical damage in monolayer cultures. PLoS One 2013, 8, e82635 10.1371/journal.pone.0082635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese C. R.; Wegener J.; Walker S. R.; Giaever I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 1554–1559. 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan M. D.; Mill C. P.; Riese D. J. 2nd; Leary J. F. A high throughput, interactive imaging, bright-field wound healing assay. Cytometry, Part A 2011, 79A, 227–232. 10.1002/cyto.a.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby W. J.; Zijlstra A. Established and novel methods of interrogating two-dimensional cell migration. Integr. Biol. 2012, 4, 1338–1350. 10.1039/c2ib20154b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. J.; Yang J. P.; Wu B. M.; Tawil B. A Novel Three-Dimensional Wound Healing Model. J. Dev. Biol. 2014, 2, 198–209. 10.3390/jdb2040198. [DOI] [Google Scholar]
- Pijuan J.; Barceló C.; Moreno D. F.; Maiques O.; Sisó P.; Marti R. M.; Macià A.; Panosa A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107 10.3389/fcell.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Sito L.; Mao M.; He J.; Zhang Y. S.; Zhao X. Current advances in skin-on-a-chip models for drug testing. Microphysiol. Syst. 2018, 1, 1–9. 10.21037/mps.2018.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. M.Histocultures and Their Use. In Encyclopedia of Life Sciences; Wiley, 2001. [Google Scholar]
- Li D.; Lin T. L.; Lipe B.; Hopkins R. A.; Shinogle H.; Aljitawi O. S. A novel extracellular matrix-based leukemia model supports leukemia cells with stem cell-like characteristics. Leuk. Res. 2018, 72, 105–112. 10.1016/j.leukres.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Michniak-Kohn B. B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. 10.3390/pharmaceutics4010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M.; Leung A.. Creation of Human Skin Equivalents for the In Vitro Study of Angiogenesis in Wound Healing. In Angiogenesis Protocols, Springer, 2009; pp 241–248. [DOI] [PubMed] [Google Scholar]
- Brohem C. A.; da Silva Cardeal L. B.; Tiago M.; Soengas M. S.; de Moraes Barros S. B.; Maria-Engler S. S. Artificial skin in perspective: concepts and applications. Pigm. Cell Melanoma Res. 2011, 24, 35–50. 10.1111/j.1755-148X.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong K.-H.; Park D.; Lee Y.-C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: a mini-review. J. Polym. Res. 2017, 24, 112 10.1007/s10965-017-1278-4. [DOI] [Google Scholar]
- Urciuolo F.; Passariello R.; Imparato G.; Casale C.; Netti P. A. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering 2022, 9, 233 10.3390/bioengineering9060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi M.; Bansal R.; Rouwkema J. Bioengineered 3D Models to Recapitulate Tissue Fibrosis. Trends Biotechnol. 2020, 38, 623–636. 10.1016/j.tibtech.2019.12.010. [DOI] [PubMed] [Google Scholar]
- Todd C.; Hewitt S.; Kempenaar J.; Noz K.; Thody A.; Ponec M. Co-culture of human melanocytes and keratinocytes in a skin equivalent model: effect of ultraviolet radiation. Arch. Dermatol. Res. 1993, 285, 455–459. 10.1007/BF00376817. [DOI] [PubMed] [Google Scholar]
- Kosten I. J.; Spiekstra S. W.; de Gruijl T. D.; Gibbs S. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol. Appl. Pharmacol. 2015, 287, 35–42. 10.1016/j.taap.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Chau D. Y. S.; Johnson C.; MacNeil S.; Haycock J. W.; Ghaemmaghami A. M. The development of a 3D immunocompetent model of human skin. Biofabrication 2013, 5, 035011 10.1088/1758-5082/5/3/035011. [DOI] [PubMed] [Google Scholar]
- Lorthois I.; Simard M.; Morin S.; Pouliot R. Infiltration of T cells into a three-dimensional psoriatic skin model mimics pathological key features. Int. J. Mol. Sci. 2019, 20, 1670. 10.3390/ijms20071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. U.; Abaci H. E.; Herron L.; Guo Z.; Sallee B.; Pappalardo A.; Jackow J.; Wang E. H. C.; Doucet Y.; Christiano A. M. Recapitulating T cell infiltration in 3D psoriatic skin models for patient-specific drug testing. Sci. Rep. 2020, 10, 4123 10.1038/s41598-020-60275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud N. N.; Alkilany A. M.; Dietrich D.; Karst U.; Al-Bakri A. G.; Khalil E. A. Preferential accumulation of gold nanorods into human skin hair follicles: Effect of nanoparticle surface chemistry. J. Colloid Interface Sci. 2017, 503, 95–102. 10.1016/j.jcis.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Mahmoud N. N.; Alhusban A. A.; Ali J. I.; Al-Bakri A. G.; Hamed R.; Khalil E. A. Preferential Accumulation of Phospholipid-PEG and Cholesterol-PEG Decorated Gold Nanorods into Human Skin Layers and Their Photothermal-Based Antibacterial Activity. Sci. Rep. 2019, 9, 5796 10.1038/s41598-019-42047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani A. Z.; Nasereddin J.; Hamed R.; Nimrawi S.; Hussein G.; Abo-Zour H.; Donnelly R. F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152 10.3390/pharmaceutics14061152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal A.; Kant V.; Gopalakrishnan A.; Tandan S. K.; Kumar D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014, 731, 8–19. 10.1016/j.ejphar.2014.02.033. [DOI] [PubMed] [Google Scholar]
- Ahmad S. U.; Binti Aladdin N.-A.; Jamal J. A.; Shuid A. N.; Mohamed I. N. Evaluation of Wound-Healing and Antioxidant Effects of Marantodes pumilum (Blume) Kuntze in an Excision Wound Model. Molecules 2021, 26, 228 10.3390/molecules26010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Garcia J.; Sebastian A.; Alonso-Rasgado T.; Bayat A. Optimization of an ex vivo wound healing model in the adult human skin: Functional evaluation using photodynamic therapy. Wound Repair Regener. 2015, 23, 685–702. 10.1111/wrr.12325. [DOI] [PubMed] [Google Scholar]
- Rakita A.; Nikolić N.; Mildner M.; Matiasek J.; Elbe-Bürger A. Re-epithelialization and immune cell behaviour in an ex vivo human skin model. Sci. Rep. 2020, 10, 1 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y.; Maibach H. I. Dermal-epidermal separation methods: research implications. Arch. Dermatol. Res. 2018, 310, 1–9. 10.1007/s00403-017-1774-8. [DOI] [PubMed] [Google Scholar]
- Menke N. B.; Cain J. W.; Reynolds A.; Chan D. M.; Segal R. A.; Witten T. M.; Bonchev D. G.; Diegelmann R. F.; Ward K. R. An in silico approach to the analysis of acute wound healing. Wound Repair Regener. 2010, 18, 105–113. 10.1111/j.1524-475X.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- Mandale V.; Thomas A.; Wavhale R.; Chitlange S. In-silico Screening of Phytoconstituents on Wound Healing Targets - Approaches and Current Status. Curr. Drug Discovery Technol. 2022, 19, e301121198426 10.2174/1570163819666211130141442. [DOI] [PubMed] [Google Scholar]
- Machado M. J. C.; Watson M. G.; Devlin A. H.; Chaplain M. A. J.; McDougall S. R.; Mitchell C. A. Dynamics of Angiogenesis During Wound Healing: A Coupled In Vivo and In Silico Study. Microcirculation 2011, 18, 183–197. 10.1111/j.1549-8719.2010.00076.x. [DOI] [PubMed] [Google Scholar]
- Grambow E.; Sorg H.; Sorg C. G. G.; Strüder D. Experimental Models to Study Skin Wound Healing with a Focus on Angiogenesis. Med. Sci. 2021, 9, 55 10.3390/medsci9030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buganza Tepole A.; Kuhl E. Systems-based approaches toward wound healing. Pediatr. Res. 2013, 73, 553–563. 10.1038/pr.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scianna M.; Bassino E.; Munaron L. A cellular Potts model analyzing differentiated cell behavior during in vivo vascularization of a hypoxic tissue. Comput. Biol. Med. 2015, 63, 143–156. 10.1016/j.compbiomed.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Ud-Din S.; Bayat A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regener. 2017, 25, 164–176. 10.1111/wrr.12513. [DOI] [PubMed] [Google Scholar]
- Al-Warhi T.; Elmaidomy A. H.; Maher S. A.; Abu-Baih D. H.; Selim S.; Albqmi M.; Al-Sanea M. M.; Alnusaire T. S.; Ghoneim M. M.; Mostafa E. M.; et al. The Wound-Healing Potential of Olea europaea L. Cv. Arbequina Leaves Extract: An Integrated In Vitro, In Silico, and In Vivo Investigation. Metabolites 2022, 12, 791 10.3390/metabo12090791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risueño I.; Valencia L.; Jorcano J. L.; Velasco D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901 10.1063/5.0046376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon V.; Berthod F.; Black A. F.; Damour O.; Germain L.; Auger F. A. A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br. J. Dermatol. 2003, 148, 1094–1104. 10.1046/j.1365-2133.2003.05298.x. [DOI] [PubMed] [Google Scholar]
- Pappalardo A.; Herron L.; Alvarez Cespedes D. E.; Abaci H. E. Quantitative Evaluation of Human Umbilical Vein and Induced Pluripotent Stem Cell-Derived Endothelial Cells as an Alternative Cell Source to Skin-Specific Endothelial Cells in Engineered Skin Grafts. Adv. Wound Care 2021, 10, 490–502. 10.1089/wound.2020.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaci H. E.; Guo Z.; Coffman A.; Gillette B.; Lee W. H.; Sia S. K.; Christiano A. M. Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells. Adv Healthcare Mater. 2016, 5, 1800–1807. 10.1002/adhm.201500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N.; Morimoto Y.; Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. 10.1016/j.biomaterials.2016.11.031. [DOI] [PubMed] [Google Scholar]
- Mori N.; Morimoto Y.; Takeuchi S. Perfusable and stretchable 3D culture system for skin-equivalent. Biofabrication 2019, 11, 011001 10.1088/1758-5090/aaed12. [DOI] [PubMed] [Google Scholar]
- Bhatia S. N.; Ingber D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- Mah C. S.; Kochhar J. S.; Ong P. S.; Kang L. A miniaturized flow-through cell to evaluate skin permeation of endoxifen. Int. J. Pharm. 2013, 441, 433–440. 10.1016/j.ijpharm.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Abaci H. E.; Guo Z.; Coffman A.; Gillette B.; Lee W.; Sia S. K.; Christiano A. M. Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv. Healthcare Mater. 2016, 5, 1800–1807. 10.1002/adhm.201500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan Q.; Ting F. C. W. In vitro micro-physiological immune-competent model of the human skin. Lab Chip 2016, 16, 1899–1908. 10.1039/c6lc00229c. [DOI] [PubMed] [Google Scholar]
- Biglari S.; Le T. Y. L.; Tan R. P.; Wise S. G.; Zambon A.; Codolo G.; De Bernard M.; Warkiani M.; Schindeler A.; Naficy S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Healthcare Mater. 2019, 8, 1801307 10.1002/adhm.201801307. [DOI] [PubMed] [Google Scholar]
- Arslan-Yildiz A.; El Assal R.; Chen P.; Guven S.; Inci F.; Demirci U. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103 10.1088/1758-5090/8/1/014103. [DOI] [PubMed] [Google Scholar]
- Weng T.; Zhang W.; Xia Y.; Wu P.; Yang M.; Jin R.; Xia S.; Wang J.; You C.; Han C.; Wang X. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574 10.1177/20417314211028574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael S.; Sorg H.; Peck C. T.; Koch L.; Deiwick A.; Chichkov B.; Vogt P. M.; Reimers K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One 2013, 8, e57741 10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayavenkataraman S.; Lu W.; Fuh J. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001 10.1088/1758-5090/8/3/032001. [DOI] [PubMed] [Google Scholar]
- Olejnik A.; Semba J. A.; Kulpa A.; Dańczak-Pazdrowska A.; Rybka J. D.; Gornowicz-Porowska J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. 10.1021/acssynbio.1c00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblete Jara C.; Nogueira G.; Morari J.; do Prado T. P.; de Medeiros Bezerra R.; Velloso L. A.; Velander W.; de Araújo E. P. An older diabetes-induced mice model for studying skin wound healing. PLoS One 2023, 18, e0281373 10.1371/journal.pone.0281373. [DOI] [PMC free article] [PubMed] [Google Scholar]