Summary
Background
The COVID-19 pandemic impacted the healthcare systems, adding extra pressure to reduce antimicrobial resistance. Therefore, we aimed to evaluate changes in antibiotic prescription patterns after COVID-19 started.
Methods
With the approval of NHS England, we used the OpenSAFELY platform to access the TPP SystmOne electronic health record (EHR) system in primary care and selected patients prescribed antibiotics from 2019 to 2021. To evaluate the impact of COVID-19 on broad-spectrum antibiotic prescribing, we evaluated prescribing rates and its predictors and used interrupted time series analysis by fitting binomial logistic regression models.
Findings
Over 32 million antibiotic prescriptions were extracted over the study period; 8.7% were broad-spectrum. The study showed increases in broad-spectrum antibiotic prescribing (odds ratio [OR] 1.37; 95% confidence interval [CI] 1.36–1.38) as an immediate impact of the pandemic, followed by a gradual recovery with a 1.1–1.2% decrease in odds of broad-spectrum prescription per month. The same pattern was found within subgroups defined by age, sex, region, ethnicity, and socioeconomic deprivation quintiles. More deprived patients were more likely to receive broad-spectrum antibiotics, which differences remained stable over time. The most significant increase in broad-spectrum prescribing was observed for lower respiratory tract infection (OR 2.33; 95% CI 2.1–2.50) and otitis media (OR 1.96; 95% CI 1.80–2.13).
Interpretation
An immediate reduction in antibiotic prescribing and an increase in the proportion of broad-spectrum antibiotic prescribing in primary care was observed. The trends recovered to pre-pandemic levels, but the consequence of the COVID-19 pandemic on AMR needs further investigation.
Funding
This work was supported by Health Data Research UK and by National Institute for Health Research.
Keywords: Antimicrobial resistance, Broad-spectrum antibiotics, COVID-19 pandemic, Primary care
Research in context.
Evidence before this study
The National Health Service (NHS) in the United Kingdom has released data on antibiotic prescribing patterns during the COVID-19 pandemic. Between April 1st and August 31st of 2020 the rate of antibiotic prescribing in primary care (after adjusting for a reduction in the number of appointments) was 6.7% higher than the same period in 2019. Additionally, there was an increase in the use of broad-spectrum antibiotics beginning in March 2020, with a peak occurring between March and May of that year. This peak was higher than previous seasonal peaks.
We searched MEDLINE for literature on the impact of COVID-19 on broad-spectrum antibiotic prescribing trends from March 2020 to December 2022, for articles with titles including (“covid∗” or “coronavirus” or “sars-cov-2”), and title or abstracts including (“antibiotic” or “broad-spectrum”) AND (“primary care” or “GP” or “general practice”)). We found no further studies that focused on changes in broad-spectrum antibiotic prescribing for specific types of infections in the UK.
Added value of this study
To our knowledge this is the first study to explore the impact of the COVID-19 pandemic on broad-spectrum antibiotic prescribing by infection types in primary care. We accessed electronic health records from over 2500 general practices and approximately 23 million individuals across England. The study included over 32 million antibiotic prescriptions from January 2019 to December 2021, with a separate investigation of the use of broad-spectrum antibiotics for uncoded or same-day infection diagnoses coded infections, including Cold, Cough, Sore throat, other upper respiratory tract infections (URTI), lower respiratory tract infections (LRTI), urinary tract infections (UTI), Otitis externa, Otitis media, Sinusitis and COPD. We found that the percentage of broad-spectrum antibiotic prescriptions increased in March 2020 for all groups except UTI and returned to pre-epidemic levels by December 2021. Within the antibiotic user cohort, we estimated the number of broad-spectrum antibiotics that would have been issued if no pandemic occurred, based on the pre-pandemic expectations. We found an additional 122,000 broad-spectrum antibiotics per million antibiotic prescriptions for LRTI diagnoses and 12,100 for patients with sore throat.
Implications of all the available evidence
Our study revealed a significant impact of the pandemic on antibiotic prescribing patterns in primary care. While this work observed a recovery in the proportion of broad-spectrum antibiotics suggests a positive impact from the rapid publication of guidelines, further monitoring is necessary to understand subsequent prescribing trends and to assess the long-term impact of the COVID-19 pandemic to aid in the management of antimicrobial resistance.
Introduction
Coronavirus disease 2019 (COVID-19) has had a strong impact on healthcare and was declared a global pandemic by the World Health Organization on 11th March 2020.1 Primary care services in England promptly responded to the COVID-19 pandemic and changed the delivery of services from face-to-face to telephone or video call.2 To avoid overuse of antibiotics under the impact of COVID-19, in March 2021 National Institute for Health and Care Excellence (NICE) published the COVID-19 rapid guideline which recommended that antibiotics are not indicated for preventing or treating COVID-19 unless there was an additional bacterial coinfection.3 Prescribing narrow-spectrum antibiotics is also important for reducing antimicrobial resistance (AMR). Overuse of broad-spectrum antibiotics (co-amoxiclav, cephalosporins, and quinolones) drives AMR with an increase in resistant bacterial infections that can be life-threatening and hard to treat.4 There have been financial incentives in primary care to reduce broad-spectrum prescribing.5
Recent evidence has demonstrated the impact of COVID-19 on antibiotic prescribing, showing that antibacterial prescribed for respiratory tract infections reduced by 12.4% per season compared with pre-COVID.6,7 In secondary care, there was widespread broad-spectrum antibiotic use in the early pandemic.7 However, little is known about broad-spectrum prescribing patterns during the pandemic. One study found that the proportion of broad-spectrum antibiotics prescribed continued to decline before COVID-19 and increased out of sync with seasonal fluctuations when the pandemic started.8
With the concomitant effects of the COVID-19 and the AMR pandemic, we face a changing healthcare environment. There is an urgent need for research to assess the changes in antibiotic prescribing during COVID-19,9,10 including a more comprehensive evaluation of the changes in the proportion of broad-spectrum antibiotic prescribing. This study aimed to describe broad-spectrum antibiotic use in English primary care during and following the initial surge in COVID-19 cases in England.
Methods
Data source
All primary care data from general practices using TPP SystmOne Software were linked, stored and analysed securely within the OpenSAFELY platform (https://opensafely.org/). To protect patient privacy, all records excluded any free text data, and include pseudonymised data such as coded diagnoses, medications, and physiological parameters. The data from OpenSAFELY-TPP were linked to the SARS-CoV-2 PCR testing results from the Second-Generation Surveillance System (SGSS) at patient level.11 All code for running the analyses is shared openly for review and re-use under MIT open license (https://github.com/opensafely/amr-uom-brit). Detailed pseudonymised patient data is potentially re-identifiable and therefore not shared.
Study population
This study analysed routinely collected primary care data from general practice electronic health records between 1 January 2019 and 31 December 2021. The primary cohort included all registered patient with at least one year of continuous follow-up. Patients with missing sex and age were excluded. For an antibiotic user cohort, all patients with antibiotic prescriptions during study follow-up (2019–2021) were selected.
Outcomes and data extraction
The primary outcome of interest in this study was the proportion of prescribed antibiotics classified as broad-spectrum. We also calculated the monthly volume and rate of all antibiotic prescribing among registered patients. As a subgroup analysis, the proportion of broad-spectrum antibiotic prescribing each month was calculated stratified by age, sex, region, ethnicity, Index of Multiple Deprivation (IMD) quintiles, and with/without same day infection coded. We defined “coded” as antibiotic prescriptions with a corresponding infection record coded in the primary care EHR system on the same day. Infections considered were urinary tract infection (UTI), lower respiratory tract infection (LRTI) and upper respiratory tract infection (URTI), sinusitis, otitis externa, otitis media, cold, chronic obstructive pulmonary disease (COPD), cough, sore throat, pneumonia, and renal (See Supplementary Table S1). Finally, we looked at the proportion of broad-spectrum prescribing among antibiotics prescribed before or after a SARS-CoV-2 positive test. The SARS-CoV-2 positive test event was defined as a positive swab test for SARS-CoV-2 recorded by the Second-Generation Surveillance System. An antibiotic given within 14 days (before or after) of a positive SARS-CoV-2 result was defined as prescribed to a patient with a COVID-19 infection.
We considered 79 types of antibiotics defined by the NHS Dictionary of Medicines and Devices (dm + d) and identified according to chapter 5.1.1 (Antibacterial drugs) of the British National Formulary. Chapter 5.1.9 and Chapter 5.1.10 (Antituberculosis drugs and Antileprotic drugs) were excluded.12,13 The list of broad-spectrum antibiotic antibiotics was based on NICE guidance note KTT9, which included co-amoxiclav, cephalosporins, and quinolones.5,14 This definition was reviewed by three specialists.15 The infection records were identified using a structured clinical vocabulary SNOMED CT.16 All the codelists used in this study can be found in Supplementary Table S1.
Statistical analysis
In the registered patient cohort, the overall number of antibiotic prescriptions was assessed monthly for antibiotic items prescribed per Specific Therapeutic group Age-sex Related Prescribing Unit (STAR-PU).17 STAR-PU is a weighting method developed to assess overall prescribing by adjusting the age and sex distribution of each practice.18 We also queried the monthly count of COVID-19 cases to present the wave of COVID-19 pandemic. In the antibiotic user cohort, we calculated the percentage of prescriptions that were broad spectrum each month. We also calculated this monthly percentage within the sub-group of individuals that had a positive SARS-Cov-2 test record within a 14-day window.
Interrupted time series (ITS) analyses assess the impact of population-level health interventions implemented at defined time points.19 Here, the intervention was identified as the start of the COVID-19 pandemic in England (See Supplementary Fig. S1). To account for diagnostic uncertainty, unexpected public activity, and practice prescribing behaviours between the first positive infection identified in England and the national lockdown restriction (23rd March 2020),20, 21, 22 we conservatively defined the start of the COVID-19 pandemic as 1st April 2020 and excluded the one-month period from 1st March to 31st March 2020 from our interrupted time series analysis.23 The period from 1st January 2019 to 29th February 2020 was defined as pre-COVID-19 and during-COVID-19 included the duration from 1st April 2020 to 31st December 2021. Following standard methodology for interrupted time series analyses, we used a binomial logistic regression model with the number of broad-spectrum antibiotic prescriptions per month weighted by the monthly count of all antibiotic prescriptions (see supplement for detailed formula).24 We fitted the model by including the following variables, a continuous monthly time variable to predict the long-term trend, a binary variable to indicate if the time was pre- or during-COVID-19 to estimate the immediate impact of the COVID-19, and an interaction term between the time variable and COVID-19 binary variable to estimate the recovery of slope after COVID-19 started. Models were also adjusted for seasonal effects (using a categorical variable of calendar month), and first order lagged residuals to adjust for autocorrelation. To adjust for overdispersion, we also scaled the standard error by using the Pearson goodness of fit statistic and divided by the degrees of freedom in the model.25
To estimate the changes in broad-spectrum antibiotic prescribing since the start of the COVID-19 pandemic, we calculated odds ratios (ORs) for the relative difference at the start of the during-COVID-19 compared with the end of the pre-COVID-19. To estimate the sustained effect of COVID-19 over time (the slope), we used the coefficients to estimate the monthly log odds of broad-spectrum prescription after April 2020. We repeated the analysis by fitting Poisson regression to estimate the broad-spectrum prescribing count if there had been no COVID-19 pandemic (by setting the COVID-19 binary variable to zero). We calculated the absolute changes in items of broad-spectrum antibiotic given in one month and three months from the start of COVID-19 by comparing the estimated number of prescriptions.
Several sensitivity analyses were conducted. The exclusion period before start of the COVID-19 pandemic was changed into two months and three months. Amoxicillin was not included as a broad-spectrum antibiotic. Therefore, we assessed the trend of the proportions of amoxicillin separately over time. To confirm that the length of time window did not influence the trend of proportions of COVID-related broad-spectrum antibiotics, we calculated the proportion within a two-day time window.
Data management was performed using Python 3.9.1, with analysis carried out using R 4.0.2.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Over 32 million antibiotic items were prescribed between January 2019 and December 2021 in 2530 practices, representing 40% of English practices. Of the total number of antibiotics, 8.7% were broad-spectrum. We observed a decline in the total number of patients receiving antibiotics during the COVID-19 pandemic (Table 1). The patient characteristics remained stable throughout the study. For patients with at least one antibiotic prescription in 2020, 1,171,553 (27.1%) antibiotic users had recorded at least one SARS-Cov-2 test, and 17.0% of them had positive results.
Table 1.
Characteristics of overall study population stratified by calendar year. N (%).
| 2019 | 2020 | 2021 | |
|---|---|---|---|
| Number of patientsa | 5,078,868 | 4,328,589 | 4,505,988 |
| Number of practices | 2531 | 2531 | 2533 |
| Age | |||
| <18 | 877,367 (17.3) | 632,635 (14.6) | 713,362 (15.8) |
| 18–64 | 2,734,655 (53.8) | 2,406,910 (55.6) | 2,529,004 (56.1) |
| >64 | 1,466,846 (28.9) | 1,289,044 (29.8) | 1,263,622 (28.0) |
| Sex | |||
| Female | 3,119,414 (61.4) | 2,728,549 (63.0) | 2,842,116 (63.1) |
| Male | 1,959,454 (38.6) | 1,600,040 (37.0) | 1,663,872 (36.9) |
| Region | |||
| East of England | 1,224,952 (24.1) | 1,035,132 (23.9) | 1,075,805 (23.9) |
| East Midlands | 891,457 (17.6) | 752,018 (17.4) | 781,568 (17.3) |
| London | 237,733 (4.7) | 202,906 (4.7) | 215,828 (4.8) |
| North East | 258,822 (5.1) | 215,502 (5.0) | 217,121 (4.8) |
| North West | 483,056 (9.5) | 419,987 (9.7) | 442,843 (9.8) |
| South East | 319,916 (6.3) | 277,702 (6.4) | 282,037 (6.3) |
| South West | 671,346 (13.2) | 587,737 (13.6) | 600,072 (13.3) |
| West Midlands | 210,697 (4.1) | 178,706 (4.1) | 187,259 (4.2) |
| Yorkshire and The Humber | 778,261 (15.3) | 656,729 (15.2) | 700,849 (15.6) |
| Unknown | 2628 (0.1) | 2170 (0.1) | 2606 (0.1) |
| Ethnicity | |||
| White | 3,716,152 (73.2) | 3,228,183 (74.6) | 3,331,125 (73.9) |
| Black | 68,439 (1.3) | 58,694 (1.4) | 63,471 (1.4) |
| South Asian | 274,492 (5.4) | 223,932 (5.2) | 246,564 (5.5) |
| Mixed | 59,908 (1.2) | 51,441 (1.2) | 58,622 (1.3) |
| Other | 79,612 (1.6) | 66,796 (1.5) | 72,743 (1.6) |
| Unknown | 880,265 (17.3) | 699,543 (16.2) | 733,463 (16.3) |
| Index of multiple deprivation (IMD) quintile | |||
| 1–most deprived | 897,996 (17.7) | 759,201 (17.5) | 774,886 (17.2) |
| 2 | 982,043 (19.3) | 833,962 (19.3) | 856,856 (19.0) |
| 3 | 1,052,727 (20.7) | 896,214 (20.7) | 925,974 (20.5) |
| 4 | 1,003,778 (19.8) | 853,066 (19.7) | 889,396 (19.7) |
| 5–least deprived | 1,061,064 (20.9) | 902,303 (20.8) | 948,083 (21.0) |
| Unknown | 81,260 (1.6) | 83,843 (1.9) | 110,793 (2.5) |
| Had SARS-CoV-2 test | |||
| No | NA | 3,157,036 (72.9) | 2,637,345 (58.5) |
| Yes | NA | 1,171,553 (27.1) | 1,868,643 (41.5) |
| Had positive SARS-CoV-2 test | |||
| No | NA | 971,994 (83.0) | 1,178,575 (63.1) |
| Yes | NA | 199,559 (17.0) | 690,068 (36.9) |
IMD (Index of Multiple Deprivation) quintile measured from patient-level address.
Number of patients: the yearly number of patients receiving antibiotics.
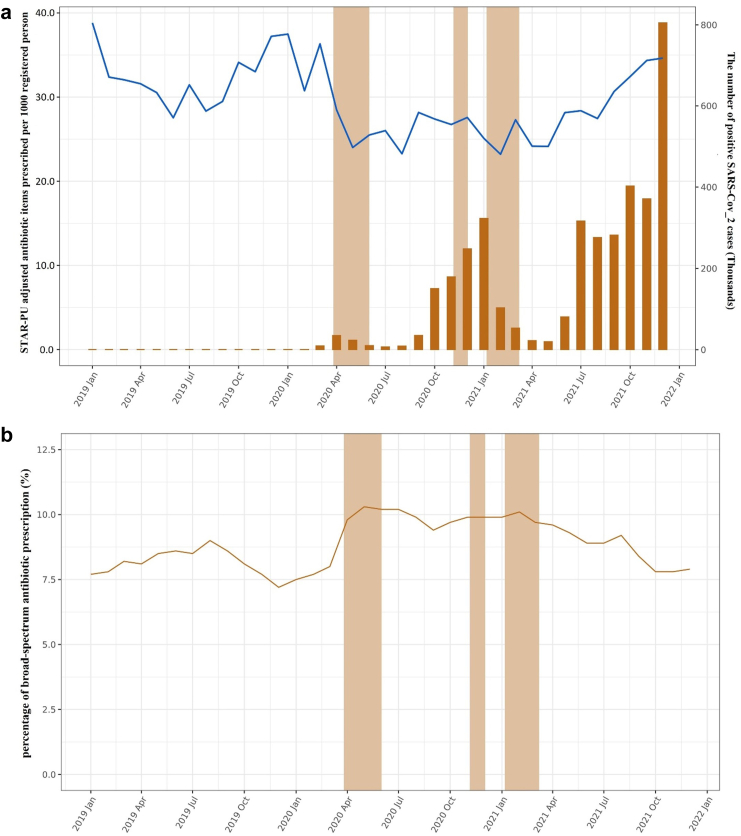
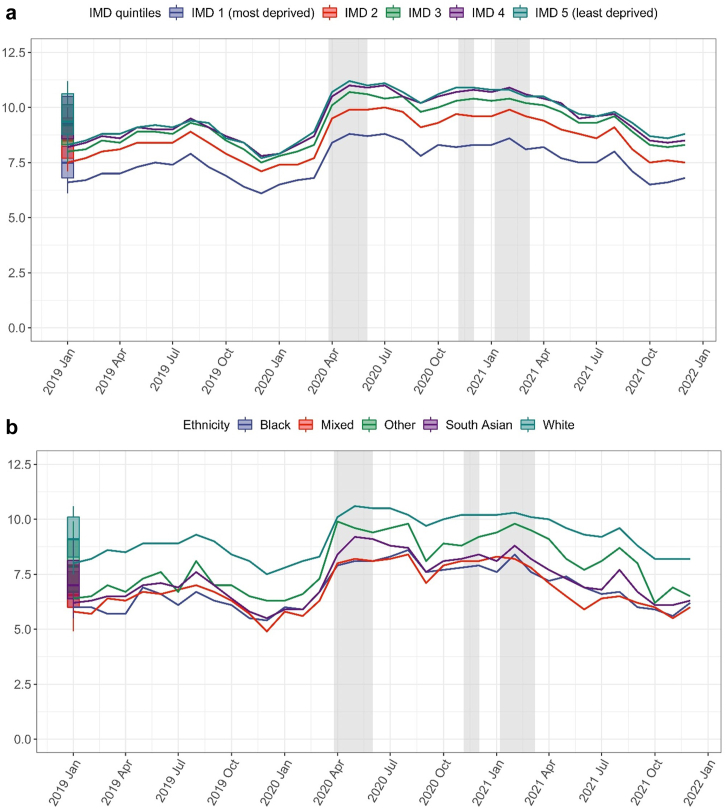
We observed a decrease in the number of STAR-PU adjusted antibiotic items prescribed per 1000 registered patient at the beginning of COVID-19 pandemic in England (Fig. 1). The percentage of broad-spectrum antibiotic prescriptions showed an opposite trend initially but with gradual recovery over time to pre-pandemic levels. There was variability in the percentage of broad-spectrum prescribed by deprivation and ethnicity, although the changes over time were consistent between subgroups (Fig. 2). Supplementary Fig. S2 shows the results stratified by age, sex, region, and antibiotic prescriptions with a corresponding same-day infection record. The percentages of amoxicillin were evaluated separately, and the trend matched what we observed in the overall antibiotic (Supplementary Fig. S3).
Fig. 1.
The overall antibiotic and broad-spectrum antibiotic prescribing trend. a. Monthly number of STAR-PU adjusted antibiotic items prescribed per 1000 registered patients and positive SARS-CoV-2 cases over calendar time. Blue line (Left vertical axis): STAR-PU adjusted antibiotic items. Yellow bars (Right vertical axis): Positive SARS-CoV-2 cases. Shadow areas indicating the periods of national lockdown. b. Monthly percentage of broad-spectrum antibiotic prescribing over calendar time.
Fig. 2.
Monthly percentage of broad-spectrum antibiotic prescribing over calendar time stratified by index of multiple deprivation (a) and ethnicity (b). IMD (Index of Multiple Deprivation) quintile measured from patient-level address. Numerator is the number of broad-spectrum antibiotic prescriptions and denominator number of all antibiotic prescriptions. Boxplots represent the historical average from January 2019 to December 2021 (median and the interquartile range (IQR)) proportion of broad-spectrum antibiotic prescribing. Shadow regions indicating the periods of national lockdown.
Supplementary Table S2 displays the estimated increase in broad-spectrum prescribing for each infection. We found the largest absolute increase in broad-spectrum antibiotic given per one million antibiotic prescriptions for LRTI and estimated that there was cumulatively 122,000 more broad-spectrum antibiotics prescribed from 1st April to 31st July 2020.
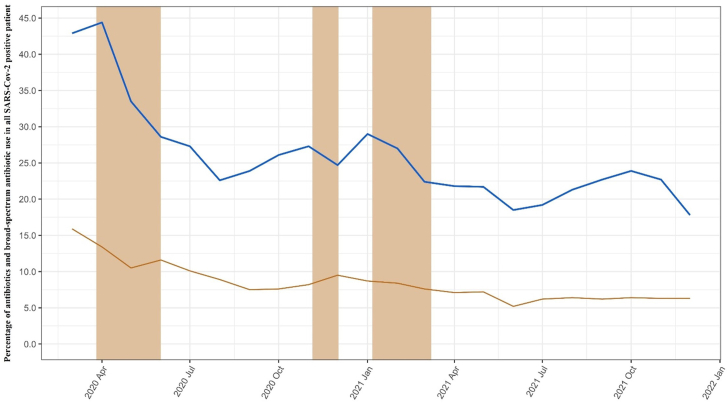
Fig. 3 shows antibiotic use among SAR-CoV-2 positive patients. Of the 889,627 patients, 23.4% of these patients were prescribed at least one antibiotic, of which 7.6% were identified as broad-spectrum. The most frequent antibiotics prescribed were amoxicillin (35.2%), doxycycline (21.2%), clarithromycin (7.7%), nitrofurantoin (7.3%), and phenoxymethylpenicillin (6.8%). The percentage of antibiotics prescribed declined over the study period, from 44% in April 2020 to 23% in August 2020, and remained relatively stable until the end of the study period in December 2021 (Fig. 3). A sensitivity analysis of 2-day time window showed a similar trend (Supplementary Fig. S4).
Fig. 3.
Percentage of overall and broad-spectrum antibiotic prescribing in COVID-19 positive patients within ± 14 days from the date of SARS-Cov-2 positive test. Blue line: Percentage of antibiotic prescribed for COVID-19 positive patients; denominator was monthly number of patients with COVID-19 positive test. Yellow line: Percentage of broad-spectrum antibiotic prescribing in those COVID-19 positive patients prescribed an antibiotic. Shadow area indicating the periods of national lockdown.
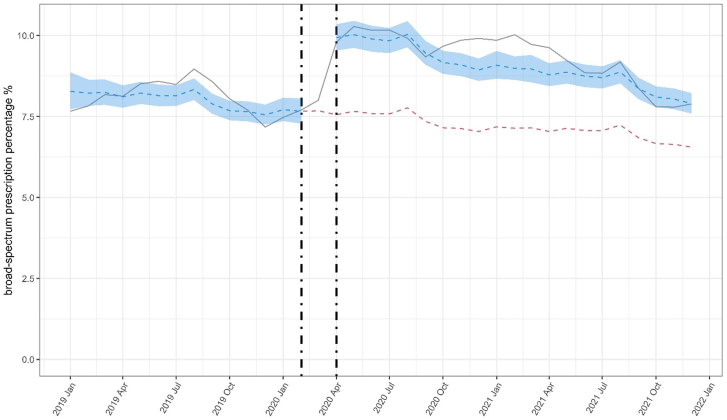
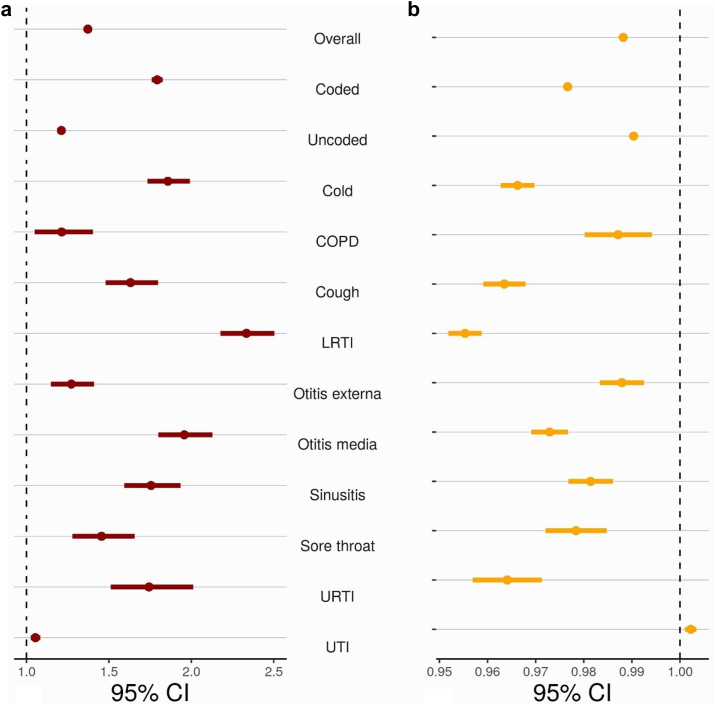
ITS analyses found that the broad-spectrum percentage was higher after COVID-19 started (Fig. 4). Patterns were broadly consistent when stratified by same day infection recording and all infection type, except UTI (Supplementary Fig. S5). There was evidence that the overall broad-spectrum percentage was higher (OR 1.37 [95% CI 1.36–1.38]) at the start of during-COVID-19 than the end of pre-COVID-19 (Fig. 5). We observed that UTI had the lowest increase in the percentage of broad-spectrum antibiotic prescribing. Broad-spectrum antibiotic prescribing returned to normal levels for all infections. Sensitivity analyses using different exclusion periods before start of COVID-19 provided broadly consistent results (Supplementary Figs. S6 and S7).
Fig. 4.
Interrupted time-series analysis of changes in percentage of broad-spectrum antibiotic prescribing pre- and during-COVID-19. Grey line indicates observed percentage of broad-spectrum antibiotics prescribing. Blue line indicates predicted percentage from interrupted time-series model and shaded blue region 95% CI. Vertical dotted lines represent calendar time excluded from analysis. Red line indicates estimated percentage if COVID-19 pandemic had not happened.
Fig. 5.
Changes in the percentage of broad-spectrum antibiotic prescribing during COVID-19 compared to pre-COVID-19 stratified by type of common infection. (a) ORs (95% CI) of percentage of broad-spectrum antibiotic prescribing overall and stratified by infection type immediately after start of COVID-19 compared to pre-pandemic (b) ORs (95% CI) of percentage of broad-spectrum antibiotic prescribing overall and stratified by infection type of monthly time trend during COVID-19 compared to pre-pandemic (OR >1 indicates a recovery trend towards pre-pandemic level, OR <1 increasing differences with pre-pandemic level). Abbreviations: COPD, chronic obstructive pulmonary disease; LRTI, lower respiratory tract infection; URTI, respiratory tract infection; UTI, urinary tract infection. Coded: Antibiotic prescription with same-day infection record in EHR. Uncoded: Antibiotic prescription without same-day infection record in EHR.
Discussion
We found a sharp increase in the proportion of broad-spectrum antibiotic prescribing at the start of the COVID-19 pandemic in England. In contrast, the overall antibiotic prescribing volume decreased at the same time. Patients tested as SAR-CoV-2 positive in primary care were more likely to have an antibiotic, and it was more likely to be a broad-spectrum during the early pandemic. However, that proportion dropped in the later part of the pandemic. The proportion of broad-spectrum prescriptions increased during the early pandemic and recovered to the pre-COVID-19 level later.
This study addresses a research gap in broad-spectrum antibiotic prescribing practices and specific trends in England by providing a comprehensive analysis of broad-spectrum antibiotic prescribing patterns for various common infection types. Furthermore, the impact of the COVID-19 pandemic on antibiotic prescribing patterns is explored in greater depth by examining the proportion of antibiotics prescribed to the population who tested positive for SARS-CoV-2. The Quality Premium was introduced in England to reward local NHS organizations for improvements in the quality of services they commission. Since its implementation in 2015, the percentage of broad-spectrum prescribing has declined by year.26,27 Other UK-based studies have investigated the impact of the COVID-19 pandemic on antibiotic prescribing patterns.6,28, 29, 30 These studies found that antibiotic prescribing initially increased during the early pandemic months before falling below expected levels during the national lockdown, with a sustained increase in antibiotic items per 1000 URTI consultations observed in England.30 The decrease in antibacterial prescribing for RTIs during the pandemic is attributed to a reduction in RTIs and presentations to primary care associated with national prevention measures for COVID-19.6,30 While overall antibacterial usage decreased in both primary and secondary care settings, there were increases in the rate of usage in hospitals in April 2020 and in specific antibacterials.29 These findings emphasise the importance of antimicrobial stewardship during pandemics to ensure appropriate prescribing and to avoid negative consequences on patient outcomes and AMR. Our study observed that although the overall volume of antibiotic items issued dropped sharply when COVID-19 pandemic started, the proportion of these which were broad-spectrum increased. This indicates that the broad-spectrum antibiotic prescription rate did not drop as quickly as narrow spectrum rates. The percentage of broad-spectrum antibiotic prescriptions increased to the highest point in May 2020, and it may have been due to diagnostic uncertainty and prescribing broad-spectrum antibiotics such as co-amoxiclav to cover a range of potential infections during the early pandemic.31 The intervention of the national lockdown policy reduced the chance for face-to-face communication in the community, which might change the epidemiological status of other infectious diseases. For example, the number of consultations was lower than expected in primary care for acute respiratory infections (including pharyngitis) in 2020.28 However, the change in the percentage of broad-spectrum antibiotic prescribing for each infection type in our study was broadly similar, and these results suggested that the changes in prevalence for a certain type of infection during the pandemic was unlikely to be a possible factor in the changes in the percentage of broad-spectrum antibiotic prescribing.32,33 Other national and regional studies also reported on the impact of the COVID-19 pandemic on antibiotic prescribing in primary care, including in the Netherlands, Italy, and the United States.34, 35, 36 For example, an Italian study looked at the tendency to prescribe more broad-spectrum antibiotics for respiratory infections during the pandemic, while total antibiotic prescribing declined significantly from March 2020 onwards.35 However, most of these studies were relatively small and focused on changes in overall antibiotic prescribing rates and the prescribing of antibiotics for respiratory infections.
This study provides a comprehensive analysis of broad-spectrum antibiotic prescribing patterns in primary care during the pandemic, which brings a better understanding of AMR surveillance before and after COVID-19. The most common antibiotics in the 14-day time window were amoxicillin and doxycycline. The changes in broad-spectrum prescribing were not greatly influenced by the introduction of the NICE guideline (in March 2021) that recommends prescribing amoxicillin and doxycycline in COVID-19 patients when the cause of pneumonia-like symptoms is unclear.37 Antibiotic prescribing choices for respiratory tract infections, such as LRTI, URTI, and cough, underwent more significant changes before and during the pandemic, which aligns with the fact that COVID-19 presents with respiratory infection-like symptoms. Furthermore, the recovery level suggests that the influence of COVID-19 on broad-spectrum antibiotic prescribing has likely diminished over time.
This study provides valuable evidence for the potential impact of the COVID-19 pandemic on antibiotic prescribing pattern in primary care using ITS analysis. Although there was an initial increase in the proportion of broad-spectrum antibiotic prescribing, that varied by infection, there were similar patterns of reduction for each infection type over the pandemic period, returning to almost pre-pandemic levels. However, the highest rates of broad-spectrum antibiotic prescribing early pandemic was found for LRTI, and may be related to the fact that coronavirus is an infectious respiratory disease.38
There are several strengths and limitations of this study. This study utilised the OpenSAFELY-TPP database, which is representative of the English population.39 The extensive dataset accessed through TPP's SystmOne clinical information system strengthens the robustness of our findings. In this study, we quantified the change in broad-spectrum antibiotic prescribing over time using an ITS analysis. This allows for a detailed assessment of the longitudinal impact of interventions.19 However, it is challenging in ITS analyses to identify causal relationships such as changes in broad-spectrum antibiotic prescribing patterns and the COVID-19 pandemic. This is because the pandemic brought about behavioural changes in healthcare delivery, clinical guidelines at the individual and societal level, and changes in mortality and health levels. Furthermore, the pandemic has led to a shift towards remote consultations, affecting the way GPs assess and manage patients, which could also have influenced antibiotic prescribing practices. A systematic review and meta-analysis by Bakhit et al. found that the impact of telehealth consultations on antibiotic prescribing varied between conditions.40 That study highlighted there was a significant decrease in antibiotic prescribing for acute rhinosinusitis during telehealth consultations. However, they observed an increase in antibiotic prescribing for acute otitis media during telehealth consultations. Additionally, several studies underscored the importance of examining the broader context of healthcare provision during the pandemic, including the rapid adoption of telemedicine, the reorganisation of primary care, and the potential impact on patient behaviour.23,40, 41, 42 Understanding these factors is crucial for developing effective strategies to ensure appropriate antibiotic prescribing and continued access to healthcare services, even in the face of new and challenging situations.
In conclusion, the COVID-19 pandemic posed challenges in the mission to tackle antimicrobial resistance, as there was an immediate increase in the proportion of broad-spectrum antibiotic prescriptions in primary care at the start of the COVID-19 pandemic. There were substantive difference in levels of broad-spectrum antibiotic prescribing with deprivation and ethnicity, although the relative differences remained stable throughout the pandemic. The recovery in the percentage of broad-spectrum antibiotics and the decrease in the proportion of antibiotics for COVID-19 patients suggest that rapid publication guidelines may have brought a positive impact. Future research should explore the potential unintended consequences of changing prescribing patterns during the COVID-19 pandemic.
Contributors
Conceptualisation: TvS, DA, KH, BMK, VP, AP; Methodology: XZ, VP, AP, YY, AF, JM, PI; Formal analysis: XZ, VP, AP, YY, AF; Diagnostic codelists: TvS; Software: JM, PI, LF, OpenSAFELY Collective, BG, BMK; Writing—original draft: XZ; Writing—revising, review and editing: all authors. All read and approved the final manuscript. TvS is the guarantor for the article, and accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
All data were linked, stored, and analysed securely within the OpenSAFELY platform. All code for running the analyses is shared openly for review and re-use under MIT open license (https://github.com/opensafely/amr-uom-brit). Detailed pseudonymised patient data is potentially re-identifiable and therefore not shared.
Information governance and ethical approval
NHS England is the data controller for OpenSAFELY-TPP; TPP is the data processor; all study authors using OpenSAFELY have the approval of NHS England. This implementation of OpenSAFELY is hosted within the TPP environment which is accredited to the ISO 27001 information security standard and is NHS IG Toolkit compliant;43
Patient data has been pseudonymised for analysis and linkage using industry standard cryptographic hashing techniques; all pseudonymised datasets transmitted for linkage onto OpenSAFELY are encrypted; access to the platform is via a virtual private network (VPN) connection, restricted to a small group of researchers; the researchers hold contracts with NHS England and only access the platform to initiate database queries and statistical models; all database activity is logged; only aggregate statistical outputs leave the platform environment following best practice for anonymisation of results such as statistical disclosure control for low cell counts.44
The OpenSAFELY research platform adheres to the obligations of the UK General Data Protection Regulation (GDPR) and the Data Protection Act 2018. In March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 (COPI) to require organisations to process confidential patient information for the purposes of protecting public health, providing healthcare services to the public and monitoring and managing the COVID-19 outbreak and incidents of exposure; this sets aside the requirement for patient consent.45 This was extended in July 2022 for the NHS England OpenSAFELY COVID-19 research platform.46 In some cases of data sharing, the common law duty of confidence is met using, for example, patient consent or support from the Health Research Authority Confidentiality Advisory Group.47
Taken together, these provide the legal bases to link patient datasets on the OpenSAFELY platform. GP practices, from which the primary care data are obtained, are required to share relevant health information to support the public health response to the pandemic, and have been informed of the OpenSAFELY analytics platform. This study was approved by the Health Research Authority and NHS Research Ethics Committee [REC reference 21/SC/0287].
Declaration of interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare the following: BG and OpenSAFELY has received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. AM has received consultancy fees (from https://inductionhealthcare.com) and is member of RCGP health informatics group and the NHS Digital GP data Professional Advisory Group that advises on access to GP Data for Pandemic Planning and Research (GDPPR). For the latter, he received payment for the GDPPR role.
Acknowledgements
We are very grateful for all the support received from the TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England and the NHS England Transformation Directorate.
This work was supported by Health Data Research UK (Better prescribing in frail elderly people with polypharmacy: learning from practice and nudging prescribers into better practice-BetterRx) and by National Institute for Health Research (NIHR130581–Cluster randomised trial to improve antibiotic prescribing in primary care: individualised knowledge support during consultation for general practitioners and patients—BRIT2).
This research used data assets made available as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant ref MC_PC_20058). In addition, the OpenSAFELY Platform is supported by grants from the Wellcome Trust (222097/Z/20/Z); MRC (MR/V015757/1, MC_PC-20059, MR/W016729/1); NIHR (NIHR135559, COV-LT2-0073), and Health Data Research UK (HDRUK2021.000, 2021.0157).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100653.
Appendix A. Supplementary data
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majeed A., Maile E.J., Bindman A.B. The primary care response to COVID-19 in England's National Health Service. J R Soc Med. 2020;113:208–210. doi: 10.1177/0141076820931452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overview | COVID-19 rapid guideline: managing COVID-19 | Guidance | NICE. https://www.nice.org.uk/guidance/ng191
- 4.Clostridium difficile infection: risk with broad-spectrum antibiotics Evidence summary Key points from the evidence Key points from the evidence. 2015. www.nice.org.uk/guidance/esmpb1pathways [Google Scholar]
- 5.NHS England » antibiotic quality premium monitoring dashboard. https://www.england.nhs.uk/anti-dash/
- 6.Andrews A., Bou-Antoun S., Guy R., Brown C.S., Hopkins S., Gerver S. Respiratory antibacterial prescribing in primary care and the COVID-19 pandemic in England, winter season 2020–21. J Antimicrob Chemother. 2022;77:799–802. doi: 10.1093/jac/dkab443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beovic B., Dousak M., Ferreira-Coimbra J., et al. Antibiotic use in patients with COVID-19: a ‘snapshot’ infectious diseases international research initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75:3386–3390. doi: 10.1093/jac/dkaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N.J., McLeod M., McNulty C.A.M., Lecky D.M., Holmes A.H., Ahmad R. Trends in antibiotic prescribing in out-of-hours primary care in England from January 2016 to June 2020 to understand behaviours during the first wave of COVID-19. Antibiotics. 2021;10:32. doi: 10.3390/antibiotics10010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight G.M., Glover R.E., McQuaid C.F., et al. Antimicrobial resistance and COVID-19: intersections and implications. Elife. 2021;10:1–27. doi: 10.7554/eLife.64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N., Aylin P., Rawson T., Gilchrist M., Majeed A., Holmes A. Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. Clin Microbiol Infect. 2021;27:762–768. doi: 10.1016/j.cmi.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laboratory reporting to UKHSA a guide for diagnostic laboratories Reporting to UKHSA: a guide for diagnostic laboratories.
- 12.What is the dm+d? The NHS dictionary of medicines and devices | bennett institute for applied data science. https://www.bennett.ox.ac.uk/blog/2019/08/what-is-the-dm-d-the-nhs-dictionary-of-medicines-and-devices/
- 13.BNF (British National Formulary) | NICE. https://bnf.nice.org.uk/
- 14.Antimicrobial stewardship: prescribing antibiotics | Advice | NICE.
- 15.Co-amoxiclav, cephalosporins and quinolones Issue #127 opensafely/codelist-development. https://github.com/opensafely/codelist-development/issues/127
- 16.SNOMED CT - NHS Digital. https://digital.nhs.uk/services/terminology-and-classifications/snomed-ct
- 17.Lloyd D.C.E.F., Harris C.M., Roberts D.J. Specific therapeutic group age-sex related prescribing units (STAR-PUs): weightings for analysing general practices' prescribing in England. BMJ. 1995;311:991–994. doi: 10.1136/bmj.311.7011.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Average daily quantity of hypnotics prescribed per specific therapeutic group age-sex related prescribing unit (STAR PU) - NHS. https://www.nhs.uk/scorecard/12030
- 19.Bernal J.L., Cummins S., Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacobucci G. Covid-19: UK lockdown is “crucial” to saving lives, say doctors and scientists. BMJ. 2020;368:m1204. doi: 10.1136/bmj.m1204. [DOI] [PubMed] [Google Scholar]
- 21.Transport use in Great Britain has decreased since the imposition of social distancing rules.
- 22.Vannoni M., Stuckler D., McKee M., Semenza J. Using volunteered geographic information to assess mobility in the COVID-19 pandemic context: cross-city time series analysis of 41 cities in 22 countries from March 2nd to 26th 2020. Global Health. 2020;16(1):85. doi: 10.1186/s12992-020-00598-9. published online July 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansfield K.E., Mathur R., Tazare J., et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health. 2021;3:e217–e230. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews A., Herrett E., Gasparrini A., et al. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ. 2016;353 doi: 10.1136/BMJ.I3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhaskaran K., Gasparrini A., Hajat S., Smeeth L., Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. 2013;42:1187–1195. doi: 10.1093/ije/dyt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NHS England » quality premium. https://www.england.nhs.uk/ccg-out-tool/qual-prem/
- 27.Balinskaite V., Johnson A.P., Holmes A., Aylin P. The impact of a national antimicrobial stewardship program on antibiotic prescribing in primary care: an interrupted time series analysis. Clin Infect Dis. 2019;69:227–232. doi: 10.1093/cid/ciy902. [DOI] [PubMed] [Google Scholar]
- 28.Rezel-Potts E., L'Esperance V., Gulliford M.C. Antimicrobial stewardship in the UK during the COVID-19 pandemic: a population-based cohort study and interrupted time-series analysis. Br J Gen Pract. 2021;71:e331–e338. doi: 10.3399/BJGP.2020.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews A., Budd E.L., Hendrick A., et al. Surveillance of antibacterial usage during the COVID-19 pandemic in England, 2020. Antibiotics. 2021;10 doi: 10.3390/ANTIBIOTICS10070841/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z., Bou-Antoun S., Gerver S., Cowling T.E., Freeman R. Sustained increases in antibiotic prescriptions per primary care consultation for upper respiratory tract infections in England during the COVID-19 pandemic. JAC Antimicrob Resist. 2023;5 doi: 10.1093/JACAMR/DLAD012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Impact of COVID-19 on prescribing in english general practice: March 2020 | bennett Institute for applied data science. https://www.bennett.ox.ac.uk/blog/2020/05/impact-of-covid-19-on-prescribing-in-english-general-practice-march-2020/
- 32.GP out-of-hours syndromic surveillance: weekly bulletins for 2020 - GOV.UK. https://www.gov.uk/government/publications/gp-out-of-hours-syndromic-surveillance-weekly-bulletins-for-2020
- 33.GP in hours: weekly bulletins for 2020 - GOV.UK. https://www.gov.uk/government/publications/gp-in-hours-weekly-bulletins-for-2020
- 34.van de Pol A.C., Boeijen J.A., Venekamp R.P., et al. Impact of the COVID-19 pandemic on antibiotic prescribing for common infections in The Netherlands: a primary care-based observational cohort study. Antibiotics. 2021;10:196. doi: 10.3390/antibiotics10020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbieri E., Liberati C., Cantarutti A., et al. Antibiotic prescription patterns in the paediatric primary care setting before and after the COVID-19 pandemic in Italy: an analysis using the AWaRe metrics. Antibiotics. 2022;11:457. doi: 10.3390/antibiotics11040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutcher L., Li Y., Lee G., Grundmeier R., Hamilton K.W., Gerber J.S. COVID-19 and antibiotic prescribing in pediatric primary care. Pediatrics. 2022;149 doi: 10.1542/PEDS.2021-053079/184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overview | COVID-19 rapid guideline: managing COVID-19 | Guidance | NICE. https://www.nice.org.uk/guidance/NG191
- 38.Harapan H., Itoh N., Yufika A., et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13:667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.TPP assists leading epidemiologists in ground-breaking COVID-19 study – TPP. https://tpp-uk.com/news/example-post/
- 40.Bakhit M., Baillie E., Krzyzaniak N., et al. Antibiotic prescribing for acute infections in synchronous telehealth consultations: a systematic review and meta-analysis. BJGP Open. 2021;5 doi: 10.3399/BJGPO.2021.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joy M., McGagh D., Jones N., et al. Reorganisation of primary care for older adults during COVID-19: a cross-sectional database study in the UK. Br J Gen Pract. 2020;70:e540–e547. doi: 10.3399/bjgp20X710933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hincapié M.A., Gallego J.C., Gempeler A., Piñeros J.A., Nasner D., Escobar M.F. Implementation and usefulness of telemedicine during the COVID-19 pandemic: a scoping review. J Prim Care Community Health. 2020;11 doi: 10.1177/2150132720980612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Data security and protection Toolkit - NHS digital. https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit
- 44.ISB1523: Anonymisation Standard for Publishing Health and Social Care Data - NHS Digital. https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb1523-anonymisation-standard-for-publishing-health-and-social-care-data
- 45.Coronavirus (COVID-19): notification to organisations to share information - GOV.UK. https://web.archive.org/web/20200421171727/https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information
- 46.Coronavirus (COVID-19): notice under regulation 3(4) of the health service (control of patient information) Regulations 2002 - GOV.UK. https://www.gov.uk/government/publications/coronavirus-covid-19-notification-to-organisations-to-share-information/coronavirus-covid-19-notice-under-regulation-34-of-the-health-service-control-of-patient-information-regulations-2002
- 47.Confidentiality Advisory Group - Health Research Authority. https://www.hra.nhs.uk/about-us/committees-and-services/confidentiality-advisory-group/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.