Abstract
Objectives
Hepatitis C virus (HCV) poses a global public health threat. Prisons are a focus of prevention efforts due to high infection burdens. Expedition of treatment for incarcerated people is critical, as many are short-term sentenced. We evaluated point-of-care (PoC) HCV RNA testing in a maximum-security Scottish prison and assessed its impact on transition to treatment. We also evaluated costs and determinants of implementation.
Design
Mixed-methods evaluation of a single-centre care pathway pilot using National Health Service (NHS) data from 2018 to 2021. Descriptive statistics and survival analysis were undertaken. Cost analysis was assessed from a provider perspective. Healthcare staff participated in semistructured interviews and thematic analysis with a deductive approach was undertaken to identify implementation determinants.
Setting
A large maximum-security Scottish prison health centre administered by the NHS.
Participants
296 incarcerated NHS patients (all men) and six NHS staff members (two men and four women).
Interventions
HCV testing using the Cepheid GeneXpert platform with Xpert HCV VL Fingerstick assay.
Outcome measures
The main outcome was survival (in days) from HCV test to treatment initiation. Secondary outcomes were cost-per-cure obtained and implementation determinants.
Results
During the pilot, 167 Xpert tests were administered, with an 84% completion rate, and treatment transition was superior for those who received it (p=0.014). Where PoC tests were administered, shorter survival to treatment was observed (19 vs 33 days: adjusted HR (aHR) 1.91 (1.03–3.55), p=0.040; 19 vs 50 days; aHR 3.76 (1.67–8.46), p=0.001). PoC was costlier than conventional testing. In qualitative analysis, most facilitators were observed among characteristics of individual domain while most barriers were noted in the inner setting.
Conclusions
Integrating PoC HCV RNA diagnosis into nurse-led HCV care in a maximum-security prison health centre shortens survival to HCV treatment. However, there are cost implications to this approach and multiple determinants that impact on implementation should be addressed.
Keywords: Organisation of health services, Public health, INFECTIOUS DISEASES, Hepatology, PUBLIC HEALTH, QUALITATIVE RESEARCH
Strengths and limitations of this study.
The study is strengthened by assessing the feasibility of point-of-care (PoC) testing from multiple angles, which address clinical impact, costs to the health service and barriers and facilitators to implementation, giving a holistic view of this approach.
In contrast to other similar work, a strength of this study is that PoC testing was administered by nurses in the prison health centre.
The study is limited by a small sample in the qualitative component, and its single-centre nature, which both restrict the generalisability of the findings.
The study is further limited by only including National Health Service (NHS) staff in the qualitative component.
Introduction
Hepatitis C virus (HCV) infection is an enduring global public health threat. For those infected, in the absence of diagnosis and linkage to treatment, it can cause long-term negative health outcomes, such as progression of liver fibrosis to eventual cirrhosis, decreased health-related quality of life and extra-hepatic sequelae such as renal or cardiovascular impairment. Prisons have been an important focus of HCV prevention efforts due to their high HCV burden relative to the general population, which intersects with the large number of people who inject drugs (PWID) who are imprisoned.1 Imprisonment rates among PWID are substantial, with up to 58% estimated to have ever been incarcerated.2 Further figures suggest that up to 38% of incarcerated people may have been exposed to HCV, due to the overlapping nature of injection drug use (IDU) and incarceration, and the absence of primary prevention measures for PWID while incarcerated.3 4 Sharing of non-sterile injecting equipment in prisons is the leading cause of HCV transmission.2 A previous study of Scottish prisons found that 32% of people in prison had a history of IDU and, among those, HCV prevalence was 53%.5
Recent data indicate that approximately 71% of individuals test positive for illicit substances on reception to Scottish prisons; of those, 29% test positive for opioids and 24% for cocaine, which are commonly injected.6 In the prison in this evaluation, approximately 38% and 18% of individuals tested positive for these, respectively, on reception, implying an ongoing risk of blood-borne virus (BBV) transmission.6 The Scottish justice system has a ‘remand problem’, defined as imprisonment awaiting sentence for 40–140 days.7 8 In recent data, which spans the pilot period of this project, those identified as being in the part-year prison population across the prison estate, that is, residing in a given establishment for less than one whole year, was estimated at 80.2%.9 Recent figures for His Majesty’s Prison (HMP) Perth, the prison in this pilot, estimated the remand population at approximately 22.8%.10 These figures suggest substantial proportions of the prison population are highly transient, at risk of HCV transmission, with a short time frame for healthcare engagement.
In the context of HCV, expedition of treatment for incarcerated persons is important to avoid loss to the system. Treating HCV-infected individuals while incarcerated has been identified as an important engagement strategy for people otherwise disconnected from conventional healthcare.11 HCV treatment duration is relatively fixed, which leaves diagnosis as the key remaining modifiable care component.12 Particularly in the absence of enhanced harm reduction supports to reduce risk, which are scarcely available in prisons.13 This is especially pertinent to Tayside because Dundee, whose population is served by the prison in this evaluation, has the highest rate of incarceration per head of population in Scotland as well as a historically high burden of HCV infection.9 14 Consequent to this historically high HCV burden, Tayside has a suite of well-developed community care pathways which offer HCV care from multiple environments. Those affected by BBVs, such as HCV, who are liberated from HMP Perth to the local area are appointed to nurse-led community outreach clinics or local pharmacies for treatment continuation or post-treatment follow-up after liberation.
In recent years, point-of-care (PoC) HCV testing platforms have become available which could ameliorate time burdens associated with existing testing methods and streamline linkage to treatment. However, the evidence documenting the impact these devices have in real-world prison contexts is nascent. Furthermore, the determinants to integrating PoC testing for HCV RNA into prison environments are unclear and there has been limited examinations of the cost implications of such interventions in UK prisons. This manuscript describes a pilot project in a Scottish prison which integrated PoC HCV RNA testing into routine on-site nurse-led care using the Cepheid GeneXpert platform with the Xpert HCV VL Fingerstick assay.15 The primary outcome of this study was to determine whether there was a difference in survival, measured in days, from a positive HCV RNA result to treatment initiation, among those who received a PoC test relative to those tested conventionally. Secondary outcomes were to: assess the cost of PoC RNA testing relative to conventional methods and evaluate the determinants to implementing the PoC RNA testing platform.
Methods
Study design
This was a mixed-methods NHS service evaluation—with retrospective analysis of routine NHS HCV testing, treatment and cost data, and prospective qualitative interviews—of a modified HCV care pathway in HMP Perth, a prison in central Scotland.16 17 Accordingly, no randomisation, masking or allocation to alternating interventions were undertaken as part of this pilot (choice of test rested with practitioner/patient). Caldicott Guardian approval was granted for data access (IGTCAL7004).18 This process reviews internal NHS evaluations, ensuring the protection and appropriate use of patient data. The evaluation was registered with the NHS clinical governance group for prison healthcare (ref: 27/19).
Patient and public involvement
Patients and members of the public were not involved in the design or conduct of this work.
Setting
NHS Tayside is a large health board area located on the East of Scotland. HMP Perth is a large maximum-security male prison in the NHS Tayside board area, which houses people on mixed duration sentences.19 Healthcare is provided by the NHS from an on-site centre. Opt-out HCV testing is in place on reception to prison and includes conventional phlebotomy and dried blood spot (DBS) methods.20 Prison staff escort individuals from the residential areas of the prison to BBV clinics. As a test of change, PoC HCV RNA testing was integrated into routine care in prison BBV clinics alongside conventional testing methods.
Participants
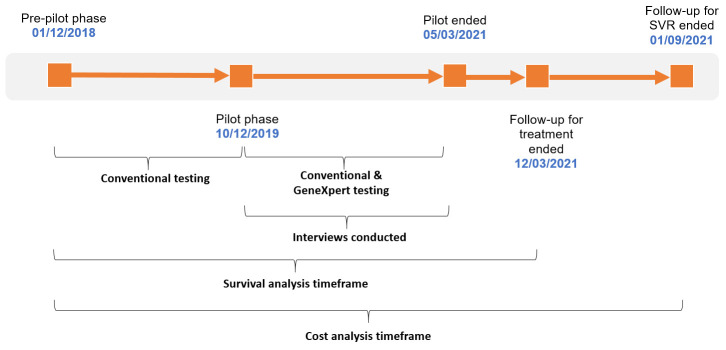
This study used existing service data for quantitative analysis. All adults (≥18 years) with detectable HCV RNA, and/or treated for HCV in HMP Perth from December 2018 to March 2021, were eligible for inclusion. The timeline for the study is shown in figure 1. Data were collected for a 1-year ‘pre-pilot’ phase, when only conventional testing was offered, to compare against the pilot phase data. In the analysis, those tested during the pilot phase were grouped by whether they received a PoC test or a conventional test, for comparison. NHS Tayside staff members involved in any stage of the implementation process were eligible to participate in the prospective qualitative strand.
Figure 1.
Summary of observation dates and study activities. Conventional testing was by whole blood sent to a laboratory for analysis and dried blood spot methods.
Clinical outcomes
Clinical outcomes were collected to inform the cost analysis. Sustained virologic response (SVR) was undetectable (<10 IU/mL) HCV RNA at least 12 weeks post-treatment. Relapse was undetectable RNA at end of treatment, but detectable prior to or at SVR; or treatment initiation and detectable RNA prior to or at SVR, if end of treatment test not conducted. Loss to follow-up (LTFU) was defined as no post-treatment RNA test on record up to and including the censor date.
Statistics
Descriptive statistics were undertaken to obtain relevant counts and proportions. To assess the primary outcome, individuals were grouped depending on their test type (conventional or PoC) and when the test was taken (prepilot or during the pilot). Kaplan-Meier failure analysis and log-rank testing were undertaken, followed by Cox proportional hazards (PH) modelling. Two PH models were fit: one comparing the PoC group to the prepilot conventionally tested group; and one comparing the PoC group to those tested conventionally during the pilot. This strategy was chosen for two reasons: (1) to account for any changes to service delivery beyond our control during the pilot period (eg, anything implemented by the prison service) and (2) the COVID-19 pandemic occurred during the pilot, which impacted on laboratory test turnaround times. We sought to ensure any effect observed was independent of this lag. Models were also adjusted for age, as a proxy for potential transience through the prison (in the absence of sentencing data and based on the experience of the project team). Limited models were also performed (online supplemental table S1) with straightforward comparisons based on test type alone. The terminating event was treatment initiation. To assess treatment opportunity loss during the pilot, equality of proportions who remained untreated between groups was tested using a two-sample test of proportions (z test). Statistical testing was undertaken using Stata BE V.17. P values of ≤0.05 were assumed to demonstrate statistical significance.
bmjopen-2022-068604supp001.pdf (978.2KB, pdf)
Cost analysis
Although healthcare cost analyses typically express outcomes in quality-adjusted life years and willingness-to-pay thresholds,21 it was not possible to collect the data for this type of analysis in this retrospective evaluation. Consequently, an incremental ‘cost-per-SVR’ approach was taken from an NHS perspective, where the costs of all HCV RNA test and treatment were summed and divided by the population benefits of linkage to care, that is, obtaining SVR. Costs for all relevant sample types were obtained from the manufacturer or NHS department. Medication costs were estimated from the British National Formulary and published sources and do not account for discounting in the primary calculations.22–24 Staff time was costed proportionately in line with NHS agenda for change.25 Estimates do not include sundry items and do not account for inflation. Those whose pretreatment HCV RNA test could not be verified were excluded. The time horizon was the study period.
Qualitative methods
A convenience sample of NHS staff members (n=8) known to the research team, and involved in implementing the GeneXpert, were invited to participate in semistructured interviews. Written informed consent was obtained. For practical reasons, focus groups were undertaken with nursing staff, while individual interviews were undertaken with others. These were recorded digitally and transcribed verbatim with identifying data censored. The Consolidated Framework for Implementation Research (CFIR) informed interview guide design and data analysis.26 The interview guides are included in online supplemental tables S2–S5. The CFIR is a meta typology composed of five major domains, which provides a structured and pragmatic approach for understanding real-world implementation initiatives.26 It was selected for its system-level approach, consistent with the NHS analytic perspective. Non-NHS staff and prison residents were not approached to participate as they were outwith the remit of NHS service evaluation.
Thematic analysis was undertaken to analyse interview data using a deductive approach.27 Transcripts were read two times by the analyst (CJB) and coded for ‘barrier’ or ‘facilitator’. A barrier was defined as any phenomenon that had an inferred negative impact on any aspect of implementation, real or abstract, conversely a facilitator was any phenomenon inferred to have had a positive impact. Once compiled, determinants were allocated to domains of the CFIR. Triangulation of determinants was performed on 20% of transcripts by an independent analyst (AM) according to a predetermined algorithm (online supplemental figure S1). Divergences were discussed until consensus was reached and this informed coding of remaining transcripts. Analysis was on screen or paper, without use of analytic software.
Results
Primary outcomes
From December 2018 to March 2021 (figure 1), 386 RNA tests were performed, which identified 91 (23.6%) RNA-positive cases requiring treatment. Of those 91, 70 (76.9%) were tested conventionally and 21 (23.1%) with the GeneXpert. Sixty-seven (73.6%) individuals started HCV treatment. Of those, seven (10.4%) had missing or unreliable testing data and were excluded, giving a total of 60 (89.6%) treated cases for the primary analysis. In total, 167 (43.3%) RNA tests conducted were administered using the Xpert HCV VL Fingerstick assay. Of all Xpert tests, 23 (13.8%) returned error and three (1.8%) returned invalid results, giving an overall test completion rate of 84.4%. The 26 failed tests occurred among 20 patients. Of those, 15 patients had evidence of retesting using the GeneXpert, consuming 18 Xpert assays (repeat errors), while five had conventional blood draw. In general, the quantity of failed tests decreased over time (online supplemental figure S2). Error rates for conventional tests were not recorded on routine systems and, therefore, are unreported. Xpert test failures were mostly related to manual handling of assay cartridges (n=24; 92.3%). Sixteen (9.6%) Xpert tests were not recorded on electronic health records at the end of the pilot, while 12 (7.2%) had some level of inaccurate information (online supplemental table S6) on the electronic report. This most frequently occurred when testing was reinitiated following a short pause on clinical activities triggered by initial COVID-19 pandemic (online supplemental figure S3).
Descriptive parameters for the analysed cohort who initiated treatment (n=60) are outlined in table 1. Median age was 39 years, and most (70%) cases were HCV treatment naïve. The most frequent infection risk factor was IDU (91.7%). Most (60%) were in receipt of opioid agonist therapy (OAT), and there were no instances of diagnosed cirrhosis. The most common genotype was one (38.3%), followed by three (26.7%).
Table 1.
Demographic and clinical characteristics of treated cases included in time-to-event analysis, 2018–21, HMP Perth, Tayside (n=60)
| Parameter | Treated cases (n=60) |
| Gender—n (%) | |
| Male | 60 (100) |
| Female | 0 (0.0) |
| Age at RNA test—median (IQR) | 39 (33.5–43.5) |
| Infection risk factor—n (%) | |
| IDU | 55 (91.7) |
| Unknown | 5 (8.3) |
| HCV genotype—n (%) | |
| 1 | 23 (38.3) |
| 2 | 1 (1.7) |
| 3 | 16 (26.7) |
| Unknown | 20 (33.3) |
| Prior HCV treatment—n (%) | |
| No | 42 (70.0) |
| Yes | 18 (30.0) |
| OAT—n (%) | |
| No | 24 (40.0) |
| Yes | 36 (60.0) |
| Fibroscan (KpA)—median (IQR)* | 5.7 (4.9–7.5) |
| Fib4 score—median (IQR)† | 0.90 (0.54–1.34) |
| Cirrhosis diagnosis‡—n (%) | |
| No | 60 (100.0) |
| Yes | 0 (0.0) |
*n=9.
†n=52.
‡Cases with Fib4 score ≤1.45 were assumed not to have cirrhosis; for cases in the indeterminate Fib4 range, or cases with a score of ≥3.25, medical notes were manually reviewed to check for a diagnosis of cirrhosis by other means. Where Fib4 was not available, but Fibroscan was available, a score of ≥14 kPa (F4) was used to define the presence of cirrhosis, scores of <14 kPa were assumed not to have cirrhosis. Cases with no assessments for liver stiffness (n=6) who commenced treatment with a standard duration (8 weeks) were assumed not to have cirrhosis.
HCV, hepatitis C virus; HMP, His Majesty’s Prison; IDU, injection drug use; KpA, kilopascals; OAT, opioid agonist therapy.
Time to treatment was 33 (IQR 22–70) days for those conventionally tested from 2018 to 2019; 50 (IQR 33–220) days for those tested conventionally during the pilot phase; and 19 (IQR 7–28) days for those tested using the GeneXpert during the pilot. These differences were statistically significant (X2=13.10, p=0.001). During the pilot phase specifically, 16 of 27 (59.3% (95% CI 40.7 to 77.8)) HCV RNA+ cases tested conventionally did not initiate treatment. Among those tested using the Xpert assay, five of 21 (23.8% (95% CI 5.59 to 42.0)) did not initiate treatment. This translated to a proportionate difference in loss to treatment of 35.5% (95% CI 9.46 to 61.43), which was statistically significant (z=2.47, p=0.014). Clinical and other outcomes are shown in figure 2.
Figure 2.
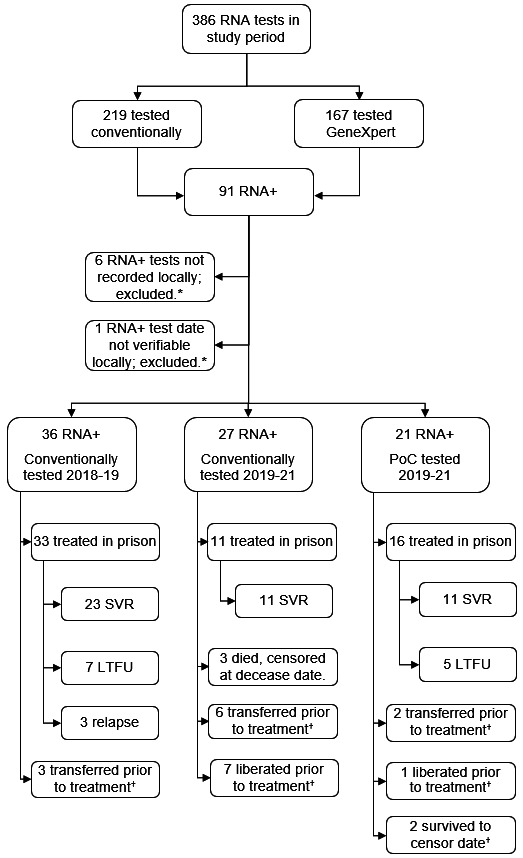
Target cohort profile with related clinical outcomes and censoring. *Cases received treatment but excluded from cost and time-to-treatment analyses, as their testing data were unavailable or unverifiable. †All censored in survival analysis at relevant decease, liberation, transfer or follow-up censor dates. Group 1 are those tested conventionally from 2018 to 19 (reference period); group 2 are those conventionally tested during the pilot phase (2019–21); group 3 are those tested with the GeneXpert during the pilot phase (2019–21). LTFU, lost to follow-up; PoC, point of care; RNA, ribonucleic acid; RNA+, RNA positive (actively infected); SVR, sustained virologic response.
PH modelling, adjusted for age, is shown in table 2. Consistent with the shorter survival time observed, the hazard of treatment was higher for those tested with the GeneXpert in both models, with a higher hazard observed when comparing cases in the pilot phase directly (model 2).
Table 2.
Proportional hazards models adjusted by age
| Variable | n (%) | aHR (95% CI) | P |
| Model 1 | |||
| Conventionally tested 2018–2019 (ref) | 36 (63.2) | … | |
| GeneXpert tested 2019–2021 | 21 (36.8) | 1.91 (1.03 to 3.55) | 0.040 |
| Age at test | … | 1.04 (1.00 to 1.08) | 0.051 |
| Model 2 | |||
| Conventional testing 2019–2021 (ref) | 27 (56.2) | … | |
| GeneXpert tested 2019–21 | 21 (43.8) | 3.76 (1.67 to 8.46) | 0.001 |
| Age at test | … | 1.02 (0.97 to 1.09) | 0.396 |
Model 1 fit: X2=8.07, p=0.017. Harrell’s C: 0.64 (95% CI 0.56 to 0.72), p <0.0001.
Model 1 survival information: n=57; failures=49; time at risk=2827 days.
Model 2 fit: X2=10.93, p=0.004. Harrell’s C: 0.68 (95% CI 0.58 to 0.78), p = <0.0001.
Model 2 survival information: n=48; failures=27; time at risk=2458 days.
aHR, adjusted HR; PH, proportional hazards.
Secondary outcomes
In the cost analysis, the price per SVR was higher (table 3) for those tested with the GeneXpert relative to conventional methods in both the prepilot phase (+£721.30, +1.9%) and the pilot phase (+£14,499.80, +60.7%). However, when maximum discount rates were applied to medication costs, and those who were LTFU post-treatment were assumed to have achieved an SVR,12 PoC testing costs became favourable per SVR achieved relative to the prepilot phase (–£148.51, –4.7%). That said, in this scenario, it remained unfavourable relative to conventional testing in the pilot phase (+£372.39, +14.1%). Retesting with the Xpert assay, following a failed test, contributed roughly £717 of additional cost.
Table 3.
Incremental cost per cure over duration of study observation period by diagnostic test type and study phase
| Parameter | Conventional (2018–2019) |
Conventional (2019–2021) |
GeneXpert (2019–2021) |
| RNA tests (n) | 164* | 55* | 167 |
| Testing† | £9140.61 | £3078.84 | £6656.62 |
| Actual cost per test | £55.74 | £55.98 | £39.86 |
| Medication‡ | £857,559.78§ | £259,866.60¶ | £415,786.56** |
| Total costs | £866 700.39 | £262 945.44 | £422 443.18 |
| Total SVR (n) | 23 | 11 | 11 |
| Proportion tests, SVR | 14% | 20% | 7% |
| Cost per SVR | £37 682.63 | £23 904.13 | £38 403.93 |
| Discounted medication rates | |||
| Per SVR/30% discount | £26 497.06 | £16 816.86 | £27 064.29 |
| Per SVR/50% discount | £19 040.02 | £12 092.01 | £19 504.54 |
| Per SVR/90% discount | £3728.52 | £2642.32 | £4385.03 |
| Discounted medication rates, all LTFU assumed cured | |||
| Per SVR/0% discount | £28 890.01 | £23 904.13 | £26 402.70 |
| Per SVR/30% discount | £20 314.42 | £16 816.86 | £18 606.70 |
| Per SVR/50% discount | £14 597.35 | £12 092.01 | £13 034.37 |
| Per SVR/90% discount | £3163.22 | £2642.32 | £3014.71 |
Costs for testing are inclusive of staff time.
*213 venepuncture samples and six dried blood spot samples sent for RNA testing.
†Combined costs for RNA samples. Note that the cost per test was calculated by dividing the testing costs by the number of tests performed in each group.
‡Combined costs at full list prices, estimated from British National Formulary online, and does not include any negotiated discounts.
§Glecaprevir/pibrentasvir, 100/40 mg at £12 993.66 per pack of 84, 8-week duration (n=32); sofosbuvir/ledipasvir 90/400 mg at £12 993.33 per pack of 28, 8-week duration (n=1).
¶Glecaprevir/pibrentasvir, 100/40 mg at £12 993.66 per pack of 84, 8-week duration (n=9); Sofosbuvir/velpatasvir, 400/100 mg at £12 993.33 per pack of 28 tablets, 8-week duration (n=1). Excludes treatment costs for one individual whose medication costs were not incurred by the health service.
**Glecaprevir/pibrentasvir, 100/40 mg at £12 993.66 per pack of 84, 8-week duration (n=16).
LTFU, loss to follow-up; RNA, ribonucleic acid; SVR, sustained virologic response.
In the qualitative analysis, six (75%) of eight invited staff involved in delivery of the prison HCV pathway participated in five semistructured interviews (two group and three individual), with representation from service leadership, laboratory and nursing staff. The largest proportion of facilitators was within the characteristics of individual CFIR domain and barriers in the inner setting domain (table 4).
Table 4.
Proportion of implementation determinants in each CFIR domain
| Determinants | CFIR domain | ||||
| Intervention characteristics | Outer setting | Inner setting | Characteristics of individuals | Process | |
| Facilitators—n (%) | 6 (20.7) | 5 (17.2) | 6 (20.7) | 8 (27.6) | 4 (13.8) |
| Barriers—n (%) | 5 (12.2) | 2 (4.9) | 13 (31.7) | 9 (21.9) | 12 (29.3) |
Notes: Percentages are proportions of all barriers/facilitators across CFIR domains.
CFIR, Consolidated Framework for Implementation Research.
In total, 41 barriers and 29 facilitators were identified (table 5). To briefly summarise some key determinants, the analysis highlighted concerns around the manual result notification process, which used an amended microbiology sample processing form to notify PoC results to central laboratory staff, for example:
Table 5.
List of all determinants to implementation of the Cepheid GeneXpert in HMP Perth identified in semi-structured staff interviews (n=70)
| Domain | Barriers (n=41) |
| Inner setting | Leadership staff felt individual custody trumped healthcare in the prison, hindering improvements to care. |
| Laboratory staff did not prioritise uploading GeneXpert results as they did not perform the test. | |
| Laboratory staff did not prioritise reporting GeneXpert results because it was a pilot project. | |
| Leadership staff felt that, as the nurses were not present for the majority of admissions, in-reach was limited. | |
| Leadership staff felt a lack of freedom to operate in the prison hindered the design of the pathway. | |
| Clinical staff felt the lack of physical space and clinic rooms adversely affected how and when the GeneXpert could be used. | |
| Clinical staff were limited to using the GeneXpert and obtaining samples for testing in specific locations in the prison. | |
| Clinical staff found it difficult to transit individuals from residential areas of the prison the health centre due to the need for intermediary ‘runners’. | |
| Clinical staff felt pressured by SPS staff (‘runners’) to finish clinic appointments quickly. | |
| Clinical staff found it difficult to implement healthcare initiatives as it was perceived as secondary to the regimental running of the prison/security. | |
| Clinical staff found it difficult to engage colleagues outside their direct team in HCV testing due to perceived lack of integrated care. | |
| The GeneXpert was seen as difficult to implement in the long-term due to high staff turnover in the prison. | |
| Laboratory staff found it difficult to log results in a timely manner due to staff turnover and training issues. | |
| Outer setting | Laboratory staff felt uncertainty around whether reporting tasks could be delegated to administrative staff due to professional regulations. |
| Laboratory staff found it difficult to manage the reporting workflow due to the pressures of the Covid-19 pandemic. | |
| Characteristics of Individuals | Leadership staff felt a lack of awareness of HCV among people in prison and prison staff hindered improvements to prison care. |
| Laboratory staff did not see administration of GeneXpert results as part of their job/in line with their skillset. | |
| Laboratory staff felt uncertain about the value of their role in the reporting process. | |
| Clinical staff felt cynical about whether SPS staff ‘runners’ actually approached individuals to inform them their attendance at the health centre was required. | |
| Clinical staff indicated a preference to obtain a venous sample to fingerprick sample due to their self-perceived proficiency at obtaining venous bloods. | |
| Clinical staff viewed fingerprick sampling method as slower than obtaining venous samples. | |
| Clinical staff often wanted to know antibody status of an individual, meaning at times they may not have prioritised PCR testing with GeneXpert. | |
| Clinical staff felt obtaining fingerpick samples using the minivette introduced infection control concerns. | |
| Laboratory staff felt unsure about the value of their role in the result reporting process. | |
| Intervention characteristics | Leadership staff felt the need to return to device to check result after 1 hour made it difficult to plan work for a clinic when they had competing priorities for their time. |
| Performing a GeneXpert test was perceived as more work than obtaining conventional samples and sending them for lab analysis, by leadership staff. | |
| Transporting GeneXpert test assays in the prison caused anxiety for clinical staff due to the sensitivity of the rear fin on the cartridge. | |
| Clinical staff felt the dexterity required to correctly insert the sample into the cartridge caused errors in results. | |
| Laboratory and clinical staff found it challenging to interpret the viral load quantification output (scientific notation) from the device. | |
| Process | Laboratory staff felt the lack of an IT link raised concerns about accurate result reporting. |
| Laboratory staff found it difficult to plan/implement an SOP for reporting results, as they were unsure what to expect in terms of volume of tests. | |
| Clinical staff had difficulty conceptualising how the device would be used due to a lack of a plan on who to target for testing and how to do so. | |
| Clinical staff found it difficult to plan a ‘1 day’ test/treat pathway due to safety concerns with the frontline medication used. | |
| Clinical staff found it difficult to transit individuals to the prison health centre due to the provision of OAT at concurrent time to BBV clinics. | |
| The GeneXpert process was viewed as time-consuming and difficult to implement systematically due to unpredictable nurse workload. | |
| Laboratory staff did not prioritise uploading test results to electronic systems because they did not perform the test themselves. | |
| The paper reporting process was felt to introduce potential for result reporting/transcription errors. | |
| Laboratory staff found it difficult to adapt to the paper/manual reporting workflow as it was unfamiliar to them. | |
| Laboratory staff felt there was poor communication between themselves and clinical staff implementing the testing. | |
| Clinical staff found it difficult to verify patients’ CHI numbers as they are not routinely used in the prison system. | |
| Clinical staff were anxious about the paper reporting process because it placed a high degree of responsibility on them not to make reporting errors. | |
| Facilitators (n=29) | |
| Inner setting | Laboratory staff were open to challenge on results incorrectly uploaded due to their perceived professional responsibility to ensure accuracy. |
| Clinical staff found it easier to plan engagement with testing by co-designing awareness materials with people in prison. | |
| Clinical staff found it easier to implement the GeneXpert pathway because of previous testing undertaken in the prison for diabetes by another team. | |
| Clinical staff found it easier to navigate the prison environment for testing after being ‘key trained’. | |
| The prison BBV nursing team’s openness to change and credibility with prison staff was perceived as helpful to implementation, by leadership staff. | |
| Clinical staff found it easier to engage patients due to the ethos of their team which values individual relationships. | |
| Outer setting | The local HCV elimination strategy was seen as facilitative of improving care by leadership staff. |
| MCN infrastructure and inter-organisational working was seen as facilitative of improving prison BBV care by leadership staff. | |
| GeneXpert was viewed as preferable for sampling in patients with difficult venous access by clinical staff. | |
| Some people in prison indicated a preference to clinical staff to be tested using the GeneXpert due to the non-invasive sampling. | |
| Clinical staff found it easier to implement the GeneXpert pathway as the virology team were perceived as supportive. | |
| Characteristics of Individuals | Laboratory staff felt prior experience with reference result reporting and prior PoC pilots for influenza were helpful in implementing the result reporting workflow for the GeneXpert. |
| Laboratory staff appreciated the unique testing challenges in the prison environment. | |
| Laboratory staff perceived GeneXpert testing in the prison as innovative. | |
| Wider knowledge of GeneXpert testing in other UK cities among laboratory staff and individual advocacy among those staff facilitated the decision to support the project. | |
| Clinical staff trusted the results from the GeneXpert due to an awareness other teams were using them. | |
| Clinical staff perceived the GeneXpert as making their job easier. | |
| New staff in the prison health centre were perceived as being open to change by existing clinical staff. | |
| Clinical staff perceived the GeneXpert as enabling quicker transition from diagnosis to treatment. | |
| Intervention characteristics | Leadership staff felt the strong existing evidence base on the clinical effectiveness of the GeneXpert and benefits of HCV treatment for PWID facilitated implementation. |
| Laboratory staff found it easier to integrate the GeneXpert as there were no financial implications to do so. | |
| Clinical staff found it easier plan their use of the GeneXpert as it was mobile (on trolley). | |
| Clinical staff could plan afternoon clinics/more flexible clinic times as the GeneXpert made the 12.30 bloods cut-off inapplicable for PCRs. | |
| Leadership staff felt that GeneXpert delivered quick, actionable, results and was easy to use. | |
| GeneXpert was perceived as preferable to conventional testing due to the speed of the results by leadership staff. | |
| Process | Laboratory staff felt existing lab systems could be easily amended to integrate the GeneXpert test platform. |
| Clinical staff found it easier to engage people in prison into testing by building rapport with and disseminating HCV information via ‘pass men’. | |
| Laboratory staff felt integrating the GeneXpert process as a whole was minimally disruptive to their usual work. | |
| Laboratory staff felt it was an easier process compared with conventional testing as they did not have to process the samples themselves. |
BBV, blood-borne virus; CHI, community health index; HCV, hepatitis C virus infection; HMP, His Majesty’s Prison Service; IT, information technology; MCN, managed care network; OAT, opioid agonist therapy; PCR, polymerase chain reaction; PoC, point-of-care; PWID, people who inject drugs; SOP, standard operating procedure; SPS, Scottish Prison Service.
If that bit of paper goes missing, the result’s missing […] there’s potential for, like, transcription errors […] there is a temptation as well, if you’re really busy, to put them on the backburner and leave them.
– Biomedical scientist
On the device itself, interpreting viral load quantification output on the GeneXpert raised issues, as it was difficult to understand:
I checked it yesterday, to go and just to see, it was like, ‘7.52e05’.
That doesn’t mean anything to anybody…
The lab phoned for me […] they couldn’t understand the result, they didn’t know what it was […] it came up ‘7.52e’, and they were querying that.
– Nurse
Also, there were perceived challenges around cooperative teamworking between NHS and prison staff in engaging prison residents in implementing HCV testing, including the PoC testing approach:
There’s a lot of ‘refusals’ […] we’ve never been able to work out what that refusal is caused by […] they’ve now got to fill in a form to say why they refused, and they’re not getting done.
– Nurse
Manual handling of assays and related consumables, particularly with respect to infection control, was a source of anxiety:
It’s messy, with the fingerprick and things, it’s messy […] from an infection control sense it’s a lot messier.
We don’t want to damage that fin [on the assay]. I’m paranoid about that, I really am.
– Nurse
Over and above these barriers, there were concerns around integrating the GeneXpert into usual workflow, task prioritisation and staff turnover (see table 5). On the other hand, the analysis uncovered multiple perceived facilitators of implementation, for example, the rapidity of results:
I was able to go and give them their results before I went home, so it’s great!
– Nurse
You’ve got the difference between getting a result you can act upon, rather than having to wait a week. So, that’s a major advantage.
– Leadership
Additionally, the increased flexibility in clinic times made possible by the option of PoC testing was viewed favourably:
That’s a good point […] because the bloods go away [are sent to the laboratory] at half past 12. So, PCR really needs to be done in the morning.
It wouldn’t have to get sent off…
It takes away all the barriers doesn’t it
…you can extend that clinic then. Into the afternoon because you’ve not got that cut-off at half past 12.
– Nurse
Perceived patient preferences, that is, preferring capillary/fingerstick sampling to conventional phlebotomy, were seen as a facilitator to implementation, as nurses could engage individuals who were otherwise disinterested:
I’ve got one guy waiting for this machine because he refuses point-blank to get, get needles in him.
– Nurse
In addition to these key facilitators, supportive colleagues, the wider evidence base for PoC testing and the mobility of the device were viewed as positively influential on implementing the GeneXpert in the prison.
Discussion
Interventions to enhance transition to HCV treatment are required for critical populations, including incarcerated people, if WHO 2030 elimination goals are to be realised.28 29 This single-site evaluation has demonstrated that it is clinically beneficial to implement on-site nurse-led PoC RNA testing for HCV in a maximum-security Scottish prison. One-hundred and sixty-seven PoC tests were administered and, among individuals who tested HCV positive, those who received one had increased likelihood of initiating treatment sooner than those tested conventionally. This effect was observed in both the main models and simplified supplementary models, though the magnitude of the effect is likely most realistic in model 1 reported here (19 vs 33 days; adjusted HR 1.91 (1.03–3.55)), which compared PoC testing to conventional service delivery unincumbered by the effects of COVID-19. However, the proportion of error/invalid tests in our pilot was higher than observed in other real-world settings. For example, an Australian study implemented PoC RNA testing in needle and syringe provision (NSP) sites, where testing was undertaken by non-healthcare staff.30 In that study, 1.4% (2/140) of all PoC RNA tests were invalid. Another study implemented the same intervention across harm reduction centres in Georgia.31 The error rate was slightly higher in this study at 3.6% (22/619)—and most were related to operator error, similar to our findings—but still much lower than the rate in our evaluation. The number of failed tests did attenuate over time (online supplemental figure S2), which suggests an association with operator proficiency (ie, a learning curve).
As noted in the qualitative results, staff turnover was an issue in this pilot, which is common in prison health services.32 This may have impacted on Xpert error rates. The laboratory services also experienced high staff turnover and difficulties managing the reporting workflow due to COVID-19 (table 5). This somewhat explains the proportion of result reporting inaccuracies which occurred, particularly the spike in June 2020 (online supplemental figure S3) and the following months as services remobilised following the initial COVID-19 outbreak. Ensuring prompt and adequate training for new staff will be critical to reducing the likelihood of errors going forward.
The cost analysis suggested that employing this PoC RNA an approach may not be cost favourable. Price differences appeared to be impacted by: (a) the significant difference in linkage-to-treatment for the GeneXpert group relative to the conventional group in the pilot phase, which incurred higher treatment costs; (b) the proportion of RNA tests in the pilot phase which were Xpert rather than conventional, meaning higher overall testing costs, despite the lower cost-per-test at the individual level and (c) LTFU among those who started treatment, which was proportionally higher in the GeneXpert group (5/16; 38%), relative to those conventionally tested in prepilot (7/33; 21%) and pilot (0/11; 0%) phases. Improved linkage-to-treatment is important to consider when choosing whether to implement such interventions because, with the high efficacy of Direct Acting Antiviral (DAA) treatment, LTFU individuals are likely to have achieved SVR.12 The costs of a relatively less efficient pathway may be higher with respect to enduring HCV transmission and its attendant consequences. In the hypothesised scenario of maximum discounting of DAAs, and cure attainment among those LTFU, the GeneXpert group costs became favourable relative to the prepilot phase. For others considering a similar approach, consideration might be given to this as well as the identified implementation determinants.
Bringing quickly actionable HCV testing closer to incarcerated people has been increasingly advocated in recent years.33–35 The primary results reported here align with recent similar research. A study undertaken in HMP Wormwood Scrubs, England, reduced time from screening to treatment from 3 months, for those tested by DBS, to 1 week, by implementing PoC RNA testing and augmenting it with a streamlined care pathway.36 However, processing of GeneXpert samples was not undertaken on site. Similarly, an Australian study reported high test uptake and shortened transition to treatment among a cohort screened on reception using a one-stop approach including PoC RNA testing with additional fast-track components. Initial results indicated that those tested by PoC had shorter time from testing to treatment (6 ver 90 days; p<0.001) as well as high treatment uptake, similar to our findings.37 Although views of imprisoned people on the acceptability of PoC testing did not form part of the work undertaken here, other studies have examined this. A qualitative substudy on the Australian project showed that the PoC intervention was highly acceptable to participants.38 Other work found testing in this manner highly acceptable, with most preferring it to venepuncture.39 Furthermore, a Canadian study found that PoC fingerprick HCV testing was highly preferred to conventional venepuncture for those with challenging venous access, which we also observed in the qualitative analysis.40
Currently, we know what works well for HCV diagnosis and treatment on a technical level.41 Therefore, we have reported multiple determinants of implementation with the intention of informing projects undertaken elsewhere (table 5). Overall, the results implied an underlying tension between individual knowledge, self-efficacy and organisational culture, with leadership, readiness to implement and prioritisation of work. In the inner setting, most barriers were associated with the constrained nature in which clinical staff could operate within the prison; the relative priority of healthcare in the prison environment; staff turnover and training issues and the relative priority of the pilot to laboratory staff. Most facilitators were in the characteristics of individuals domain. They predominantly spanned existing knowledge of the GeneXpert platform; prior experience with PoC testing for other clinical indications; a perception of the GeneXpert as innovative and easing workloads and a perceived openness to change among nursing staff. In intervention characteristics, staff felt the need to return to the device after 60 min to conclude that the process was inconvenient relative to conventional methods. Furthermore, the sensitivity of the assays to external forces; the way the device reports viral load quantification and obtaining fingerprick samples were all observed as challenges.
Conversely, the evidence around the GeneXpert; the mobility of the device; its impact on clinic planning and the rapidity of test results were all positive influences. In the process domain, the result-reporting procedure, although effective, was less attractive than an automated electronic system. Of the PoC tests with some level of inaccurate information on electronic health records (online supplemental table S6 and figure S3), most, if not all, could have been avoided with an automated electronic result reporting system. Other programmes integrating PoC RNA testing into routine BBV care should give serious consideration to developing such a link. Finally, in the outer setting, professional regulations made it difficult to delegate certain tasks to alternative staff members and impacts related to COVID-19 made the reporting workflow challenging to manage within the laboratory. Overall, in the wider regional context, the HCV elimination strategy in Tayside, along with the organisational structures which govern it, were seen as facilitative. We hope that by reporting the determinants of implementation against a recognised transferrable framework, we can increase their relevance across divergent settings and contribute to programme design elsewhere.
This evaluation has multiple limitations. We did not seek the views of patients on the acceptability of PoC RNA testing, due to the nature of the work which was focused primarily on implementation from a health systems perspective and inherently limited in scope. Beyond this, the qualitative analysis used convenience sampling, which is non-random and prone to motivation bias and limited generalisability.42 Determinants reported here may, thus, not be representative of other jurisdictions that have run similar projects, and future comparative studies would be valuable to determine this when the literature base is more robust. Additionally, in the qualitative work, diverging interview methods were used (focus group and one-to-one), with attendant strengths and weaknesses. The focus group approach, for example, could have led to hesitance in expressing views in the presence of staff of differing seniority, minimal expression of deviating opinions, limited discussion due to confidentiality or disclosure issues or bias from moderator intervention.43 44 Conversely, individual interviews may have generated interviewee self-consciousness; lacked the spontaneity of group discussion and struggled to describe the commonness of issues raised.45 These biases and issues will inherently have affected the quality of data. All interviews may have been biased by interviewee familiarity with the interviewer/facilitator, and the interviewer’s existing knowledge of the intervention. Furthermore, the sample who participated in qualitative interviews was limited—almost all relevant staff (nurses, biomedical staff, service leadership, commissioning staff) participated—but may raise concerns regarding ‘saturation’. With respect to this, given the specialised knowledge of participants; their relevance to the pathway; the use of an established theoretical framework and a prespecified analysis strategy, the concept of ‘information power’ is relevant.46 This suggests the more information a sample holds, relevant to the evaluation, and where scrutiny is informed by a theoretical framework, the fewer participants are required to ‘saturate’ the analysis. In taking an approach conceptually aligned with this view, we hoped to ameliorate some of the challenges associated with the qualitative strand of the evaluation. Other limitations include the impact of COVID-19 on laboratory testing turnaround times during the pilot, which may have disadvantaged the conventional group in the survival analysis, and the rudimentary approach to the cost analysis, which only included direct costs. Finally, the survival data frame was right censored for some cases, meaning their exact survival time was uncertain.
Conclusions
The results suggest that integrating the Cepheid GeneXpert platform into routine nurse-led HCV care in a maximum-security prison health centre improves linkage to treatment in the Scottish context. Our data augment the available literature with respect to the benefits of this approach on linkage to care, but reports gains which are more modest, possibly driven by the absence of additional care pathway changes reported by others. Multiple determinants to implementation were highlighted, which may inform similar pilots in other prisons. The new platform was less favourable in cost terms than conventional testing; however, this was affected by several factors (linkage to treatment, LTFU), and in realistic hypothesised scenarios, multiple favourable cost outcomes were observed. Consequent to this pilot, we are now undertaking further research informed by this work with this testing platform in local NSP sites, and a comparable analysis is planned.
Supplementary Material
Acknowledgments
The authors acknowledge the patients who contributed their data and the staff who participated.
Footnotes
Twitter: @Byrne_C_J
Contributors: CJB, SKI and JFD conceptualised the evaluation. CJB curated data; selected the methodologies employed; undertook qualitative and quantitative analyses; visualised data; and wrote the original draft. SKI and JFD provided supervision. AM undertook qualitative data analysis. All authors contributed to revisions and approved the final version of the manuscript. The guarantor (CJB) had full access to the data and takes responsibility for the integrity of the data and the accuracy of analysis.
Funding: Cepheid UK Ltd provided diagnostic platforms and test assays free-of-charge and provided funding for a fixed-term part-time coordinator to facilitate the pilot. Cepheid UK Ltd played no role in design of the evaluation; data collection, analysis, or interpretation; the writing of the manuscript; or the decision to submit the manuscript for publication. Award number: N/A.
Competing interests: CJB, AM and SKI have no disclosures. JFD reports grants and personal fees from AbbVie; grants and personal fees from Gilead; and grants and personal fees from MSD, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Quantitative data underpinning this study were obtained from routinely updated NHS health records in line with approval granted by the NHS Caldicott Guardian. The individuals to whom the data pertains did not explicitly consent to its use for research purposes. Therefore, it is not possible for the authors to share this data. However, interested parties can make specific requests to NHS Tayside Information Governance by email on: informationgovernance.tayside@nhs.scot. Consideration will be given to sharing qualitative data upon receipt of a methodologically sound proposal and will be subject to the agreement of interview participants.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
As this was a retrospective service evaluation—such evaluations may include access to patient data, as well as provision of questionnaires and interviews without NHS ethical review (as such work is not classed as ‘research’ by the NHS Research Ethics Service)—NHS ethical review was not required. Instead, Caldicott Guardian approval was obtained for data access (IGTCAL7004). This process reviews internal NHS evaluations, ensuring the protection and appropriate use of patient data. The evaluation was also registered with the NHS Tayside clinical governance group for prison healthcare (ref: 27/19).
References
- 1.Gallacher J, McPherson S. Progress towards micro-elimination of hepatitis C in the custodial setting. J Viral Hepat 2021;28:300–1. 10.1111/jvh.13428 [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017;5:e1192–207. 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronfli N, Linthwaite B, Kouyoumdjian F, et al. Interventions to increase testing, linkage to care and treatment of hepatitis C virus (HCV) infection among people in prisons: a systematic review. Int J Drug Policy 2018;57:95–103. 10.1016/j.drugpo.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and central Asia. Lancet 2016;388:1228–48. 10.1016/S0140-6736(16)30856-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor A, Munro A, Allen E, et al. Low incidence of hepatitis C virus among prisoners in Scotland. Addiction 2013;108:1296–304. 10.1111/add.12107 [DOI] [PubMed] [Google Scholar]
- 6.Scottish Public Health Observatory . SPS addiction prevalence testing stats 2018/19. 2021. Available: https://www.scotpho.org.uk/behaviour/drugs/data/availability-and-prevalence/ [Accessed 26 Apr 2021].
- 7.Lightowler C, Hare D. Prisons and sentencing reform: developing policy in Scotland. 2009. Available: http://www.sccjr.ac.uk/wp-content/uploads/2012/11/Report_2009__02_-_Prisons_and_Sentencing_Reform.pdf [Accessed 27 Apr 2021].
- 8.Scottish Parliament Justice Committee . An inquiry into the use of remand in scotland. Scottish Parliamentary Corporate Body, 2018: 5. [Google Scholar]
- 9.Scottish Government . Scottish prison population statistics 2019-20. 2020. Available: www.gov.scot/publications/scottish-prison-population-statistics-2019-20 [Accessed 27 Apr 2021].
- 10.HM Inspectorate of Prisons for Scotland . 2018. Available: www.prisonsinspectoratescotland.gov.uk [Accessed 27 Apr 2021].
- 11.Stöver H, Meroueh F, Marco A, et al. Offering HCV treatment to prisoners is an important opportunity: key principles based on policy and practice assessment in Europe. BMC Public Health 2019;19:30. 10.1186/s12889-018-6357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C: final update of the series☆. J Hepatol 2020;73:1170–218. 10.1016/j.jhep.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 13.Stöver H, Tarján A, Horváth G, et al. The state of harm reduction in prisons in 30 European countries with a focus on people who inject drugs and infectious diseases. Harm Reduct J 2021;18:67. 10.1186/s12954-021-00506-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Protection Scotland . The needle exchange surveillance initiative: prevalence of blood-borne viruses and injecting risk behaviours among people who inject drugs attending injecting equipment provision services in scotland, 2008-09 to 2015-16. Glasgow: Health Protection Scotland; 2017. [Google Scholar]
- 15.Lamoury FMJ, Bajis S, Hajarizadeh B, et al. Evaluation of the Xpert HCV viral load finger-stick point-of-care assay. J Infect Dis 2018;217:1889–96. 10.1093/infdis/jiy114 [DOI] [PubMed] [Google Scholar]
- 16.Health Research Authority . Is my study research? HRA decision tools. Available: http://www.hra-decisiontools.org.uk/ [Accessed 26 Jan 2022].
- 17.NHS National Patient Safety Agency . Defining research [NHS Research Ethics Service]. 2007. Available: http://www.pi.nhs.uk/data/consent/NRES_leaflet_Defining_Research.pdf [Accessed 26 Jan 2022].
- 18.NHS Tayside Information Governance Group . Caldicott approval procedure. 2010. Available: https://www.ahspartnership.org.uk/images/cmsimages/pdf/Caldicott%20Approval%20Procedure.pdf [Accessed 27 Apr 2021].
- 19.Scottish Prison Service . HMP perth. 2015. Available: https://www.sps.gov.uk/Corporate/Prisons/Perth/HMP-Perth.aspx [Accessed 26 Jan 2022].
- 20.Health Protection Scotland . Guidance to support opt-out Blood Borne Virus (BBV) testing in Scottish prisons. 2019. Available: https://www.hps.scot.nhs.uk/web-resources-container/guidance-to-support-opt-out-blood-borne-virus-bbv-testing-in-scottish-prisons/ [Accessed 26 Apr 2021].
- 21.Snell M. Cost-benefit analysis: A practical guide. 2nd ed. London: Thomas Telford, 2011: 91. 10.1680/cba.41349 [DOI] [Google Scholar]
- 22.British National Formulary . Sofosbuvir with Velpatasvir. 2021a. Available: https://bnf.nice.org.uk/medicinal-forms/sofosbuvir-with-velpatasvir.html [Accessed 19 Oct 2021].
- 23.British National Formulary . Glecapravir with Pibrentasvir. 2021b. Available: https://bnf.nice.org.uk/medicinal-forms/sofosbuvir-with-velpatasvir.html [Accessed 19 Oct 2021].
- 24.Myring G, Lim AG, Hollingworth W, et al. Cost-effectiveness of pharmacy-led versus conventionally delivered antiviral treatment for hepatitis C in patients receiving opioid substitution therapy: an economic evaluation alongside a pragmatic cluster randomised trial. International Network on Hepatitis and Health in Substance Users (INHSU), 2021. 10.2139/ssrn.4019338 [DOI] [PubMed] [Google Scholar]
- 25.NHS Employers . National job profiles. 2021. Available: https://www.nhsemployers.org/pay-pensions-and-reward/job-evaluation/national-job-profiles [Accessed 10 May 2021].
- 26.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 28.World Health Organization . Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. Geneva: WHO Document Production Services; 2016. Available: https://apps.who.int/iris/handle/10665/246177 [Accessed 27 Jan 2022]. [Google Scholar]
- 29.Lazarus JV, Safreed-Harmon K, Thursz MR, et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis 2018;38:181–92. 10.1055/s-0038-1666841 [DOI] [PubMed] [Google Scholar]
- 30.Williams B, Howell J, Doyle J, et al. Point-of-care hepatitis C testing from needle and syringe programs: an Australian feasibility study. Int J Drug Policy 2019;72:91–8. 10.1016/j.drugpo.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 31.Shilton S, Markby J, Japaridze M, et al. Feasibility and effectiveness of HCV viraemia testing at harm reduction sites in Georgia: a prospective three-arm study. Liver Int 2022;42:775–86. 10.1111/liv.15191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chafin WS, Biddle WL. Nurse retention in a correctional facility: a study of the relationship between the nurses’ perceived barriers and benefits. J Correct Health Care 2013;19:124–34. 10.1177/1078345812474643 [DOI] [PubMed] [Google Scholar]
- 33.Akiyama MJ, Kronfli N, Cabezas J, et al. Hepatitis C elimination among people incarcerated in prisons: challenges and recommendations for action within a health systems framework. Lancet Gastroenterol Hepatol 2021;6:391–400. 10.1016/S2468-1253(20)30365-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crespo J, Llerena S, Cobo C, et al. HCV management in the incarcerated population: how do we deliver on this important front? Curr Hepatology Rep 2019;18:259–67. 10.1007/s11901-019-00472-2 [DOI] [Google Scholar]
- 35.Cunningham EB, Hajarizadeh B, Bretana NA, et al. Ongoing incident hepatitis C virus infection among people with a history of injecting drug use in an Australian prison setting, 2005-2014: the HITS-p study. J Viral Hepat 2017;24:733–41. 10.1111/jvh.12701 [DOI] [PubMed] [Google Scholar]
- 36.Mohamed Z, Al-Kurdi D, Nelson M, et al. Time matters: point of care screening and streamlined linkage to care dramatically improves hepatitis C treatment uptake in prisoners in England. Int J Drug Policy 2020;75:102608. 10.1016/j.drugpo.2019.102608 [DOI] [PubMed] [Google Scholar]
- 37.Sheehan Y, Cunningham E, Cochrane A, et al. A one-stop-shop intervention integrating point-of-care HCV RNA testing to enhance hepatitis C testing and treatment uptake among new receptions to prison: the PIVOT study. International Network on Health and Hepatitis In Substance Users (INHSU), 2021. [Google Scholar]
- 38.Lafferty L, Cochrane A, Sheehan Y, et al. “That was quick, simple, and easy”: patient perceptions of acceptability of point-of-care hepatitis C RNA testing at a reception prison. Int J Drug Policy 2022;99:103456. 10.1016/j.drugpo.2021.103456 [DOI] [PubMed] [Google Scholar]
- 39.Bajis S, Maher L, Treloar C, et al. Acceptability and preferences of point-of-care finger-stick whole-blood and venepuncture hepatitis C virus testing among people who inject drugs in Australia. Int J Drug Policy 2018;61:23–30. 10.1016/j.drugpo.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 40.Kronfli N, Dussault C, Chalifoux S, et al. A randomized pilot study assessing the acceptability of rapid point-of-care Hepatitis C Virus (HCV) testing among male inmates in Montreal, Canada. Int J Drug Policy 2020;85:102921. 10.1016/j.drugpo.2020.102921 [DOI] [PubMed] [Google Scholar]
- 41.Lazarus JV, Pericàs JM, Picchio C, et al. We know daas work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J Intern Med 2019;286:503–25. 10.1111/joim.12972 [DOI] [PubMed] [Google Scholar]
- 42.Robinson OC. Sampling in interview-based qualitative research: a theoretical and practical guide. Qual Res Psychol 2014;11:25–41. 10.1080/14780887.2013.801543 [DOI] [Google Scholar]
- 43.Tausch AP, Menold N. Methodological aspects of focus groups in health research. Glob Qual Nurs Res 2016;3:233339361663046. 10.1177/2333393616630466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acocella I. The focus groups in social research: advantages and disadvantages. Qual Quant 2012;46:1125–36. 10.1007/s11135-011-9600-4 [DOI] [Google Scholar]
- 45.Ryan F, Coughlan M, Cronin P. Interviewing in qualitative research: the one-to-one interview. Int J Ther Rehabil 2009;16:309–14. 10.12968/ijtr.2009.16.6.42433 [DOI] [Google Scholar]
- 46.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies. Qual Health Res 2016;26:1753–60. 10.1177/1049732315617444 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-068604supp001.pdf (978.2KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Quantitative data underpinning this study were obtained from routinely updated NHS health records in line with approval granted by the NHS Caldicott Guardian. The individuals to whom the data pertains did not explicitly consent to its use for research purposes. Therefore, it is not possible for the authors to share this data. However, interested parties can make specific requests to NHS Tayside Information Governance by email on: informationgovernance.tayside@nhs.scot. Consideration will be given to sharing qualitative data upon receipt of a methodologically sound proposal and will be subject to the agreement of interview participants.