Abstract
Background
Despite chronic obstructive pulmonary disease (COPD) being a major global cause of mortality and hospitalisation, it is often undiagnosed or inaccurately diagnosed in clinical settings.
Objective
To systematically synthesise all peer-reviewed papers from primary healthcare settings that have reported data on: (1) undiagnosed COPD, that is, patients with respiratory symptoms and postbronchodilator airflow obstruction consistent with COPD, without a formal clinician’s diagnosis of COPD either documented in health records or reported by patients and (2) ‘overdiagnosed COPD’, that is, clinician’s diagnosis without postbronchodilator airflow obstruction.
Methods
Studies investigating these diagnostic metrics in patients from primary healthcare clinics (according to predefined inclusion/exclusion criteria) were sourced from Medline and Embase and assessed for bias (Johanna Briggs Institute tools for prevalence studies and case series). Meta-analyses of studies of adequate sample size used random effect modelling stratified by risk factor categories.
Results
Of 26 eligible articles, 21 cross-sectional studies investigated 3959 cases of spirometry-defined COPD (with or without symptoms), and 5 peer-reviewed COPD case series investigated 7381 patients. The prevalence of spirometry-confirmed COPD without a diagnosis documented in their health records was 14%–26% in studies of symptomatic smokers (N=3). 1 in 4 patients taking inhaled therapies (25% (95% CI 22% to 28%), N=2) and 1 in 6 smokers irrespective of symptoms (16% (95% CI 14% to 18%), N=6) fulfilled diagnostic spirometry criteria but did not report receiving a COPD-related diagnosis. In an adequately powered series of COPD cases documented in primary healthcare records (N=4), only between 50% and 75% of subjects had any airflow obstruction on postbronchodilator spirometry performed by study researchers, therefore, COPD was clinically ‘overdiagnosed’ in 25%–50% of subjects.
Discussion
Although data were heterogeneous and of modest quality, undiagnosed COPD was common in primary healthcare, especially for symptomatic smokers and patients treated with inhaled therapies. In contrast, frequent COPD ‘overdiagnosis’ may represent treatment of asthma/reversible component or another medical diagnosis.
PROSPERO registration number
CRD42022295832.
Keywords: COPD epidemiology, Clinical epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Inadequate diagnosis of chronic obstructive pulmonary disease (COPD) is common, however, previous reviews have only narratively synthesised the evidence and not focused on primary healthcare (eg, general practice) patient populations in the ‘real world’; nor have they calculated the absolute proportions undiagnosed with clinical COPD in the community.
WHAT THIS STUDY ADDS
This first systematic review and meta-analysis of studies investigating COPD diagnosis in primary healthcare settings has documented the prevalence of undiagnosed COPD in 14%–26% of symptomatic smokers and in one-quarter of patients taking inhaled therapies. In contrast, there was a 25%–50% prevalence of COPD ‘overdiagnosis’ where clinician-labelled patients having COPD did not have objective evidence of postbronchodilator airflow obstruction, also highlighting the known underutilisation of spirometry globally.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The summary prevalence estimates of undiagnosed (or unrecognised) COPD inform clinicians in primary healthcare of the potential opportunities to uncover COPD cases and implement secondary preventive strategies and other important aspects of COPD management. On the other hand, to minimise COPD ‘overdiagnosis’ and prescribing of non-indicated inhaled therapies, patients first presenting with suspected COPD should be further investigated to elucidate the underlying true diagnosis.
Introduction
Accurately diagnosing chronic obstructive pulmonary disease (COPD)—a leading cause of global mortality1 non-fatal respiratory health burden and risk of lung cancer2—is needed for optimal disease management. As the most widely used strategic report for COPD management, the Global Initiative for Chronic Obstructive Lung Diseases (GOLD) states that the presence of postbronchodilator (post-BD) airflow obstruction is mandatory for establishing the diagnosis of COPD.3 Clinical suspicion is raised in patients who have respiratory symptoms (eg, shortness of breath, chronic cough or sputum production, recurrent lower respiratory tract infections) and/or a known risk factor (eg, history of smoking ≥10 pack-years, occupational exposure to vapour/gas/dust/fumes, familial COPD or low birth weight). Other local guidelines may be subtly different, but in general concern adult patients (>35–40 years) with respiratory symptom(s), a known risk factor and for whom post-BD spirometry is recommended to confirm the clinical diagnosis.4–7
The recent Lancet Commission has re-emphasised spirometry as the first confirmatory test to guide diagnosis,8 but its consistent underuse remains a global problem.9 For many patients with COPD, the diagnosis is first made after admission to hospital during an acute exacerbation,10–12 usually when there has already been substantial lung damage and chronic treatments are poorly effective. Concerningly, opportunities for these symptomatic patients to have the diagnosis confirmed at an earlier stage are commonly missed13 so their COPD has potentially remained unrecognised for many years. Conversely, symptomatic individuals may be managed as having COPD but do not have obstructed spirometry—this has been termed ‘COPD overdiagnosis’ by the respiratory field.14
Narrative reviews have attempted to contextualise the magnitude of these diagnostic issues globally.15–17 However, these reviews mainly focused on studies surveying participants from general community populations when there was some uncertainty as to whether such people had the opportunity to be assessed by a clinician from a healthcare service to confirm the diagnosis.18 The reviews also did not focus on the absolute proportions of subjects undiagnosed in the whole population sample who had spirometry-defined COPD measured by researchers but no clinician diagnosis of COPD on their health record and/or by self-report. Nor did they separate this metric from relative COPD underdiagnosis among only subjects with spirometry-defined COPD, which could seem inflated in the setting of a relatively low COPD prevalence. To illustrate both underdiagnosis figures, for a primary healthcare (PHC) population in which the prevalence of spirometry-defined COPD turned out to be 6.0%, if 95% of the 6.0% were undiagnosed (relative underdiagnosis), then this means that 5.75% of that PHC population did not have a clinician diagnosis of COPD (absolute percentage undiagnosed).19
Furthermore, previous reviews15–17 did not investigate the hypothesis that high-risk populations such as smokers, and those on inhaled medicines and/or who have respiratory symptoms may differ in their estimates of COPD underdiagnosis and overdiagnosis. It is important to estimate the prevalence of undiagnosed COPD within these at-risk populations to better understand the potential extent of undermanaged disease in patients of PHC settings. While conversely, the occurrence of COPD ‘overdiagnosis’ highlights the issues of empirically treating patients and risk of prescribing non-indicated medicines.
Therefore, using post-BD airflow obstruction as the mandatory criterion to establish a diagnosis of COPD,3 20 our overall aim was to systematically synthesise the evidence in peer-reviewed publications from PHC settings to quantify the issues of COPD underdiagnosis (absolute and relative proportions) and COPD overdiagnosis. Specifically, we aimed to estimate the prevalence of: (1) undiagnosed COPD in the whole samples based on the presence of post-BD airflow obstruction, and in the presence of respiratory symptom(s) and/or risk factor(s); (2) relative COPD underdiagnosis only among those found by researchers to have spirometric evidence of COPD and (3) COPD ‘overdiagnosis’ in patients labelled as having COPD, but without airflow obstruction on post-BD spirometry. We also aimed to describe the characteristics of participants who were underdiagnosed and/or overdiagnosed, where such data were available.
Methods
A systematic review protocol was developed and registered in PROSPERO: the international prospective register of systematic reviews (registration number CRD42022295832).21 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklists and flow diagram were used for reporting.22 The research question was formulated using the SPIDER tool: Sample (general practice populations); Phenomenon of Interest (COPD prevalence and its underdiagnosis and overdiagnosis); Design (either cross-sectional prevalence study or COPD case series); Evaluation (proportions underdiagnosed and overdiagnosed) and Research type (quantitative).
Search strategy
Two electronic databases, Medline (OVID) and Embase, were systematically searched up to 4 January 2022. The search strategy was codeveloped with an experienced librarian consisting of MeSH terms and keywords for “COPD”, “under-diagnosis”, “over-diagnosis” and “misdiagnosis” including synonyms and acronyms (online supplemental methods S1). Summary tables and reference lists in articles were manually searched for further eligible studies. The inclusion and main exclusion criteria were developed based on the knowledge gaps identified and the most widely accepted method for confirming a COPD diagnosis, that is, fixed airflow obstruction on post-BD spirometry. We excluded non-English papers (given time constraints/costs) and conference abstracts (given limited information and inability to perform detailed risk assessments).
bmjresp-2022-001478supp001.pdf (773.4KB, pdf)
Inclusion criteria
PHC (ie, general practice, family health, community health) settings.
COPD defined using post-BD spirometry as the diagnostic criterion; synonymous with the term ‘spirometry-defined COPD’.
-
Study contained at least one prevalence estimate of COPD underdiagnosis/overdiagnosis/misdiagnosis including:
Prevalence undiagnosed among those found by researchers to have spirometry-defined COPD (also referred to as COPD underdiagnosis).
Prevalence overdiagnosed among those labelled by the treating clinician as having COPD, but without obstructive post-BD spirometry when tested by researchers.
Exclusion criteria
Post-BD forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) not presented or was not the diagnostic criterion.
Not a PHC setting (ie, general population, specialised populations, specialised clinics or hospital settings).
Study analysed prior to 2001 (first publication of GOLD guidelines).20
Narrative reviews, case reports, study design protocol or no primary data.
Articles published in languages other than English.
Animal experiments, conference abstracts and unpublished studies.
Article selection
Following duplicate-publication removal, two authors (SWSY and JP) independently screened all titles and abstracts in Covidence systematic review software.23 Articles selected for full-text review were retrieved and assessed against the inclusion/exclusion criteria (SWSY and NSI). All disagreements were resolved through discussions between SWSY, NSI, NW and JP.
There were efforts to include all available studies where prevalence data were reported, even if COPD underdiagnosis and/or overdiagnosis was not the main research question.
Quality assessment
The quality of included articles was assessed using the Johanna Briggs Institute (JBI) quality assessment tool checklist for the methodology of prevalence studies24 and case series.25 These tools enabled the identification of poorer quality studies across specific categories including sampling frame and recruitment, sample size, measurement reliability and clarity of reporting. A calculation was performed to assess the adequacy of the sample size used to estimate COPD prevalence, which could bias the estimates.26 27
Data extraction (and calculations)
Two authors (SWSY and JP) independently extracted and discussed the following information from the papers selected: first author, publication year, study name, country, study design, participant number and % of eligible, sampling/selection, number/type of PHC clinics/general practices, gender/sex/age, prevalence of spirometry-defined COPD, prevalence of undiagnosed COPD in the study population and also relative to those found to have spirometry-defined COPD, prevalence overdiagnosed in those labelled with COPD and qualitative descriptions of characteristics of those underdiagnosedor overdiagnosed, where available.
While many underdiagnosis/overdiagnosis figures could be directly extracted from the individual studies themselves, some figures needed to be calculated from the data reported (as indicated in tables). SWSY and JP calculated the absolute prevalence of undiagnosed COPD from the entire population studied (see definitions). Furthermore, the percentage diagnosed was calculated to be 100% minus the percentage of COPD underdiagnosis (ie, 25% relative undiagnosed would equate to 75% diagnosed accurately).17 Contact with authors of eligible studies was not required for this purpose.
After preliminary data extraction, the studies were classified by design. The cross-sectional prevalence studies were further divided into the following broad risk-indicator categories given the heterogeneity: adults ≥40 years, smokers with symptoms, smokers irrespective of symptoms, respiratory symptoms irrespective of smoking and other high-risk groups. These factors were important to consider as they highlighted subsets of individuals who were more prone to developing COPD or potentially being prescribed non-indicated therapies.
Definitions used to extract data from research studies*
Prevalence of spirometry-defined COPD
Prevalence of undiagnosed COPD in entire study population (absolute proportion)
Prevalence of underdiagnosis in those with spirometry-defined COPD (relative proportion)
Prevalence of ‘overdiagnosis’ in those with a prior clinician’s diagnosis of COPD
Consistent with global guidelines,20 post-BD spirometry defined the lung function feature of COPD based on FEV1/FVC ratio, either using a fixed cut-off of 0.720 and/or lower limit of statistical normal (LLN). Clinical COPD was further defined by a combination of post-BD airflow obstruction and respiratory symptom(s)±known risk factor(s).20
Data analysis
Statistical analysis used the ‘metaprop’ command28 within Stata SE V.16 software (Stata), which required data on percentage, case numbers and total subject numbers for the prevalence figures studied.
A meta-analysis was conducted using random effect modelling stratified by risk factor categories. While overall and subgroup estimates with 95% CIs were calculated, a pooled estimate was only reported if the degree of heterogeneity (I2) was <75% or if the 95% CI of all studies within a risk category clearly overlapped. Studies that had smaller sample sizes than those expected to estimate COPD prevalence accurately were omitted in a sensitivity analysis and these forest plots have been presented. These calculations26 27 performed as part of the risk-of-bias assessment (JP), retrospectively looked at the number of participants required to observe the prevalence found by the study researchers with a predefined precision.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
Of 1294 titles and abstracts screened, 207 full-text articles were reviewed, and 181 records excluded based on inclusion/exclusion criteria (figure 1). Of 26 remaining articles, 21 were cross-sectional prevalence studies with 3959 cases of spirometry-defined COPD from a total of 19 423 subjects tested.19 29–48 Just one additional study was identified from a manual search of the articles,39 and another was excluded as spirometry was part of the diagnostic workup and informed the clinician’s diagnosis.49 All 21 articles provided data on undiagnosed COPD, while 9 also provided data on COPD ‘overdiagnosis’. There were eight studies assessing patients with symptoms and/or taking inhaled therapies (table 1), seven assessing spirometry-defined COPD in adult smokers irrespective of symptoms (table 2), four in patient populations ≥40 years (online supplemental table S1), one reporting on a registry and another which assessed higher-risk patients identified by a screening survey (table 2). In addition, there were 5 COPD case series reporting 7381 confirmed cases from 10 142 unique subjects,50–54 which provided data related only to COPD ‘overdiagnosis’ (table 3).
Figure 1.
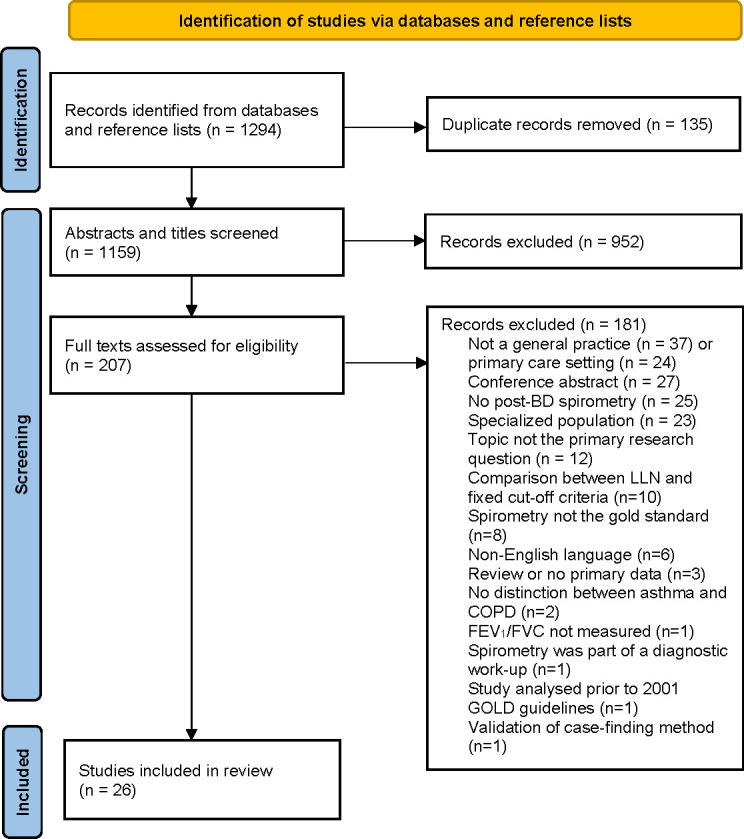
PRISMA flow diagram.22 BD, bronchodilator; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Diseases; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Undiagnosed clinical COPD* and its overdiagnosis using postbronchodilator spirometry: cross-sectional studies of symptomatic adults or adults taking inhaler therapies in primary healthcare settings
| Author year citation | Study name location | Participant no† (%eligible‡), Sampling, selection, no/type of GP practices |
Gender Age (mean (SD) years) |
Prevalence of spirometry-defined COPD (%, (n/N))§ | Underdiagnosis in COPD subpopulation (%, (n/N))§ |
Undiagnosed COPD in studied population (%, (n/N))*§ |
Overdiagnosis in population labelled with COPD (%, (n/N))§ |
| Smokers with symptoms | |||||||
| Liang, 201829 | RADICALS study Australia |
N=1050 (9.8%) Consecutive; adults >40 years, symptomatic ever-smokers with ≥2 GP visits in last 12 months; 43 urban practices |
60%–63% male Undiagnosed: 62 (10) years Diagnosed: 67(10) years |
25.9% (272/1050) | 52.2% (142/272) |
13.5% (142/1050)** | 41.2% (91/221)** |
| Løkke, 201237 | Denmark, Sweden | N=4049 (n/a) Adults ≥35 years Ever-smoker or occupational exposure plus ≥1 respiratory symptom No previous lung disease; 241 GPs |
51% male 58 (male) 57 (female) |
21.7% (878/4049) excluding BDR>500 mL |
¶ | 21.7% (878/4049) |
NA |
| Sandelowsky, 201140 | Stockholm, Sweden | N=138 (69.7%)† Adults 40–75 years Ever-smoker (current or past) with an LRTI, without prior lung disease No of suburban clinics unclear |
44% male 55 years (95% CI 54 to 57) |
27.5% (38/138) spirometry 4–5 weeks post-LRTI |
¶ | 27.5% (38/138) |
NA |
| Yawn, 200943 | USA | N=1201 (93.6%) Non-consecutive; adults >40 years Chronic bronchitis and >10 pack-years without inhaler use or COPD confirmed by spirometry; 50 practices |
45% male 52.9 (9.1) years 80% white |
25.6% (308/1201) |
¶** | 25.6% (308/1201) |
NA |
| Inhalers including asthma | |||||||
| Abramson, 201235 | SPIRO-GP, VIC Australia, prior to randomisation | N=199 (12.1%) Adults 18–70 years Prescribed inhaled medication from 31 urban GP practices |
33% males 54 (13) years |
45.7% (91/199) (COPD+ACO) |
58.2% (53/91)** (COPD+ACO) |
26.6% (53/199)** (COPD+ACO) |
32.1% (18/56)** (COPD+ACO) |
| Tinkelman, 200648 | Aberdeen UK, Denver, USA | N=597 (1–10%)‡ Representative; adults ≥40 years with history of OLD and/or receipt of inhalers last 12 months Query no of GP practices |
38.3% male 58.7 (11) years | 39.4% (235/597)** (COPD+ACO) 28.6% (171/597)** (COPD only) |
62.1% (146/235)** (COPD+ACO) 68.4% (117/171)** (COPD only) |
24.5% (146/597)** (COPD+ACO) 19.6% (117/597)** (COPD only) |
51.6% (95/184)** (COPD+ACO) 60.6% (83/137)** (COPD only) |
| Symptoms-risk factors | |||||||
| Frank, 200646 | MAGIC study, Manchester, UK | N=825 (≈14%) Consecutive; adults ≥30 years Ever-smokers and/or ≥4 symptoms or risk factors ††; 2 GP practices |
45.3% male 55.5 years |
19.8% (163/825) (GOLD stage II–IV) |
63.2% (103/163) |
12.5% (103/825)** |
NA |
| Hamers, 200647 | Brazil | N=142 (40.6%)† ≥15 years old with symptoms of shortness of breath and/or cough 34 urban and rural GP practices |
45.1% male 46.8 (18.7) years |
25.4% (36/142) |
75% (27/36) |
19.0% (27/142)** |
70% (21/30)** |
*Clinical COPD if patient subjects had symptoms and spirometric evidence of COPD; undiagnosed based on an absent diagnosis in medical records except for Tinkelman et al.48
†COPD prevalence could be inaccurate due to insufficient sample size (online supplemental results E4), so have been omitted from the forest plots in a sensitivity analysis.
‡All % eligible figures were calculated, except when mentioned in the discussion text by Tinkelman et al.48
§COPD was defined using the GOLD criterion, postbronchodilator forced expiratory ratio <0.70, except where indicated by § when the only criterion was the LLN.
¶Percentage undiagnosed in the COPD subpopulation was 100% as the population excluded patients with a history of COPD (3 of total of 7 studies).
**Data needed to be deduced and calculated from the figures given (by the present authors).
††Includes wheezing; woken by cough, chest tightness, dyspnoea; hay fever or familial asthma.48
ACO, asthma-COPD overlap; BDR, bronchodilator response; COPD, Chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; LLN, lower limit of statistical normal; LRTI, lower respiratory tract infection; NA, not available; OLDs, obstructive lung diseases; PC, primary care.
Table 2.
Undiagnosed COPD* and its overdiagnosis using postbronchodilator spirometry: cross-sectional studies of smokers (irrespective of symptoms) and other high-risk patients in primary healthcare settings
| Author, year, citation | Study name location | Participant No† (%eligible‡), Sampling, selection, no/type of GP practices |
Gender Age (mean (SD) years) |
Prevalence of spirometry-defined COPD (%, (n/N))§ | Underdiagnosis in COPD subpopulation (%, (n/N))§ |
Undiagnosed COPD in studied population (%, (n/N))*§ |
Overdiagnosis in population labelled with COPD (%, (n/N))§ |
| Smoking per se (regardless of symptoms) | |||||||
| Stafyla, 201830 | Thessaly, Greece | N=186 (91.2%)† Convenience; adults ≥40 years Current or ex-smokers living near 10 urban/rural practices |
68% males 62.3 (13) years |
17.8% (33/186) |
42.4% (14/33) |
7.5% (14/186)¶ |
NA |
| Casas Herrera, 2016 31 | PUMA study (PLATINO) four countries South America | N=1540 (80.8%) Representative; adults ≥40 years Ever-smoker and/or biomass exposure 57 urban/rural centres |
49.7% male 18.8% 40–49 33.8% 50–59 47.4% 60+ yrs |
20.1% (309/1540) |
77.0% (238/309)§ (range: 63%–90%) |
15.5% (238/1540) § (range: 10%–23%) |
30.4% (31/102) |
| Llordés, 201533 | Spain | N=1738 (70.5%) Adults >45 years, smoking history on medical records; one primary care centre |
84.3% male 59.9 (10) years |
24.3% (422/1738) (95% CI 22 to 26) |
56.6% (239/422) |
13.8% (239/1738)¶ | 15.7% (34/217) |
| Queiroz, 201236 | Brazil | N=200 (80%) Adults ≥40 years ≥20 pack-years or ≥80 hour-year biomass smoke exposure; 39 urban practices |
60% male 65.9 (11) years |
31.5% (63/200) |
71.4% (45/63) |
22.5% (45/200)¶ |
NA |
| Al Ghobain, 201138 | Saudi Arabia | N=501 (36.3%) Non-consecutive; adults ≥40 years old Ever-smokers >5 years duration without prior lung disease; 60 private PC clinics |
89.6% male 47.9 (6.9) years |
14.2% (71/501) |
** | 14.2% (71/501) |
NA |
| Hill et al42 | Ontario CAN | N=1003 (15.3%) Consecutive Adults >40 years ≥20 pack-years 3 GP practices (urban/rural) |
No COPD: 52.6% male, 59 (11) years; COPD: 49.5% male, 65 (9) years |
20.7% (208/1003) | 67.3% 138/205)¶ |
13.8% (138/1003)¶ |
43.7% (45/103) |
| Tinkelman, 200745 | Aberdeen UK, Denver, USA | N=818 (6%–24%)‡ Representative; adults >40 years, current or past smokers, no prior OLDs, heart disease or inhaler use Query no of practices |
49.3% male 58.2 (11) years | 18.9% (155/818) |
¶** | 18.9% (155/818) |
NA |
| Asthma-COPD registry | |||||||
| Melbye, 201139 | DIOLUP, Norway | N=376 (21.1%) Adults ≥40 years registered with a diagnosis of asthma and/or COPD from 7 practices; latest diagnosis used |
38.0% male 62 years (median) |
39.6% (149/376) (COPD only) |
36.2% (54/149)¶ (COPD only) |
14.3% (54/376)¶ (COPD only) |
25.8% (33/128)¶ (COPD only) |
| Medium-high COPD-risk | |||||||
| Dirven, 201041 | Netherlands | n=147 (11.7%) undergoing spirometry† Adults 40–75 years without prior OLD at medium-to-high risk for COPD on screening survey; 1 GP at rural practices |
48.7% male 55.2 (9.2) years |
24.5% (37/147)†† |
¶** | 24.5% (37/147)†† |
NA |
*Spirometry-defined COPD based on postbronchodilator spirometry.
†COPD prevalence could be inaccurate due to insufficient sample size (online supplemental results E4), so have been omitted from the forest plots in a sensitivity analysis.
‡All % eligible figures were calculated, except when mentioned in the discussion text by Tinkelman et al.48
§COPD was defined using the GOLD criterion, postbronchodilator forced expiratory ratio <0.70; the LLN was also reported by Casas Herrera et al31 (data not shown).
¶Data needed to be deduced and calculated from the figures given (by the present authors).
**Percentage undiagnosed in the COPD subpopulation was 100% as the population excluded patients with a history of COPD (3 of total of 7 studies).
††Data from the manuscript did not quite add up to the percentages given.31 45
ACO, asthma-COPD overlap; BDR, bronchodilator response; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; LLN, lower limit of normal; LRTI, lower respiratory tract infection; NA, not available; OLDs, obstructive lung diseases; PC, primary care.
Table 3.
COPD* and its potential overdiagnosis using postbronchodilator spirometry: COPD case series in primary healthcare settings
| Author year Citation |
Study name location | Participant no† (%eligible) ‡, Selection, no/type of GP practices |
Gender Age (mean (SD) years) |
COPD cases (COPD±ACO) | Prevalence of spirometry-defined COPD (%, (n/N))§ | Overdiagnosis in those labelled with COPD (%, (n/N))§ |
| Fisk et al, 201950 | Welsh COPD Primary Care Audit, UK | N=8957 (18.6%) Patients on the QOF COPD register used by 280 (61%) of 462 Welsh GP practices who had post-BD FEV1/FVC read-coded* |
54% male 72 (12) years range 36–105 |
COPD | 74.8% (6702/8957) |
25.2% (2255/8957) |
| Ghattas et al, 201351 | USA | N=80† Consecutive patients either with COPD or clinically managed for COPD symptoms; Single health centre |
40.0% male 52.9 (7.7) years |
COPD and ACO | 35.0% (28/80) |
65.0% (52/80)¶ |
| Walters et al 201152 | TAS, Australia | N=341 (28%) Non-consecutive adult patient 40–80 years managed for COPD with >10 pack-years and ≥1 GP visit last 12 months; from 31 urban/rural practices |
48.8% male Non-COPD 59 (9) COPD 64 (8) years |
COPD | 68.6% (234/341) | 31.4% (107/341) |
| Zwar et al 201153 | PELICAN study, NSW Australia (prior to randomisation) | N=445 (38.9%) Non-consecutive adults 40–80 years clinically managed for COPD; 56 GPs from 44 practices |
No COPD: 44% males; 62 (11) COPD±asthma: 52% males; 67 (10) |
COPD and ACO | 57.8% (257/445) | 42.2% (188/445)¶ |
| Sichletidis et al, 200754 | Northern Greece | N=319 (87.4%) Non-consecutive adult patients >40 years treated with a COPD diagnosis; 8 health centres |
Symptomatic COPD cases 83.8% male Age-N/A |
COPD and ACO (n=5) | 50.1% (160/319) | 49.9% (159/319) |
*COPD as defined by post-BD airflow obstruction on spirometry testing.
†COPD prevalence could be inaccurate due to insufficient sample size (online supplemental results E4), so study has been omitted from the forest plot in a sensitivity analysis.
‡All % eligible figures were calculated.
§COPD was defined by airflow obstruction detected by postbronchodilator spirometry using the GOLD criterion (forced expiratory ratio <0.70)
¶Data needed to be deduced and calculated from the figures given (by the present authors).
ACO, asthma-COPD overlap; BD, bronchodilator; COPD, Chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; QOF, quality and outcomes framework.
Description of studies
Study origin
Of 21 cross-sectional prevalence studies, 10 were from Europe/UK,19 30 33 34 37 39–41 44 46 3 from South America,31 36 47 2 from Australia,29 35 2 from Asia,32 38 2 from North America,42 43 and 2 from Scotland and the USA45 48 (online supplemental figure S1). Of five COPD case series, two were from Europe/UK,50 54 two from Australia52 53 and one from the USA.51
Ages of participants
Almost all studies recruited adults aged ≥40 years (N=23), with an averaged mean study age of 59.1 years (95% CI 47.9 to 68.5, range 46.8–72 years).
Diagnostic criteria
All studies used the GOLD criterion for post-BD spirometry to define COPD (ie, FEV1/FVC<0.7), except one cross-sectional study from Poland which used the FEV1/FVC<LLN criterion.44 The source of doctor diagnosis for symptomatic patients and COPD case series/registries was health records. This contrasts with patient-reported diagnoses in studies of smokers (irrespective of symptoms), and mixed reporting for other risk categories. This and the adequacy of the sample size are summarised in online supplemental figure S2.
COPD underdiagnosis
Absolute proportion of undiagnosed COPD within the total population sample
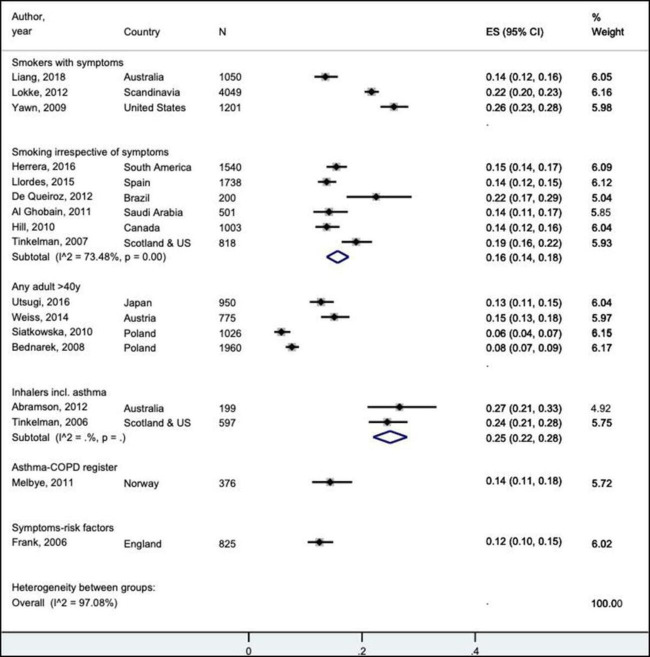
The baseline prevalence of spirometry-defined COPD in all cross-sectional studies (N=21) was variable (online supplemental figure S3). The proportion of spirometry-defined COPD that was undiagnosed in these population samples substantially varied by risk category (I2=97.1%, figure 2). For three adequately sized studies of symptomatic smokers, 14%–26% of these patients had spirometry-confirmed COPD which was not documented in their health records, that is, they had clinical COPD defined by post-BD airflow obstruction and symptom(s)±risk factor(s).20 This was especially more for smokers who had only a clinician diagnosis of chronic bronchitis (25.6%, 95% CI 23.2% to 28.1%), that is, a concurrent COPD diagnosis was not documented in a quarter of medical records.43 Furthermore, undiagnosed COPD was seen for 27.5% of smokers who were treated for a recent lower respiratory tract infection (95% CI 20.7 to 35.5, n=38/138), although this study was excluded from the forest plot in the sensitivity analysis given the small sample size.40 Notably, 14.2% (95% CI 11.5% to 15.7%) of subjects in one Australian study had their COPD undiagnosed, despite attending a PHC clinic at least twice within the last 12 months.29
Figure 2.
Undiagnosed patients with postbronchodilator airflow obstruction in the total population studied (ES) relating to cross-sectional prevalence studies of sufficient sample size, grouped by risk categories. A pooled estimate was only reported in the main text if the degree of heterogeneity (I2) was <75% or if the 95% CIs of all studies within a risk category clearly overlapped. Undiagnosed clinical COPD could be confirmed in the subgroups of symptomatic persons (ie, smokers with symptoms or with symptoms/risk factors) and registries, and these patients did not have a diagnosis of COPD listed in their primary healthcare record. The abbreviation of ‘N’ refers to the total number of participants in the study. COPD, chronic obstructive pulmonary disease.
For studies of smokers who had spirometric evidence of COPD (irrespective of symptoms) but did not report receiving a diagnosis, the pooled prevalence estimate was 16% (95% CI 14% to 18%, I2=73.5%, figure 2). This contrasted with studies of patients taking inhaled therapies for obstructive lung diseases in which COPD and/or fixed airflow obstruction in people with asthma was undiagnosed in 25% (95% CI 22% to 28%, I2=n/a, figure 2).
Relative proportion underdiagnosed among only those with spirometric evidence of COPD
Compared with general populations of adults >40 years, overtly at-risk populations with a higher prevalence of COPD confirmed by the researchers’ post-BD spirometry (online supplemental figure S3) generally had a lower proportion of subjects who were underdiagnosed (online supplemental figure S4 and associated text). Even so, more than half of the symptomatic adults shown to have spirometry-confirmed COPD in these studies did not have the diagnosis entered in their health records, that is, 52%–63% were underdiagnosed despite having symptoms, and 37%–48% were formally diagnosed.29 46 Over one-third of Norwegian subjects listed on an asthma-COPD registry who were found to have post-BD airflow obstruction by researchers did not have a current clinician diagnosis of COPD (36.2%).39
Of these studies, seven reported quantitative data on participant characteristics of patients undiagnosed compared with those doctor diagnosed (online supplemental table S2). Having fewer respiratory symptoms,29 30 36 37 42 as well as milder disease (ie, better FEV1/FVC),29 31 were consistent features for underdiagnosis. Predisposing features also included younger age,29 30 37 fewer pack-years,37 but paradoxically, also those currently smoking.29 30
COPD ‘overdiagnosis’
Prevalence of absent post-BD airflow obstruction among those labelled with COPD
From eight heterogeneous cross-sectional studies of adequate sample size, six reported 26%–52% of patients did not have post-BD airflow obstruction on spirometry, despite probable intent to manage for COPD (online supplemental figure S5). The other two individual studies included one Spanish study which had a predominance of male smokers (84%) and reported the lowest proportion ‘overdiagnosed’ (15.7%).33 The other was an Austrian general adult population study which considered patient self-reported chronic bronchitis/emphysema as COPD. It adopted a stricter spirometric cut-off criterion (GOLD stage II with FEV1<80%20 which could explain why it reported the highest proportion ‘overdiagnosed’ (77%).34
The baseline prevalence of spirometry-confirmed COPD among the adequately powered COPD case series varied between 50% and 75%.50 52–54 Therefore, 25%–50% of these patients had no evidence of airflow obstruction on post-BD spirometry and would be considered ‘overdiagnosed’ (table 3 and online supplemental figure S6). Two of the prevalence studies also investigated a trial of 4–6 weeks of inhaled corticosteroid (ICS)-combination inhalers and documented a normalisation of post-BD airflow obstruction in 16%–19% of treated patients.33 37
Quantitative data on predisposing factors for ‘overdiagnosis’ (online supplemental table S3) suggested such participants had fewer symptoms,42 52 were prescribed less respiratory medication,48 younger52 53 and more obese than those who had post-BD spirometric airflow obstruction.50 52
Risk of bias: JBI critical appraisal tools
All studies used valid identification methods, that is, post-BD spirometry, appropriate definitions and population-specific reference values. However, the overall quality was moderate at best, since 17 of 26 studies did not satisfy at least 1 of the required criteria. Specifically, some cross-sectional prevalence studies (online supplemental table S4) had limited sampling frames,19 33 41 44 46 recruited convenience samples,19 30 35 36 43 had low recruitment rates,29 35 42 46 48 an inadequate sample size to accurately estimate COPD prevalence,30 40 41 47 used a combination of microspirometry and spirometry29 or the prevalence figures for COPD underdiagnosis were difficult to find.19 34 41 43 Only one prevalence study provided 95% CIs33; another reported that most testing staff were not trained.29 Similarly of the five COPD case series (online supplemental table S5), three recruited participants in a non-consecutive manner52–54 and another was underpowered to estimate true COPD prevalence.51 Further details including the sample size estimations are reported in online supplemental results S4. None of the studies were excluded from this review based on low quality; however, those with small sample sizes relative to COPD prevalence were part of a sensitivity analysis and were not shown in the illustrative forest plots. Their absence made little difference to the message.
Discussion
Our systematic review has found that both undiagnosed and ‘overdiagnosed’ COPD were common in PHC settings when COPD was defined by post-BD airflow obstruction20 as determined by the study researchers. For the first time, we have reworked the definition of undiagnosed COPD to estimate that 14%–26% of all symptomatic smokers attending a PHC clinic have spirometry-confirmable COPD which was not documented in their health records. This review also estimates that one in four patients taking inhaled therapies have fixed obstructive lung function consistent with COPD that was not formally documented by the treating clinician35 and/or recognised by the patient.48 While knowledge of the relative prevalence of undiagnosed COPD is difficult to translate to a clinical context, we estimated that less than half of symptomatic adults who had spirometry-confirmable COPD had this diagnosis entered into their medical records.
In contrast, around 25%–50% of people labelled or managed as having COPD had no airflow obstruction on spirometry when tested by study researchers, which has been described by the field as COPD ‘overdiagnosis’. Quotation marks were used as spirometry is relatively insensitive at detecting COPD in its early stages,55 so it is possible that some patients taking corticosteroid (ICS)-combination inhalers could have been treated for chronic airflow limitation at this potentially reversible stage33 37 56 and this needs testing in prospective trials. Although the studies included in this review were limited, heterogeneous and variable in quality, the findings raise important issues around the accuracy of COPD diagnosis in PHC settings.
Undiagnosed COPD is common in at-risk populations
In this review, we have shown that many symptomatic smokers who had attended their PHC clinic even multiple times within the last 12 months,29 had spirometry-confirmable COPD that was not documented by their treating clinician. However, the exact reasons for their attendances and whether they were treated with inhaled therapies were not specified. In another small study, a quarter of smokers treated for a recent lower respiratory tract infection had undiagnosed COPD,40 and we note some major guidelines suggest that recurrent infections should raise clinical suspicion for COPD.3 4
While under-reporting can occur if individuals normalise their symptoms as simply a ‘smokers’ cough’ or ‘old age’,57 our findings likely reflect an underinterrogation and/or investigation of COPD by clinicians for this high-risk group. It is possible that many primary care clinicians are unaware that adults with undiagnosed COPD are at much higher risk for acute exacerbations, pneumonia or death, even if they are asymptomatic.58 Confirmation of a COPD diagnosis with spirometry has been associated with fewer hospital admissions and reduced COPD-related mortality,59 most likely because of more appropriate clinical management. Importantly, we highlight at-risk groups of patients in PHC for whom COPD is commonly unrecognised and an accurate diagnosis might well improve health outcomes.
Internationally recognised experts,60 expert bodies61 62 and guidelines4–7 20 advocate for active COPD case-finding by objectively testing high-risk groups. While systematic case-finding by inviting smokers to undergo serial spirometry has potential to be cost-effective,63 64 in practice, clinicians tend not to record patients with less severe symptoms/disease within a COPD registry65 which would facilitate best practice care.
COPD ‘overdiagnosis’ is a complex issue
Several studies and quality improvement activities from PHC settings in both high-income66–68 and low-income and middle-income countries (LMICs)69 have shown that fewer than 40% of patients labelled as having COPD had had their diagnosis confirmed objectively by spirometry, that is, with likely substantial overdiagnosis. This is consistent with large numbers of patients being prescribed respiratory medicines including BD on a presumption that they have COPD.70 Interestingly, a large randomised controlled trial of symptomatic smokers with no airflow obstruction on spirometry found symptoms were improved similarly in both treatment and placebo arms (56.4% improved following 12 weeks of dual BD therapy compared with 59.0% taking a placebo).71 This suggests that symptoms can vary over time,55 and this treatment itself has limited efficacy for such patients.
Given the potential for diagnostic confusion, clinicians may undertreat other medical conditions such as asthma, heart failure72 and/or bronchiectasis. Symptoms such as wheezing, breathlessness and cough/sputum are non-specific and overlap with those of COPD. This is also the case for people who are obese and present with breathlessness on exertion.50 52 While we cannot elucidate the true reasons why the studied patients may have been ‘overdiagnosed’, this systematic review has highlighted issues of empirically treating early COPD and the potential for inappropriate prescribing of medicines.
Underutilisation of objective testing: a global problem
A central issue to both COPD underdiagnosis and overdiagnosis35 45 46 52 53 is the underuse of objective testing,20 when spirometry performed well is diagnostic, reliable and non-invasive. However, even in the pre-COVID-19 era, access to this point-of-care test was limited in many PHC settings for multiple reasons. These included a lack of testing expertise and training, time allocation and/or low financial incentives. In addition, uncertainty in interpreting spirometry can be a major barrier to best practice.73 In these situations, establishing an accurate diagnosis, therefore, currently relies on the availability of laboratory-based spirometry, but long waiting lists, out-of-pocket expenses and excessive travel time/distances are often barriers. This is especially so in LMICs.74
Potential facilitators of improved guideline adherence could include professional development for doctors and practice nurses, integrating lung function testing reminders into electronic health records and/or developing decision support technology and algorithms to increase awareness and instil confidence in clinicians to test and more accurately assess patients.75 However, these initiatives would only partly address the additional clinician-based factors of: over-reliance and/or ‘overconfidence’ in clinical-only diagnostic skills76; mislabelling in hospital discharge summaries being carried forward into a PHC diagnosis; and frequently an attitude of nihilism73 and viewing the spirometer as a means of convincing the patient to stop smoking rather than a tool to aid accurate diagnosis.35
While local diagnostic protocols may differ between geographical regions, PHC services and stages of the SARS-CoV-2 pandemic77 a detailed discussion of these protocols is beyond the scope of this review but is unlikely to change the clinical picture presented. However, we note that all studies included in this review were from the pre-COVID-19 era. During the pandemic, the number of primary care spirometry tests in particular fell dramatically from prolonged disruptions that has now led to the deskilling of staff with probable loss of confidence in performing spirometry. This has only been partially reversed in the ‘living-with-COVID-19’ era78 due to ongoing concerns about the potential spread of SARS-CoV-2 virus to healthcare workers and patients through performing spirometry which can generate aerosol droplets, especially by inducing coughing.79 80
Professional societies have provided evidence-based protocols of infection prevention control for spirometry testing at the point of care in PHC settings that include adequate ventilation in testing spaces, cleaning of room and equipment, single-use in-line antimicrobial filters and full personal protective equipment.79–83 Complying with the ventilatory requirements can be particularly difficult as most practices do not have the resources or expertise to estimate the adequacy of ventilation provided, and rooms may need to be reconfigured or else vacated for a period between testing patients. Together with staff re-engagement and retraining, the costs associated with these COVID-19 safe protocols can be prohibitive in PHC services. This poses a serious new challenge for primary care clinicians when trying to follow clinical pathways that align with the recommendations of COPD guidelines,4–7 20 given that access to public and private respiratory laboratories can be limited by either availability or patient out-of-pocket expenses, respectively. A potential way forward is developing prediction tools that risk-stratify patients,8 84 and/or establishing diagnostic ‘hubs or hublets’ to provide a good-quality diagnostic spirometry service at a local network level in the community.81 85
Strengths and limitations
This first systematic review has intentionally studied ‘real-world’ PHC clinic samples, employing a widely accepted objective diagnostic criterion for confirming a COPD diagnosis,20 while minimising the confounding potential of predominant asthma if only pre-BD spirometry was assessed.
We acknowledge that excluding articles published only in languages other than English at the title/abstract phase may have missed some evidence and could have limited the generalisability of findings. We also could not directly assess the included studies for publication bias, as there is no well-accepted equivalent of a funnel or Egger’s plot for prevalence studies. However, our sample size calculations confirmed that approximately one-fifth of studies (n=5) had been published despite low subject numbers.26 27
The risk of bias was at least moderate, with substantial heterogeneity between included studies such that pooled estimates were uncommonly reported. In all but two studies,19 31 the age spectrum of COPD was not considered, despite COPD being more common in the elderly. There were only limited studies from Asia and none from Africa, so the findings should not be generalised to these continents and more research is needed there.
Summary
In this systematic review, we have estimated the absolute proportions of undiagnosed spirometry-confirmed COPD (ie, symptomatic patients who were not documented by primary care clinicians to have COPD but had post-BD airflow obstruction when studied) and thus the potential extent of disease undermanagement in PHC patient populations based on their underlying risk profiles. Furthermore, this review has revealed that many patients who do not have spirometric evidence of COPD when studied have a clinical label of COPD in their health record. Other than a lack of spirometry to confirm or refute a COPD diagnosis accurately, potential explanations also include clinicians not accurately documenting patient diagnoses in the PHC health record, patients not recalling receiving their COPD diagnosis accurately, and patients being already treated prior to spirometry.
Nonetheless, this review highlights that underdiagnosis and overdiagnosis of COPD is a complex and multifaceted problem, and the magnitude of this issue is expected to worsen given even further reductions in spirometry use post-COVID-19. There is an underlying need for greater awareness and action by both at-risk patients and healthcare providers about reporting and documenting symptoms and then objectively investigating for a potential COPD diagnosis. However, more robust scientific and economic evidence of benefit of earlier diagnosis is also needed for public health systems to increase funding and support that might improve the culture of active case-finding for COPD and enhance use of spirometry in PHC settings.
Footnotes
Contributors: JP, KH, EHW and MJA conceptualised the study. SWSY mapped the literature then JP/EHW designed the study. Overseen by NW and JP registered the protocol, SWSY/JP reviewed titles/abstracts, SWSY/NSI reviewed full texts and SWSY/JP extracted the full data. JP performed the data analysis and led the interpretation, supervised by SCD and NW. JP drafted the manuscript which was revised by SWSY/EHW and critically assessed for important intellectual content by all authors. JP revised the manuscript after peer review with input from all authors, especially KH. All authors approved the final version. JP submitted the study and has taken responsibility for the overall content as guarantor.
Funding: JP and SCD were supported by the NHRMC of Australia APP1159090 and APP1193993 respectively.
Competing interests: JP, SCD, EHW and MJA hold an investigator-initiated grant from GlaxoSmithKline for unrelated research, and SCD and JP have an investigator-initiated partnership grant with AstraZeneca for unrelated research. MJA additionally holds investigator-initiated grants from Pfizer, Boehringer-Ingelheim and Sanofi for unrelated research; has undertaken an unrelated consultancy for Sanofi; and received a speaker’s fee from GlaxoSmithKline. KH has received personal fees and non-financial support from Astra Zeneca, GlaxoSmithKline, Novartis, Chiesi, Boehringer Ingelheim and Teva outside the submitted work. KH has also received fees for performing spirometry and for training health professionals in spirometry testing.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the present systematic review/meta-analysis are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
As this systematic review analysed publicly available, aggregated, and non-identifiable patient data, this study did not require ethical approval as there was no risk to participants.
References
- 1.World Health Organization . The top 10 causes of death. Fact sheet. 2020. Available: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Accessed 3 Apr 2021].
- 2.Young RP, Hopkins RJ, Christmas T, et al. Copd prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380–6. 10.1183/09031936.00144208 [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2023. Available: https://goldcopd.org/2023-gold-report-2 [Accessed 23 Jan 2023].
- 4.NICE . Chronic obstructive pulmonary disease in over 16s: diagnosis and management. London, 2019. [PubMed] [Google Scholar]
- 5.Yang IA, Dabscheck E, George J, et al. The COPD-X plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease. version 2. Lung Foundation Australia, 2021. Available: https://copdx.org.au/wp-content/uploads/2021/04/COPDX-V2-63-Feb-2021_FINAL-PUBLISHED.pdf [Google Scholar]
- 6.Chronic Obstructive Pulmonary Disease (COPD) . Diagnosis and management. Effective. 2017. Available: https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/copd_full_guideline.pdf [Accessed 24 Jan 2023].
- 7.Park YB, Rhee CK, Yoon HK, et al. Revised (2018) COPD clinical practice guideline of the Korean academy of tuberculosis and respiratory disease: A summary. Tuberc Respir Dis (Seoul) 2018;81:261–73. 10.4046/trd.2018.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a lancet commission. Lancet 2022;400:921–72. 10.1016/S0140-6736(22)01273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open 2018;8:e018557. 10.1136/bmjopen-2017-018557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calverley PM. COPD: early detection and intervention. Chest 2000;117:365S–71S. 10.1378/chest.117.5_suppl_2.365s [DOI] [PubMed] [Google Scholar]
- 11.Hunter LC, Lee RJ, Butcher I, et al. Patient characteristics associated with risk of first hospital admission and readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) following primary care COPD diagnosis: a cohort study using linked electronic patient records. BMJ Open 2016;6:e009121. 10.1136/bmjopen-2015-009121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balcells E, Antó JM, Gea J, et al. Characteristics of patients admitted for the first time for COPD exacerbation. Respir Med 2009;103:1293–302. 10.1016/j.rmed.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 13.Jones RCM, Price D, Ryan D, et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med 2014;2:267–76. 10.1016/S2213-2600(14)70008-6 [DOI] [PubMed] [Google Scholar]
- 14.Thomas ET, Glasziou P, Dobler CC. Use of the terms “overdiagnosis” and “misdiagnosis” in the COPD literature: a rapid review. Breathe (Sheff) 2019;15:e8–19. 10.1183/20734735.0354-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho T, Cusack RP, Chaudhary N, et al. Under- and over-diagnosis of COPD: a global perspective. Breathe (Sheff) 2019;15:24–35. 10.1183/20734735.0346-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koblizek V, Novotna B, Zbozinkova Z, et al. Diagnosing COPD: advances in training and practice-a systematic review. Adv Med Educ Pract 2016;7:219–31. 10.2147/AMEP.S76976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018;198:1130–9. 10.1164/rccm.201804-0621CI [DOI] [PubMed] [Google Scholar]
- 18.Petrie K, Toelle BG, Wood-Baker R, et al. Undiagnosed and misdiagnosed chronic obstructive pulmonary disease: data from the BOLD Australia study. Int J Chron Obstruct Pulmon Dis 2021;16:467–75. 10.2147/COPD.S287172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siatkowska H, Kozielski J, Ziora D. Chronic obstructive pulmonary disease patients in the general practice. Pneumonol Alergol Pol 2010;78:112–20. 10.5603/ARM.27736 [DOI] [PubMed] [Google Scholar]
- 20.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease. 2021. Available: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf [Accessed 21 May 2021].
- 21.Perret J, Yip S, Waidyatillake N, et al. Chronic obstructive pulmonary disease prevalence and its underdiagnosis and overdiagnosis in primary care settings. PROSPERO 2022 CRD42022295832. 2022. Available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022295832
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veritas Health Innovation . Covidence systematic review software, Melbourne, Australia. n.d. Available: www.covidence.org
- 24.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147–53. 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 25.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth 2020;18:2127–33. 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 26.Daniel WW. Biostatistics: a foundation for analysis in the health sciences. 7th ed. John Wiley & Sons, 1999. [Google Scholar]
- 27.Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench 2013;6:14–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang J, Abramson MJ, Zwar NA, et al. Diagnosing COPD and supporting smoking cessation in general practice: evidence-practice gaps. Med J Aust 2018;208:29–34. 10.5694/mja17.00664 [DOI] [PubMed] [Google Scholar]
- 30.Stafyla E, Kotsiou OS, Deskata K, et al. Missed diagnosis and overtreatment of COPD among smoking primary care population in central Greece: old problems persist. Int J Chron Obstruct Pulmon Dis 2018;13:487–98. 10.2147/COPD.S147628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casas Herrera A, Montes de Oca M, López Varela MV, et al. Copd underdiagnosis and misdiagnosis in a high-risk primary care population in four Latin American countries. A key to enhance disease diagnosis: the PUMA study. PLoS One 2016;11:e0152266. 10.1371/journal.pone.0152266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utsugi H, Nakamura H, Suzuki T, et al. Associations of lifelong cigarette consumption and hypertension with airflow limitation in primary care clinic outpatients in Japan. Respir Investig 2016;54:35–43. 10.1016/j.resinv.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 33.Llordés M, Jaén A, Almagro P, et al. Prevalence, risk factors and diagnostic accuracy of COPD among smokers in primary care. COPD 2015;12:404–12. 10.3109/15412555.2014.974736 [DOI] [PubMed] [Google Scholar]
- 34.Weiss G, Steinacher I, Lamprecht B, et al. Detection of chronic obstructive pulmonary disease in primary care in Salzburg, Austria: findings from the real world. Respiration 2014;87:136–43. 10.1159/000354796 [DOI] [PubMed] [Google Scholar]
- 35.Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J 2012;21:167–73. 10.4104/pcrj.2011.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Queiroz M de C de, Moreira MAC, Rabahi MF. Underdiagnosis of COPD at primary health care clinics in the city of aparecida de Goiânia, Brazil. J Bras Pneumol 2012;38:692–9. 10.1590/s1806-37132012000600003 [DOI] [PubMed] [Google Scholar]
- 37.Løkke A, Ulrik CS, Dahl R, et al. Detection of previously undiagnosed cases of COPD in a high-risk population identified in general practice. COPD 2012;9:458–65. 10.3109/15412555.2012.685118 [DOI] [PubMed] [Google Scholar]
- 38.Al Ghobain M, Al-Hajjaj MS, Wali SO. Prevalence of chronic obstructive pulmonary disease among smokers attending primary healthcare clinics in Saudi Arabia. Ann Saudi Med 2011;31:129–33. 10.4103/0256-4947.77485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melbye H, Drivenes E, Dalbak LG, et al. Asthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or more. Int J Chron Obstruct Pulmon Dis 2011;6:597–603. 10.2147/COPD.S25955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandelowsky H, Ställberg B, Nager A, et al. The prevalence of undiagnosed chronic obstructive pulmonary disease in a primary care population with respiratory tract infections-a case finding study. BMC Fam Pract 2011;12:122. 10.1186/1471-2296-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dirven JAM, Muris JWM, van Schayck CP. Copd screening in general practice using a telephone questionnaire. COPD 2010;7:352–9. 10.3109/15412555.2010.510547 [DOI] [PubMed] [Google Scholar]
- 42.Hill K, Goldstein RS, Guyatt GH, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ 2010;182:673–8. 10.1503/cmaj.091784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yawn B, Mannino D, Littlejohn T, et al. Prevalence of COPD among symptomatic patients in a primary care setting. Curr Med Res Opin 2009;25:2671–7. 10.1185/03007990903241350 [DOI] [PubMed] [Google Scholar]
- 44.Bednarek M, Maciejewski J, Wozniak M, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008;63:402–7. 10.1136/thx.2007.085456 [DOI] [PubMed] [Google Scholar]
- 45.Tinkelman DG, Price D, Nordyke RJ, et al. Copd screening efforts in primary care: what is the yield? Prim Care Respir J 2007;16:41–8. 10.3132/pcrj.2007.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank TL, Hazell ML, Linehan MF, et al. The diagnostic accuracies of chronic obstructive pulmonary disease (COPD) in general practice: the results of the MAGIC (manchester airways group identifying COPD) study. Prim Care Respir J 2006;15:286–93. 10.1016/j.pcrj.2006.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamers R, Bontemps S, van den Akker M, et al. Chronic obstructive pulmonary disease in brazilian primary care: diagnostic competence and case-finding. Prim Care Respir J 2006;15:299–306. 10.1016/j.pcrj.2006.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma 2006;43:75–80. 10.1080/02770900500448738 [DOI] [PubMed] [Google Scholar]
- 49.Heffler E, Crimi C, Mancuso S, et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med 2018;142:48–52. 10.1016/j.rmed.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 50.Fisk M, McMillan V, Brown J, et al. Inaccurate diagnosis of COPD: the welsh national COPD audit. Br J Gen Pract 2019;69:e1–7. 10.3399/bjgp18X700385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghattas C, Dai A, Gemmel DJ, et al. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int J Chron Obstruct Pulmon Dis 2013;8:545–9. 10.2147/COPD.S45693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walters JA, Walters EH, Nelson M, et al. Factors associated with misdiagnosis of COPD in primary care. Prim Care Respir J 2011;20:396–402. 10.4104/pcrj.2011.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zwar NA, Marks GB, Hermiz O, et al. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med J Aust 2011;195:168–71. 10.5694/j.1326-5377.2011.tb03271.x [DOI] [PubMed] [Google Scholar]
- 54.Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J 2007;16:82–8. 10.3132/pcrj.2007.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sin DD. RETHINCking COPD-bronchodilators for symptomatic tobacco-exposed persons with preserved lung function? N Engl J Med 2022;387:1230–1. 10.1056/NEJMe2210347 [DOI] [PubMed] [Google Scholar]
- 56.Global Initiative for Asthma . Global strategy for asthma management and prevention. 2022. Available: www.ginasthma.org [Accessed 24 Jun 2022].
- 57.Pinnock H, Sohanpal R. Chronic obstructive pulmonary disease: reduced nihilism, but there is still a ways to go. Chronic Obstr Pulm Dis 2016;3:605–9. 10.15326/jcopdf.3.3.2016.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in denmark: a prospective cohort study. Lancet Respir Med 2017;5:426–34. 10.1016/S2213-2600(17)30119-4 [DOI] [PubMed] [Google Scholar]
- 59.Gershon A, Mecredy G, Croxford R, et al. Outcomes of patients with chronic obstructive pulmonary disease diagnosed with or without pulmonary function testing. CMAJ 2017;189:E530–8. 10.1503/cmaj.151420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price D, Freeman D, Cleland J, et al. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J 2011;20:15–22. 10.4104/pcrj.2010.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Heart, Lung and Blood Institute . A case-finding strategy for moderate-to-severe COPD in the United States. 2008. Available: https://www.nhlbi.nih.gov/events/2008/case-finding-strategy-moderate-severe-copd-united-states [Accessed 30 Sep 2019].
- 62.Lung Foundation Australia . Position paper. COPD case finding in community settings. Available: https://lungfoundation.com.au/wp-content/uploads/2018/11/Information-Paper-COPD-Case-Finding-position-paper-Oct2019.pdf [Accessed 15 Nov 2019].
- 63.Jordan RE, Adab P, Sitch A, et al. Targeted case finding for chronic obstructive pulmonary disease versus routine practice in primary care (targetcopd): a cluster-randomised controlled trial. Lancet Respir Med 2016;4:720–30. 10.1016/S2213-2600(16)30149-7 [DOI] [PubMed] [Google Scholar]
- 64.Lambe T, Adab P, Jordan RE, et al. Model-based evaluation of the long-term cost-effectiveness of systematic case-finding for COPD in primary care. Thorax 2019;74:730–9. 10.1136/thoraxjnl-2018-212148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haroon S, Adab P, Dickens AP, et al. Impact of COPD case finding on clinical care: a prospective analysis of the targetCOPD trial. BMJ Open 2020;10:e038286. 10.1136/bmjopen-2020-038286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.COPD . MedicineInsight post-market surveillance report number 11: NPS medicinewise: Sydney. n.d. Available: https://www.nps.org.au/assets/NPS/pdf/MedicineInsight-COPD-Post-Market-Surveillance-2017-Report.pdf
- 67.Decramer M, Brusselle G, Buffels J, et al. Copd awareness survey: do Belgian pulmonary physicians comply with the gold guidelines 2010? Acta Clin Belg 2013;68:325–40. 10.2143/ACB.3403 [DOI] [PubMed] [Google Scholar]
- 68.Echazarreta AL, Arias SJ, Del Olmo R, et al. Prevalence of COPD in 6 urban clusters in Argentina: the EPOC.AR study. Arch Bronconeumol (Engl Ed) 2018;54:260–9. 10.1016/j.arbres.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 69.Kaur I, Aggarwal B, Gogtay J. Understanding perception of chronic obstructive pulmonary disease among general practitioners, physicians, and pulmonologists in India: results from a face-to-face survey. Perspect Clin Res 2016;7:100–5. 10.4103/2229-3485.179438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016;374:1811–21. 10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han MK, Ye W, Wang D, et al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med 2022;387:1173–84. 10.1056/NEJMoa2204752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong CW, Tafuro J, Azam Z, et al. Misdiagnosis of heart failure: a systematic review of the literature. J Card Fail 2021;27:925–33. 10.1016/j.cardfail.2021.05.014 [DOI] [PubMed] [Google Scholar]
- 73.Walters JA, Hansen EC, Johns DP, et al. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of COPD. Thorax 2008;63:408–14. 10.1136/thx.2007.082859 [DOI] [PubMed] [Google Scholar]
- 74.World Health Organisation . General availability of peak flow measurement spirometry at the primary health care level. 2020. Available: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/general-availability-of-peak-flow-measurement-spirometry-at-the-primary-health-care-level
- 75.Overington JD, Huang YC, Abramson MJ, et al. Implementing clinical guidelines for chronic obstructive pulmonary disease: barriers and solutions. J Thorac Dis 2014;6:1586–96. 10.3978/j.issn.2072-1439.2014.11.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med 2008;121:S2–23. 10.1016/j.amjmed.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 77.ARTP COVID19 Group . Association for respiratory technology and physiology (ARTP) - guidelines for recommencing physiological services during the coronovirus disease 2019 (COVID-19) endemic phase. Version 5.1 (August 2020 update). 2020.
- 78.Saunders MJ, Haynes JM, McCormack MC, et al. How local SARS-CoV-2 prevalence shapes pulmonary function testing laboratory protocols and practices during the COVID-19 pandemic. Chest 2021;160:1241–4. 10.1016/j.chest.2021.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borg BM, Osadnik C, Adam K, et al. Pulmonary function testing during SARS-cov-2: an ANZSRS/TSANZ position statement. Respirology 2022;27:688–719. 10.1111/resp.14340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stanojevic S, Beaucage F, Comondore V, et al. Resumption of pulmonary function testing during the COVID-19 pandemic. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine 2022;6:78–81. 10.1080/24745332.2021.2010478 [DOI] [Google Scholar]
- 81.Primary Care Respiratory Society (PCRS) . Spirometry in primary care. guidance on reinstating spirometry in England. Available: https://www.artp.org.uk/write/MediaUploads/Standards/COVID19/ARTP_PCRS_spiro_re-start_FINAL2.pdf [Accessed 23 Jan 2023].
- 82.Association for Respiratory Technology and Physiology (ARTP) . Statement for the NHS national respiratory programme. Task and finish group. Recommendations for undertaking risk-managed spirometry. Available: https://www.artp.org.uk/write/MediaUploads/Standards/COVID19/ARTP_PCRS_spiro_re-start_FINAL2.pdf [Accessed 23 Jan 2023].
- 83.Recommendation from ERS group 9.1 (respiratory function technologists/ scientists). Lung function testing during COVID-19 pandemic and beyond. Available: https://medicine.usask.ca/respiratoryresearch/documents/ers-9.1-statement-on-lung-function-during-covid-19-final-with-contributors.pdf [Accessed 3 Feb 2023].
- 84.Perret JL, Vicendese D, Simons K, et al. Ten-year prediction model for post-bronchodilator airflow obstruction and early detection of COPD: development and validation in two middle-aged population-based cohorts. BMJ Open Respiratory Research 2021;8:e001138. 10.1136/bmjresp-2021-001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lung Foundation Australia . Transforming the agenda for COPD: a path towards prevention and lifelong lung health - Lung Foundation Australia’s Blueprint for Action on Chronic Obstructive Pulmonary Disease (COPD) 2022-2025. Milton, Queensland: Lung Foundation Australia, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001478supp001.pdf (773.4KB, pdf)
Data Availability Statement
All data relevant to the present systematic review/meta-analysis are included in the article or uploaded as online supplemental information.