Abstract
Objectives
Registries have been highlighted as means to improve quality of care. Here, we describe temporal trends in risk factors, lifestyle and preventive medication for patients after myocardial infarction (MI) registered in the quality registry Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART).
Design
A registry-based cohort study.
Setting
All coronary care units and cardiac rehabilitation (CR) centres in Sweden.
Participants
Patients attending a CR visit at 1-year post-MI 2006–2019 were included (n=81 363, 18–74 years, 74.7% men).
Outcome measures
Outcome measures at 1-year follow-up included blood pressure (BP) <140/90 mm Hg, low-density lipoprotein-cholesterol (LDL-C)<1.8 mmol/L, persistent smoking, overweight/obesity, central obesity, diabetes prevalence, inadequate physical activity, and prescription of secondary preventive medication. Descriptive statistics and testing for trends were applied.
Results
The proportion of patients attaining the targets for BP<140/90 mmHg increased from 65.2% (2006) to 86.0% (2019), and LDL-C<1.8 mmol/L from 29.8% (2006) to 66.9% (2019, p<0.0001 both). While smoking at the time of MI decreased (32.0% to 26.5%, p<0.0001), persistent smoking at 1 year was unchanged (42.8% to 43.2%, p=0.672) as was the prevalence of overweight/obesity (71.9% to 72.9%, p=0.559). Central obesity (50.5% to 57.0%), diabetes (18.2% to 27.2%) and patients reporting inadequate levels of physical activity (57.0% to 61.5%) increased (p<0.0001 for all). From 2007, >90.0% of patients were prescribed statins and approximately 98% antiplatelet and/or anticoagulant therapy. Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker prescription increased from 68.7% (2006) to 80.2% (2019, p<0.0001).
Conclusions
While little change was observed for persistent smoking and overweight/obesity, large improvements were observed for LDL-C and BP target achievements and prescription of preventive medication for Swedish patients after MI 2006–2019. Compared with published results from patients with coronary artery disease in Europe during the same period, these improvements were considerably larger. Continuous auditing and open comparisons of CR outcomes might possibly explain some of the observed improvements and differences.
Keywords: Myocardial infarction, REHABILITATION MEDICINE, Quality in health care, Coronary heart disease, Risk management
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The major strengths of the study are the broad representability and national coverage of data including all patients<75 years of age who suffered a myocardial infarction (MI) and were followed in the Swedish quality registry SWEDEHEART.
Major modifiable cardiovascular risk factors were included; blood pressure levels, low-density lipoprotein cholesterol levels, smoking habits, self-reported physical activity, overweight, obesity, central obesity, as well as prescription of secondary preventive medication.
The major limitations of the study are the lack of data on MI patients not attending CR and on those≥75 years of age.
Also, comparing our data with other survey and audit data is limited by differences in patient selections, different rates of CR participation, time of follow-up, and differences in measurement methods.
Introduction
Treating cardiovascular risk factors and adopting healthy behaviours after myocardial infarction (MI) is the most effective way to reduce recurrent cardiovascular events.1 2 Based on abundant and continuously accumulating evidence, the European Society of Cardiology (ESC) regularly publishes guidelines on cardiovascular disease prevention in clinical practice.3 Secondary prevention is usually provided through cardiac rehabilitation (CR)—a complex intervention entailing the optimal use of cardio-protective medication, exercise training, behavioural modification, patient education, and psychosocial counselling.4 In the latest ESC prevention guidelines, participation in CR post-MI is given the highest possible recommendation and level of evidence.3 Still, implementing the guidelines in clinical practice has proven to be a challenge, with goal attainment in CR being far from optimal.5 6 Especially it seems challenging to reach lifestyle associated targets such as being adequately physically active and active smokers at the time of the MI being abstinent from smoking. Furthermore, only marginal improvements have been observed in goal attainment for blood pressure (BP) and low-density lipoprotein cholesterol (LDL-C) during the last 10 years despite increasing availability of more effective pharmacotherapy.5
Systematically monitoring quality of care, structure and process of delivery within CR has been highlighted as a possible way to increase prevention target attainment.7–11 The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) is a nationwide quality registry that records patient characteristics, treatments and outcomes of consecutive patients with MI admitted to coronary care units in Sweden.12 Registration of CR quality and process-based metrics for patients after an MI started in 2005. Since 2006, data have been collected for patients under the age of 75 at two routine follow-up visits within CR—at 2 months and 1 year post-MI.13 14 Referrals to CR are automatically generated through the electronic registry system for all MI patients and since 2016 more than 75% of all eligible patients, who are alive at 1 year after the acute event, attend the 1-year CR follow-up visit.15 Data from SWEDEHEART are available online and are updated continuously, facilitating open comparisons between CR programmes in the country.16
The objective of this study was to describe temporal trends 2006–2019 in risk factor prevalence, lifestyle and prescription of secondary preventive medication at 1 year after MI for patients attending CR in Sweden, hypothesising that a national quality registry can contribute to improving outcomes in CR.
Methods
Patient population and settings
In this retrospective registry-based cohort study, data on all patients (1) with a Swedish national identification number, (2) aged 18–74 years, (3) admitted for a first time or recurrent MI (ICD codes I21, I22 or I23) and (4) having a 1-year CR follow-up visit registered in SWEDEHEART between 1 January 2006 and 31 December 2019 were used. Since patients with recurrent MI are included in SWEDEHEART, the same patient can be registered on several occasions, although not more than once per year since each individual patient can only generate one SWEDEHEART-based follow-up per year. Until 2018, it was mandatory to register patients <75 years of age, while registration of those 75 years or older was optional. For this reason, we chose to apply the age limit of 18–74 years throughout the whole period in the current study. No other exclusion criteria were applied.
Patient and public involvement statement
Patients were not involved in the design or conduct of the current study. The SWEDEHEART registry’s steering group has, however, included a patient representative for many years. The steering group is involved in decisions concerning variables included in the registry and how results generated from registry data are disseminated to the general public.
Data collection
Hospitalisation data
Detailed description of the SWEDEHEART registry has previously been published.12 13 In short, the registry includes more than 100 variables collected during hospitalisation, describing patient characteristics and acute MI care.12 These include age, sex, smoking status (current smoker, previous smoker (stopped smoking >1 month) or never smoker), history of diabetes, hypertension, atherosclerotic cardiovascular disease (MI, percutaneous coronary intervention (PCI), coronary artery by-pass grafting (CABG) or stroke), and current pharmacotherapy, collected from electronic medical records and by self-report. Data on race/ethnicity are not available in SWEDEHEART. Height (cm) and weight (kg) are collected, measured during hospitalisation or self-reported, and body mass index (BMI, kg/m2) is calculated. Waist circumference is not measured during hospitalisation. Systolic and diastolic BP (mm Hg) are registered. Blood samples collected include total cholesterol, triglycerides, LDL-C, high-density lipoprotein cholesterol (HDL-C), fasting plasma glucose and HbA1c (for patients with diabetes only). In SWEDEHEART, estimated LDL-C according to the Friedewald formula: LDL-C=total cholesterol−HDL-C−(0.45×triglycerides) is used to minimise interlaboratory differences in LDL-C.17 In case of triglycerides >4.5 mmol/L or missing values on total cholesterol, HDL-C or triglycerides, directly measured LDL-C, is used instead. In the SWEDEHEART user manual, it is recommended that laboratory measures are performed according to local laboratory routines.
CR data
Approximately 80 variables are collected at CR visits at 2 months (time frame 6–10 weeks) and 1 year (time frame 11–13 months) post-MI.13 These include weight and waist circumference, systolic and diastolic BP, blood samples (lipids, fasting plasma glucose, and in patients with diabetes HbA1c), smoking status and current pharmacotherapy. Additionally, patients report how many days during the last week they have been physically active for a minimum of 30 min (at least 10 min at a time) at an intensity that will induce shortness of breath and a slightly increased pulse, corresponding to a brisk walk.
All data in SWEDEHEART are registered online. Data validity is continuously monitored, with sampling confirming >95% agreement with data from medical records.12 13
Exposure and outcome variables
Exposure in this study was defined as the calendar year of the 1-year follow-up visit. Outcome variables at 1-year follow-up included the following: BP <140/90 mm Hg (both systolic and diastolic BP targets fulfilled, same goal irrespective of diabetes status); LDL-C <1.8 mmol/L; diabetes prevalence; persistent smoking (proportion of smokers at the time of MI who were still smoking at 1-year follow-up); inadequate physical activity (being physically active (as defined above) <5 days/week); overweight/obesity (BMI ≥25 kg/m2); central obesity (waist circumference ≥102 cm for men and ≥88 cm for women); prescription of secondary preventive medication: lipid-lowering drugs (statins and/or ezetimibe), ACE inhibitors (ACEi) or angiotensin receptor blockers (ARB), beta blockers, antiplatelet agents (acetylsalicylic acid and/or P2Y12-receptor antagonists) and anticoagulants (warfarin or direct oral anticoagulants). Registration of the use of proprotein convertase subtilisin/kexin 9 inhibitors started in SWEDEHEART in 2017. As information on use prior to 2017 was not available as well as the use being minimal in the first years (0.5%–1.5%), we decided to include only statins and ezetimibe in the definition of lipid-lowering therapy.15
Statistical analysis
The distribution of continuous variables was assessed by visual inspection of histograms and Q-Q plots. Most continuous variables were non-normally distributed and are presented as medians (quartile 1, quartile 3), apart from delta values which are presented as means±SD. Data for categorical variables are presented as percentages. Trend tests were performed using Cochrane-Armitage trend test for categorical variables and Wilcoxon type test for continuous variables. To compare data between years 2006 and 2019, χ2 test was used for categorical variables and Wilcoxon rank sum test for continuous variables. Outcomes were analysed as dichotomised variables. Median values for continuous outcome variables and mean delta values between baseline (time of index event) were also analysed. For waist circumference, delta was based on the 2-month and 1-year follow-up visit measurements. No imputation was performed on missing data. Data were analysed using SAS V.9.4 (SAS Institute, Cary, North Carolina). A two-sided p value of<0.05 was considered statistically significant.
Results
Patient characteristics during hospitalisation
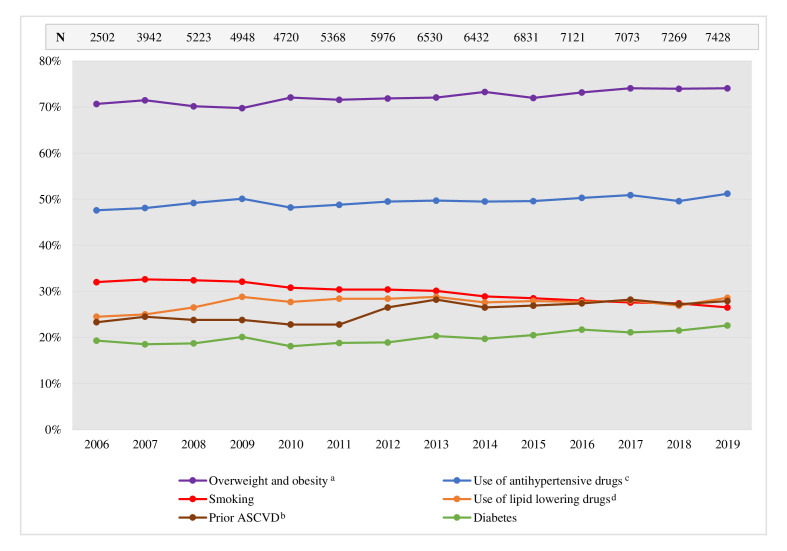
Between 2006 and 2019, 81 363 MI cases were registered in SWEDEHEART, representing 78 679 individual patients 18–74 years of age at the time of the acute event who subsequently attended a 1-year follow-up registry visit within CR. Patients were predominantly men, the proportion increasing slightly during the period from 73.5% in 2006 to 75.2% in 2019 (p trend <0.0001). The median (q1, q3) age was 63.0 (57.0, 69.0) years in 2006 and 65.0 (58.0–70.0) years in 2019 (p trend <0.0001). Further patient characteristics are seen in figure 1 and online supplemental table S1–S3. The most prominent changes observed during the period were a decrease in the proportion of smokers from 32.0% to 26.5% (p trend <0.0001), an increase in the proportion of overweight and obese patients (BMI ≥25 kg/m2) from 70.7% to 74.1% (p trend <0.0001), and an increase in the use of lipid-lowering drugs (statins and/or ezetimibe) (24.5% to 28.6%, p trend=0.004) or antihypertensive drugs (ACEi/ARB, beta blockers, diuretics and/or calcium channel blockers) (47.6% to 51.2%, p trend <0.0001) prior to admission (figure 1). The proportion of patients being revascularised (by PCI or CABG) during hospitalisation and the proportion being prescribed statins, ezetimibe, ACEi/ARB and P2Y12-receptor antagonist therapy at discharge increased during the observed period (p trend <0.0001 for all), while the proportion receiving beta blockers at discharge was decreased (p trend <0.0001) (online supplemental table S3).
Figure 1.
Patient characteristics as registered during MI hospitalisation for patients attending the 1-year follow-up visit within CR in Sweden 2006–2019. aBMI≥25 kg/m2; bprior MI, PCI, CABG or stroke; cACE inhibitors/ARB, beta blockers, diuretics and/or calcium channel blockers; dstatins and/or ezetimibe. ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease (MI, PCI, CABG or stroke); BMI, body mass index; CABG, coronary artery bypass grafting; MI, myocardial infarction; N, number; PCI, percutaneous coronary intervention.
bmjopen-2022-069770supp001.pdf (218.7KB, pdf)
BP, lipids and diabetes
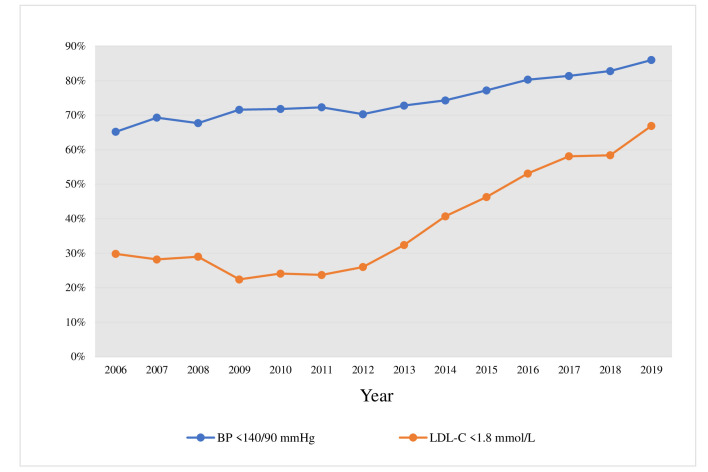
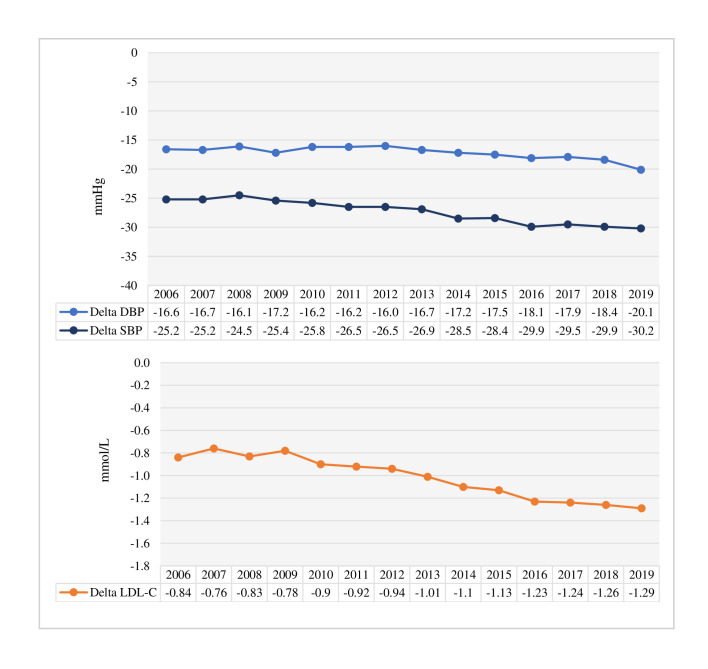
The proportion of patients achieving BP <140/90 mm Hg at the 1-year follow-up visit increased from 65.2% in 2006 to 86.0% in 2019 (p trend <0.0001) (figure 2). Regarding LDL-C, 29.8% were treated to the <1.8 mmol/L target in 2006, increasing to 66.9% in 2019 (p trend <0.0001), with 30.4% having an LDL-C of <1.4 mmol/L in 2019 (figure 2). Mean delta values for systolic and diastolic BP and LDL-C between hospitalisation and 1-year follow-up also increased during the observed period (p for trend <0.0001 for all) (figure 3). The 1-year median systolic blood pressure, diastolic blood pressure, total cholesterol, LDL-C and triglycerides decreased over the period, while HDL-C remained unchanged (online supplemental table S4).
Figure 2.
Proportion of patients achieving targets for BP and LDL-C at the 1-year follow-up visit 2006–2019. The p value for trend from 2006 to 2019 was <0.0001 for both BP and LDL-C. BP, blood pressure; LDL-C, low-density lipoprotein cholesterol.
Figure 3.
Mean delta values between hospitalisation and the 1-year CR follow-up visit for systolic and diastolic BP (upper panel) and LDL-C (lower panel) by year 2006–2019. The p value for the trend from 2006 to 2019 was <0.0001 for all. DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
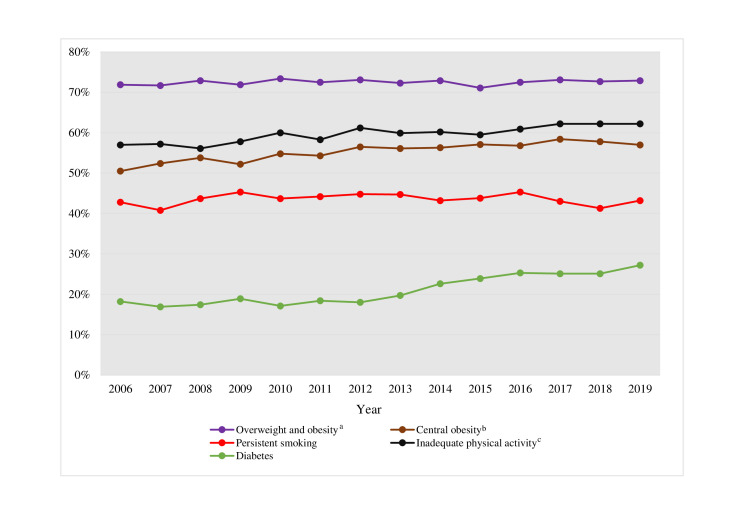
The prevalence of diabetes at the 1-year follow-up increased from 18.2% in 2006 to 27.2% in 2019 (p trend <0.0001) (figure 4). Between 2006 and 2013, there was a minimal difference between the prevalence of diabetes at hospitalisation and at 1-year follow-up (±1%-point). Since 2014, however, the difference increased, in 2019, being 4.6%-points higher at the 1-year follow-up. HbA1c (patients with diabetes only) at the 1-year follow-up decreased from 56 mmol/mol to 52 mmol/mol (p trend <0.0001) while the delta value between hospitalisation and 1 year remained unchanged (online supplemental tables S4–S5). Fasting glucose at 1-year (all patients) increased from 5.7 mmol/L to 6.0 mmol/L over the period (p trend <0.0001) (online supplemental table S4).
Figure 4.
Prevalence of persistent smoking (proportion of active smokers at the time of MI who were still smoking), inadequate physical activity, overweight/obesity, and diabetes at 1-year post-MI. aBMI≥25 kg/m2; bwaist circumference ≥102 cm for men and ≥88 cm for women; cphysically active ≥30 min for less than 5 days a week. BMI, body mass index; MI, myocardial infarction.
Lifestyle
The prevalence of persistent smoking, inadequate physical activity, overweight/obesity and central obesity at 1-year post-MI is seen in figure 4. Persistent smoking, that is, the proportion of smokers at the time of MI who were still smoking at the one-year follow-up, remained unchanged over the period (42.8% in 2006 and 43.2% 2019, p trend=0.672). The proportion of patients reporting inadequate physical activity increased during the observed period from 57.0% to 61.5% (p trend <0.0001). While the prevalence of patients who were overweight or obese at hospitalisation increased, the proportion at 1-year follow-up was similar (71.9% to 72.9%, p trend=0.559). In 2006–2015, an increase in BMI between baseline and 1-year follow-up was observed (between 0.04 kg/m2 and 0.30 kg/m2), while in 2016–2019, the difference was negative (between −0.01 kg/m2 and −0.15 kg/m2, p trend <0.0001) (online supplemental table S5). The prevalence of central obesity increased from 50.5% in 2006 to 57.0% in 2019 (p trend <0.0001). Yearly median values at 1-year follow-up for number of days during the last week, the patients had been physically active, BMI and waist circumference are shown in online supplemental table S4.
Secondary preventive medication
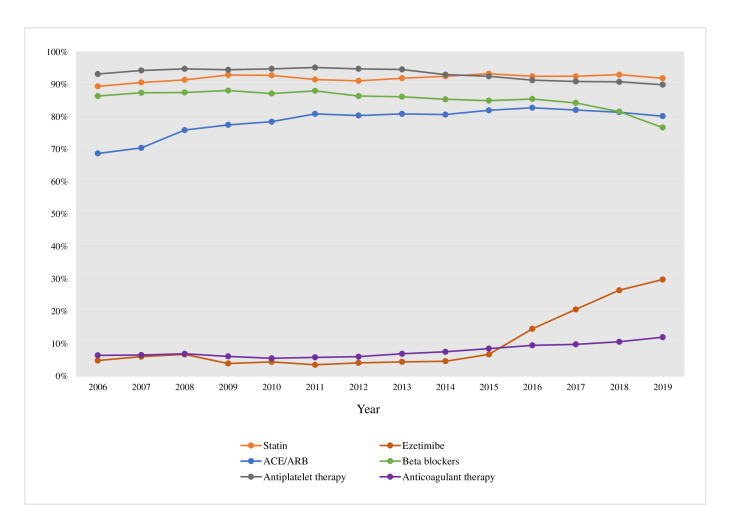
The use of secondary preventive medication at the 1-year follow-up visit is seen in figure 5. Since 2007, more than 90% of all patients were prescribed statins. Between 4% and 6% of the patients were prescribed ezetimibe prior to 2014 where after its use increased successively to 29.8% in 2019 (p trend <0.0001). Approximately 98% were prescribed either an antiplatelet or anticoagulant therapy throughout the period, with the proportion of patients receiving anticoagulant therapy doubling from 6.4% in 2006 to 12.0% in 2019 (p trend <0.0001). ACEi/ARB prescription increased from 68.7% to 80.2% (p trend <0.0001) while the use of beta blockers decreased from 86.4% to 76.7% (p trend <0.0001). The decrease was mostly driven by a decrease in use among patients with preserved ejection fraction (from 85.1% in 2006 to 70.5% in 2019, p for difference <0.0001), while the use in patients with reduced ejection fraction was unchanged (87.8% in 2006 compared with 88.6% in 2019, p for difference=0.540).
Figure 5.
Proportion of patients at 1-year follow-up for each year 2006–2019 treated with statins, ezetimibe, ACEi or ARB, beta blockers, antiplatelet (acetylsalicylic acid or P2Y12-receptor antagonists) or anticoagulant therapy (warfarin or direct oral anticoagulants). ACEi, ACE inhibitor; ARB, angiotensin receptor blocker.
Discussion
In this study of temporal trends in risk factor control and use of secondary preventive medication in post-MI patients attending CR in Sweden 2006–2019, a considerable improvement in BP and LDL-C goal achievement and use of evidence-based pharmacotherapy was observed. On the other hand, changes in lifestyle were less encouraging, with the proportion of persistent smokers at 1-year remaining unchanged, and prevalence of inadequate physical activity, central obesity as well as diabetes increasing.
BP, lipids and diabetes
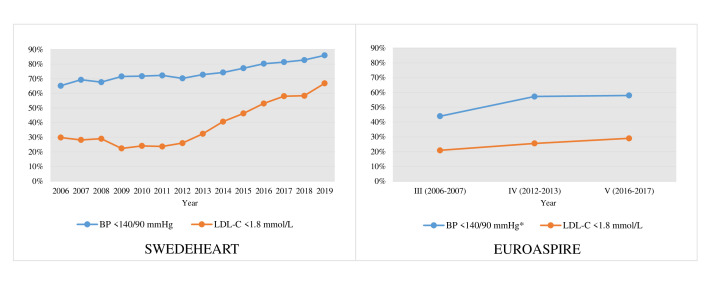
In the EUROASPIRE surveys patients aged 18–79 years with coronary artery disease (CAD) were interviewed and examined at approximately 1 year after a first or recurrent coronary event (acute MI, unstable angina or revascularisation), to determine whether guidelines on CR were followed in clinical practice.5 6 The III–V surveys were conducted over a period approximately matching our current study period (EUROASPIRE III 2006–2007, IV 2012–2013 and V 2016–2017), the patients had similar initiating events, and the mean age and gender proportions were comparable to the SWEDEHEART population (online supplemental table S6), giving a good opportunity to compare our results to European data. In our study, the proportion of patients achieving the BP goal of <140/90 mm Hg increased from 65.2% to 86.0% between 2006 and 2019, compared with an increase from 44.0% to 58.0% between EUROASPIRE III and V (figure 6).5 6 As such, the proportion of patients achieving the BP goal was considerably higher (approximately 20% points) during the whole period in SWEDEHEART. There was an even larger difference in the proportion of patients reaching the LDL-C target of <1.8 mmol/L, increasing from 29.8% (2006) to 66.9% (2019) (37% point improvement) in SWEDEHEART, compared with 20.9% versus 29.0% (8% point improvement) between EUROASPIRE III and V (figure 6). One reasonable explanation for the large difference in proportion of patients achieving treatment targets for BP and LDL-C in SWEDEHEART compared with EUROASPIRE could be that all patients in our study participated in CR to some extent, compared with 35%–40% in the EUROASPIRE cohorts.18 19 Participation in CR has been shown to increase adherence to secondary preventive medication and the proportion of patients reaching risk factor goals20 as well as improving prognosis.2 Somewhat contradictory though, data from EUROASPIRE IV on risk factor target achievement showed no difference in the proportion of patients reaching targets for BP and LDL-C when comparing attenders and non-attenders in CR.19 Another possible explanation could be the higher proportion of patients being prescribed lipid-lowering therapies in our study as compared with EUROASPIRE. Between 2015 and 2019, 94%–95% of patients were prescribed statins and/or ezetimibe, with the corresponding proportion in EUROASPIRE V (2016–2017) being 84%, out of which only 50% were prescribed high-intensity lipid-lowering drugs.5 In a study using Swedish registry data, the proportion of AMI patients receiving high-intensity statins post-MI during 2014–2016 was 91.3%.21 An additional explanation for the more pronounced improvement in target attainment in SWEDEHEART compared with EUROASPIRE, as well as a more pronounced use of potent lipid-lowering therapy, might be the possibility of continuous self-audit of publicly available data for CR centres reporting to SWEDEHEART, as only a minority of the countries participating in EUROASPIRE had quality registries or audits comparable to SWEDEHEART. Among patients with CAD attending CR in Austria, where a well-functioning CR registry has been in use since 2001,7 85% of patients between 2005 and 2015 reached the systolic BP goal of <140 mm Hg.22 Similarly, according to annual reports from the Danish CR Database on patients with CAD attending CR, which started in 2015, the proportion of patients reaching the LDL-C goal of <1.8 mmol/L increased from 54% in 2015 to 63% in 2019,23 24 figures aligning well with our results for the same years. The joint observations from these three registries (SWEDEHEART, Austrian registry and Danish Registry) support the conclusion that benchmarking at a local and national level, and providing opportunities for open comparisons between centres, can positively impact quality of care.7 25 26
Figure 6.
Management of BP and LDL in SWEDEHEART (left panel) and EUROASPIRE (right panel).5 6 *Different definitions of BP treatment goals for patients with diabetes were adapted in the EUROASPIRE surveys (III<130/80 mmHg, IV 140/80 mmHg, V<140/85 mmHg), while the definition<140/90 mmHg was adapted for patients with and without diabetes in SWEDEHEART. BP, blood pressure; LDL-C, low-density lipoprotein cholesterol.
An interesting observation in our data was the increased difference in diabetes prevalence between hospitalisation and 1-year follow-up towards the end of the observed period. Also, median HbA1c values among patients with diabetes decreased. This possibly reflects heightened awareness and more structured routines for diagnosing diabetes in patients after an MI, with patients with milder forms of glucose disturbances being diagnosed. More patients being diagnosed should in the long term positively impact prognosis.27 28 The increase in fasting glucose values in the whole population, paralleled by increased prevalence of central obesity, further underlines the importance of vigilant screening and treatment of diabetes in the post-MI population.
Lifestyle
Approximately, 30% of patients were smokers at the time of the index event in both the SWEDEHEART registry and the EUROASPIRE surveys. The proportion of persistent smokers at 1-year after the event, however, was generally higher in EUROASPIRE than in SWEDEHEART (online supplemental figure S1).5 29 The fact that Sweden has the lowest proportion of daily smokers in Europe might partly explain the higher success rate for smoking cessation in our data. In contrary with the lack of difference in BP and LDL-C target achievement between CR attenders and non-attenders in EUROASPIRE, there was a substantial difference between attenders and non-attenders in smoking cessation rates, with 47% and 43% of CR attenders being persistent smokers in EUROASPIRE III (2006–2007) and IV (2011–2012), compared with 54% and 53% of the non-attenders.18 19 The corresponding figures in SWEDEHEART (all patients defined as attenders) during the same years were 42% (2006–2007) and 45% (2011–2012). In both cohorts, however, there was no improvement in smoking cessation rates during the observed periods. The same can be seen in the British National Audits for CR (NACR) 2016–201930 and the Danish CR Database 2015–2019.24 Observational studies have shown that smoking cessation post-MI results in a 36% relative risk reduction in total mortality.31 The smoking cessation rates among CR attenders in the EUROASPIRE surveys and patients registered in SWEDEHEART, when compared with the considerably higher figures for non-attenders from EUROASPIRE, underline the importance of CR attendance for supporting tobacco abstinence. At the same time, it is discouraging to see no improvement in smoking cessation rates in any of the reviewed data sets.
The proportion of patients reporting insufficient physical activity at the 1-year follow-up increased during the observed period. As different questionnaires for assessing physical activity have been used in the surveys and audits cited here, direct comparisons cannot be made. In general, though, in EUROASPIRE, the level of physical activity in all surveys was suboptimal and did not improve between surveys,5 6 while the proportion of patients classified as physically active increased somewhat in the National Audit for CR (NACR) reports 2016–2019.30
While the prevalence of overweight/obesity at the time of the MI increased during the study period, the proportion of overweight/obese patients at the 1-year follow-up visit remained unchanged (72%–73%). This might partly be explained by a slight weight gain between hospitalisation and 1-year follow-up during the first half of the observed period, while a minimal weight loss was observed during the latter half. The clinical relevance of this observation is, however, uncertain. No change in the proportion of obese patients was observed in NACR 2016–201930 or the EUROASPIRE surveys, where just over 80% were overweight or obese5 6 (online supplemental figure S1). The prevalence of central obesity was similar in our study and in EUROASPIRE and increased to the same extent (by approximately 10% points) during the observed period.5 6
In a recently published paper based on data from EUROASPIRE IV and V, poor adherence to lifestyle changes was addressed.20 The authors concluded that while adherence to lifestyle advice was better among patients who had attended CR, an increased focus on behavioural change within CR to address unhealthy lifestyles is strongly needed. With all patients in our cohort having participated in CR to some extent, data on lifestyle being monitored and openly compared annually in the SWEDEHEART registry, and no visible change for the better seen for more than a decade, our results strongly support this conclusion.
Cardioprotective medication
According to our study, the use of lipid lowering drugs was high during the whole period. More than 90% of the patients were prescribed statins at the 1-year follow-up visit throughout the observed period and ezetimibe use increased rapidly after 2015, reaching 29.8% in 2019. In 2015–2109, more than 94% of all patients were prescribed statins and/or ezetimibe. Meanwhile, the use of lipid-lowering therapy, including statins, ezetimibe, fibrates, bile acid sequestrants and nicotinic acid, increased from approximately 80% of patients in EUROASPIRE III to 84% in EUROASPIRE V5 29 32 (Online supplemental figure S2). In the CR attendance analyses from the EUROASPIRE III and IV surveys, compared with non-attenders, the proportion of CR attenders on lipid-lowering therapy was considerably higher, or 83% versus 78% (EAIII) and 88% versus 85% (EAIV), respectively.18 19 Data on the use of cardioprotective medication from the Austrian registry or the British NACR have to our knowledge not been published. In annual reports from the Danish CR database, during 2016–2019, between 93% and 96% of CAD patients were prescribed statins at the end of CR.24 According to our study, the use of ACEi/ARB increased from 64.9% in 2006 to 79.5% in 2019 while patients prescribed ACEi/ARB in EUROASPIRE III was 71% and 75% in EUROASPIRE V (online supplemental figure S2).5 29 32 In the EUROASPIRE III and IV, the use of ACEi/ARB and BP-lowering medication was significantly higher in CR attenders than in non-attenders, although the difference was not as large as for lipid-lowering treatment.18 19 While conclusions about the influence of auditing on cardioprotective medication prescription in Sweden are hard to draw, generally it can be concluded that the use of cardioprotective medication in our and other surveys has been high and has increased both in Sweden and Europe in general during the observed period.
Strengths and limitations
The major strength of this study is the broad representability and national coverage of data, with more than 75% of all MI patients under the age of 75 being registered in SWEDEHEART and attending a 1-year CR follow-up visit since 2016. At the same time, a major limitation is the lack of data describing MI patients not attending CR and on those ≥75 years of age and results cannot be generalised to these groups. Even though the mean age in our data was similar to EUROASPIRE, the age range differed somewhat (our data 18–74 years vs 18–79 years in EUROASPIRE), which might have led to a slight overestimation of the results. Also, the coverage on centre level during the first years was low and representability, therefore, not as extensive. Comparing our data with other survey and audit data are limited by differences in patient selections, different rates of CR participation, time of follow-up, differences in measurement methods (ie, questionnaires, self-report) and definitions (ie, physical inactivity).
Conclusion
Between 2006 and 2019, an increasing proportion of patients in Sweden reached secondary preventive goals for BP and LDL-C 1 year after an MI. The proportion of patients treated with evidence-based secondary preventive medication also increased. Both levels of BP and LDL-C as well as use of pharmacological treatment were comparable with data from other similar European quality registries and national level audits used for benchmarking. The trends were more favourable than those observed in EUROASPIRE, data from which represents several European countries where audits were not widely available. The results may indicate that national quality registries can contribute to improving outcomes in CR and add evidence to the importance of auditing and benchmarking as means to improve quality of care. Less encouraging, no changes were seen the proportion of current smokers at the time of the MI who are abstinent at 1-year, more patients reported inadequate levels of physical activity, and the proportion of patients with central obesity and diabetes increased, as was observed in EUROASPIRE. These observations bare witness of a large unmet need to prioritise patient lifestyle support after an MI, which should be improved to provide patients with adequate risk reduction.
Supplementary Material
Footnotes
Contributors: ML, EH and MB contributed to the conception and design of the work. AN, BL, KH and TJ offered medical expertise and guidance. NH conducted all data analysis. ML drafted and as guarantor was responsible for the overall content of the manuscript and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All other authors critically revised the manuscript. All approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: All authors except NH are or have been engaged in the SWEDEHEART registry. Otherwise, the authors have no conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data used in this study is based on the SWEDEHEART registry. Access to data from the registry needs to be applied for and third-party data usage is not allowed, irrespective of whether the data contain potentially identifying or sensitive data or not. Instead, given ethical study approval from the Swedish Ethical Review Authority, access to SWEDEHEART data supporting the present findings can be applied for from the Uppsala Clinical Research Center (UCR) in Sweden. Further information can be found on the UCR https://www.ucr.uu.se/en/ and Swedish Ethical Review Authority https://etikprovningsmyndigheten.se/websites.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The need for signed informed consent by patients for inclusion in Swedish quality registries has collectively been waived in Sweden. Upon hospital admission, MI patients are informed verbally and in writing by a nurse or physician about data being collected and entered in the registry. However, all patients have the right to deny registration and the right upon request to be removed from the registry at any time. Opt-out is extremely rare, counting less than ten cases per year. The study complies with the Declaration of Helsinki and was approved by The Swedish Ethical Review Authority (registration number: 2019-04277).
References
- 1. Björck L, Rosengren A, Bennett K, et al. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur Heart J 2009;30:1046–56. 10.1093/eurheartj/ehn554 [DOI] [PubMed] [Google Scholar]
- 2. Salzwedel A, Jensen K, Rauch B, et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the cardiac rehabilitation outcome study (CROS-II). Eur J Prev Cardiolog 2020;27:1756–74. 10.1177/2047487320905719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 4. Ambrosetti M, Abreu A, Corra U, et al. n.d. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation 2020. Eur J Prev Cardiol;2020:2047487320913379. [DOI] [PubMed] [Google Scholar]
- 5. Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiolog 2019;26:824–35. 10.1177/2047487318825350 [DOI] [PubMed] [Google Scholar]
- 6. Kotseva K, De Bacquer D, Jennings C, et al. Time trends in lifestyle, risk factor control, and use of evidence-based medications in patients with coronary heart disease in Europe: results from 3 EUROASPIRE surveys, 1999-2013. Glob Heart 2017;12:315–22.:S2211-8160(15)00295-1. 10.1016/j.gheart.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 7. Poffley A, Thomas E, Grace SL, et al. A systematic review of cardiac rehabilitation registries. Eur J Prev Cardiol 2017;24:1596–609. 10.1177/2047487317724576 [DOI] [PubMed] [Google Scholar]
- 8. Aktaa S, Batra G, Wallentin L, et al. European Society of cardiology methodology for the development of quality indicators for the quantification of cardiovascular care and outcomes. Eur Heart J Qual Care Clin Outcomes 2022;8:4–13. 10.1093/ehjqcco/qcaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aktaa S, Gencer B, Arbelo E, et al. European Society of cardiology quality indicators for cardiovascular disease prevention: developed by the Working group for cardiovascular disease prevention quality indicators in collaboration with the European association for preventive cardiology of the European Society of cardiology. Eur J Prev Cardiol 2022;29:1060–71. 10.1093/eurjpc/zwab160 [DOI] [PubMed] [Google Scholar]
- 10. Thomas RJ, Balady G, Banka G, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of cardiology/american heart association Task force on performance measures. J Am Coll Cardiol 2018;71:1814–37.:S0735-1097(18)30025-1. 10.1016/j.jacc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 11. Brittish Association for Cardiovascular Prevention and Rehabilitation . The BACPR standards and core components for cardiovascular disease prevention and rehabilitation. London, England, 2017. [Google Scholar]
- 12. Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–21. 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 13. Bäck M, Leosdottir M, Hagström E, et al. The SWEDEHEART secondary prevention and cardiac rehabilitation registry (SWEDEHEART Cr registry). Eur Heart J Qual Care Clin Outcomes 2021;7:431–7. 10.1093/ehjqcco/qcab039 [DOI] [PubMed] [Google Scholar]
- 14. Hambraeus K, Tydén P, Lindahl B. Time trends and gender differences in prevention guideline adherence and outcome after myocardial infarction: data from the SWEDEHEART registry. Eur J Prev Cardiol 2016;23:340–8. 10.1177/2047487315585293 [DOI] [PubMed] [Google Scholar]
- 15. Jernberg T, Boberg B, Back M, et al. SWEDEHEART annual report 2019. Uppsala, Sweden: Uppsala Clinical Research Center; 2019. [Google Scholar]
- 16. Vasko P, Alfredsson J, Back M, et al. SWEDEHEART annual report 2020. annual report. Uppsala, Sweden: Uppsala Clinical Research Center (UCR); 2021. [Google Scholar]
- 17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 18. Kotseva K, Wood D, Backer GD, et al. Use and effects of cardiac rehabilitation in patients with coronary heart disease: results from the EUROASPIRE III survey. Eur J Prev Cardiolog 2013;20:817–26. 10.1177/2047487312449591 [DOI] [PubMed] [Google Scholar]
- 19. Kotseva K, Wood D, De Bacquer D. Determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiolog 2018;25:1242–51. 10.1177/2047487318781359 [DOI] [PubMed] [Google Scholar]
- 20. De Bacquer D, Astin F, Kotseva K, et al. Poor adherence to lifestyle recommendations in patients with coronary heart disease: results from the EUROASPIRE surveys. Eur J Prev Cardiol 2022;29:383–95. 10.1093/eurjpc/zwab115 [DOI] [PubMed] [Google Scholar]
- 21. Svensson MK, Sorio Vilela F, Leósdóttir M, et al. Effects of lipid-lowering treatment intensity and adherence on cardiovascular outcomes in patients with a recent myocardial infarction: a Swedish register-based study. Ups J Med Sci 2022;127. 10.48101/ujms.v127.8296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reich B, Benzer W, Harpf H, et al. Efficacy of extended, comprehensive outpatient cardiac rehabilitation on cardiovascular risk factors: a nationwide registry. Eur J Prev Cardiolog 2020;27:1026–33. 10.1177/2047487319898958 [DOI] [PubMed] [Google Scholar]
- 23. Zwisler A-D, Rossau HK, Nakano A, et al. The Danish cardiac rehabilitation database. Clin Epidemiol 2016;8:451–6. 10.2147/CLEP.S99502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomsen KK. Danish cardiac rehabilitation registry annual report. 2019.
- 25. Paton JY, Ranmal R, Dudley J, et al. Clinical audit: still an important tool for improving healthcare. Arch Dis Child Educ Pract Ed 2015;100:83–8. 10.1136/archdischild-2013-305194 [DOI] [PubMed] [Google Scholar]
- 26. Abreu A, Frederix I, Dendale P, et al. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: the avenue towards EAPC accreditation programme: a position statement of the secondary prevention and rehabilitation section of the European association of preventive cardiology (EAPC). Eur J Prev Cardiol 2021;28:496–509. 10.1177/2047487320924912 [DOI] [PubMed] [Google Scholar]
- 27. Feldman AL, Griffin SJ, Fhärm E, et al. Screening for type 2 diabetes: do screen-detected cases fare better? Diabetologia 2017;60:2200–9. 10.1007/s00125-017-4402-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ögmundsdottir Michelsen H, Sjölin I, Schlyter M, et al. Cardiac rehabilitation after acute myocardial infarction in Sweden-evaluation of programme characteristics and adherence to European guidelines: the perfect cardiac rehabilitation (perfect-CR) study. Eur J Prev Cardiol 2020;27:18–27. 10.1177/2047487319865729 [DOI] [PubMed] [Google Scholar]
- 29. Kotseva K, Wood D, Backer GD, et al. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. European Journal of Cardiovascular Prevention & Rehabilitation 2009;16:121–37. 10.1097/HJR.0b013e3283294b1d [DOI] [PubMed] [Google Scholar]
- 30. Doherty P. National audit of cardiac rehabilitation-annual statistical reports York, U.K;
- 31. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86–97. 10.1001/jama.290.1.86 [DOI] [PubMed] [Google Scholar]
- 32. Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: a European Society of cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636–48. 10.1177/2047487315569401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069770supp001.pdf (218.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data used in this study is based on the SWEDEHEART registry. Access to data from the registry needs to be applied for and third-party data usage is not allowed, irrespective of whether the data contain potentially identifying or sensitive data or not. Instead, given ethical study approval from the Swedish Ethical Review Authority, access to SWEDEHEART data supporting the present findings can be applied for from the Uppsala Clinical Research Center (UCR) in Sweden. Further information can be found on the UCR https://www.ucr.uu.se/en/ and Swedish Ethical Review Authority https://etikprovningsmyndigheten.se/websites.