Abstract
Background
Respiratory syncytial virus (RSV) is the most common cause of hospitalisation for lower respiratory tract infection (LRTI) in children. RSV LRTI during early childhood may increase susceptibility to recurrent wheezing and asthma.
Research question
The aim of this study was to describe the pulmonary sequelae at 1 and 2 years of age following RSV LRTI hospitalisation during the first year of life in term infants.
Study design and methods
A longitudinal case–control study was undertaken from April 2016 to December 2019. Cases constituted children hospitalised with PCR-confirmed RSV LRTI during infancy and controls were children not previously hospitalised with LRTI. A questionnaire detailing environmental and medical history, as well as a modified International Study of Asthma and Allergies (ISAAC) questionnaire, was administered, and pulmonary function testing, including oscillometry, tidal breath flow-volume loops and multiple breath wash-out, was performed, at one and two years of age.
Results
One (n=308) and two-year-old (n=214) cases were more likely than one (n=292) and two-year-old (n=209) controls to have experienced clinical pulmonary symptoms, including wheezing ((55% vs 24%; p<0.001) and (61% vs 16%; p<0.001)), received treatment for wheezing ((17 vs 8%; p<0.001) and (51 vs 6%; p<0.001)) and had any admissions for wheezing ((31 vs 6%; p<0.001) and (46 vs 1.4%; p<0.001)) or any LRTI ((24 vs 2%; p<0.001) and (32 vs 1.4%; p<0.001)), after the initial RSV hospitalisation. RSV LRTI during infancy was associated with an increase in airway resistance by two years (22.46 vs 20.76 hPa.s.l-1 (p=0.022)), along with a decrease in compliance at both one (−4.61 vs −3.09 hPa.s/l (p<0.001)) and two years (−0.99 vs 0.33 hPa.s/l1 (p<0.001)). There was an increased work of breathing at one year, but this was no longer present at two years.
Interpretation
RSV LRTI during infancy in cases was associated with more clinical and pulmonary function sequelae through to two years of age.
Keywords: Paediatric Lung Disaese, Respiratory Infection, Respiratory Measurement, Viral infection
WHAT IS ALREADY KNOWN ABOUT THIS TOPIC
Respiratory syncytial virus is the most common cause of lower respiratory tract infections (LRTI) and hospital admission during early childhood and may lead to long-term pulmonary symptoms. In infants and toddlers, respiratory questionnaires and infant pulmonary function techniques, such as oscillometry, tidal breath flow volume loops and multiple breath inert gas washout technique are feasible and can be used to measure pulmonary function sequelae.
WHAT THIS STUDY ADDS
In this longitudinal, descriptive case–control study, we report that children with an admission for RSV LRTI during infancy have increased symptoms and lung function abnormalities, including increased resistance and decreased compliance, up until at least two years of age.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study adds valuable data describing early life pulmonary sequelae after RSV LRTI. Further research is needed to better delineate the long-term effects of RSV LRTI on the developing lung as well as research describing contributory factors, such as genetic, environmental and socioeconomic, and the impact on lung function trajectories.
Introduction
Lower respiratory tract infections (LRTI) are the leading cause of death in children less than 5 years outside the neonatal period; the majority occurring in low and middle-income countries.1 While childhood respiratory syncytial virus (RSV) LRTI mortality is relatively low (66 000 to 1 98 000 deaths per year), it is the leading cause of LRTI hospitalisation2 and has been associated with long-term pulmonary sequelae.3 RSV LRTI affects the developing lung and may decrease growth velocity of the small airways manifesting as recurrent wheezing and LRTIs, diminished lung function and chronic respiratory symptoms.4
Long-term pulmonary sequelae of RSV infection can be assessed through administering questionnaires to caregivers or objectively through pulmonary function tests (PFT). Paediatric PFT techniques, especially spirometry, are well established in children older than 6 years of age.5 In infants and toddlers, respiratory oscillometry (Osc), tidal breath flow volume loops (TBFVL) and multiple breath inert gas washout technique (MBW) can be used. There is a paucity of studies describing pulmonary sequelae following RSV LRTI, and only one from Africa that described lung function impairment in children at 2 years following an RSV LRTI.6 In a recent systematic review, we identified 31 studies with marked heterogeneity; obstructive airway disease with no bronchial hyper-responsiveness was the most common sequelae of RSV infection, although conflicting results from studies.4
We undertook a descriptive, observational case–control study to describe the clinical and pulmonary function sequelae through to 2 years of age, in infants who were hospitalised for RSV LRTI in infancy and matched controls.
Methods
The study was undertaken at the Chris Hani Baragwanath Academic Hospital (CHBAH), a secondary-tertiary level hospital in Johannesburg, serving the peri-urban mainly black-African community of Soweto and surrounding areas, from 1 April 2016 to 31 December 2019. South Africa is classified an upper middle-income country, but there are huge disparities between upper and lower social quintiles (Gini coefficient 0.63 (63%)). Soweto has an estimated population of 1.3 million with a substantially lower household income than the national average.7
Infants were hospitalised at CHBAH for acute LRTI at the discretion of the attending physician, which is generally premised on fulfilling WHO case definition for severe pneumonia.8 Acute LTRI was defined as acute onset of cough or difficulty in breathing, with fast breathing for age, whereas severe LRTI was defined as acute onset of cough or difficulty in breathing, with lower chest wall indrawing, with or without fast breathing. This case definition was broad to include cases of bronchiolitis or pneumonia. Only first episodes of admission for LRTI were included. The standard-of-care for the management of LRTI during the study period was supplemental oxygen in infants with hypoxia or signs of severe respiratory distress. In infants with bronchiolitis, beta2-agonists, corticosteroids and antibiotics were not routinely administered; whereas, antibiotics were routinely administered in infants with pneumonia.
Two surveillance studies (undertaken by the Respiratory and Meningeal Pathogens Research Unit at CHBAH from December 2014) of hospitalised children were used to identify RSV LRTI cases for eligibility into our study: Surveillance of pathogen-specific causes of pneumonia and diarrhoea hospitalisation in children (HREC: 131109) and Surveillance of Severe Childhood Illness in Soweto, South Africa/Babies of Soweto study (HREC: 140904). Cases were defined as term infants hospitalised for severe or very severe RSV LRTI (online supplemental appendix 1). Infants hospitalised with LRTI had a flocked nasopharyngeal swab (FLOQS, Copan Flock Technologies, Brescia, Italy) administered within 72 hours of symptom onset, for the identification of respiratory pathogens. Total nucleic acid extraction using the automated NucliSENS easyMAG nucleic acid extraction machine was performed. A one-step multiplex PCR assay, which detects RSV A, RSV B, Human metapneumovirus, Influenza A, Influenza B and Bordetella pertussis (using IS481), was performed.9 Daily in-hospital laboratory audits were conducted to identify infants with confirmed RSV LRTI. Cases were term infants hospitalised for severe or very severe RSV LRTI. Exclusion criteria were a birth weight less than 2500 g or an underlying medical diagnosis that may affect respiratory function, such as significant cardiac or respiratory disease as well as cases with documented coinfections. Parents of eligible cases were informed about the study and their children invited to participate in the longitudinal follow-up at 12 (10–14) and 24 (22–26) months of age. PFT were deferred if participants had symptoms of an LRTI within 1 month before testing.
bmjresp-2023-001618supp001.pdf (233.4KB, pdf)
Controls, matched at a 1:1 ratio for sex and chronological age (±2 weeks) at the time of pulmonary function testing (10–14 or 22–26 months), were healthy term infants not hospitalised with an LRTI in the first year of life, from the same community. Controls were identified through a birth cohort study of 35 000 mother–newborn dyads (V98_28OB study; HREC: 140203) from 1 April 2016 until 31 December 2016, and Surveillance of Severe Childhood Illness in Soweto, South Africa/Babies of Soweto study (HREC: 140904) 1 January until 31 March 2019. The sample function in R V.3.5.1. was used for random selection of blocks of 100 matched controls, which would be completed before a further list would be generated.
Study procedures
Demographic and clinical data were collected. A modified International Study of Asthma and Allergies in Childhood Phase Three Core Questionnaire 6–7 years was administered to the caregiver,10 assessing the prevalence and severity of respiratory symptoms and PFTs (using Osc, including the novel intrabreath modality of Osc, TBFVL and MBW) were performed in that order, at one and two years of age, during behaviourally assessed quiet sleep, according to acceptability and repeatability criteria as per American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines unless otherwise specified.11–13
For Osc, a custom-built set-up (University of Szeged, Hungary) consisting of a loudspeaker generating small amplitude (0.1–0.2 kPa) pressure oscillations, a wavetube-pneumometer measurement head connected to an antibacterial filter (No. 11012, Humid-Vent Filter Pedi Clean, Teleflex Medical) and a facemask covering the nose and mouth (No. 415802, Anaesthetic Mask Infant size 2, Teleflex Medical) was used. A 16 Hz tracking signal was used for measurements of respiratory impedance (Zrs) for 120 s. Real-time quality control was done visually throughout the recordings. A minimum of 20 regular breathing periods with no artefacts were collected for each infant for intrabreath analysis, and the recording was repeated if necessary. Details of equipment, signal processing and Zrs analysis are previously described.14 Zrs was characterised by resistance (Rrs) and reactance (Xrs) at end expiration (ReE and XeE, respectively) and at end inspiration (ReI and XeI, respectively). The corresponding tidal changes in Zrs were calculated as ΔR=ReE ReI and ΔX=XeE XeI as well as their values normalised by tidal volume (VT). The conventional total breath averages of Zrs at 16 Hz (Rmean and Xmean) were also computed. TBFVL and MBW were performed with the EXHALYSER D and the accompanying SPIROWARE V.3.2.1 software package supplied by ECO MEDICS AG (Duernten, Switzerland), using sulphur hexafluoride (SF6) as the tracer gas. All measurements were done according to ATS/ERS guidelines, apart from TBFVL, where 100 consecutive breaths were measured, and all breaths meeting acceptability criteria of ±10% from the median VT were included in the analysis (minimum of 30 required for a successful test). TBFVL measured the tidal breathing parameters of volume and flow. MBW measured the indices of functional residual capacity (FRC) and lung clearance index (LCI).
Statistical analysis
Basic descriptive and comparative analyses were performed using using Stata V.13 (StataCorp (Texas, USA)). ORs with 95% CIs were reported, and a p value <0.05 was considered statistically significant. Conditional logistic regression was used to determine the odds of developing clinical pulmonary sequelae; we adjusted for variables with a p value <0.20 in the univariate analysis. Linear regression analysis was used for continuous outcome variables to determine associations between risk factors and the development of pulmonary function sequelae. All linear regression models were adjusted for age and height at time of pulmonary function testing and gender. Coefficients were reported with 95% CIs.
Patient and public involvement
Patients were not involved in the planning and design of the study.
Results
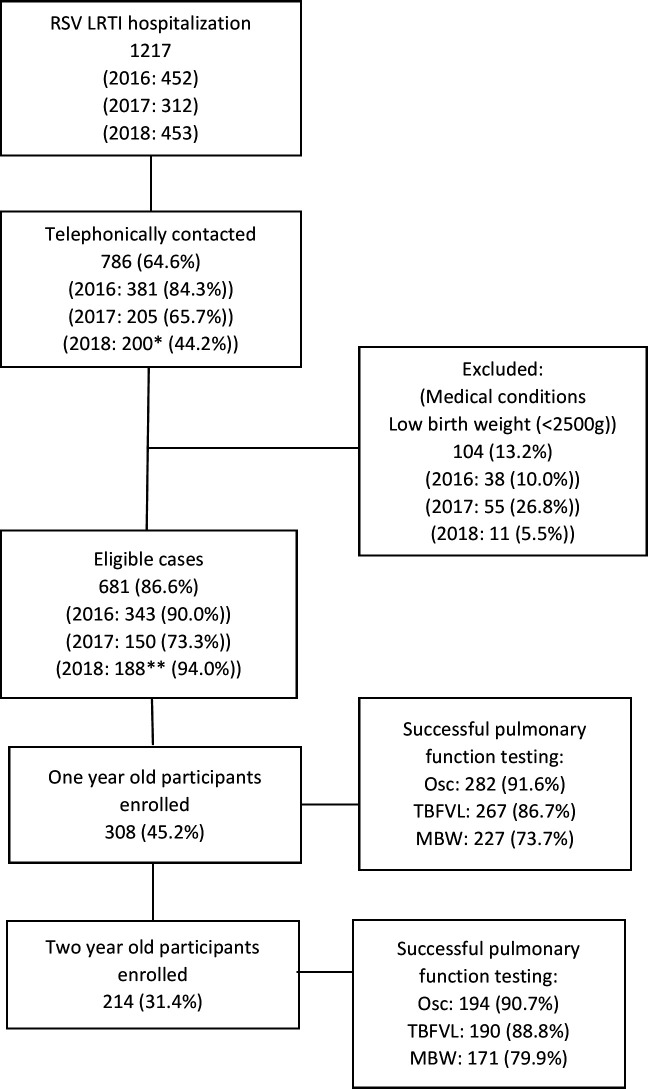
We identified 964 hospitalised RSV cases (figure 1). There were 308 one-year-old (57% male; median age 12.4 months) and 214 two-year-old (54% men; median age 24.6) cases, and 292 one-year-old (53% men; median age 12.5 months) and 209 two-year-old (46% men; median age 23.9) controls enrolled into the study (table 1). Birth weight was similar between both one (3095 vs 3138 g (p=0.124)) and two-year-old (3102 vs 3175 g (p=0.319)) cases and controls. The median age of RSV hospitalisation for cases was 4.5 months, and the time since RSV hospitalisations was 7.6 months at the one-year visit and 20.8 months at the two-year visit. As compared with controls, one-year-old cases had increased odds of attending crèche (adjusted OR (aOR): 2.52; 95% CI 1.51 to 4.18) and to have received oxygen therapy post-delivery (aOR: 2.81; 95% CI 1.33 to 5.97); while two-year-old cases had decreased odds of having attended crèche (aOR: 0.49; 95% CI 0.30 to 0.80) and increased odds of being exposed to tuberculosis in the household (aOR: 10.58; 95% CI 1.33 to 84.32) (table 2).
Figure 1.
Flow diagram of study participant inclusion *Only partial RSV season included. **2018 cohort not invited for 2-year pulmonary function testing. LRTI, lower respiratory tract infection; MBW, multiple breath wash-out; Osc, oscillometry; RSV, respiratory syncytial virus; TBFVL, tidal breath flow-volume loops.
Table 1.
Demographic characteristics of 1-year and 2-year-old participants
| Variable | 1-year-old participants | 2-year-old participants | ||||
| Cases (n*=308) | Controls (n=292) | P-value | Cases (n=214) | Controls (n=209) | P-value | |
| Age (months) (median/IQR) | 12.41 (11.48–13.66) | 12.48 (11.72–13.07) | 0.820 | 24.63 (23.87–25.46) | 23.90 (23.18–24.73) | <0.001 |
| Gender male (n/%) | 174 (56.5) | 155 (53.1) | 0.401 | 116 (54.2) | 95 (45.5) | 0.072 |
| Black African Race (n/%) | 308 (100) | 292 (100) | 1.00 | 214 (100.0) | 209 (100.0) | 1.00 |
| Weight (kg) (median/IQR) | 9.9 (9.0–10.6) | 9.8 (8.8–10.5) | 0.118 | 12.0 (10.9–13.3) | 11.6 (10.6–12.6) | 0.007 |
| Height (cm) (median/IQR) | 74 (72–77), 54–89 | 74 (72–77), 62–88 | 0.621 | 87 (85–90) | 86 (83–89) | 0.051 |
| Birth weight (g) (median/IQR) | 3095 (2840–3400) | 3138 (2900–3380) | 0.124 | 3102 (2895–3400) | 3175 (2855–3445) | 0.319 |
| HIV status (n/%) | ||||||
| Unexposed | 229 (74.4) | 214 (73.3) | 157 (73.4) | 150 (71.8) | ||
| Exposed uninfected | 67 (21.7) | 74 (25.3) | 0.101 | 45 (21.0) | 52 (24.9) | 0.531 |
| Infected | 11 (3.6) | 4 (1.3) | 3 (1.4) | 1 (0.5) | ||
| Unknown | 1 (0.3) | 0 (0.0) | 9 (4.2) | 6 (2.9) | ||
| Underlying medical conditions (n/%) | 0 (0.0) | 0 (0.0) | N/A | 0 (0.0) | 0 (0.0) | N/A |
| Age (months) at RSV† admission (median/IQR) | 4.48 (2.26–6.85) | N/A | N/A | 4.48 (2.26–6.85) | N/A | N/A |
| Time (months) between RSV admission and visits (median/IQR) |
7.60 (4.77–10.68) | N/A | N/A | 20.82 (16.51–22.79) | N/A | N/A |
| Duration of RSV admission (days) (median/IQR) | 2 (1-4) | N/A | N/A | 2 (1-5) | N/A | N/A |
| Received supplemental oxygen during RSV admission (n/%) | 103 (34.7) | N/A | N/A | 69 (34.9) | N/A | N/A |
*n: number.
†Underlying medical conditions: congenital (ex. congenital cardiac disease, hydrocephalus), genetic (ex. trisomy 21) or medical diagnosis (ex. neurological disability, such as neuromuscular disease or cerebral palsy, hepatic abnormalities such as biliary atresia, and musculoskeletal disorders such as osteogenesis imperfecta) that may affect respiratory function.
RSV, respiratory syncytial virus.
Table 2.
Risk factors for respiratory sequelae in 1-year and 2-year-old participants
| Variable | 1-year-old participants | 2-year-old participants | ||||
| Cases (n*=308) |
Controls (n=292) | aOR* (95% CI) | Cases (n=214) |
Controls (n=209) | aOR (95% CI) | |
| Infant factors | ||||||
| Neonatal admission post-delivery (n/%) | 47 (15.3) | 24 (8.2) | 1.34 (0.74 to 2.44) | 31 (14.5) | 32 (15.3) | 0.79 (0.40 to 1.54) |
| Duration of admission post-delivery (days) (median/IQR) |
3 (2-7) | 3 (2-7) | 3 (2-7) | 5 (2-8) | ||
| Required oxygen post-delivery (n/%) | 28 (9.1) | 10 (3.4) | 2.81 (1.33 to 5.97) | 18 (8.4) | 16 (7.7) | 1.41 (0.58 to 3.43) |
| Duration of oxygen post-delivery (days) (median/IQR) |
3 (1-10) | 2 (1-4) | 2 (1-7) | 6 (2-7) | ||
| Tuberculosis contact (n/%) | 14 (4.5) | 17 (5.9) | 0.83 (0.40 to 1.74) | 10 (4.7) | 1 (0.5) | 10.58 (1.33 to 84.32) |
| Type of feeding during first 4 months (n/%) | ||||||
| Breastfeeding | 199 (64.6) | 187 (64.0) | 1.10 (0.78 to 1.54) | 136 (63.6) | 115 (55.0) | 1.53 (0.97 to 2.10) |
| Formula | 70 (22.7) | 61 (20.9) | 1.19 (0.81 to 1.76) | 51 (23.8) | 56 (26.8) | 0.85 (0.55 to 1.33) |
| Mixed feeding | 39 (12.7) | 44 (15.1) | 0.82 (0.51 to 1.30) | 27 (12.6) | 38 (18.2) | 0.65 (0.38 to 1.11) |
| Parental factors | ||||||
| Mother’s HIV status: positive (n/%) | 71 (24.1) | 85 (29.6) | 0.73 (0.48 to 1.11) | 48 (23.6) | 58 (29.6) | 0.75 (0.46 to 1.24) |
| Intrauterine tobacco smoke exposure (n/%) | 12 (3.9) | 8 (2.7) | 2.3 (0.84 to 6.31) | 6 (2.8) | 8 (3.8) | 0.70 (0.24 to 2.06) |
| Maternal diagnosed asthma (n/%) | 11 (3.6) | 7 (2.4) | 0.76 (0.37 to 1.57) | 7 (3.3) | 8 (3.8) | 1.84 (0.67 to 5.07) |
| Paternal diagnosed asthma (n/%) | 8 (2.6) | 8 (2.7) | 0.66 (0.36 to 1.20) | 6 (2.8) | 3 (1.4) | 1.19 (0.37 to 3.87) |
| Environmental factors | ||||||
| Smoke exposure within home (n/%) | 48 (15.6) | 58 (19.9) | 0.71 (0.43 to 1.15) | 42 (19.6) | 39 (18.7) | 0.94 (0.39 to 2.26) |
| Total rooms in the house (median/IQR) | 4 (2-5) | 4 (1-4) | 4 (2-4) | 3 (1-4) | ||
| Number people sleeping in the same room as the child (mean/SD) | 2.28 (0.91) | 2.05 (0.95) | 2.33 (0.91) | 2.17 (0.86) | ||
| Total number of people living in the house (mean/SD) | 5.51 (2.63) | 5.17 (2.35) | 5.52 (2.36) | 5.09 (2.17) | ||
| Under-5 children attending crèche in the household (mean/SD) | 0.52 (0.77) | 0.31 (0.58) | 0.50 (0.67) | 0.38 (0.56) | ||
| Index child attends crèche (n/%) | 80 (26.0) | 36 (12.3) | 2.52 (1.51 to 4.18) | 61 (28.5) | 79 (37.8) | 0.49 (0.30 to 0.80) |
| Pets in the household (n/%) | 57 (18.5) | 45 (15.4) | 1.15 (0.62 to 2.11) | 47 (22.0) | 26 (12.4) | 1.58 (0.81 to 3.08) |
*aOR: adjusted odds ratio (adjusted for variables with p-value <0.20 on univariate analysis).
†Parental reporting.
n, number.
International study of asthma and allergies in childhood phase three core questionnaire
By one year of age, cases (subsequent to hospital discharge after their index RSV-LRTI episode) compared with controls had increased odds of wheezing or whistling in the chest (aOR: 2.78; CI 1.75 to 4.44), hospitalisation for wheezing or whistling in the chest (aOR: 5.66; CI 3.17 to 10.09) or for any LRTI (aOR: 10.43; CI 4.39 to 24.77) and to have received treatment for wheezing or whistling in the chest (aOR: 3.05; CI 1.70 to 5.47). Furthermore, cases compared with controls were also more likely to have reported sleep disturbance due to wheezing or whistling in the chest (aOR: 8.36; CI 5.42 to 12.90) and having a dry cough at night (aOR: 4.37; CI 3.02 to 6.33) at 1 year of age (table 3).
Table 3.
Clinical pulmonary sequelae after RSV LRTI during infancy in 1-year and 2-year-old participants
| Variable | 1-year-old participants | 2-year-old participants | ||||
| Cases (n*=308) | Controls (n=292) | aOR† (95% CI) | Cases (n=214) | Controls (n=209) | aOR (95% CI) | |
| Wheezing or whistling in the chest over the past year‡ (n/%) | 170 (55.2) | 69 (23.6) | 2.78 (1.75 to 4.44) | 131 (61.2) | 34 (16.3) | 8.11 (5.04 to 13.04) |
| If yes, number of episodes per year (n/%) | ||||||
| −1–2 | 115 (37.3) | 50 (17.1) | 3.42 (2.32 to 5.03) | 87 (40.7) | 26 (12.4) | 7.14 (4.37 to 11.66) |
| –>2 | 55 (17.9) | 19 (6.5) | 6.33 (2.57 to 15.58) | 44 (20.6) | 8 (3.8) | 19.62 (5.37 to 71.70) |
| Admissions for wheezing or whistling in the chest in the past year‡ (n/%) | 95 (30.8) | 16 (5.5) | 5.66 (3.17 to 10.09) | 99 (46.3) | 3 (1.4) | 58.08 (17.98 to 187.59) |
| If yes, number of episodes per year (n/%) | ||||||
| −1–2 | 64 (20.8) | 15 (5.1) | 9.56 (3.96 to 23.05) | 76 (35.5) | 3 (1.4) | 39.73 (12.18 to 129.52) |
| –>2 | 31 (10.1) | 1 (0.3) | 33.35 (5.46 to 1363.42) | 23 (10.7) | 0 (0.0) | 25.04 (3.97 to 1035.97) |
| Admissions for a chest infection in the past year‡ (n/%) | 73 (23.7) | 6 (2.1) | 10.43 (4.39 to 24.77) | 69 (32.2) | 3 (1.4) | 33.89 (10.35 to 110.96) |
| If yes, number of episodes per year (n/%) | ||||||
| −1–2 | 49 (15.9) | 6 (2.1) | 9.59 (3.98 to 23.13) | 44 (20.6) | 2 (1.0) | 39.30 (5.27 to 293.09) |
| –>2 | 24 (7.8) | 0 (0.0) | 24.59 (3.94 to 1014.49) | 25 (11.7) | 1 (0.5) | 40.32 (5.29 to 307.27) |
| Sleep disturbance for wheezing or whistling in the chest in the past year‡ (n/%) | 161 (52.3) | 37 (12.7) | 8.36 (5.42 to 12.90) | 148 (69.2) | 20 (9.6) | 21.63 (12.47 to 37.59) |
| If yes, number of times per week during last year (n/%) | ||||||
| −1–2 | 50 (16.2) | 15 (4.9) | 4.60 (2.13 to 9.93) | 52 (24.3) | 13 (6.2) | 10.80 (5.47 to 21.31) |
| –>2 | 111 (36.0) | 22 (7.6) | 8.14 (4.15 to 15.97) | 96 (44.9) | 7 (3.3) | 38.09 (16.80 to 86.37) |
| If yes, number of times per week during last year (Mean/SD) | 3.0 (1.1) | 2.8 (1.1) | 3.0 (1.0) | 2.35 (1.2) | ||
| Dry cough at night, apart from during a cold or an infection, during the past year‡ (n/%) | 189 (61.3) | 76 (26.0) | 4.37 (3.02 to 6.33) | 142 (66.4) | 42 (20.1) | 8.37 (5.36 to 13.06) |
| If yes, number of nights per week, during last year (n/%) | ||||||
| −1–2 | 53 (17.2) | 38 (13.0) | 2.62 (1.41 to 4.88) | 34 (15.9) | 23 (11.0) | 3.30 (1.77 to 6.15) |
| –>2 | 136 (44.2) | 37 (12.7) | 6.90 (4.45 to 10.70) | 108 (50.5) | 19 (9.1) | 13.18 (7.53 to 23.09) |
| If yes, number of nights per week during last year (mean/SD) | 3.09 (0.99) | 2.55 (1.13) | 3 (3-4) | 2 (2-4) | ||
| Wheezing or whistling in the chest during activity in the past year (n/%) | 107 (34.7) | 15 (5.1) | 11.40 (6.11 to 21.27) | 97 (45.3) | 12 (5.7) | 14.21 (7.46 to 27.08) |
| Received treatment for wheezing or whistling in the chest in the past year (n/%) | 53 (17.2) | 22 (7.5) | 3.05 (1.70 to 5.47) | 110 (51.4) | 12 (5.7) | 21.11 (10.76 to 41.45) |
*n: number.
†aOR: adjusted OR (adjusted for all variables with p value <0.20 on univariate analysis).
‡For 1-year-old cases questions apply to the remainder of the year after the RSV admission.
LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus.
Between one and two years of age, cases remained more likely to experience wheezing or whistling in the chest (aOR: 8.11; CI 5.04 to 13.04), to be hospitalised for wheezing or whistling in the chest (aOR: 58.08; CI 17.98 to 187.59 or a LRTI (aOR: 33.89; CI 10.35 to 110.96) and to have received treatment for wheezing or whistling in the chest (aOR: 21.11; CI 10.76 to 41.45). Also, cases remained more likely to have sleep disturbance due to wheezing or whistling in the chest (aOR: 21.63; CI 12.47 to 37.59), a dry cough at night (aOR: 8.37; CI 5.36 to 13.06) and experience wheezing or whistling in the chest during activity (aOR: 14.21; CI 7.46 to 27.08) in the past year (table 3).
PFTs: success rate
PFTs were successfully performed in more than two-thirds of participants at one and two years of age (table 4). Highest success rates were seen for Osc at both one and two years of age, followed by TBFVL and MBW.
Table 4.
Pulmonary function testing success rates for 1- and 2-year-old participants
| 1-year-old participants | 2-year-old participants | |||
| Cases (n*=308) | Controls (n=292) | Cases (n=214) | Controls (n=209) | |
| Osc completed (n/%) | 282 (91.6) | 276 (94.5) | 194 (90.7) | 184 (88.0) |
| TBFVL completed (n/%) | 267 (86.7) | 268 (91.8) | 190 (88.8) | 176 (84.2) |
| MBW completed (n/%) | 227 (73.7) | 201 (68.8) | 171 (79.9) | 153 (73.2) |
*n=number.
MBW, multiple breath washout; Osc, oscillometry; TBFVL, tidal breath flow-volume loops.
Respiratory Osc
At one year of age, Rmean was similar between cases and controls (26.34 vs 26.32 hPa.s/l1 (p=0.976)); but higher in cases by two years of age (22.46 vs 20.76 hPa.s/l1 (p=0.022)) (table 5). The values or ReE and ReI were increased in cases compared with controls at both one (21.28 vs 19.76 hPa.s/l1 (p=0.003) and 24.53 vs 22.41 hPa.s/l (p=0.003) respectively) and two years of age (17.42 vs 15.82 hPa.s/l1(p=0.001) and 19.80 vs 18.17 hPa.s/l (p=0.007) respectively), whereas the tidal changes in Rrs (ΔR) and their values normalised by VT were not significantly different between groups at either age.
Table 5.
Pulmonary function testing data for 1-year and 2-year-old participants*
| Osc | 1-year-old participants | 2-year-old participants | ||||||
| Cases (n*=282) |
Controls (n=276) |
Coefficient (95% CI) |
P-value | Cases (n=214) |
Controls (n=209) |
Coefficient (95% CI) |
P-value | |
| Rmean (hPa.s.l−1) Median (95% CI) |
26.34 (25.27 to 27.42) | 26.32 (25.23 to 27.41) | 0.02 (−1.51 to 1.56) |
0.976 | 22.46 (21.47 to 23.46) | 20.76 (19.73 to 21.79) | 1.70 (0.24 to 3.16) | 0.022 |
| ReE (hPa.s.l−1) Median (95% CI) |
21.28 (20.58 to 21.98) |
19.76 (19.05 to 20.47) |
1.521 (0.520 to 2.522) | 0.003 | 17.42 (16.78 to 18.06) | 15.82 (15.15 to 16.48) | 1.60 (0.66 to 2.54) | 0.001 |
| ReI (hPa.s.l−1) Median (95% CI) |
24.53 (23.56 to 25.51) |
22.41 (21.42 to 23.40) |
2.12 (0.73 to 3.51) |
0.003 | 19.80 (18.99 to 20.60) | 18.17 (17.34 to 19.02) | 1.63 (0.45 to 2.80) | 0.007 |
| ΔR (hPa.s.l−1) Median (95% CI) |
−3.25 (−3.80 to −2.71) |
−2.65 (−3.20 to −2.11) |
−0.60 (−1.37 to 0.17) |
0.126 | −2.36 (−2.76 to −1.94) |
−2.36 (−2.78 to −1.94) |
−0.02 (−0.62 to 0.57) |
0.939 |
| ΔR/VT (hPa.s.l−2) Median (95% CI) |
−36.05 (−44.16 to −27.93) |
−45.12 (−53.33 to −36.90) |
9.07 (−2.49 to 20.62) |
0.124 | −20.10 (−23.80 to −16.39) |
−20.13 (−23.97 to −16.28) |
0.03 (−5.41 to 5.47) |
0.992 |
| Xmean (hPa.s.l−1) Median (95% CI) |
−4.61 (−5.10 to −4.11) |
−3.09 (−3.60 to −2.59) |
−1.51 (−2.22 to −0.80) |
<0.001 | −0.99 (−1.31 to −0.67) |
0.33 (−0.00 to 0.66) |
−1.32 (−1.79 to −0.85) |
<0.001 |
| XeE (hPa.s.l−1) Median (95% CI) |
−2.91 (−3.33 to −2.49) |
−0.83 (−1.26 to −0.41) |
−2.07 (−2.68 to −1.47) |
<0.001 | 0.08 (−0.21 to 0.37) |
1.34 (1.04 to 1.65) |
−1.27 (−1.70 to −0.84) |
<0.001 |
| XeI (hPa.s.l−1) Median (95% CI) |
−2.73 (−3.16 to −2.29) |
−0.88 (−1.32 to −0.44) |
−1.85 (−2.47 to −1.22) |
<0.001 | 0.44 (0.21 to 0.68) |
1.22 (0.97 to 1.46) |
−0.78 (−1.12 to −0.43) |
<0.001 |
| ΔX (hPa.s.l−1) Median (95% CI) |
−0.18 (−0.57 to 0.21) |
0.04 (−0.35 to 0.44) |
−0.23 (−0.78 to 0.33) |
0.421 | −0.36 (−0.57 to −0.16) |
0.13 (−0.09 to 0.34) |
−0.49 (−0.79 to −0.19) |
0.001 |
| ΔX/VT (hPa.s.l−2) Median (95% CI) |
−1.99 (−7.26 to 3.28) |
1.84 (−3.50 to 7.18) |
−3.83 (−11.33 to 3.68) |
0.317 | −2.86 (−4.68 to −1.05) |
1.48 (−0.40 to 3.36) |
−4.34 (−7.00 to −1.68) |
0.001 |
| TBFVL | 1-year-old participants | 2-year-old participants | ||||||
|
Cases (n=267) |
Controls (n=268) |
Coefficient (95% CI) |
P-value |
Cases (n=190) |
Controls (n=176) |
Coefficient (95% CI) |
P-value | |
| RR (b/min) Median (95% CI) | 29.22 (28.65 to 29.78) | 27.64 (27.09 to 28.21) | 1.57 (0.77 to 2.37) | <0.001 | 25.42 (24.83 to 26.00) | 24.76 (24.15 to 25.37) | 0.65 (−0.21 to 1.51) |
0.136 |
| VT (ml) Median (95% CI) | 96.80 (95.09 to 98.52) | 99.40 (97.69 to 101.11) | −2.60 (−5.02 to −0.17) |
0.036 | 126.30 (123.97 to 128.64) | 125.06 (122.63 to 127.49) | 1.24 (−2.20 to 4.69) |
0.478 |
| VT (ml/kg) Median (95% CI) |
9.89 (9.69 to 10.09) | 10.22 (10.02 to 10.42) | −0.33 (−0.61 to −0.04) |
0.024 | 10.51 (10.28 to 10.74) | 10.76 (10.52 to 11.00) | −0.25 (−0.58 to 0.09) |
0.149 |
| Breath time (s) Median (95% CI) | 2.12 (2.08 to 2.17) |
2.24 (2.20 to 2.28) |
−0.11 (−0.17 to −0.05) |
<0.001 | 2.43 (2.38 to 2.49) |
2.49 (2.43 to 2.54) |
−0.05 (−0.13 to 0.03) |
0.180 |
| Tinsp (s) Median (95% CI) |
0.98 (0.96 to 0.99) |
1.01 (0.99 to 1.03) |
−0.03 (−0.06 to −0.01) |
0.013 | 1.11 (1.09 to 1.14) |
1.12 (1.09 to 1.14) |
−0.00 (−0.04 to 0.03) |
0.801 |
| Texp (s) Median (95% CI) |
1.15 (1.12 to 1.18) |
1.23 (1.20 to 1.25) |
−0.08 (−0.12 to −0.04) |
<0.001 | 1.32 (1.29 to 1.36) |
1.37 (1.34 to 1.41) |
−0.05 (−0.10 to 0.00) |
0.061 |
| MIF (ml/s) Median (95% CI) |
98.55 (96.83 to 100.27) | 98.29 (96.57 to 100.01) | 0.26 (−2.18 to 2.69) |
0.836 | 114.26 (112.09 to 116.43) | 111.59 (109.34 to 113.85) | 2.67 (−0.53 to 5.86) |
0.101 |
| PIF (ml/s) Median (95% CI) | 128.71 (126.26 to 131.16) |
128.51 (126.06 to 130.96) | 0.20 (−3.27 to 3.67) |
0.910 | 147.56 (144.52 to 150.60) | 143.80 (140.63 to 146.96) | 3.76 (−0.72 to 8.24) |
0.100 |
| MEF (ml/s) Median (95% CI) | 88.82 (87.11 to 90.54) | 84.51 (82.79 to 86.22) | 4.32 (1.89 to 6.74) | 0.001 | 99.04 (96.91 to 101.18) | 94.51 (92.28 to 96.73) | 4.54 (1.39 to 7.69) | 0.005 |
| PEF (ml/s) Median (95% CI) | 128.82 (126.14 to 131.50) | 120.51 (117.84 to 123.19) | 8.31 (4.52 to 12.10) | <0.001 | 139.50 (136.42 to 142.58) | 130.55 (127.34 to 133.75) | 8.95 (4.41 to 13.49) | <0.001 |
| TPIF (s) Median (95% CI) |
0.49 (0.47 to 0.50) |
0.50 (0.49 to 0.52) |
−0.02 (−0.04 to 0.00) |
0.085 | 0.53 (0.52 to 0.55) |
0.55 (0.53 to 0.57) |
−0.01 (−0.04 to 0.01) |
0.358 |
| TPEF (sec) Median (95% CI) | 0.41 (0.39 to 0.43) |
0.48 (0.46 to 0.50) |
−0.07 (−0.10 to −0.04) |
<0.001 | 0.48 (0.46 to 0.50) |
0.54 (0.52 to 0.56) |
−0.06 (−0.09 to −0.24) |
0.001 |
| TPEF/Texp (%) Median (95% CI) | 36.24 (34.82 to 37.67) | 39.19 (37.76 to 40.61) | −2.94 (−4.96 to −0.93) |
0.004 | 36.72 (35.26 to 38.17) | 39.55 (38.03;41.07) | −2.84 (−4.99;−0.69) |
0.010 |
| MBW | 1-year-old participants | 2-year-old participants | ||||||
|
Cases (n=227) |
Controls (n=201) |
Coefficient (95% CI) |
P-value |
Cases (n=171) |
Controls (n=153) |
Coefficient (95% CI) |
P-value | |
| FRC (ml) median (95% CI) | 187.95 (184.27;191.64) | 180.87 (177.01;184.75) | 7.07 (1.73;12.42) | 0.010 | 243.61 (237.99;249.24( | 243.87 (237.89;249.86) | −0.25 (−8.61;8.09) |
0.951 |
| FRC (ml/kg) median (95% CI) | 19.22 (18.75;19.70) | 18.64 (18.15;19.13) | 0.58 (0.10;1.27) | 0.098 | 20.27 (19.70;20.85) | 20.79 (20.18;21.40) | −0.52 (−1.37;0.33) |
0.233 |
| LCI2.5‡ median (95% CI) | 6.94 (6.83;7.05) |
7.41 (7.30;7.52) |
−0.47 (−0.63;−0.32) |
<0.001 | 6.86 (6.75;6.96) |
6.70 (6.59;6.81) |
0.15 (0.00;0.31) | 0.047 |
| O2 saturation (%) median (95% CI) |
95.75 (95.51;96.00) | 95.88 (95.61;96.14) | −0.12 (−0.49;0.24) |
0.510 | 95.48 (95.19;95.76) | 95.81 (95.52;96.10) | −0.34 (−0.75;0.08) |
0.111 |
*n: number.
†Analyses using linear regression modelling adjusted for age and height at time of pulmonary function testing and sex. Reported as medians with 95% CI and coefficients with 95% CI and p-values.
‡LCI2.5: Lung clearance index at 2.5% of initial tracer gas concentration.
FRC, functional residual capacity; MEF, mean tidal expiratory flow; MIF, mean tidal inspiratory flow; MV, minute ventilation; Osc, airway oscillometry; PEF, peak tidal expiratory flow; PIF, peak tidal inspiratory flow; ReE, End-expiratory resistance; ReI, end-inspiratory resistance; Rmean, mean resistance; RR, respiratory rate; TBFVL, tidal breath flow-volume loops; Texp, expiratory time; Tinsp, inspiratory time; VT, tidal volume; XeE, end-expiratory reactance; XeI, end-inspiratory reactance; Xmean, mean reactance; ΔR, difference between end-expiratory and end-inspiratory resistance; ΔX, difference between end-expiratory and end-inspiratory reactance.
The Xmean was more negative in cases compared with controls at both one (−4.61 vs −3.09 hPa.s/l (p<0.001)) and two years (−0.99 vs 0.33 hPa.s/l (p<0.001)). The tidal changes in Xrs (ΔX) and their values normalised for VT were significantly different between cases and controls only in the two-year-old group (−0.36 vs 0.13 hPa.s/l (p=0.001)).
Tidal breath flow volume loops
At one year of age, cases had an increased respiratory rate (29.22 vs 27.64/min (p<0.001)), a decreased VT (96.80 vs 99.40 mL (p=0.036)) and VT/kg (9.89 vs 10.22 mL (p=0.024)) and an increased minute ventilation (2775 vs 2703 mL (p=0.021)) compared with controls (table 5). The respiratory rate (25.42 vs 24.76/min (p=0.136)), VT (126.30 vs 125.06 (p=0.478)) and the VT/kg (10.51 vs 10.76 (p=0.149)) were similar between cases and controls at two years of age, while the minute ventilation remained increased in cases (3159 vs 3053 mL (p=0.011)).
The total breath time was decreased in cases compared with controls (2.12 vs 2.24 s (p<0.001)), with both the inspiratory (0.98 vs 1.01 s (p=0.013)) and expiratory (1.15 vs 1.23 s (p<0.001)) times decreased at one year of age. These changes were not significant at two years of age.
Mean (98.55 vs 98.29 mL/s (p=0.836)) and peak (128.71 vs 128.51 mL/s (p=0.910)) inspiratory flows were similar between cases and controls at one year of age, and at two years of age (114.26 vs 111.59 mL/s (p=0.101) and 147.56 vs 143.80 mL/s (p=0.100) respectively). Mean and peak expiratory flows were increased in cases compared with controls at both one (88.82 vs 84.51 mL/s (p=0.001) and 128.82 vs 120.51 mL/s (p<0.001), respectively) and two years of age (99.04 vs 94.51 mL/s (p=0.005) and 139.50 vs 130.55 mL/s (p<0.001), respectively). The time to peak expiratory flow as a ratio of expiratory time was decreased in cases compared with controls at both one (36.24 vs 39.19 (p=0.004)) and two years of age (36.72 vs 39.55) (p=0.010)).
Multiple breath inert gas washout technique
FRC (187.95 vs 180.87 mL (p=0.010)) was increased and LCI (6.94 vs 7.41 (p<0.001)) decreased in cases compared with controls at one year of age, but not so at two years of age, where the FRC was similar (243.6 vs 243.9 (p=0.951)) and LCI was higher in cases (6.82 vs 6.70 (p=0.047)). Also, oxygen saturation was similar between cases and controls at both one and two years of age (95.75 vs 95.88% (p=0.510) and 95.48 vs 95.81% (p=0.111), respectively).
Discussion
In this study, we report an increased risk of pulmonary sequelae at one and two years of age in South African children after severe and very severe RSV LRTI during infancy. Importantly, one-year and two-year old cases were more likely to have wheezing, admissions for wheezing or chest infections or a dry cough at night than controls. In addition, the magnitudes of mean airway reactance and resistance, as well as their intrabreath values, were increased in both one-year and two-year-old cases compared with controls, consistent with narrower, less compliant airways. Furthermore, the TPEF/TE ratio was decreased in both one-year and two-year-old cases, suggesting the presence of expiratory airflow obstruction. These findings highlight that despite the relatively low RSV LRTI associated mortality in this setting, RSV hospitalisation is associated with long-term morbidity, and an urgent need for RSV preventative strategies, including maternal RSV vaccination or prophylactic monoclonal antibodies.15–17
Multiple studies have investigated the association between RSV LRTI during infancy and subsequent clinical pulmonary sequelae up until and beyond adolescence, using the same questionnaire-based approach—the overall conclusions similar to ours.6 18–23 Additionally, there have been a number of studies investigating pulmonary function in children after six years of age using spirometry as the main investigational tool.24 In a systematic review, we reported that children with RSV LRTI during the first three years of life had obstructive lung disease with no bronchodilator response, although there being conflicting results.4 There is, however, a paucity of studies investigating lung function parameters in the early stages of lung development. More longitudinal data with age-appropriate PFTs are required, to better delineate the progression of lung disease during this period and provide valuable data on whether children with subsequent prolonged wheezing have an underlying predisposition to acute viral LRTI with wheezing episodes, and long-term sequelae, or whether the acute viral LRTI itself causes the long-term pulmonary function sequelae. The contribution of other individual factors such as the presence of atopy, environmental tobacco smoke exposure, altered airway microbiome and genetic predisposition, on pulmonary resistance and compliance, in addition to that of acute LRTI needs to be delineated, before causation can be proven. These factors may explain the apparent improvement of pulmonary function indices towards the two-year-old testing point in our study.
Our study reported no difference in resistance between children who have RSV-LRTI during infancy compared with healthy controls when measured by Osc at one year of age, but resistance was increased in the cases at two years of age. There was, however, an increase in reactance magnitude (ie, more negative Xrs values) among cases at one and two years of age, indicating decreased pulmonary compliance post severe RSV LRTI. This is similar to another South African study that reported a decrease in compliance in two-year-old children, after all-cause LRTI during infancy, an effect that was independent of premorbid lung function.6 Other studies reporting Osc data, performed at older ages, report conflicting data to our study, with no evidence of lung function sequelae at six and eight years in children with mild RSV LRTI, or at eight years in those with severe RSV-LRTI.25 26 This difference could be due to age of the participants and the severity of the initial LRTI.
We performed pulmonary function testing on approximately 1000 one and two-year-old children, with an overall good success rate for both cases and controls at one-year and two-year testing. This compares well to the success rates obtained by Zar et al. reporting on data, using similar methods to our study, from the Drakenstein Child Health study, with an overall success rate, of 87% at one year and 83% at two years.6 Success rates for PFTs in children are influenced by the age of the child, and the corresponding sleep pattern, if done in the sleep state, the presence of underlying lung pathology as well as the duration and complexity of the techniques performed.5 6 27 28 This increasing complexity and duration of the testing would explain the decreasing success rate between Osc and MBW and highlight the feasibility of performing infant PFTs.
To our knowledge, this is the first report in early childhood on post-RSV LRTI sequelae using the recently developed intra-breath Osc measurements, which follow the dynamic changes in impedance and identify their values at the zero flow points of respiration (ReE, ReI, XeE, XeI) and the corresponding tidal changes (ΔR and ΔX), thereby minimising the influence of flow-dependent non-linearities arising mostly in the upper airways (nasal pathways and the glossopharyngeal area) and providing a more accurate reflection of the dynamic changes of resistance and reactance of the lower airways, through out the breath cycle.29 Given the large contribution of the upper airways in the nasal breathing infants, these intrabreath values were more accurate in delineating a difference in resistance and reactance.
TBFVL data indicated an increase in respiratory rate in the cases at one year, but this was no longer present at two years, when an increase in tidal volume was observed. The inspiratory flow indices were not affected at either one or two years of age, whereas the expiratory flow indices were increased in both one and two-year-old cases, coupled with a decreased time to reach peak expiratory flow as a percentage of the expiratory time. The only other study to present results on any TBFVL indices in children with all-cause LRTI and to compare RSV LRTI and non-RSV LRTI was from Cape Town, South Africa.6 The authors reported limited TBFVL data; with all-cause LRTI causing an increase in respiratory rate at two years, with recurrent LRTI further increasing the difference between cases and controls; however, this was not specific to RSV, with no significance when comparing RSV LRTI with non-RSV LRTI. There was no difference in tidal volume or in TPEF/TE between two-year-old children with or without prior LRTI, nor between those with prior RSV LRTI versus non-RSV LRTI. These results are in contrast to ours, possibly due to the smaller number of RSV cases in the Cape Town cohort and the inclusion of all-cause and less severe ambulatory LRTI as well as wheezing episodes.
At one year, there was a significant increase in FRC, an average 8 mL higher in cases, which at two years was no longer significant. This initial increase in FRC could be due to residual postinfectious RSV small airway obstruction, leading to air-trapping or dynamic hyperinflation, which gradually diminishes with time and, therefore, is due to the acute event and does not equate to long-term postinfectious abnormalities, however, this would need to be substantiated with a prolonged follow-up of the cohort. Similar findings have also been reported. Broughton et al observed no difference in FRC results, measured by body plethysmography and helium gas dilution technique, in infants born before 32 weeks gestational age with and without RSV LRTI.30
There were limitations to our study. Only one and two year-old African children with severe or very severe RSV-associated LRTI were included in our study, therefore the results are not generalisable to all populations and ages, or to children with mild LRTI. There is a possibility that controls may have had an LRTI but that parents had not sought medical attention, thereby incorrectly including these patients as controls. Reliance on caretaker recall for reporting clinical sequelae may lead to under or over reporting of symptoms. No baseline PFT were performed before RSV-associated hospitalisation, and, therefore, it would not be possible to ascribe causation between RSV and pulmonary function abnormalities, but rather an association. One-year-old cases were more likely to have received supplementary oxygen postdelivery; this might suggest that they had a higher innate propensity to poor lung function or that later pulmonary deficits may be due to peri-partum insults.
The strengths of the study were that three consecutive RSV seasons were included, correcting for potential variations in RSV genotype and serotype. We used strict definitions for severe LRTI, the diagnosis of RSV and for inclusion and exclusion criteria. Importantly, we used both subjective and objective measurable assessments on almost 1000 children, making this a very large pool of data. Furthermore, we used two different assessment approaches and children were tested at one and two years of age, where there is generally a paucity of data, thereby adding valuable data to the ever-growing pool.
In conclusion, one and two-year-old children are more likely to experience clinical pulmonary symptoms and have further admissions for wheezing and LRTI after an initial RSV LRTI during infancy. Furthermore, RSV LRTI is associated with an increase in resistance and decrease in compliance at two years as well as an increase in work of breathing and FRC at one year. These results highlight the importance of further investment into RSV vaccine production, through which substantial long-term pulmonary morbidity could be prevented.
Footnotes
Contributors: CV conceived and designed the study; acquired, analysed and interpreted the data; drafted all manuscripts and approved the final manuscript; CV is the author acting as guarantor. LR, ML, VB, MN, DG, ZH, ZD and SM made substantial contributions to the conception and design of the study; analysed and interpreted the data and approved the final manuscript.
Funding: This study was funded through the Vaccines and Infectious Diseases Analytics research unit and through funding by the South African Medical Research Council (SAMRC) through its SAMRC/WITS Clinician Researcher Programme PhD Scholarships in Clinical Research. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by University of the Witwatersrand Human Research Ethics Committee (Medical): Numbers M160224, M131109, M140904 and M140203. Participants gave informed consent to participate in the study before taking part.
References
- 1.Levels Trends in Child Mortality: Report 2020, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation . New York: United Nations Children’s Fund, 2020. [Google Scholar]
- 2.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390:946–58. 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll AJ, Arshad SH, Bont L, et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? critical review of the evidence and guidance for future studies from a world health organization-sponsored meeting. Vaccine 2020;38:2435–48. 10.1016/j.vaccine.2020.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verwey C, Nunes MC, Dangor Z, et al. Pulmonary function sequelae after respiratory syncytial virus lower respiratory tract infection in children: a systematic review. Pediatr Pulmonol 2020;55:1567–83. 10.1002/ppul.24804 [DOI] [PubMed] [Google Scholar]
- 5.Beydon N, Davis SD, Lombardi E, et al. An official American thoracic society/european respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007;175:1304–45. 10.1164/rccm.200605-642ST [DOI] [PubMed] [Google Scholar]
- 6.Zar HJ, Nduru P, Stadler JAM, et al. Early-Life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: epidemiology and effect on lung health. Lancet Glob Health 2020;8:e1316–25. 10.1016/S2214-109X(20)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statistics South Africa . Community Survey 2016, Statistical release P0301. In: Africa SS. 2016. [Google Scholar]
- 8.Rudan I, O’Brien KL, Nair H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013;3:010401. 10.7189/jogh.03.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soofie N, Nunes MC, Kgagudi P, et al. The burden of pertussis hospitalization in HIV-exposed and HIV-unexposed South African infants. Clin Infect Dis 2016;63(suppl 4):S165–73. 10.1093/cid/ciw545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellwood P, Asher MI, Beasley R, et al. The International study of asthma and allergies in childhood (Isaac): phase three rationale and methods. Int J Tuberc Lung Dis 2005;9:10–6. [PubMed] [Google Scholar]
- 11.Frey U, Stocks J, Coates A, et al. Specifications for equipment used for infant pulmonary function testing. ERS/ATS Task force on standards for infant respiratory function testing. European respiratory society/ American thoracic Society. Eur Respir J 2000;16:731–40. 10.1034/j.1399-3003.2000.16d28.x [DOI] [PubMed] [Google Scholar]
- 12.Robinson PD, Latzin P, Verbanck S, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J 2013;41:507–22. 10.1183/09031936.00069712 [DOI] [PubMed] [Google Scholar]
- 13.Bates JH, Schmalisch G, Filbrun D, et al. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task force on standards for infant respiratory function testing. European respiratory society/american thoracic Society. Eur Respir J 2000;16:1180–92. 10.1034/j.1399-3003.2000.16f26.x [DOI] [PubMed] [Google Scholar]
- 14.Radics BL, Gyurkovits Z, Makan G, et al. Respiratory oscillometry in newborn infants: conventional and intra-breath approaches. Front Pediatr 2022;10:867883. 10.3389/fped.2022.867883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin MP, Yuan Y, Takas T, et al. Single-Dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020;383:415–25. 10.1056/NEJMoa1913556 [DOI] [PubMed] [Google Scholar]
- 16.Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022;386:837–46. 10.1056/NEJMoa2110275 [DOI] [PubMed] [Google Scholar]
- 17.Simões EAF, Center KJ, Tita ATN, et al. Prefusion F protein–based respiratory syncytial virus immunization in pregnancy. N Engl J Med 2022;386:1615–26. 10.1056/NEJMoa2106062 [DOI] [PubMed] [Google Scholar]
- 18.Schauer U, Hoffjan S, Bittscheidt J, et al. Rsv bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J 2002;20:1277–83. 10.1183/09031936.02.00019902 [DOI] [PubMed] [Google Scholar]
- 19.Escobar GJ, Masaquel AS, Li SX, et al. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr 2013;13:97. 10.1186/1471-2431-13-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escobar GJ, Ragins A, Li SX, et al. Recurrent wheezing in the third year of life among children born at 32 weeks’ gestation or later: relationship to laboratory-confirmed, medically attended infection with respiratory syncytial virus during the first year of life. Arch Pediatr Adolesc Med 2010;164:915–22. 10.1001/archpediatrics.2010.177 [DOI] [PubMed] [Google Scholar]
- 21.Henderson J, Hilliard TN, Sherriff A, et al. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005;16:386–92. 10.1111/j.1399-3038.2005.00298.x [DOI] [PubMed] [Google Scholar]
- 22.Coutts J, Fullarton J, Morris C, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol 2020;55:1104–10. 10.1002/ppul.24676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–5. 10.1016/S0140-6736(98)10321-5 [DOI] [PubMed] [Google Scholar]
- 24.Beydon N. Pulmonary function testing in young children. Paediatr Respir Rev 2009;10:208–13. 10.1016/j.prrv.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 25.Lauhkonen E, Koponen P, Nuolivirta K, et al. Lung function by impulse oscillometry at age 5-7 years after bronchiolitis at age 0-6 months. Pediatr Pulmonol 2015;50:389–95. 10.1002/ppul.23039 Available: http://doi.wiley.com/10.1002/ppul.v50.4 [DOI] [PubMed] [Google Scholar]
- 26.Juntti H, Kokkonen J, Dunder T, et al. Association of an early respiratory syncytial virus infection and atopic allergy. Allergy 2003;58:878–84. 10.1034/j.1398-9995.2003.00233.x [DOI] [PubMed] [Google Scholar]
- 27.Gray D, Willemse L, Visagie A, et al. Determinants of early-life lung function in African infants. Thorax 2017;72:445–50. 10.1136/thoraxjnl-2015-207401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KF, Parker KP, Montgomery GL. Sleep in infants and young children: Part one: normal sleep. J Pediatr Health Care 2004;18:65–71. 10.1016/s0891-5245(03)00149-4 [DOI] [PubMed] [Google Scholar]
- 29.Czövek D, Shackleton C, Hantos Z, et al. Tidal changes in respiratory resistance are sensitive indicators of airway obstruction in children. Thorax 2016;71:907–15. 10.1136/thoraxjnl-2015-208182 [DOI] [PubMed] [Google Scholar]
- 30.Broughton S, Sylvester KP, Fox G, et al. Lung function in prematurely born infants after viral lower respiratory tract infections. Pediatr Infect Dis J 2007;26:1019–24. 10.1097/INF.0b013e318126bbb9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001618supp001.pdf (233.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request.