Abstract
Objectives
Pharmacological venous thromboembolism (VTE) prophylaxis is recommended in the vast majority of trauma patients. The purpose of this study was to characterize current dosing practices and timing of initiation of pharmacological VTE chemoprophylaxis at trauma centers.
Methods
This was an international, cross-sectional survey of trauma providers. The survey was sponsored by the American Association for the Surgery of Trauma (AAST) and distributed to AAST members. The survey included 38 questions about practitioner demographics, experience, level and location of trauma center, and individual/site-specific practices regarding the dosing, selection, and timing of initiation of pharmacological VTE chemoprophylaxis in trauma patients.
Results
One hundred eighteen trauma providers responded (estimated response rate 6.9%). Most respondents were at level 1 trauma centers (100/118; 84.7%) and had >10 years of experience (73/118; 61.9%). While multiple dosing regimens were used, the most common dose reported was enoxaparin 30 mg every 12 hours (80/118; 67.8%). The majority of respondents (88/118; 74.6%) indicated adjusting the dose in patients with obesity. Seventy-eight (66.1%) routinely use antifactor Xa levels to guide dosing. Respondents at academic institutions were more likely to use guideline-directed dosing (based on the Eastern Association of the Surgery of Trauma and the Western Trauma Association guidelines) of VTE chemoprophylaxis compared with those at non-academic centers (86.2% vs 62.5%; p=0.0158) and guideline-directed dosing was reported more often if the trauma team included a clinical pharmacist (88.2% vs 69.0%; p=0.0142). Wide variability in initial timing of VTE chemoprophylaxis after traumatic brain injury, solid organ injury, and spinal cord injuries was found.
Conclusions
A high degree of variability exists in prescribing and monitoring practices for the prevention of VTE in trauma patients. Clinical pharmacists may be helpful on trauma teams to optimize dosing and increase prescribing of guideline-concordant VTE chemoprophylaxis.
Keywords: VTE prophylaxis, VTE chemoprophylaxis, enoxaparin, venous thromboembolism, trauma
Background
Venous thromboembolism (VTE) prophylaxis with either low-dose unfractionated heparin (UFH) or low molecular weight heparin (LMWH) is recommended for hospitalized trauma patients, with LMWH being preferred for most patients.1–5 Ongoing debate exists regarding the optimal dosing of LMWH, timing of initiation in patients at high risk of hemorrhage, and duration of VTE chemoprophylaxis after discharge.3–18
Enoxaparin is the LMWH currently recommended and the most common agent in the USA.3 4 Previous studies have demonstrated that ‘standard’ enoxaparin doses do not achieve target prophylactic antifactor Xa (anti-Xa) levels to prevent VTE complications in trauma patients.6 Therefore, many practitioners have adopted practices to monitor anti-Xa levels and provide dose adjustments to achieve anti-Xa targets. Additionally, patients with obesity are at high risk for failure of thromboprophylaxis and some clinicians opt for higher, initial, fixed-dose enoxaparin or weight-based regimens in this patient population.7 While higher alternative enoxaparin dosing regimens have demonstrated improved attainment of target anti-Xa levels, the addition of routine monitoring can be costly and the impact on clinical outcomes is controversial, making adoption into standard practice variable.8–11 16
Timing of initiation, duration of VTE chemoprophylaxis, holding doses for procedures, and screening for VTE are other practices that may vary significantly. Ideally, most trauma patients should receive pharmacological VTE chemoprophylaxis within 24 hours of admission and in an uninterrupted fashion. In some cases, delaying initiation of VTE chemoprophylaxis is necessary due to active bleeding, coagulopathy, traumatic brain injury (TBI), spinal cord injury (SCI), solid organ injury, epidural catheter placement, or hemorrhage risk.3 4 18 In these circumstances, less guidance is available regarding optimal timing of chemoprophylaxis which can lead to significant delays.3 4 12–14 Moreover, the optimal agent and duration of VTE chemoprophylaxis postdischarge and routine screening practices are controversial.15 17
Previously, a survey of a small cohort of trauma surgeon leaders demonstrated that variation exists in clinical practice for VTE chemoprophylaxis.19 A larger survey of trauma surgeons has also shown wide variation in screening for deep vein thrombosis after trauma.15 Since that time, updated clinical practice algorithms have been published to influence practice patterns; yet, discrepancies and gaps in guideline recommendations remain that may cause variation in practices to persist.3 4 12 14 Therefore, the purpose of this study was to characterize the current VTE chemoprophylaxis practices in trauma patients through a survey of a broad group of trauma providers and compare responses with the current guideline recommendations.
Methods
We conducted an international, cross-sectional survey of trauma providers. The survey was sponsored by the American Association for the Surgery of Trauma (AAST). It was developed and disseminated using Qualtrics (Provo, Utah, USA), an online survey and distribution software.
A convenience sample from the AAST mailing list was included. Participants were invited to complete the survey using an anonymous, electronic link sent via the AAST listserv in March 2021. One reminder email was sent in April 2021. The survey link was also available to be accessed via the AAST webpage. Participation in the survey was voluntary and respondents consented to participate by completing and submitting the survey. Incomplete responses were excluded from analysis. The survey was estimated to take 5–10 min to complete.
Two trauma physicians and one clinical pharmacist initially developed the survey items based on guideline recommendations and gaps. Five trauma physicians and two advanced practice providers who were not study investigators completed pilot testing to review content, determine ease of use, and identify errors. Feedback from testers was then incorporated into the final version and the changes were approved by study investigators.
The survey was a 38-item questionnaire (online supplemental appendix A). It was separated into six sections, including: demographics, preferred pharmacological drug and dose, monitoring practices, screening of asymptomatic patients, duration of VTE chemoprophylaxis postdischarge, and timing to initiation of chemoprophylaxis in patients with TBI, solid organ injury, and SCI. Display logic was used for 17 questions based on previous responses. For example, if respondents answered they did not routinely dose adjust enoxaparin based on anti-Xa levels, questions on anti-Xa monitoring were not displayed and participants were automatically advanced to the next section.
tsaco-2022-001070supp001.pdf (271.7KB, pdf)
At the time of first distribution, Practice Management Guidelines from the Eastern Association of the Surgery of Trauma and the Western Trauma Association (WTA) were available.1 3 Respondents were asked to select their standard regimen (agent and dose) for VTE chemoprophylaxis in trauma patients without obesity (body mass index <30) with normal renal function (creatinine clearance ≥30 mL/min). Guideline-directed therapy in this patient population was defined as enoxaparin 30 mg subcutaneously every 12 hours, enoxaparin 40 mg subcutaneously every 12 hours, or enoxaparin 0.5 mg/kg subcutaneously every 12 hours. Comparisons of responses based on trauma center type, trauma-level designation, presence of a clinical pharmacist, and institutional VTE protocol were done.
Responses were analyzed using descriptive and frequency statistics. Comparisons between groups were analyzed using a two-tailed, Fisher’s exact test. Data analyses were performed using SPSS V.27.0 (SPSS, Chicago, Illinois, USA).
Results
One hundred thirty-one trauma providers responded. Thirteen responses were excluded due to incomplete data, leaving 118 responses for analysis. The invitation to participate was sent to 1466 AAST members. The response rate was estimated to be 8.0%.
The 118 respondents represented 98 institutions from widely distributed geographic locations. Respondent demographics are reported in table 1. Seven (5.9%) respondents were affiliated with an international trauma center. Most respondents were at level 1 trauma centers (100/118; 84.7%) and had an institutional protocol for VTE prophylaxis approved (103/118; 87.3%).
Table 1.
Respondent and institution demographics
| Demographic (n=118) | N (%) |
| Type of institution | |
| Academic | 94 (79.7) |
| Community/Other | 24 (20.3) |
| Physician (MD/DO) respondent | 117 (99.2) |
| State/Regional trauma designation | |
| Level I | 100 (84.7) |
| Level II | 17 (14.4) |
| Level III | 1 (0.8) |
| ACS-certified trauma center | 91 (77.1) |
| Geographic location | |
| Midwest | 20 (16.9) |
| Northeast | 32 (27.1) |
| South | 37 (31.4) |
| West | 22 (18.6) |
| International | 7 (5.9) |
| Years in practice | |
| <2 | 20 (16.9) |
| 5–6 | 9 (7.6) |
| 6–10 | 16 (13.6) |
| >10 | 73 (61.9) |
| Protocol for VTE prophylaxis | 103 (87.3) |
| Trauma team includes a clinical pharmacist | 76 (64.4) |
ACS, American College of Surgeons; DO, Doctor of Osteopathic Medicine; MD, Doctor of Medicine; VTE, venous thromboembolism.
Table 2 lists the respondents’ standard dosing regimens for VTE chemoprophylaxis and comparisons between groups. The most common medication and dose reported was enoxaparin 30 mg subcutaneously two times per day (80/118; 67.8%). Guideline-directed dosing of VTE chemoprophylaxis was the standard for 81.4% (96/118) of the cohort. The majority indicated adjusting the dose in patients with obesity (88/118; 74.6%). Additionally, 78 (66.1%) routinely use anti-Xa levels to guide dosing. Most respondents use UFH (5000 units every 8 hours or every 12 hours) for patients with renal dysfunction (81/118; 68.6%).
Table 2.
Standard medication regimen, routine anti-Xa level monitoring, and dose adjustment in obesity
| Question response | Total cohort (n=118) | Academic institution (n=94) | Non-academic institution (n=24) | P value | Clinical pharmacist on trauma team (n=76) | No clinical pharmacist (n=42) | P value | Level I trauma designation (n=100) | Level II/III trauma designation (n=18) | P value | Protocol for VTE prophylaxis (n=103) | No protocol (n=15) | P value |
| Guideline-directed regimen, n (%) | 96 (81.4) | 81 (86.2) | 15 (62.5) | 0.0158 | 67 (88.2) | 29 (69.0) | 0.0142 | 83 (83.0) | 13 (72.2) | 0.3246 | 84 (81.6) | 12 (80.0) | 1 |
| Standard regimen*, n (%) | |||||||||||||
| Enoxaparin 40 mg subcutaneously every 24 hours | 14 (11.9) | 6 (6.4) | 8 (33.3) | 6 (7.9) | 8 (19.0) | 11 (11.0) | 3 (16.7) | 12 (11.7) | 2 (13.3) | ||||
| Enoxaparin 30 mg subcutaneously every 12 hours | 80 (67.8) | 68 (72.3) | 12 (50.0) | 55 (72.4) | 25 (59.5) | 71 (71.0) | 9 (50.0) | 70 (68.0) | 10 (66.7) | ||||
| Enoxaparin 40 mg subcutaneously every 12 hours | 8 (6.8) | 7 (6.4) | 1 (4.2) | 5 (6.6) | 3 (7.1) | 5 (5.0) | 3 (16.7) | 6 (5.8) | 2 (13.3) | ||||
| Enoxaparin 0.5 mg/kg subcutaneously every 12 hours | 8 (6.8) | 6 (6.4) | 2 (8.3) | 7 (9.2) | 1 (2.4) | 7 (7.0) | 1 (5.6) | 8 (7.8) | 0 (0.0) | ||||
| UFH 5000 units subcutaneously every 8 hours | 2 (1.7) | 2 (2.1) | 0 (0.0) | 1 (1.3) | 1 (2.4) | 1 (1.0) | 1 (5.6) | 1 (1.0) | 1 (6.7) | ||||
| Other | 6 (5.1) | 5 (5.3) | 1 (4.2) | 2 (2.6) | 4 (9.5) | 5 (5.0) | 1 (5.6) | 6 (5.8) | 0 (0.0) | ||||
| Standard regimen in renal dysfunction†, n (%) | |||||||||||||
| Enoxaparin 40 mg subcutaneously every 24 hours | 9 (7.6) | 5 (5.3) | 4 (16.7) | 5 (6.6) | 4 (9.5) | 7 (7.0) | 2 (11.1) | 8 (7.8) | 1 (6.7) | ||||
| Enoxaparin 30 mg subcutaneously every 12 hours | 11 (9.3) | 8 (8.5) | 3 (12.5) | 7 (9.2) | 4 (9.5) | 9 (9.0) | 2 (11.1) | 10 (9.7) | 1 (6.7) | ||||
| Enoxaparin 40 mg subcutaneously every 12 hours | 3 (2.5) | 3 (3.2) | 0 (0.0) | 2 (2.6) | 1 (2.4) | 2 (2.0) | 1 (5.6) | 2 (1.9) | 1 (6.7) | ||||
| Enoxaparin 0.5 mg/kg subcutaneously every 12 hours | 3 (2.5) | 2 (2.1) | 1 (4.2) | 2 (2.6) | 1 (2.4) | 3 (3.0) | 0 (0.0) | 3 (2.9) | 0 (0.0) | ||||
| Enoxaparin 30 mg subcutaneously every 24 hours | 6 (5.1) | 5 (5.3) | 1 (4.2) | 4 (5.3) | 2 (4.8) | 4 (4.0) | 2 (11.1) | 6 (5.8) | 0 (0.0) | ||||
| UFH 5000 units subcutaneously every 8 hours | 72 (61.0) | 58 (61.7) | 14 (58.3) | 48 (63.2) | 24 (57.1) | 62 (62.0) | 10 (55.6) | 60 (58.3) | 12 (80.0) | ||||
| UFH 5000 units subcutaneously every 12 hours | 9 (7.6) | 8 (8.5) | 1 (4.2) | 5 (6.6) | 4 (9.5) | 8 (8.0) | 1 (5.6) | 9 (8.7) | 0 (0.0) | ||||
| Other | 5 (4.2) | 5 (5.3) | 0 (0.0) | 3 (3.9) | 2 (4.8) | 5 (5.0) | 0 (0.0) | 5 (4.9) | 0 (0.0) | ||||
| Routinely dose adjust enoxaparin in overweight or obese, n (%) | 88 (74.6) | 75 (79.8) | 13 (54.2) | 0.0168 | 62 (81.6) | 26 (61.9) | 0.0268 | 78 (78.0) | 10 (55.6) | 0.0736 | 79 (76.7) | 9 (60.0) | 0.2048 |
| Routinely monitor anti-Xa levels, n (%) | |||||||||||||
| Yes | 78 (66.1) | 62 (66.0) | 16 (66.7) | 0.5798 | 46 (60.5) | 32 (76.2) | 0.0688 | 64 (64.0) | 14 (77.8) | 0.2254 | 65 (63.1) | 13 (86.7) | 0.3464 |
| No | 29 (24.6) | 25 (26.6) | 4 (16.7) | 23 (30.3) | 6 (14.3) | 27 (27.0) | 2 (11.1) | 27 (26.2) | 2 (13.3) | ||||
| No response | 11 (9.3) | 7 (7.4) | 4 (16.7) | 7 (9.2) | 4 (9.5) | 9 (9.0) | 2 (11.1) | 11 (10.7) | 0 (0.0) | ||||
*Non-obese (body mass index <30) and normal renal function (creatinine clearance ≥30 mL/min).
†Creatinine clearance ≤30 mL/min.
Anti-Xa, antifactor Xa; UFH, unfractionated heparin; VTE, venous thromboembolism.
Respondents at academic institutions were more likely to use guideline-directed dosing of VTE chemoprophylaxis compared with those at non-academic centers (86.2% vs 62.5%; p=0.0158) and guideline-directed dosing was reported more often if the trauma team included a clinical pharmacist (88.2% vs 69.0%; p=0.0142). Respondents at academic centers and/or those who rounded with a clinical pharmacist were more likely to dose adjust VTE chemoprophylaxis for patients with obesity (79.8% vs 54.2%; p=0.0168 and 81.6% vs 61.9%; p=0.0268, respectively). No differences were detected in findings from respondents at level I versus level II/III trauma centers or those that had a VTE protocol adopted versus not (table 2).
Most respondents indicated not routinely screening asymptomatic patients for VTE with duplex ultrasound (90/118; 76.3%). Of those who do routinely screen patients for VTE, 60% (9/15) responded that screening occurred every 7 days. Others reported various timeframes for screening, such as twice weekly, on day 3 if not on pharmacological prophylaxis then weekly, once at hospital day 10–14, or based on thromboelastography assay.
Seventy-three participants (61.9%) reported using pharmacological VTE prophylaxis postdischarge in high-risk patients. The most common medications reported following discharge were enoxaparin 30 mg subcutaneously two times per day (52/73; 71.2%) and aspirin 81 mg oral daily (11/73; 15.1%). Non-weight-bearing status to bilateral lower extremities was the indication most agreed on for postdischarge chemoprophylaxis (60/73; 82.2%), followed by pelvic fractures (51/73; 69.9%), and SCI (43/73; 58.9%). Most commonly, respondents indicated that the duration of postdischarge chemoprophylaxis varied based on the indication (27/73; 37.0%) (table 3).
Table 3.
Postdischarge venous thromboembolism prophylaxis practices
| Question response | N (%) |
| Discharge high-risk patients on VTE prophylaxis (n=118) | |
| Yes | 73 (61.9) |
| No | 33 (28.0) |
| No response | 12 (10.2) |
| Injuries for postdischarge VTE prophylaxis (n=73)* | |
| Spinal column injuries with neurological deficits | 43 (58.9) |
| Non-weight-bearing status to bilateral lower extremities | 60 (82.2) |
| Non-weight-bearing status to one lower extremity | 29 (39.7) |
| Pelvic fracture | 51 (69.9) |
| Other | 7 (9.6) |
| No response | 8 (11.0) |
| Agents used for postdischarge VTE prophylaxis (n=73)* | |
| Aspirin 81 mg orally daily | 11 (15.1) |
| Aspirin 81 mg orally two times per day | 4 (5.5) |
| Aspirin 325 mg orally daily | 6 (8.2) |
| Aspirin 325 mg orally two times per day | 3 (4.1) |
| Enoxaparin 30 mg subcutaneously two times per day | 52 (71.2) |
| Enoxaparin 40 mg subcutaneously daily | 7 (9.6) |
| Warfarin | 7 (9.6) |
| DOAC | 8 (11.0) |
| Other | 6 (8.2) |
| No response | 8 (11.0) |
| Duration of postdischarge VTE prophylaxis (n=73) | |
| 7 days | 0 (0.0) |
| 14 days | 1 (1.4) |
| 21 days | 13 (17.8) |
| 1 month | 14 (19.2) |
| 3 months | 1 (1.4) |
| Varies based on the indication | 27 (37.0) |
| Other | 5 (6.8) |
| No response | 12 (16.4) |
*Select all that apply.
DOAC, direct oral anticoagulant; VTE, venous thromboembolism.
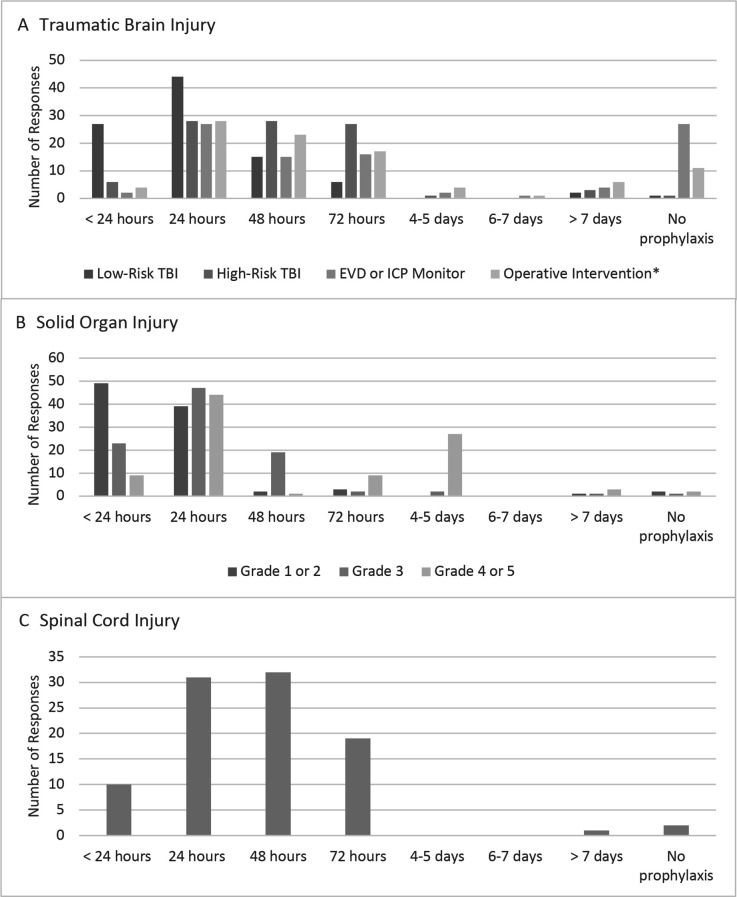
Wide variability in initial timing of VTE chemoprophylaxis after TBI, solid organ injury, and SCI was found. Figure 1 demonstrates the timing to initiation of pharmacologic VTE prophylaxis (either LMWH or UFH) in patients with TBI, solid organ injury, and operative SCI post-fixation. Most participants had an institutional protocol approved for VTE prophylaxis in patients with TBI (69/118; 58.5%), while others indicated that the decision to initiate chemoprophylaxis was not standardized and based on consultant recommendation (22/118; 18.6%). Fewer respondents indicated having an institutional VTE prophylaxis protocol for patients with solid organ injuries (45/118; 38.1%) or SCI (46/118; 39.0%).
Figure 1.
Timing of initiation of pharmacological venous thromboembolism prophylaxis in patients with (A) traumatic brain injury (TBI) poststable head CT, (B) solid organ injury postinjury with no active or ongoing bleeding, or (C) operative spinal cord injury postfixation. EVD, external ventricular drain; ICP, intracranial pressure. *Operative intervention was defined as postcraniotomy or craniectomy. Solid organ injury based on the American Association for the Surgery of Trauma Organ Injury Scale.
Discussion
While the majority of trauma providers use enoxaparin as the standard medication for VTE chemoprophylaxis following trauma, survey results indicate that significant variation exists regarding the optimal dose, dose adjustment, and monitoring strategies. Multiple indications, medications, and durations of treatment were identified for VTE chemoprophylaxis postdischarge. Additionally, wide variability in initial timing of VTE chemoprophylaxis after TBI, solid organ injury, and SCI existed.
Clinical practice guidelines are aimed at standardizing and improving patient outcomes. Barriers to implementation of guidelines are multifactorial. Personal beliefs, disagreement among providers, system-based barriers, and lack of awareness may lead to deviations in clinical practice.20 21 Similar to previous surveys, the results of this study reaffirm that variation continues to exists regarding VTE chemoprophylaxis practices, despite new practice guidelines.15 22 Factors identified that may improve adoption of clinical practice guideline recommendations for VTE prevention were practice at an academic institution and inclusion of a clinical pharmacist on the trauma team. This is likely due to increased communication, routine educational opportunities, and enthusiasm from learners to incorporate recommendations into practice at academic centers. In addition, clinical pharmacists provide medication education, develop evidence-based institutional protocols, enact changes to the electronic health record to influence prescribing practices, and provide medication monitoring and dose recommendations to achieve drug targets that may be impactful to the provision of guideline-concordant VTE chemoprophylaxis and increased monitoring.23
Agreement across clinical practice guidelines and surgical specialties is needed to standardize VTE chemoprophylaxis in trauma patients. Despite clear guideline recommendations, disagreement among trauma and consulting providers regarding the risk of bleeding versus the risk of thrombosis can lead to deviation from practice guidelines or delays in care. In addition, while the WTA and AAST/American College of Surgeons (ACS)-Committee on Trauma (COT) guidelines share similar recommendations, they are not identical and, due to a lack of robust evidence, each set of guidelines does not address all aspects of VTE prevention, such as timing of initiation in high-risk patient populations, specifics on how and when to monitor LMWH, and recommendations for selection of agent and duration of VTE chemoprophylaxis postdischarge.3 4 Therefore, despite a large amount of cohort data describing VTE practice innovations and outcomes, there remains a paucity of high-quality data to drive standard practices for all aspects of VTE prevention. Recommendations for VTE chemoprophylaxis span multiple specialties (neurosurgery, orthopedics, ACS) and efforts need to be focused on aligning practice recommendations among surgical specialties. To promote this goal, a National Institutes of Health sponsored conference titled ‘2022 Consensus Conference to Implement Optimal VTE Prophylaxis in Trauma’ was held in May 2022 with the initial aim of bringing together a multidisciplinary cohort of trauma experts to review the current state of the science, identify gaps in the literature, and prioritize a future research agenda.16–18 21 24
Limitations for this study include decreased generalizability of results due to the low response rate and high percentage of respondents at level 1 and academic institutions. Additionally, display logic decreased the response rates for some items. Despite these limitations, respondents were widely distributed geographically which supports the conclusion that broad variability exists and does not change the interpretation of findings. Survey questions asked participants to describe ‘standard’ practices for patients; therefore, the results do not represent patient-specific recommendations. In addition, the AAST/ACS-COT clinical protocol for inpatient VTE prophylaxis was released during the survey time period with the majority of responses collected prior to the publication of the AAST/ACS-COT recommendations. Therefore, practice changes as a result of the AAST/ACS-COT clinical protocol are not reflected in this study.4 Finally, providers may have different definitions for ‘low’-risk or ‘high’-risk TBI regarding the timing of initiation in these populations.
Conclusion
A high degree of variability exists in prescribing, dose adjustment, and monitoring practices for VTE prevention in trauma patients. Clinical pharmacists may be helpful on trauma teams to optimize dosing and increase prescribing of guideline-concordant VTE chemoprophylaxis. Future efforts should focus on implementation of guideline recommendations into practice, aligning practice recommendations among surgical specialties to rectify inconsistencies, and promoting the importance of quality VTE prevention.
Footnotes
Twitter: @Kalexander4218
Contributors: All authors contributed to the methods, interpretation of results and construction of this manuscript. Study design: KA, Y-LLL, MEK, CCB, NP, ERH, DS, AEB, TWC, JDS; data collection: KA; data analysis and interpretation: KA, Y-LLL, MEK, CCB, NP, ERH, DS, AEB, TWC, JDS; writing and critical revision: KA, Y-LLL, MEK, CCB, NP, ERH, DS, AEB, TWC, JDS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: The research of JDS is supported by funding from the American the American College of Surgeons (George H. A. Clowes Memorial Research Career Development Award 2014–2019) and the National Institutes of Health (1 K08 GM109113-01). ERH reports research funding from the Patient-Centered Outcomes Research Institute (PCORI), the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health National Heart, Lung, and Blood Institute (NIH/NHLBI), and the DOD/Army Medical Research Acquisition Activity. All other authors have no other financial disclosures regarding this topic.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study obtained Institutional Review Board approval prior to survey distribution.
References
- 1.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the East practice management guidelines work group. J Trauma 2002;53:142–64. 10.1097/00005373-200207000-00032 [DOI] [PubMed] [Google Scholar]
- 2.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTe in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis. Chest 2012;141:e227S. 10.1378/chest.11-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, Sperry JL, Rizzo AG, Rosen NG, Brasel KJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: a Western trauma association critical decisions algorithm. J Trauma Acute Care Surg 2020;89:971–81. 10.1097/TA.0000000000002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yorkgitis BK, Berndtson AE, Cross A, Kennedy R, Kochuba MP, Tignanelli C, Tominaga GT, Jacobs DG, Marx WH, Ashley DW, et al. American association for the surgery of trauma/american College of surgeons-committee on trauma clinical protocol for inpatient venous thromboembolism prophylaxis after trauma. J Trauma Acute Care Surg 2022;92:597–604. 10.1097/TA.0000000000003475 [DOI] [PubMed] [Google Scholar]
- 5.Tran A, Fernando SM, Carrier M, Siegal DM, Inaba K, Vogt K, Engels PT, English SW, Kanji S, Kyeremanteng K, et al. Efficacy and safety of low molecular weight heparin versus unfractionated heparin for prevention of venous thromboembolism in trauma patients: a systematic review and meta-analysis. Ann Surg 2022;275:19–28. 10.1097/SLA.0000000000005157 [DOI] [PubMed] [Google Scholar]
- 6.Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, Kong A, Lekawa ME, Dolich MO, Cinat ME, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma 2010;68:874–80. 10.1097/TA.0b013e3181d32271 [DOI] [PubMed] [Google Scholar]
- 7.Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F, Dodek P, McIntyre L, Hall R, Heels-Ansdell D, et al. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients*. Crit Care Med 2015;43:401–10. 10.1097/CCM.0000000000000713 [DOI] [PubMed] [Google Scholar]
- 8.Kopelman TR, O’Neill PJ, Pieri PG, Salomone JP, Hall ST, Quan A, Wells JR, Pressman MS. Alternative dosing of prophylactic enoxaparin in the trauma patient: is more the answer? Am J Surg 2013;206:911–5. 10.1016/j.amjsurg.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 9.Berndtson AE, Costantini TW, Lane J, Box K, Coimbra R. If some is good, more is better: an enoxaparin dosing strategy to improve pharmacologic venous thromboembolism prophylaxis. J Trauma Acute Care Surg 2016;81:1095–100. 10.1097/TA.0000000000001142 [DOI] [PubMed] [Google Scholar]
- 10.Costantini TW, Min E, Box K, Tran V, Winfield RD, Fortlage D, Doucet J, Bansal V, Coimbra R. Dose adjusting enoxaparin is necessary to achieve adequate venous thromboembolism prophylaxis in trauma patients. J Trauma Acute Care Surg 2013;74:128–35. 10.1097/TA.0b013e3182788fa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko A, Harada MY, Barmparas G, Chung K, Mason R, Yim DA, Dhillon N, Margulies DR, Gewertz BL, Ley EJ. Association between enoxaparin dosage adjusted by anti–factor Xa Trough level and clinically evident venous thromboembolism after trauma. JAMA Surg 2016;151:1006. 10.1001/jamasurg.2016.1662 [DOI] [PubMed] [Google Scholar]
- 12.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017;80:6–15. 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 13.Nyquist P, Bautista C, Jichici D, Burns J, Chhangani S, DeFilippis M, Goldenberg FD, Kim K, Liu-DeRyke X, Mack W, et al. Prophylaxis of venous thrombosis in neurocritical care patients: an evidence-based guideline: a statement for healthcare professionals from the neurocritical care Society. Neurocrit Care 2016;24:47–60. 10.1007/s12028-015-0221-y [DOI] [PubMed] [Google Scholar]
- 14.Rappold JF, Sheppard FR, Carmichael Ii SP, Cuschieri J, Ley E, Rangel E, Seshadri AJ, Michetti CP. Venous thromboembolism prophylaxis in the trauma intensive care unit: an American association for the surgery of trauma critical care Committee clinical consensus document. Trauma Surg Acute Care Open 2021;6:e000643. 10.1136/tsaco-2020-000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haut ER, Schneider EB, Patel A, Streiff MB, Haider AH, Stevens KA, Chang DC, Neal ML, Hoeft C, Nathens AB, et al. Duplex ultrasound screening for deep vein thrombosis in asymptomatic trauma patients: a survey of individual trauma surgeon opinions and current trauma center practices. J Trauma 2011;70:27–34. 10.1097/TA.0b013e3182077d55 [DOI] [PubMed] [Google Scholar]
- 16.Teichman AL, Cotton BA, Byrne J, Dhillon NK, Berndtson AE, Price MA, Johns TJ, Ley EJ, Costantini T, Haut ER. Approaches for optimizing venous thromboembolism prevention in injured patients: findings from the consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg 2023;94:469–78. 10.1097/TA.0000000000003854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhillon NK, Haut ER, Price MA, Costantini TW, Teichman AL, Cotton BA, Ley EJ. Novel therapeutic medications for venous thromboembolism prevention in trauma patients: findings from the consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg 2023;94:479–83. 10.1097/TA.0000000000003853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schellenberg M, Costantini T, Joseph B, Price MA, Bernard AC, Haut ER. Timing of venous thromboembolism prophylaxis initiation after injury: findings from the consensus conference to implement optimal VTe prophylaxis in trauma. J Trauma Acute Care Surg 2023;94:484–9. 10.1097/TA.0000000000003847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandle J, Shackford SR, Sise CB, Knudson MM, CLOTT Study Group . Variability is the standard: the management of venous thromboembolic disease following trauma. J Trauma Acute Care Surg 2014;76:213–6. 10.1097/TA.0b013e3182aa2fa9 [DOI] [PubMed] [Google Scholar]
- 20.Correa VC, Lugo-Agudelo LH, Aguirre-Acevedo DC, Contreras JAP, Borrero AMP, Patiño-Lugo DF, Valencia DAC. Individual, health system, and contextual barriers and facilitators for the implementation of clinical practice guidelines: a systematic metareview. Health Res Policy Syst 2020;18:74. 10.1186/s12961-020-00588-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratnasekera A, Geerts W, Haut ER, Price M, Costantini T, Murphy P. Implementation science approaches to optimizing venous thromboembolism prevention in patients with traumatic injuries: findings from the 2022 consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg 2023;94:490–4. 10.1097/TA.0000000000003850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeill SE, Alexander KM, Ladd K, Liu‐DeRyke X, Smith C, Hobbs B. A survey of critical care pharmacists on venous thromboembolism prophylaxis dosing practices with enoxaparin in adult trauma patients. J Am Coll Clin Pharm 2023;1:8. 10.1002/jac5.1758 [DOI] [Google Scholar]
- 23.Lat I, Paciullo C, Daley MJ, MacLaren R, Bolesta S, McCann J, Stollings JL, Gross K, Foos SA, Roberts RJ, et al. Position paper on critical care pharmacy services: 2020 update. Crit Care Med 2020;48:e813–34. 10.1097/CCM.0000000000004437 [DOI] [PubMed] [Google Scholar]
- 24.Haut ER, Byrne JP, Price MA, Bixby P, Bulger EM, Lake L, Costantini T. Proceedings from the 2022 consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg 2023;94:461–8. 10.1097/TA.0000000000003843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tsaco-2022-001070supp001.pdf (271.7KB, pdf)