Summary
Background
Bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) is a single-tablet regimen recommended for HIV-1 treatment. The safety and efficacy of B/F/TAF as initial therapy was established in two Phase 3 studies: 1489 (vs dolutegravir [DTG]/abacavir/lamivudine) and 1490 (vs DTG + F/TAF). After 144 weeks of randomized follow-up, an open-label extension evaluated B/F/TAF to 240 weeks.
Methods
Of 634 participants randomized to B/F/TAF, 519 completed the double-blinded treatment, and 506/634 (80%) chose the 96-week open-label B/F/TAF extension, which was completed by 444/506 (88%) participants. Efficacy was based on the secondary outcome of the proportion of participants with HIV-1 RNA <50 copies/mL at Week 240 by missing = excluded and missing = failure methods. All 634 participants who were randomized to B/F/TAF and received at least one dose of B/F/TAF were included in efficacy and safety analyses. (Study 1489: ClinicalTrials.govNCT02607930; EudraCT 2015-004024-54. Study 1490: ClinicalTrials.govNCT02607956; EudraCT 2015-003988-10).
Findings
Of those with available virologic data, 98.6% (95% CI [97.0%–99.5%], 426/432) maintained HIV-1 RNA <50 copies/mL at Week 240 (missing = excluded); when missing virologic data were considered as failure, 67.2% (95% CI [63.4%–70.8%], 426/634) maintained HIV-1 RNA <50 copies/mL. Mean (SD) change in CD4+ count from baseline was +338 (236.2) cells/μL. No treatment-emergent resistance to B/F/TAF was detected. Adverse events led to drug discontinuation in 1.6% (n = 10/634) of participants (n = 5 with events considered drug-related). No discontinuations were due to renal adverse events. Median (IQR) total cholesterol increased 21 (1,42) mg/dL from baseline; the change in total cholesterol:HDL was 0.1 (−0.5,0.6). Median (IQR) weight change from baseline was +6.1 kg (2.0, 11.7) at Week 240. In Study 1489, hip and spine bone mineral density mean percent changes from baseline were ≤0.6%.
Interpretation
Through 5 years of follow-up, B/F/TAF maintained high rates of virologic suppression with no treatment-emergent resistance and rare drug discontinuations due to adverse events. These results demonstrate the durability and safety of B/F/TAF in people with HIV.
Funding
Gilead Sciences.
Keywords: Antiretroviral therapy, Integrase strand transfer inhibitor, Long-term, Renal safety, Bone safety
Research in context.
Evidence before this study
The combination of bictegravir, emtricitabine, and tenofovir alafenamide for initial treatment of HIV-1 has showed non-inferiority to standard-of-care regimens and a similar renal, bone, and lipid safety profile to comparators after 144 weeks. Longer-term data remain necessary to inform clinical care.
Added value of this study
In an integrated analysis of two phase 3 registrational studies evaluating the safety and efficacy of bictegravir, emtricitabine, and tenofovir alafenamide as initial therapy, after 240 weeks 98.6% (426/432) of those with available virologic data maintained HIV-1 RNA <50 copies/mL (missing = excluded) and 67.2% (426/634) did when missing virologic data were considered as failure (missing = failure). No treatment-emergent resistance to bictegravir, emtricitabine, and tenofovir alafenamide was detected through 240 weeks. After an initial small decline, stability in estimated glomerular filtration rate was observed, and there were no cases of proximal renal tubulopathy or renal-related adverse events through 5 years. Longitudinal changes in spine and hip bone mineral density from baseline were small, with mean percent decreases of 0.23% for spine and 0.29% for hip over 5 years.
Implications of all the available evidence
Coformulated bictegravir, emtricitabine, and tenofovir alafenamide can be administered once daily and is guideline-recommended for treating HIV, including for rapid initiation of therapy. Data from this integrated analysis extend findings from analyses of bictegravir, emtricitabine, and tenofovir alafenamide in HIV treatment-naïve individuals out to 5 years and provide long-term efficacy and safety data to guide treatment decisions for persons living with HIV.
Introduction
Integrase strand transfer inhibitors (INSTIs) are recommended in initial antiretroviral therapy for HIV-1 infection in multiple guidelines including the Department of Health and Human Services, International Antiviral Society-USA, and European AIDS Clinical Society.1, 2, 3 The recommended INSTIs, bictegravir (B or BIC) and dolutegravir (DTG), have excellent efficacy and tolerability, once-daily dosing, few drug interactions, and high barriers to resistance.
BIC is available as a coformulated single-tablet regimen with tenofovir alafenamide (TAF) and emtricitabine (F or FTC). This combination, B/F/TAF, was compared to two guidelines-recommended DTG-based 3-drug regimens when these studies were initiated in two Phase 3 randomized, double-blind, active-controlled trials.4,5 B/F/TAF was compared to DTG/abacavir/lamivudine4 (DTG/ABC/3TC) in one study and to DTG plus F/TAF in the other5; both studies found B/F/TAF to be non-inferior to the DTG-based regimens, with high rates of viral suppression and no treatment-emergent resistance through 144 weeks.4, 5, 6, 7, 8
After all participants completed at least 144 weeks of blinded study drug, unblinding occurred, and participants were offered the opportunity to switch to open-label B/F/TAF for an additional 96 weeks, totaling 240 weeks of follow-up on study. In order to inform the safety and efficacy profile of B/F/TAF over 5 years, here we report the results of this long-term study for those originally assigned to B/F/TAF, including the percentage of participants randomized to B/F/TAF who maintained HIV-1 suppression <50 copies/mL, emergent resistance, adverse events, CD4+ cell counts response, renal and bone biomarkers, and weight change over the 240 weeks of follow-up.
Methods
Study design and participants
The two randomized, double-blind, active-controlled Phase 3 studies (GS-US-380-1489 and GS-US-380-1490) were conducted in 10 countries. Detailed methods have been previously published.4,5 Both studies enrolled adults living with HIV-1 with no prior treatment experience and HIV-1 RNA viral levels ≥500 copies/mL. Participants in Study 1489 were negative for hepatitis B virus. Chronic hepatitis B infection was permitted in Study 1490 because both study regimens included F/TAF. Participants in Studies 1489 and 1490 had an estimated glomerular filtration rate (eGFRCG) of at least 50 and 30 mL/min (Cockcroft–Gault equation), respectively. Both studies required virological resistance testing showing sensitivity to FTC, tenofovir, and ABC. Baseline INSTI resistance testing was not required.
In study 1489, participants were randomized (1:1) to once daily treatment with either B/F/TAF (50/200/25 mg) or DTG/ABC/3TC (50/600/300 mg), stratified by HIV-1 RNA level and CD4+ T cell count as well as by region (USA or non-USA). The primary efficacy analysis was noninferiority of B/F/TAF compared to DTG/ABC/3TC for the proportion with HIV-1 RNA <50 copies/mL at Week 48.4
Similarly, in study 1490 participants were randomized (1:1) to once daily treatment with B/F/TAF (50/200/25 mg) or DTG 50 mg + F/TAF (200/25 mg), stratified by HIV-1 RNA level and CD4+ T cell count, as well as by region (USA vs. non-USA). The primary efficacy analysis was noninferiority of B/F/TAF compared with DTG + F/TAF for the proportion with HIV-1 RNA <50 copies/mL at Week 48.5
For both studies, 48-week,4,5 96-week6,7 and 144-week8 efficacy and safety data have previously been reported. After 144 weeks, all participants in both studies were offered an optional 96-week study extension with open-label B/F/TAF, with study visits occurring every 12 weeks. To summarize long-term outcomes for B/F/TAF, this analysis focuses on participants from both studies who were originally assigned to B/F/TAF through 240 weeks (144 weeks of blinded phase + 96 weeks of open-label phase), i.e., 5 years of treatment.
The studies were undertaken in accordance with the Declaration of Helsinki and were approved by central or site-specific review boards or ethics committees. All participants provided written informed consent. Study 1489: ClinicalTrials.gov NCT02607930; EudraCT 2015-004024-54. Study 1490: ClinicalTrials.gov NCT02607956; EudraCT 2015-003988-10.
Procedures
In both studies laboratory tests included haematological analyses, serum chemistry tests and eGFRCG (Covance Laboratories, Indianapolis, IN, USA), fasting lipids, CD4+ T cell counts, and HIV-1 RNA plasma concentration (Roche TaqMan 2.0; Roche Diagnostics, Rotkreuz, Switzerland). HIV-1 protease and reverse transcriptase genotyping were assessed at screening (Monogram Biosciences; San Francisco, CA, USA).9 Baseline HIV-1 integrase genotyping was retrospectively performed by deep sequencing (Seq-IT; Kaiserslautern, Germany). Post-baseline resistance testing (Monogram Biosciences; San Francisco, CA, USA) was performed on samples from participants who had HIV-1 RNA ≥50 copies/mL with a confirmed HIV-1 RNA of at least 200 copies/mL at or after Week 8 or who had an HIV-1 RNA of ≥200 copies/mL at Weeks 48, 96, or 144 or the last visit on randomized treatment after Week 8 and who did not subsequently re-suppress HIV-1 RNA to <50 copies/mL while on treatment.
Safety was assessed by physical examinations, laboratory tests, 12-lead electrocardiograms, concomitant medications, and evaluation of adverse events, which were coded using the Medical Dictionary for Regulatory Activities (MedDRA, version 24.0). Investigators indicated the relatedness of adverse events to study medications in a binary manner (yes or no). Dual-energy x-ray absorptiometry scans were also performed in study 1489 to measure hip and lumbar spine bone mineral density. All scans were read at a centralised centre that was masked to treatment group assignment (BioClinica, Newtown, Pennsylvania, USA).
Outcomes
In both studies the secondary efficacy outcomes at Week 240 (144 weeks of blinded phase plus 96 weeks of open-label phase) were the percentages of participants with available data with virologic suppression (HIV-1 RNA <50 copies/mL) at Week 240 using missing = excluded and missing = failure methods. Other secondary efficacy outcomes included changes from baseline in CD4+ cell count at Week 240. Percentage of participants with virologic response based on initial CD4+ levels was also evaluated.
In Study 1489, the percentage changes from baseline in hip and lumbar spine bone mineral density were assessed at Week 240. Renal safety assessments included the change from baseline in serum creatinine and eGFRCG at Week 240. Adverse event incident rates through Week 240 and changes in fasting lipids at Week 240 were assessed.
Statistical analysis
634 participants who were randomized to B/F/TAF and received at least one dose of B/F/TAF were included in efficacy and safety analyses. We assessed plasma HIV-1 RNA less than 50 copies per mL at Week 240 (between Days 1639 and 1722, inclusive). Baseline characteristics and efficacy and safety outcomes were analyzed using descriptive statistics. We used SAS® Software Version 9.4 (SAS Institute Inc., Cary, NC, U.S.) for all analyses.
Role of the funding source
The funder of the study had a role in study design, data collection, data analysis, data interpretation, and writing the report.
Results
Participant characteristics and disposition
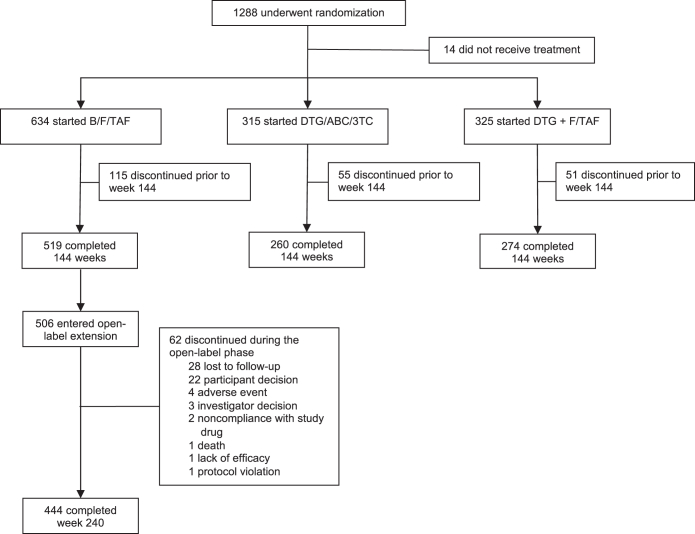
Screening for both studies began in November 2015, with final participant visits for the 240-week analysis occurring in July 2021. In all, 634 participants were randomized to and started treatment with B/F/TAF; 506/634 (80%) entered the optional open-label extension phase; and 444/506 (88%) completed 240 weeks of treatment (Fig. 1). In the combined analysis, 565/634 (89.1%) were male, the median age at baseline was 32 years (range, 18–71 years), one-third were Black, and one-quarter were Latinx (Table 1). At baseline, there were 119/634 (18.8%) that had HIV-1 RNA >100,000 copies/mL and 80/634 (12.6%) with a CD4+ T cell count <200 cells/μL.
Fig. 1.
Participant flow diagram. B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; DTG/ABC/3TC, dolutegravir/abacavir/lamivudine.
Table 1.
Baseline demographic and disease characteristics.
| B/F/TAF 240 weeks n = 634 |
|
|---|---|
| Median age, y (range) | 32 (18–71) |
| Sex at birth, n (%) | |
| Male | 565 (89.1) |
| Female | 69 (10.9) |
| Race, n (%) | |
| White | 363 (57.2) |
| Black or African descent | 211 (33.3) |
| Asian | 13 (2.1) |
| Ethnicity, n (%) | |
| Hispanic/Latinx | 155 (24.4) |
| HIV disease status, n (%) | |
| Asymptomatic | 572 (90.2) |
| Symptomatic | 26 (4.1) |
| AIDS | 36 (5.7) |
| HIV-1 RNA, median (IQR) log10 copies/mL | 4.4 (4.0, 4.9) |
| HIV-1 RNA >100,000 copies/mL, n (%) | 119 (19) |
| CD4+ cells/μL, median (IQR) | 442 (293, 590) |
| CD4+ count, n (%) | |
| <200 cells/μL | 80 (12.6) |
| ≥200–<500 cells/μL | 314 (49.5) |
| ≥500 cells/μL | 240 (37.9) |
| eGFRCG, median (IQR) mL/min | 122 (104, 143) |
| Body weight, median (IQR) kg | 77 (68, 88) |
| Body mass index, median (IQR) kg/m2 | 25.1 (22.3, 28.6) |
| Primary resistance-associated mutations, n (%)a | |
| INSTI | 7 (1.1) |
| NRTI | 16 (2.5) |
| NNRTI | 77 (12.1) |
| PI | 16 (2.5) |
| HIV-HBV coinfected, n (%) | 8 (1.3) |
| HIV-HCV coinfected, n (%) | 5 (0.8) |
B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; eGFRCG, estimated glomerular filtration rate using the Cockcroft-Gault formula; HBV, hepatitis B virus; HCV, hepatitis C virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NRTI, nucleotide reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Primary INSTI substitutions observed were T97A (n = 6) and Q148H (n = 1). Primary NRTI substitutions observed were M41L (n = 4), D67N (n = 3), K70R (n = 2), L74V (n = 1), and K219N/Q/R (n = 9). Primary NNRTI substitutions observed were L100I (n = 3), K101E (n = 5), K103 N/S (n = 41), E138 A/G/K/Q (n = 25), Y181C (n = 3), Y188L (n = 1), G190 A/S (n = 5), H221Y (n = 1), P225H (n = 3), and M230I (n = 1). Primary PI substitutions observed were D30N (n = 2), V32I (n = 1), M46I/L (n = 6), I50L (n = 1), Q58E (n = 3), V82 A/L (n = 3), L90M (n = 3).
Of the 506 participants randomized to B/F/TAF who entered the open-label extension, 62/506 (12.3%) discontinued B/F/TAF during the 96-weeks of open-label treatment (approximately 6% discontinuations annually), compared with 115/634 (18.1%) in the first 144 weeks (also about 6% annual discontinuation rate) when the studies were double-blinded. Reasons for B/F/TAF discontinuation in the open-label extension included loss to follow-up (n = 28), participant decision (n = 22), adverse event (n = 4), investigator decision (n = 3), non-adherence with study drug (n = 2), death (n = 1), lack of efficacy (n = 1), and protocol violation (n = 1).
Efficacy
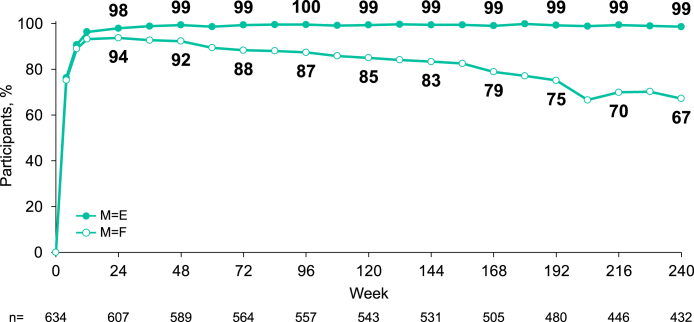
At Week 240, among 432 participants with available virologic data, 98.6% (95% CI [97.0%–99.5%], 426/432) had HIV-1 RNA <50 copies/mL (missing = excluded). Using a missing = failure analysis at Week 240, among 634 participants who initiated B/F/TAF treatment, 67.2% (95% CI [63.4%–70.8%], 426/634) maintained HIV-1 RNA <50 copies/mL (M = F); for the remaining 208 participants, 202 were no longer in follow-up or had missing virologic data, and 6 had HIV-1 RNA ≥50 copies/mL. Three of these 6 participants had confirmed virologic failures, and 1 had HIV-1 RNA >200 copies/mL at the last study visit, which all occurred during the open-label extension. Among those with baseline CD4+ <200 cells/μL, 49/50 (98.0%) had HIV-1 RNA <50 copies/mL at Week 240 (missing = excluded). Most participants treated with B/F/TAF achieved <50 copies/mL of HIV-1 RNA by Week 8 (Fig. 2).
Fig. 2.
Virologic outcomes through 240 weeks (missing = excluded and missing = failure). Percent of participants with plasma HIV-1 RNA <50 copies/mL; n = participants with non-missing HIV-RNA value.
At Week 240, the mean (SD) CD4+ count among 415 participants evaluated was 785 (282.4) cells/μL. The mean (SD) change from baseline was an increase of 338 cells/μL (236.2).
Resistance analyses
Nine participants met criteria for resistance testing, none of whom developed resistance to any component of B/F/TAF in the final resistance analysis population. Of the 9, 7 participants discontinued (investigator discretion/participant decision) and 2 were lost to follow-up.
Safety
The median time of study follow-up was 252.3 weeks. Among all participants who initiated B/F/TAF, the most common adverse events were diarrhea (136/634; 21.5%), headache (117/634; 18.5%), and nasopharyngitis (115/634; 18.1%) (Table 2). Adverse events considered by the investigator to be related to B/F/TAF occurred in 178/634 (28.1%) participants, with 9/634 (1.4%) experiencing a Grade 3 or 4 study drug-related adverse event. The most common study drug-related adverse events were headache (31/634; 4.5%) and diarrhea (30/634; 4.7%), most frequently occurring in the first 24 weeks and then waning over time. Among B/F/TAF-treated participants, 10/634 (1.6%) experienced an adverse event leading to drug discontinuation, 6 and 4 during the randomized and open-labelled phases, respectively. Five of these participants discontinued due to an adverse event considered related to study drug. Serious adverse events (SAE) occurred in 136/634 (21.5%), with 5 or more participants experiencing individual SAEs of cellulitis (8/634; 1.2%), appendicitis (6/634; 0.9%), pneumonia (5/634; 0.8%), and suicide attempt (5/634; 0.8%). Five participants (5/634; 0.8%) experienced a study-drug related SAE, including chest pain, generalized tonic-clonic seizure, spontaneous abortion, and suicide attempt (each in a single participant), as well as dizziness, acute pancreatitis, and atrial flutter (all 3 in a single participant).
Table 2.
Adverse events through week 240.
| No. (%) of participants reporting | B/F/TAF |
|
|---|---|---|
| Overall population (Baseline to Week 240) n = 634 | Open-label extension populationa n = 506 | |
| Any adverse event | 604 (95.3) | 416 (82.2) |
| Adverse events present in ≥10% in overall population | ||
| Diarrhea | 136 (21.5) | 21 (4.2) |
| Headache | 117 (18.5) | 24 (4.7) |
| Nasopharyngitis | 115 (18.1) | 37 (7.3) |
| Upper respiratory tract infection | 107 (16.9) | 31 (6.1) |
| Syphilis | 105 (16.6) | 42 (8.3) |
| Back pain | 91 (14.4) | 33 (6.5) |
| Arthralgia | 90 (14.2) | 29 (5.7) |
| Cough | 82 (12.9) | 29 (5.7) |
| Nausea | 81 (12.8) | 16 (3.2) |
| Fatigue | 70 (11.0) | 11 (2.2) |
| Insomnia | 69 (10.9) | 16 (3.2) |
| Anxiety | 68 (10.7) | 20 (4.0) |
| Influenza | 66 (10.4) | 20 (4.0) |
| Grade 3 or 4 adverse events | 132 (20.8) | |
| Serious adverse events | 136 (21.5) | |
| Study-drug related adverse events | 178 (28.1) | |
| Study-drug related adverse events (present in ≥2%) | ||
| Headache | 31 (4.7) | |
| Diarrhea | 30 (4.7) | |
| Nausea | 28 (4.4) | |
| Fatigue | 17 (2.7) | |
| Dizziness | 15 (2.4) | |
| Insomnia | 13 (2.1) | |
| Study-drug related serious adverse eventsb | 5 (0.8) | |
| Any adverse event leading to study drug discontinuationc | 10 (1.6) | |
| Any study-drug related adverse event leading to discontinuation | 5 (0.8) | |
| Deathd | 9 (1.4) | |
COVID-19 was reported in 50 participants (9.9%) in the open-label extension population.
Chest pain (n = 1), generalized tonic-clonic seizures (n = 1), spontaneous abortion (n = 1), suicide attempt (n = 1), each occurring in a single participant. Acute pancreatitis (n = 1), dizziness (n = 1), and atrial flutter (n = 1), all in a single participant.
Cardiac arrest (n = 1), chest pain (n = 1), ∗ abdominal distension (n = 1), ∗ paranoia (n = 1), depression (n = 1), ∗ intervertebral discitis (n = 1), toxicity to various agents (n = 1), obesity (n = 1), ∗ and COVID-19 (n = 1), each occurring in a single participant. Sleep disorder, ∗ dyspepsia, ∗ tension headache,∗ depressed mood, ∗ and insomnia,∗ all occurred in 1 participant.∗Considered drug-related.
Cardiac arrest following appendicitis and septic shock (day 28, n = 1), gastric adenocarcinoma (day 376, n = 1), hypertensive heart disease and congestive heart failure (day 412, n = 1), recreational drug overdose (days 771 and 1549, n = 2), suicide (day 656, n = 1), sudden cardiac death (day 1060, n = 1), unknown cause (day 1697, n = 1), and COVID-19 (day 1759, n = 1).
The most common Grade 3 or 4 laboratory abnormalities were increased creatinine kinase (n = 22) and increased fasting low-density lipoprotein (LDL; n = 11). The elevations in creatinine kinase commonly occurred postexercise, were asymptomatic, and were considered clinically insignificant by the investigator. Eight and seven participants had aspartate transaminase and alanine transaminase elevations, respectively, but no drug-related hepatitis cases were reported. Eight participants had amylase elevations. One case of pancreatitis occurring on Day 572 was attributed to study drug per the investigator, and this resolved 2 days later without discontinuation of B/F/TAF.
Lipids
Median lipid (Q1, Q3) changes from baseline to Week 240 were 21 (1, 42) mg/dL for total cholesterol, 19 (2, 40) mg/dL for low density lipoprotein (LDL) cholesterol, 4 (−2, 11) mg/dL for high density lipoprotein (HDL) cholesterol, and 10 (−16, 46) mg/dL for triglycerides. The median (Q1, Q3) change in total cholesterol:HDL ratio at Week 240 was 0.1 (−0.5, 0.6) mg/dL. There were 47/634 (7.4%) participants who initiated lipid-lowering agents between baseline and Week 240, and about 1–2% per year of study.
Renal
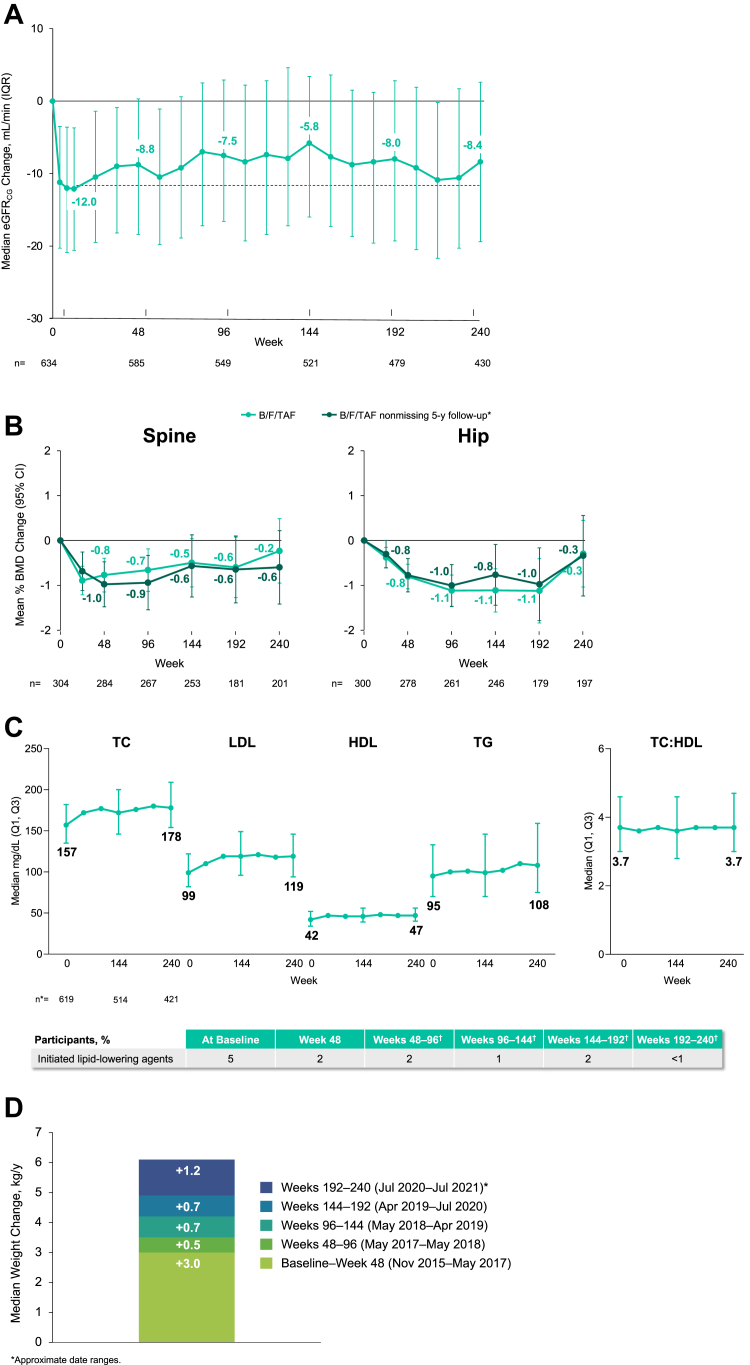
Through 240 weeks of B/F/TAF treatment, there were no reported cases of proximal renal tubulopathy or discontinuations due to renal adverse events during 2635.6 person-years of follow-up (incidence rate: 0 per 100 person-years, 95% CI; 0–0.140). Consistent with BIC inhibition of organic cation transporter-2 and tubular creatinine secretion, small declines in eGFRCG occurred in the first 4 weeks after B/F/TAF initiation.8 The median (Q1, Q3) change at Week 240 in eGFRCG was −8.4 (−19.4, 2.6) mL/min (Fig. 3).
Fig. 3.
Changes from baseline in estimated GFRCG, bone mineral density, fasting lipids, and weight through 240 weeks. A. Median change in estimated glomerular filtration rate (eGFRCG). Dotted line indicates median change at week 8. n = numbers of participants with non-missing eGFRCG change values at the visit. Bars indicate interquartile range (IQR). B. Mean Percent Change in Bone Mineral Density (BMD). Measured by dual-energy x-ray absorptiometry in Study 1489 only; n = numbers for participants with non-missing % BMD change values at the visit. ∗151 participants with spine and 147 with hip bone mineral density (BMD) data from baseline through Week 240 with non-missing follow-up. Bars indicate 95% confidence interval (CI). C. Median Change in Fasting Lipids (mg/dL). n = number of participants with non-missing metabolic assessment values at the visit. ∗For all measures, n's were 619 at Week 0, 514 at Week 144, and 421 at Week 240. †Calculated as differences from Weeks 48–96, 96 to 144, 144 to 192, and 192 to 240. HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides. Bars indicate interquartile range (IQR). D. Median Weight Change, kg/year. ∗Approximate date ranges. A. Median Change in Estimated Glomerular Filtration Rate (eGFRCG). B. Mean Percent Change in Bone Mineral Density (BMD). C. Median Change in Fasting Lipids (mg/dL). D. Median Weight Change, kg/year.
Bone
As described previously, initial declines in mean spine and hip bone mineral density were observed during the first year of the double-blinded phase, with a mean percent change from baseline of −0.37% for spine and −1.02% for hip at Week 144.8 Additional follow-up from the open-label phase showed a mean percent change from baseline of −0.23% in spine and −0.29% in hip at Week 240 (Fig. 3). The majority of participants had less than a 3% change (increase or decrease) in hip BMD: 28.4% of participants had >0% to <3% decreases and 26.4% of participants had ≥0% to <3% increases in hip BMD at Week 240, while 39.7% had ≥3% decreases and 20.7% had ≥3% increases. An additional BMD analysis looking only at non-missing data through Week 240 showed longitudinal trends that did not exceed 0.6% change from baseline in spine or hip (Fig. 3).
Weight
Median weight change from baseline to Week 240 was +6.1 (interquartile range, IQR; 2.0, 11.7) kg. The greatest change was in Year 1, with a median increase of 3.0 kg (IQR; 0.3, 5.8), followed by a 0.5–1.2 kg change per year (Fig. 3).
Discussion
Until there is a viable curative strategy, HIV therapy will need to be lifelong. As a result, long-term studies of the safety and efficacy of antiretroviral regimens are necessary. In this integrated analysis of two large Phase 3 studies in persons who initiated B/F/TAF for HIV-1 infection, we observed virologic suppression in 98.6% (426/432) of participants who continued on assigned treatment and 1.4% (6/432) with virologic failure (missing = excluded), with no cases of treatment-emergent resistance and <1% (5/634) study-drug related adverse events leading to discontinuation through 5 years of follow-up. Of the entire population who initiated B/F/TAF, including those who discontinued prior to 5 years, 67.2% (426/634) had documented virologic suppression through 5 years (missing = failure). For those who were lost to follow-up, it is unknown how many continued to experience virologic suppression. Importantly, no unanticipated safety signals emerged with long-term treatment with B/F/TAF.
Tenofovir disoproxil fumarate can cause renal toxicity and loss of bone density, especially with long-term use. Tenofovir alafenamide, the component used in B/F/TAF, has substantially lower plasma concentrations and little observed renal or bone toxicity in prospective clinical trials or observational studies.10, 11, 12, 13 From a renal safety perspective, stability in eGFRCG was observed after an initial small decline, consistent with inhibition of tubular creatinine secretion via organic cation transporter-2, and there were no cases of proximal renal tubulopathy or renal-related adverse events through 5 years (2635.6 person-years, incidence rate: 0 per 100 person-years, 95% CI; 0–0.140). Longitudinal changes in spine and hip BMD from baseline were small. After initial declines observed at the initiation of therapy, which was seen with all 3 regimens in Studies 1489 and 1490,4, 5, 6, 7, 8 BMD was stable, with mean percent decreases of 0.23% for spine and 0.29% for hip over 5 years, consistent with the general population without HIV-1.14
The small changes in fasting lipids reported in the randomized phase were similar in long-term follow-up, including stable TC:HDL ratios, and few participants (≤2% per year) initiated lipid-lowering agents between Week 48 and 240. Weight gain after HIV treatment initiation includes a component of return to health as HIV-1 replication is stopped, immunologic recovery occurs, and potential HIV-related symptoms reversed. However, for regimens such as B/F/TAF that include an INSTI and do not have TDF (which suppresses weight), additional weight gain has been observed.15, 16, 17 The observed average weight gain in participants treated with long-term B/F/TAF is consistent with these other trials, with most participants experiencing the greatest weight in the first year after initiating treatment. Weight gain in subsequent years is generally consistent with that seen in the general population,18 but with some outliers gaining excess weight independent of HIV status; the mechanism behind HIV-treatment associated excess weight gain remains unknown. In our study, there was additional weight gain concurrent with the onset of the COVID-19 pandemic, reflective of population-based changes.19
Clinical trials of novel HIV therapies generally follow participants through to standard primary and secondary endpoints, i.e., 48 and 96 weeks, respectively. Thus, these results demonstrating durable efficacy, safety, and tolerability of B/F/TAF through 5 years of follow-up are clinically important, as they reinforce the findings from the group that was randomized to B/F/TAF through Week 144. Our analysis of the safety data focused on those remaining in the study for the entire 5-year period, as our goal was to demonstrate the effects of long-term exposure to this regimen.
For both studies included in this analysis, it is unclear whether similar efficacy would be seen in real-world populations initiating HIV treatment, including individuals who have eGFR <30 mL/min where B/F/TAF is not recommended except in those with end-stage renal disease on hemodialysis; however, analyses of real-world studies indicate effectiveness of B/F/TAF is similar in clinical practice as in phase 3 studies.20 In comparison to the blinded phase, the open-label phase was optional for investigators and participants, leading to the potential for selection bias. Most participants (79.8%, 506/634) completed the blinded phase and enrolled in the open-label extension, and there were high rates of continued study follow-up throughout despite the concurrent COVID-19 pandemic, which occurred in the final year of the protocol and triggered restrictions that affected clinical services. In addition, by its nature the open-label phase may also have altered the reporting or attribution of events by participants or investigators. However, previously reported 3 years of blinded comparison data in the longitudinal analysis of safety helps to prevent subjective bias (i.e., reporting bias) in this dataset, particularly in comparison to observational studies on longitudinal outcomes. Notably, measures of efficacy, safety, and tolerability in the open-label extension were similar to those in the blinded phase, inclusive of people who initiated treatment with high viral loads and/or low CD4 counts at baseline.21
In conclusion, treatment of HIV-1 with B/F/TAF over 5 years achieved and maintained high levels of viral suppression without occurrence of drug resistance. B/F/TAF was well tolerated, with few drug-related treatment discontinuations, expected changes in eGFRCG, and minimal impact on total cholesterol:HDL ratio and bone mineral density. These finding support the use of B/F/TAF as a durable and safe long-term HIV treatment.
Contributors
Hailin Huang and Hal Martin contributed to the conception and design of the study. Paul Sax, Jose Arribas, Chloe Orkin, Adriano Lazzarin, Anton Pozniak, Edwin DeJesus, Franco Maggiolo, Hans-Jürgen Stellbrink, and Yazdan Yazdanpanah contributed to the collection of data. Paul Sax and Hailin Huang accessed and verified the data. All authors contributed to the interpretation of data and drafting or revising the manuscript. All authors approved the final version of the manuscript.
Data sharing statement
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science's discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
Declaration of interests
Paul Sax has received grants or contracts support from Gilead Sciences and ViiV Healthcare; consulting fees from Gilead Sciences, ViiV Healthcare, Janssen Pharmaceuticals, and Merck; and has served on board(s) for Merck. José R. Arribas has received grants or contracts and support for travel from ViiV Healthcare and Gilead Sciences, consulting fees from ViiV Healthcare, Gilead Sciences, MSD, Janssen Pharmaceuticals, and Aelix, and speaking honoraria from ViiV Healthcare, Gilead Sciences, and MSD; Chloe Orkin has received grants or contracts from Gilead Sciences, MSD, GSK, Janssen Pharmaceuticals, ViiV Healthcare, and AstraZeneca, speaking honoraria from Gilead Sciences, MSD, GSK, Janssen Pharmaceuticals, and ViiV Healthcare, and travel support from ViiV Healthcare and has served as president of the Medical Womens Federation and a governing council member for the International AIDS Society; Adriano Lazzarin and Yazdan Yazdanpanah have declared no interests; Anton Pozniak has received grant support from Gilead Sciences, Janssen Pharmaceuticals, ViiV Healthcare, and Merck, consulting fees from ViiV Healthcare, Merck, and Gilead Sciences, speaking honoraria from Gilead Sciences, Janssen Pharmaceuticals, ViiV Healthcare, and Merck, and travel support from Gilead Sciences and has served on boards for MRC Penta Studies, BHIV A Guidelines, and EACS Guidelines. Edwin DeJesus has participated in clinical trials for Gilead Sciences, ViiV Healthcare, Merck, AbbVie, and TheraTechnologies/Taimed. Franco Maggiolo has received consulting fees from ViiV Healthcare, Gilead Sciences, MSD, and Janssen Pharmaceuticals. Hans-Jürgen Stellbrink has received grants or contracts support, consulting fees, and speaking honoraria from Gilead Sciences, ViiV Healthcare, Janssen-Cilag, and MSD Sharp & Dohme; travel support from Gilead Sciences; has served on boards for Gilead Sciences, ViiV Healthcare, and MSD Sharp & Dohme; and has received non-financial support for either equipment, materials, drugs, medical writing, gifts, or other services from Gilead Sciences. Rima Acosta, Hailin Huang, Jason T. Hindman, Hal Martin, and Jared M. Baeten are current or former employees of Gilead Sciences and hold stock in the company. David Wohl has received support for the present manuscript from Gilead Sciences, grants or contracts support from Gilead Sciences, ViiV Healthcare, and Merck; and consulting fees and speaking honoraria from Gilead Sciences, ViiV Healthcare, Janssen Pharmaceuticals, and Merck.
Acknowledgements
This study was sponsored by Gilead Sciences. We thank the study participants, all participating investigators and support staff, and the Gilead Sciences study staff.
Writing assistance was provided by Jennifer King, PhD, on behalf of Gilead Sciences. Clinical virology scientific support was provided by Kristen Andreatta, MS, and additional biostatistics support was provided by Cindy Liu, PhD, both of Gilead Sciences.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101991.
Appendix A. Supplementary data
References
- 1.Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services; 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv Available at: [Google Scholar]
- 2.Saag M.S., Gandhi R.T., Hoy J.F., et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324(16):1651–1669. doi: 10.1001/jama.2020.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryom L., De Miguel R., Cotter A.G., et al. Major revision version 11.0 of the European AIDS clinical society guidelines 2021. HIV Med. 2022;23(8):849–858. doi: 10.1111/hiv.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallant J., Lazzarin A., Mills A., et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–2072. doi: 10.1016/S0140-6736(17)32299-7. [DOI] [PubMed] [Google Scholar]
- 5.Sax P.E., Pozniak A., Montes M.L., et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–2082. doi: 10.1016/S0140-6736(17)32340-1. [DOI] [PubMed] [Google Scholar]
- 6.Wohl D.A., Yazdanpanah Y., Baumgarten A., et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355–e363. doi: 10.1016/S2352-3018(19)30077-3. [DOI] [PubMed] [Google Scholar]
- 7.Stellbrink H.J., Arribas J.R., Stephens J.L., et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364–e372. doi: 10.1016/S2352-3018(19)30080-3. [DOI] [PubMed] [Google Scholar]
- 8.Orkin C., DeJesus E., Sax P.E., et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV. 2020;7(6):e389–e400. doi: 10.1016/S2352-3018(20)30099-0. [DOI] [PubMed] [Google Scholar]
- 9.Acosta R.K., Willkom M., Martin R., et al. Resistance analysis of bictegravir-emtricitabine-tenofovir alafenamide in HIV-1 treatment-naive patients through 48 weeks. Antimicrob Agents Chemother. 2019;63(5) doi: 10.1128/AAC.02533-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S.K., Post F.A., Arribas J.R., et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33(9):1455–1465. doi: 10.1097/QAD.0000000000002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggiolo F., Rizzardini G., Raffi F., et al. Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: a multicentre, open-label, phase 3b, randomised trial. Lancet HIV. 2019;6(10):e655–e666. doi: 10.1016/S2352-3018(19)30195-X. [DOI] [PubMed] [Google Scholar]
- 12.Trottier B., Antinori A., De Wet J., et al. HIV Glasgow; 2022. Bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) for the treatment of people living with HIV: 24-month analyses by age, race, adherence and late diagnosis in a multi-country cohort study. Virtual. Poster presentation P067. [Google Scholar]
- 13.Ambrosioni J., Rojas Liévano J., Berrocal L., et al. Real-life experience with bictegravir/emtricitabine/tenofovir alafenamide in a large reference clinical centre. J Antimicrob Chemother. 2022;77(4):1133–1139. doi: 10.1093/jac/dkab481. [DOI] [PubMed] [Google Scholar]
- 14.Alswat K.A. Gender disparities in osteoporosis. J Clin Med Res. 2017;9(5):382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venter W.D.F., Moorhouse M., Sokhela S., et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 16.Sax P.E., Erlandson K.M., Lake J.E., et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2019;71(6):1379–1389. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch B., Akpomiemie G., Chandiwana N., et al. 2023. Weight loss and metabolic changes after switching from TAF/FTC+DTG to TDF/3TC/DTG. Conference on Retroviruses and Opportunistic Infections. Seattle, USA. Abstract 671. [Google Scholar]
- 18.Hill J.O., Wyatt H.R., Reed G.W., Peters J.C. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 19.Chew H.S.J., Lopez V. Global impact of COVID-19 on weight and weight-related behaviors in the adult population: a scoping review. Int J Environ Res Publ Health. 2021;18(4) doi: 10.3390/ijerph18041876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters E., Iwuji A.C. Efficacy, safety and tolerability of Biktarvy in HIV-1 infection: a scoping review. Antivir Ther. 2023;28(1) doi: 10.1177/13596535231159030. [DOI] [PubMed] [Google Scholar]
- 21.Ramgopal M., Wurapa A., Baumgarten A., et al. 2022. 5-year outcomes of bictegravir/emtricitabine/tenofovir alafenamide as initial treatment of HIV-1 in adults with high baseline HIV-1 RNA and/or low CD4 count in two phase 3 randomized clinical trials. IDWeek 2022. Washington, DC, USA. October 19-23. Abstract 1251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.