Abstract
Introduction
We aimed to compare the proportions of patients with newly diagnosed psoriatic arthritis (PsA) and rheumatoid arthritis (RA) remaining on methotrexate (regardless of other disease-modifying antirheumatic drug (DMARD)-changes), and proportions not having started another DMARD (regardless of methotrexate discontinuation), within 2 years of starting methotrexate, as well as methotrexate effectiveness.
Methods
Patients with DMARD-naïve, newly diagnosed PsA, starting methotrexate 2011–2019, were identified from high-quality national Swedish registers and matched 1:1 to comparable patients with RA. Proportions remaining on methotrexate and not starting another DMARD were calculated. For patients with disease activity data at baseline and 6 months, response to methotrexate monotherapy was compared through logistic regression, applying non-responder imputation.
Results
In total, 3642/3642 patients with PsA/RA were included. Baseline patient-reported pain and global health were similar, whereas patients with RA had higher 28-joint scores and evaluator-assessed disease activity. Two years after methotrexate start, 71% of PsA vs 76% of patients with RA remained on methotrexate, 66% vs 60% had not started any other DMARD, and 77% vs 74% had not started specifically a biological or targeted synthetic DMARD. At 6 months, the proportions of patients with PsA versus RA achieving pain-scores ≤15 mm were 26% vs 36%; global health ≤20 mm: 32% vs 42%; evaluator-assessed ‘remission’: 20% vs 27%, with corresponding adjusted ORs (PsA vs RA) of 0.63 (95% CI 0.47 to 0.85); 0.57 (95% CI 0.42 to 0.76) and 0.54 (95% CI 0.39 to 0.75).
Discussion
In Swedish clinical practice, methotrexate use is similar in PsA and RA, both regarding initiation of other DMARDs and methotrexate retention. On a group level, disease activity improved during methotrexate monotherapy in both diseases, although more so in RA.
Keywords: Arthritis, Psoriatic; Arthritis, Rheumatoid; Methotrexate
WHAT IS ALREADY KNOWN ON THIS TOPIC
Methotrexate is widely used as first-line therapy in psoriatic arthritis, despite only weak to moderate evidence supporting its effectiveness in this diagnosis.
WHAT THIS STUDY ADDS
In this nationwide, observational study, patients with newly diagnosed psoriatic arthritis starting methotrexate displayed a similar treatment retention of methotrexate, as well as similar rates of escalation with other disease-modifying antirheumatic drugs, as compared with patients with newly diagnosed rheumatoid arthritis. Disease activity in psoriatic arthritis also decreased during methotrexate monotherapy, although outcomes were not as good as in rheumatoid arthritis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study supports the continued place for methotrexate in the treatment algorithm of psoriatic arthritis.
Introduction
The use of methotrexate in psoriatic arthritis (PsA) was challenged 10 years ago, when a large randomised controlled trial (RCT) failed to demonstrate any statistically significant benefit over placebo.1 A second challenge came with a 2019 RCT (SEAM-PsA), indicating that etanercept was superior to methotrexate, and that combining etanercept with methotrexate conferred no benefit over etanercept monotherapy.2 As a result, the latest American College of Rheumatology (ACR) guidelines bypasses methotrexate and suggests tumour necrosis factor inhibitors as first-line disease modifying antirheumatic drugs (DMARDs) in PsA.3
In contrast, methotrexate is recommended as first-line DMARD for PsA with peripheral arthritis by the European alliance of associations for rheumatology (EULAR).4 The positioning of methotrexate in the EULAR recommendations reflects expert opinion, but also refers to data from trials not primarily designed to compare methotrexate with placebo. Specifically, they too mention the SEAM-PsA RCT, where 51% of patients on methotrexate achieved an ACR20-response (compared with 61% on etanercept monotherapy), and also a treat-to-target-trial (TICOPA), where 27% maintained minimal disease activity (MDA)5 over 48 weeks with methotrexate monotherapy.6 Some support for methotrexate can also be found from smaller studies,7 8 and in the treatment of psoriasis, where RCTs have demonstrated a benefit over placebo.9
A few observational studies have also suggested an effect of methotrexate in PsA.10 11 Lie et al compared the effectiveness and retention of methotrexate in PsA to that in a reference population with rheumatoid arthritis (RA), enrolled in a Norwegian rheumatology register before 2007.11 After 6 months of methotrexate treatment, 24% of PsA and 27% of patients with RA had achieved a 28-joint Disease Activity Score (DAS28) of <2.6. However, since 2007, the treatment strategy in PsA has shifted towards more liberal escalation, and several new therapeutic options have become available. Since there are no further ongoing placebo-controlled trials of methotrexate in PsA,12 and in light of the diverging opinions regarding its effectiveness, in the current work, we aimed to revisit the questions of retention (a commonly used surrogate measure of treatment efficacy in observational studies) and response to methotrexate monotherapy in PsA, when used in clinical practice. To do so, we compared the situation in PsA to that among a matched reference population of patients with RA, where the evidence supporting the efficacy of methotrexate monotherapy is much more robust.13
Our primary objective was to compare the proportions of patients with newly diagnosed PsA or RA who had not started another DMARD within 2 years of their first initiation of methotrexate. Secondary objectives were to compare: (1) the proportions who had specifically not started a biological or targeted synthetic DMARD (b/tsDMARD) within 2 years; (2) the proportions remaining on methotrexate treatment (regardless of starting other DMARDs) and (3) methotrexate monotherapy treatment effectiveness, between PsA and RA.
Methods
Study setting and data sources
This is an observational study using prospectively collected data from national healthcare registers in Sweden. PsA cases and RA comparator-subjects were identified via International Classification of Diseases codes in the National Patient Register (NPR), with an almost complete coverage of inpatient care from 1987 onwards, and since 2001 also encompassing specialised (non-primary) outpatient care. The validity of PsA and RA diagnoses in the NPR is high, with 86% and 91%, respectively, fulfilling classification or clinical criteria.14 15 Clinical data, such as disease activity measures, were retrieved from the Swedish Rheumatology Quality register (SRQ), and prescription dispensation data from the Prescribed Drugs Register (PDR), with a complete national coverage of prescribed drugs dispensed since 2006. Information on place of residence, used for matching, and level of formal education, was collected from the Total Population Register. See legend of table 1 and online supplemental table S1 for definitions and codes used to identify cases, comparator subjects and comorbidities.
Table 1.
Baseline characteristics of PsA cases and matched RA comparators.
| Baseline characteristics | PsA | RA |
| No | 3642 | 3642 |
| Age, mean (SD) | 54.2 (14.1) | 54.5 (14.4) |
| Sex, N (%) men | 1813 (50) | 1813 (50) |
| Days from first PsA/RA diagnosis to start of methotrexate, median (IQR) | 5 (46) | 1 (15) |
| Level of formal education, years | ||
| <10, N (%) | 704 (19) | 837 (23) |
| 10–12, N (%) | 1890 (52) | 1765 (48) |
| >12, N (%) | 1040 (29) | 1025 (28) |
| Missing, N (%) | 8 (0) | 15 (0) |
| Diabetes2, N (%) | 255 (7.0) | 265 (7.3) |
| Chronic lung disease1, N (%) | 50 (1.4) | 78 (2.1) |
| Myocardial infarction1, N (%) | 58 (1.6) | 59 (1.6) |
| Cancer1, N (%) | 178 (4.9) | 178 (4.9) |
| Congestive heart disease1, N (%) | 7 (0.2) | 9 (0.3) |
| Depression/anxiety3, N (%) | 562 (15.4) | 441 (12.1) |
| DAS28-CRP, mean (SD) | 4.1 (1.1) | 4.6 (1.2) |
| Missing % | 73 | 46 |
| DAS28, mean (SD) | 4.2 (1.3) | 4.9 (1.3) |
| Missing % | 74 | 48 |
| Patient-reported pain, mean (SD) | 53 (23) | 54 (25) |
| Missing % | 70 | 45 |
| Patient-reported global health, mean (SD) | 51 (24) | 51 (25) |
| Missing % | 70 | 44 |
| Evaluator’s assessment of disease activity | ||
| Remission, N (%) | 7 (1) | 13 (1) |
| Low, N (%) | 221 (19) | 324 (16) |
| Moderate, N (%) | 720 (63) | 1096 (53) |
| High, N (%) | 195 (20) | 617 (30) |
| Maximal, N (%) | 7 (1) | 37 (2) |
| Missing % | 68 | 43 |
| CRP, median (IQR) | 7 (15) | 9 (19) |
| Missing % | 67 | 39 |
| Swollen 28-joint count, mean (SD) | 4.2 (4.3) | 6.9 (5.2) |
| Missing % | 68 | 40 |
| Tender 28-joint count, mean (SD) | 5.3 (5.1) | 7.1 (5.7) |
| Missing % | 68 | 40 |
| Swollen 66-joint count, mean (SD) | 5.8 (5.7) | NA |
| Missing % | 74 | – |
| Tender 68-joint count, mean (SD) | 8.0 (7.0) | NA |
| Missing % | 74 | – |
(1) Based on a registered diagnosis within 5 years prior to baseline. (2) Based on a diagnosis within 5 years or a prescription within 1 year, (3) Based on a prescription within 1 year. See online supplemental table S1 for codes.
CRP, C reactive protein; DAS28, 28-joint Disease Activity Score; NA, not available; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
rmdopen-2022-002883supp001.pdf (220.7KB, pdf)
Cases and comparator subjects
PsA cases were identified through the NPR and PDR, aiming to include all newly diagnosed patients with PsA in Sweden, starting methotrexate as their first DMARD during 2011–2019. Thus, cases were required to have collected a first prescription of methotrexate in 2011–2019. Further, they were required to have received a first ever PsA diagnosis within 2 years prior to starting methotrexate, with at least one main PsA diagnosis from a department of rheumatology or internal medicine at age 16 years or older during this time period, while having collected no prescriptions for any other DMARD (or an intravenous DMARD in the SRQ) within 5 years before methotrexate initiation.
Cases with a prior main diagnosis of RA, or a diagnosis indicating axial spondyloarthritis, were excluded, as were cases immigrating to Sweden within 5 years before start of methotrexate. In order to primarily include patients with PsA starting methotrexate due to inflammatory arthritis, cases were also required to have a registered visit to a rheumatology or internal medicine department, but no visit to dermatology, within 6 weeks before collecting their first methotrexate prescription.
Each included individual with PsA was matched 1:1 to a comparator-subject with RA, based on sex, age, year of methotrexate start and region of residence. The same inclusion and exclusion criteria were applied for the comparator subjects with RA as for the individuals with PsA, with the exception of excluding comparator subjects with a prior main PsA diagnosis instead of an RA diagnosis.
Follow-up
Follow-up started at the first dispensation of methotrexate (baseline) and ended at the first of: the respective outcome (primary or secondary), death, emigration, PsA cases receiving an RA diagnosis or vice versa, or end of study (31 December 2021).
Outcomes
The primary outcome was the proportion of patients not having started any other DMARD within 2 years after methotrexate start. For oral or subcutaneous DMARDs, starting another DMARD was defined as the date of collecting such a prescription from a pharmacy, while for intravenous DMARDs it was defined as the date when such a treatment was registered in SRQ. The time until starting any other DMARD was compared between PsA cases and RA comparator subjects by crude Kaplan-Meier (KM) curves and through Cox regression (see the Statistics section), including both the full follow-up period and restricted to the first 2 years. Proportions not having started any other DMARD at 1 and 2 years of follow-up were reported as the survival distribution estimates (95% CI) at those time points to account for censoring. In all these analyses, methotrexate discontinuation was disregarded (ie, censoring was not performed if a patient stopped methotrexate), since start of another DMARD, regardless of continued methotrexate treatment, indicate treatment failure with methotrexate as monotherapy.
The secondary outcome of starting specifically a b/tsDMARD was assessed similarly, but restricting the endpoint to start of any b/tsDMARD.
The secondary outcome of overall methotrexate retention (‘drug survival’, ie, regardless of adding any additional DMARDs) was assessed as the proportions (ie, the survival distribution estimates) of patients remaining on methotrexate after 1 and 2 years, and also presented as KM-curves with adjusted HRs for discontinuation, comparing PsA to RA (see the Statistics section).
The secondary objective of comparing the effectiveness of methotrexate monotherapy between PsA cases and RA was assessed in the patient subsets having a registered methotrexate start date in SRQ and a baseline visit in this register within 6 weeks prior to the start date. The reason for this restriction was that the SRQ is the sole available source of disease activity data. For these patients, disease activity at baseline and at 6 months were described. Further, the proportions of patients achieving patient-reported pain of ≤15 mm, global health of ≤20 mm (on 0–100 mm Visual Analogue Scales (VAS)), and evaluator’s assessment of ‘remission’ (score 0 on a 0–5 Likert scale) after 6 months were compared for those with complete data (complete case analysis) for each outcome at both baseline and 6 months. These outcomes were also compared using logistic regression to adjust for baseline characteristics (see the Statistics section). The three outcome measures were chosen to avoid comparing PsA and RA with disease-specific measures such as DAS28 or DAPSA (Disease Activity in PSoriatic Arthritis score), while the selected cut-offs for pain and global health are those included in the MDA.5 The 6-month visit was defined as the visit closest to day 180 after baseline, within ±90 days.
Statistics
Comparative risk of initiating treatment with any other DMARD or a b/tsDMARD, respectively, were presented as KM-curves and estimated through conditional (based on matched pairs) Cox models, crude as well as adjusted for baseline level of formal education (as presented in table 1), time from first PsA (or RA) diagnosis to start of methotrexate (quartiles), history of comorbidities (0/1 for each separate comorbidity, as presented in table 1) and patient global health (quartiles and a missing category). The proportional hazards assumption was evaluated through insertion of an interaction term with time.
Methotrexate discontinuation was defined based on the number of collected ‘defined daily doses’ (DDD) in the PDR. The defined methotrexate DDD corresponds to a weekly dose of 17.5 mg. The discontinuation date was thus set as the day the last collected prescription would have run out, assuming a weekly dose of 17.5 mg, and adding another 90 days to accommodate for potential differences in methotrexate doses in PsA and RA and shorter treatment interruptions.
ORs for achieving patient-reported pain ≤15 mm, global health ≤20 mm and evaluator’s assessment of ‘remission‘ at 6 months were estimated in separate unconditional logistic regression models, adjusted for age, sex, year of methotrexate start, region of residence, level of formal education, time from first PsA (or RA) diagnosis to start of methotrexate (quartiles), baseline comorbidity (0/1 for each separate comorbidity, as in the Cox regression above), having collected a prescription of oral prednisolone from baseline until the 6-month visit (0/1), and the baseline status for the respective outcome. Non-responder imputation was performed for patients who started any other DMARD or discontinued methotrexate before their 6-month visit due to adverse events or inefficacy. In these analyses, patients who discontinued methotrexate before 6 months for other reasons (eg, pregnancy) or who were censored (death, emigration, PsA cases receiving an RA diagnosis (or vice versa) or end of study) were excluded.
For patients initiating treatment with a b/tsDMARD, disease activity was also assessed at that time point (defined as a visit at this date or within 6 weeks before).
Patient participation
A patient representative has been involved in the planning and design of the current study.
Results
In total, 3925 PsA cases fulfilled the inclusion but not the exclusion criteria. Of these 3642 (93%) were successfully matched 1:1 to a comparator-subject with RA. Baseline comorbidity burden, level of formal education, and patient-reported pain and global health were similar between the groups (table 1), but the patients with RA had higher swollen and tender 28-joint counts, DAS28 and median C reactive protein (CRP), as well as worse evaluator’s assessment of disease activity. The mean time from first PsA or RA diagnosis until start of methotrexate was also shorter for RA, 23 days, compared with 59 days for PsA, although highly skewed towards zero (median (25th/75th/90th percentiles), PsA: 5 (0/46/182) days; RA: 1 (0/15/48) days).
The 283 PsA cases for whom no suitable RA comparator subject could be found were younger (mean age 36 years) and almost exclusively male (98%), but their disease activity measures were comparable with the matched PsA cases (see online supplemental table S2).
Time to initiating another DMARD
The survival distribution estimates (95% CI) 2 years after methotrexate initiation for not having started any other DMARD were 0.66 (0.65 to 0.68) in the PsA and 0.60 (0.59 to 0.62) in the RA group, while the corresponding figures for not having started specifically a b/tsDMARD were 0.77 (0.75 to 0.80) and 0.74 (0.73 to 0.76), respectively. Survival estimates at 1 year after methotrexate start are provided in online supplemental table S3. The crude KM-curves for time until start of any other DMARD (figure 1A) also suggested a higher risk of initiating another treatment for patients with RA, whereas the curves for time until start of a b/tsDMARD were more similar for PsA and RA (figure 1B). The adjusted HR (95% CI) for starting any other DMARD was 0.86 (0.77 to 0.96) for PsA compared with RA for the whole follow-up period and 0.91 (0.81 to 1.02) for the first 2 years. The corresponding HRs (95% CI) for starting a b/tsDMARD were 0.95 (0.84 to 1.07) and 0.99 (0.87 to 1.14), respectively. The mean DAS28 at the time of starting a b/tsDMARD (available for 439 of 1329 PsA cases and 671 of 1450 RA comparator subjects) was 4.1 for PsA and 4.4 for RA, while the mean VAS pain was 57 and 52, and VAS global 56 and 51, respectively. Stratifying the study period at the median methotrexate start-year suggested a trend for more rapid starts of other DMARDs in both diseases in later years, but with PsA closing in on RA in particular in terms of b/tsDMARDs (online supplemental figure S1).
Figure 1.
Kaplan-Meier curves indicating time from start of methotrexate until start of another DMARD. Time to starting any other DMARD: Person-years at risk for patients wih PsA/RA for the whole follow-up period, 13 985/12 792 years; for the first 2 years, 5639/5422 years. (B) Time to starting a b/tsDMARD: Person-years at risk for patients with PsA/RA for the whole follow-up period, 16 339/15 874 years; for the first 2 years, 6175/6157 years. b/tsDMARD, biological or targeted synthetic DMARD; DMARD, disease-modifying antirheumatic drugs; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
The specific DMARDs started, either as first DMARD after methotrexate, or as the first b/tsDMARD, are shown in table 2. Of the conventional synthetic DMARDs (csDMARDs), hydroxychloroquine and sulfasalazine were more commonly used in RA.
Table 2.
Frequencies of first DMARD (regardless of type) and b/tsDMARD, respectively, started after methotrexate at any time point during the follow-up
| First DMARD (any) after methotrexate | First b/tsDMARDs | |||
| DMARD | PsA | RA | PsA | RA |
| Sulfasalazine, N (%) | 540 (32) | 601 (30) | – | – |
| Etanercept, N (%) | 367 (22) | 352 (18) | 498 (37) | 572 (39) |
| Adalimumab, N (%) | 336 (20) | 240 (12) | 450 (34) | 348 (24) |
| Leflunomid, N (%) | 154 (9) | 136 (7) | – | – |
| Hydroxychloroquine, N (%) | 21 (1) | 264 (13) | – | – |
| Infliximab, N (%) | 95 (6) | 135 (7) | 126 (9) | 175 (12) |
| Golimumab, N (%) | 52 (3) | 62 (3) | 66 (5) | 80 (6) |
| Certolizumab pegol, N (%) | 23 (1) | 53 (3) | 37 (3) | 89 (6) |
| Apremilast, N (%) | 74 (4) | 0 (0) | 116 (9) | 0 (0) |
| Abatacept, N (%) | 2 (0) | 29 (1) | 3 (0) | 47 (3) |
| Tocilizumab, N (%) | 1 (0) | 30 (2) | 1 (0) | 41 (3) |
| Baricitinib, N (%) | 1 (0) | 20 (1) | 1 (0) | 31 (2) |
| Azathioprine, N (%) | 5 (0) | 14 (1) | – | – |
| Rituximab, N (%) | 0 (0) | 35 (2) | 1 (0) | 58 (5) |
| Secukinumab, N (%) | 8 (0) | 0 (0) | 12 (1) | 0 (0) |
| Tofacitinib, N (%) | 4 (0) | 3 (0) | 6 (0) | 4 (0) |
| Chloroquine, N (%) | 0 (0) | 4 (0) | – | – |
| Anakinra, N (%) | 1 (0) | 2 (0) | 1 (0) | 2 (0) |
| Ciklosporine A, N (%) | 3 (0) | 0 (0) | – | – |
| Mycophenolate mofetil, N (%) | 1 (0) | 2 (0) | – | – |
| Upadacitinib, N (%) | 2 (0) | 1 (0) | 3 (0) | 1 (0) |
| Ustekinumab, N (%) | 4 (0) | 0 (0) | 5 (0) | 0 (0) |
| Ixekizumab, N (%) | 2 (0) | 0 (0) | 2 (0) | 0 (0) |
| Auranofin, N (%) | 0 (0) | 1 (0) | – | – |
| Sodium aurothiomalate, N (%) | 0 (0) | 1 (0) | – | – |
| Sarilumab, N (%) | 0 (0) | 0 (0) | 0 (0) | 2 (0) |
| Guselkumab, N (%) | 0 (0) | 0 (0) | 1 (0) | 0 (0) |
b/tsDMARD, disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
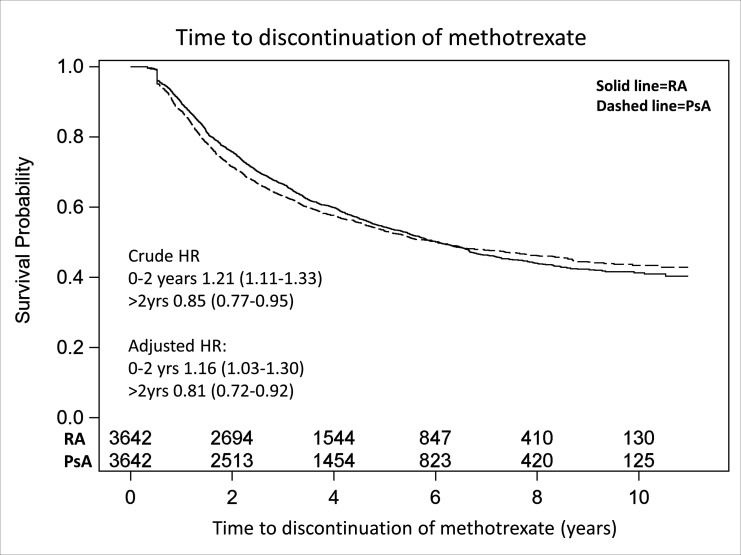
Overall methotrexate retention
The survival distribution estimates (95% CI) 2 years after starting methotrexate for not having discontinued this therapy (regardless of having added any additional DMARDs or not) were 0.71 (0.70 to 0.73) for the PsA and 0.76 (0.74 to 0.77) for the RA group (corresponding estimates at 1 year are also provided in online supplemental table S3). Over the whole follow-up period, the crude methotrexate retention curves for PsA and RA were, however, highly similar (figure 2). The proportional hazards assumption was violated in this analysis, but not after stratifying the follow-up at 2 years. The adjusted HR of methotrexate discontinuation during the first 2 years was 1.16 (1.03 to 1.30) indicating a higher risk for PsA, and in the following years 0.81 (0.72 to 0.92) indicating a higher risk for RA. The mean/median methotrexate dose (according to SRQ) for patients remaining on treatment after 2 years was 18.6/20 mg for PsA (available data N=1281) and 18.8/20 mg for RA (available N=2133).
Figure 2.
Kaplan-Meier curve indicating time from start of methotrexate until discontinuation. Person-years at risk for patients with PsA/RAs for the whole follow-up period, 14 159/14 701 years; for the first 2 years, 6231/6342 years. PsA, psoriatic arthritis; RA, rheumatoid arthritis.
Methotrexate treatment response
Of the PsA cases, 1174 (32%) had a registered methotrexate start date and baseline visit in SRQ, while the corresponding figure for RA was 2135 (59%). Of these, 42 PsA cases and 49 RA comparator subjects were excluded from the response analyses due to either being censored or discontinuing methotrexate before 6 months due to other reasons than inefficacy or adverse events.
Through comparison of baseline demographics (online supplemental table S4), the subsets of patients included in the response analyses were found to be largely representative of the whole PsA and RA cohorts in terms of age, sex and education.
Patients later imputed as non-responders (152 PsA and 292 RA) were excluded in the following description (table 3). Overall, the baseline DAS28, CRP, evaluator’s assessment of disease activity and number of swollen and tender joints were higher for RA than among the patients with PsA, while the patient-reported pain and global health scores were similar (table 3). At 6 months (mean (SD) time from methotrexate start for all patients: 158 (46) days), both PsA cases and RA comparator subjects had (on a group level) achieved a reduction in all disease activity measures, but the reductions were numerically greater for all measures in RA (table 3).
Table 3.
Disease activity at baseline and after 6 months of methotrexate monotherapy
| PsA N=980 |
RA N=1794 |
|||
| Baseline | 6 months | Baseline | 6 months | |
| DAS28-CRP, mean (SD) | 4.1 (1.1) | 2.8 (1.1) | 4.5 (1.2) | 2.6 (1.1) |
| Available % | 80 | 63 | 86 | 73 |
| DAS28, mean (SD) | 4.2 (1.3) | 2.9 (1.2) | 4.8 (1.3) | 2.7 (1.3) |
| Available % | 76 | 58 | 84 | 68 |
| Patient-reported pain, mean (SD) | 53 (23) | 32 (25) | 52 (25) | 26 (24) |
| Available % | 86 | 67 | 88 | 75 |
| Patient-reported global health, mean (SD) | 50 (24) | 33 (25) | 50 (25) | 27 (24) |
| Available % | 87 | 67 | 89 | 76 |
| CRP, median (IQR) | 7.0 (13.8) | 4.0 (5.0) | 9.0 (18) | 4.0 (4.4) |
| Available % | 95 | 70 | 98 | 78 |
| Swollen 28-joint count, mean (SD) | 4.1 (4.2) | 1.4 (2.5) | 6.8 (5.2) | 1.5 (2.6) |
| Available % | 93 | 70 | 97 | 78 |
| Tender 28-joint count, mean (SD) | 5.3 (5.1) | 2.2 (3.5) | 7.0 (5.6) | 2.0 (3.5) |
| Available % | 93 | 70 | 97 | 78 |
| Evaluator’s assessment of disease activity, Likert scale | ||||
| Remission, N (%) | 5 (1) | 162 (25) | 10 (1) | 452 (33) |
| Low, N (%) | 179 (20) | 349 (53) | 265 (16) | 639 (47) |
| Moderate, N (%) | 567 (63) | 131 (20) | 890 (54) | 226 (17) |
| High, N (%) | 139 (15) | 17 (3) | 462 (28) | 34 (3) |
| Maximal, N (%) | 7 (1) | 0 | 31 (2) | 0 |
| Available % | 92 | 67 | 92 | 75 |
CRP, C reactive protein; DAS28, 28-joint Disease Activity Score; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
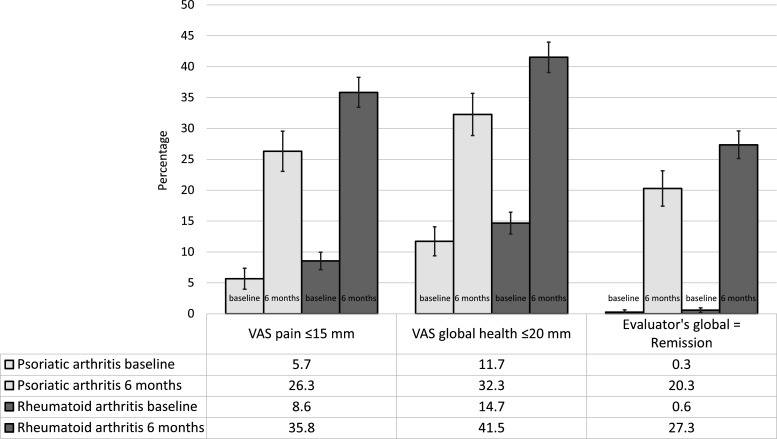
Figure 3 indicates the proportions of patients with a patient-reported VAS pain ≤15 mm, VAS global health ≤20 mm and evaluator’s assessment of ‘remission’, at baseline and at 6 months. Only patients with the outcome measure under study available at both baseline and 6 months were included, and patients starting any other DMARD or stopping methotrexate due to adverse events or inefficacy before 6 months were imputed as non-responders. The change from baseline to 6 months suggested an improvement in both groups, although greater in RA. The adjusted ORs (95% CI) (PsA compared with RA) for achieving VAS pain ≤15 mm, VAS global health ≤20 mm and evaluator’s assessment of ‘remission’ at 6 months were: 0.63 (0.47 to 0.85); 0.57 (0.42 to 0.76); 0.54 (0.39 to 0.75), suggesting a higher chance of achieving all outcomes in RA. The corresponding crude ORs were: 0.64 (95% CI 0.53 to 0.78); 0.67 (95% CI 0.56 to 0.81); 0.68 (95% CI 0.55 to 0.83). A number of patients included in each of the complete case analyses are presented in the legend of figure 3.
Figure 3.
Proportions of patients with favourable patient-reported and evaluator-reported disease measures at baseline and after 6 months of methotrexate monotherapy. Error bars indicate 95% CIs. The numbers of PsA cases and RA comparator-subjects, respectively, included in these complete case analyses were 707 and 1496 for VAS pain; 716 and 1527 for VAS global health; 759 and 1532 for evaluator’s assessment of disease activity. PsA, psoriatic arthritis; RA, rheumatoid arthritis; VAS, Visual Analogue Scale.
Discussion
Main findings
In this study of patients in Sweden starting methotrexate as their first DMARD in clinical practice, we found that patients with RA more rapidly initiated another csDMARD compared with PsA, but that the time to initiating a b/tsDMARD was similar. The treatment retention of methotrexate (regardless of adding other DMARDs) was also equivalent. However, while both groups responded to methotrexate monotherapy, the RA comparator subjects were about 40%–50% more likely to achieve a patient-reported pain ≤15 mm, global health of ≤20 mm or an evaluator’s assessment of ‘remission’, after 6 months.
Previous research
A 2019 Cochrane review illustrated the weak evidence for methotrexate in PsA,16 including only eight studies and concluding that there was only low-quality evidence suggesting methotrexate to be slightly more effective than placebo.16 This conclusion was primarily based on the largest available RCT, in which methotrexate was not found to be superior to placebo.1 One limitation that has been pointed out regarding this RCT is that only 11% were treated with a methotrexate dose above 15 mg per week, which is below contemporary clinical target doses.17 In the 2019 SEAM-PsA RCT, comparing etanercept with methotrexate,2 the methotrexate target dose was 20 mg (the mean dose used during weeks 4–24 was >18.8 mg, with a median of 20 mg).
The 2015 treat-to-target study in PsA (TICOPA),6 which included patients with a similar demographic profile as in our study, aimed at a methotrexate dose of 25 mg per week in the tight-control arm, and treatment was intensified with the target of reaching and maintaining MDA. Throughout the 48-week study, 27% of patients in the tight-control arm maintained methotrexate monotherapy, and 73% thus received other DMARDs (39% bDMARDs), while in the standard care arm 60% maintained methotrexate monotherapy and 39% received other DMARDs (7% bDMARDs). In our study, only 22% of the patients with PsA had started any other DMARD by week 48, but 14% had started a b/tsDMARD, suggesting that the treatment approach in Sweden leans towards a less active escalation of treatment overall, but a more frequent use of b/tsDMARDs, compared with the standard care arm of the TICOPA-trial (probably partly driven by differing regulatory requirements for b/tsDMARD start between Sweden and the UK, where the TICOPA trial was performed, while calendar time trends could also be involved). In our study, for patients remaining on methotrexate at 2 years, the mean methotrexate dose of 18.6 mg for PsA and 18.8 mg for RA (regardless of comedication), would be consistent with most patients receiving a dose above 15 mg per week. In the Norwegian register-based comparison of patients with PsA and RA starting methotrexate before 2007 (mean methotrexate dose at 6 months 14 mg),11 both the methotrexate retention rate and the response patterns were similar to our study, that is, both PsA and RA responding to methotrexate but RA more so. The retention and effect size of methotrexate monotherapy in our study could thus be considered to be in line with that observed in the 2015 treat-to-target study (26%/32% achieving pain ≤15/global health ≤20 vs 27% maintaining MDA on methotrexate monotherapy) and the 2010 register-based study.
The lower rates for achieving favourable patient-reported pain/global health and evaluators’ assessment of ‘remission’ with methotrexate in PsA compared with RA could have several explanations. First, the efficacy of methotrexate in PsA could be lower than in RA, which would be consistent with much of the previously published data. Second, the different disease-domains in PsA may respond differently to methotrexate, and a patient with an oligoarticular PsA could respond less favourably than a patient with a polyarthritic disease.1 18 Unfortunately, the limitations in our data do not allow for any stratifications by PsA phenotype.
Limitations and strengths
This study has several limitations, mostly related to the data available in the registers. The registers contain limited data on PsA-features other than joint disease (eg, on cutaneous psoriasis, enthesitis, dactylitis and axial disease), which is a limitation in itself, but also makes comparisons to other studies difficult. This means that we cannot determine to what extent residual extra-articular disease activity may have contributed to the lower proportions of patients with PsA reaching favourable patient-reported and evaluator-reported disease activity states at 6 months. Furthermore, without placebo groups for comparison, and in light of the relatively high numbers of missing data for disease activity measures overall, as well as their higher missingness in PsA than RA, the results of the treatment response analyses should be interpreted with some caution. The considerably higher missingness in PsA could also imply that the patients with PsA, for whom these data are recorded, may have a more severe disease phenotype than patients with missing data, although this cannot be assessed. If this is the case, the results of the current response analyses (regarding VAS pain/VAS global/Evaluator’s global) could be most representative for a PsA population with a more severe (and perhaps RA-like) phenotype.
Further, the prescription data doesn’t allow a reliable assessment of the effect of different methotrexate doses, and the means used to define methotrexate discontinuation may be unprecise. Moreover, we cannot be sure that methotrexate was prescribed primarily for PsA rather than cutaneous psoriasis, and the treatment effect on psoriasis may introduce a bias. Further, as a consequence of matching patients with RA to the PsA cases, the RA cohort will not be representative of patients with RA in general, and a small minority of PsA cases (7.2%) also had to be excluded in the absence of suitable RA comparator subjects.
The study also has several strengths. It is a large, nationwide study using prospectively collected, contemporary, real-world data. This confers a low risk of bias, since the NPR and PDR are more or less complete. Further, the matched design offers a comparison with RA, where the efficacy of MTX is well documented.
Conclusions
In demographically comparable patients with DMARD-naïve newly diagnosed PsA and RA, Swedish rheumatologists started methotrexate later in PsA, and more actively added csDMARDs in RA, while frequencies of b/tsDMARD starts and methotrexate retention were similar. Further, while acknowledging the non-placebo controlled setting, disease activity in both PsA and RA decreased during methotrexate monotherapy. Taken together, the current observational results support that methotrexate can be an effective treatment in PsA, although not as effective as in RA, and as such should continue to have a place in the treatment algorithm of PsA.
Footnotes
Contributors: All coauthors have contributed to the conception ot the study, study design, interpretation of results and writing of manuscript. UL accepts full responsibility for the study acting as guarantor.
Funding: The study was financed by grants from the Swedish Rheumatism Association, Skåne University Hospital and from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement.
Competing interests: SE: Consultant of AbbVie, Amgen, Janssen, Novartis, UCB Pharma. TO: consulting tasks for Eli Lilly, and Merck Sharp & Dohme unrelated to the present work. EK: lecture fees from Pfizer, Eli-Lily, UCB Pharma and Janssen. LJ: lecture and consulting fees from Pfizer, Novartis, Eli-Lily and Janssen. JA: Agreements between Karolinska Institutet (with JA as PI) and Abbvie, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, mainly for the national safety monitoring of rheumatology immunomodulators in Sweden (ARTIS). JKW: Speakers bureau fees from AbbVie, Amgen. Research support from AbbVie, Amgen, Eli Lilly, Novartis, Pfizer. The remaining authors have no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and ethical approval was granted by the Regional Ethics Committee in Stockholm, Sweden (Dnr. 2015/1844-31/2). Individual patient consent was not required by the ethical approval, not required in register studies.
References
- 1. Kingsley GH, Kowalczyk A, Taylor H, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51:1368–77. 10.1093/rheumatology/kes001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24. 10.1002/art.40851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of rheumatology/national psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. 10.1002/art.40726 Available: https://onlinelibrary.wiley.com/toc/23265205/71/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. 10.1136/ard.2008.102053 [DOI] [PubMed] [Google Scholar]
- 6. Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. 10.1016/S0140-6736(15)00347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scarpa R, Peluso R, Atteno M, et al. The effectiveness of a traditional therapeutical approach in early psoriatic arthritis: results of a pilot randomised 6-month trial with methotrexate. Clin Rheumatol 2008;27:823–6. 10.1007/s10067-007-0787-7 [DOI] [PubMed] [Google Scholar]
- 8. Willkens RF, Williams HJ, Ward JR, et al. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis & Rheumatism 1984;27:376–81. 10.1002/art.1780270403 Available: http://doi.wiley.com/10.1002/art.v27:4 [DOI] [PubMed] [Google Scholar]
- 9. Saurat J-H, Stingl G, Dubertret L, et al. n.d. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (champion). British Journal of Dermatology;158:558–66. 10.1111/j.1365-2133.2007.08315.x [DOI] [PubMed] [Google Scholar]
- 10. Heiberg MS, Kaufmann C, Rødevand E, et al. The comparative effectiveness of anti-TNF therapy and methotrexate in patients with psoriatic arthritis: 6 month results from a longitudinal, observational, multicentre study. Ann Rheum Dis 2007;66:1038–42. 10.1136/ard.2006.064808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lie E, van der Heijde D, Uhlig T, et al. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2010;69:671–6. 10.1136/ard.2009.113308 [DOI] [PubMed] [Google Scholar]
- 12. Clinicaltrials.Gov. 2022. Available: https://www.clinicaltrials.gov/
- 13. Lopez-Olivo MA, Siddhanamatha HR, Shea B, et al. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst Rev 2014;2014:CD000957. 10.1002/14651858.CD000957.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waldenlind K, Eriksson JK, Grewin B, et al. Validation of the rheumatoid arthritis diagnosis in the swedish national patient register: a cohort study from stockholm county [BMC musculoskeletal disorders]. BMC Musculoskelet Disord 2014;15. 10.1186/1471-2474-15-432 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallman JK, Alenius G-M, Klingberg E, et al. Validity of clinical psoriatic arthritis diagnoses made by rheumatologists in the Swedish national patient register. Scand J Rheumatol 2022:1–11. 10.1080/03009742.2022.2066807 [DOI] [PubMed] [Google Scholar]
- 16. Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev 2019;1:CD012722. 10.1002/14651858.CD012722.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coates LC, Helliwell PS. Methotrexate efficacy in the tight control in psoriatic arthritis study. J Rheumatol 2016;43:356–61. 10.3899/jrheum.150614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ye W, Coates LC. Should methotrexate have any place in the treatment of psoriatic arthritis? Rheumatic Disease Clinics of North America 2019;45:325–39. 10.1016/j.rdc.2019.04.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002883supp001.pdf (220.7KB, pdf)
Data Availability Statement
Data are available on reasonable request.