Abstract
Background
Patients with T1 stage early colorectal cancer (CRC) can be treated with radical surgery or endoscopic surgery. Endoscopic surgery has a number of advantages, including minimal trauma and a rapid recovery. However, it cannot remove regional lymph nodes to assess whether there is lymph node metastasis. Thus, the analysis of the risk factors of lymph node metastasis in patients with T1 stage CRC is of great significance in the selection of appropriate treatment methods. Although previous studies have explored the risk factors for lymph node metastasis in T1 stage CRC patients, the number of cases were relatively insufficient, and further exploration is necessary.
Methods
A total of 2,085 patients who had been pathologically diagnosed with CRC from 2015 to 2017 from the Surveillance, Epidemiology, and End Results (SEER) database. Among the patients, 324 had lymph node metastasis. A multivariate logistic regression analysis was conducted to analyze the risk factors of lymph node metastasis in patients with T1 stage CRC. Next, we established a prediction model to predict lymph node metastasis in patients with T1 stage CRC.
Results
The results of the multivariate logistic regression analysis showed that age at diagnosis, rectosigmoid cancer, poorly differentiated or undifferentiated tumor cells, and distant metastasis were independent factors of lymph node metastasis in patients with T1 stage CRC (P<0.05). This study used the R4.0.3 statistical software for the statistical analysis. The data set was randomly divided into a training set and verification set. The training set comprised 1,460 patients, and the verification set comprised 625 patients. The area under the receiver operating characteristic curve (AUC) of the training set was 0.675 [95% confidence interval (CI): 0.635–0.714], and the AUC of the verification set was 0.682 (95% CI: 0.617–0.747). In the validation set, the model was tested by the Hosmer-Lemeshow Goodness-of-Fit Test (χ2=4.018, P=0.855), and the results showed that the model was reliable at predicting lymph node metastasis in patients with T1 stage CRC.
Conclusions
For CRC patients with high risk factors of lymph node metastasis, endoscopic physicians should carefully evaluate the advantages and disadvantages of the endoscopic surgery before deciding whether to perform this surgery.
Keywords: Colorectal cancer (CRC), lymph node metastasis, prediction model
Highlight box.
Key findings
• This study identified the risk factors of lymph node metastasis in patients with T1 stage CRC.
What is known and what is new?
• Endoscopic surgery for T1 stage CRC has a number of advantages, including minimal trauma, a rapid recovery, and a low incidence of complications, but it also has its disadvantages, including, an inability to assess whether there is lymph node metastasis.
• This study explored the risk factors of lymph node metastasis in patients with T1 stage CRC.
What is the implication, and what should change now?
• For CRC patients with high risk factors of lymph node metastasis, endoscopic physicians should carefully evaluate the advantages and disadvantages of endoscopic surgery before deciding whether to perform this surgery.
Introduction
Colorectal cancer (CRC) is the third most common in malignant tumor, and accounts for about 8% of all cancer-related deaths (1). With the development and popularization of endoscopic technology, the early diagnosis rate of CRC has increased significantly (2). Endoscopic technology is not only a means of diagnosis but is also used to treat early-stage CRC. Endoscopic submucosal dissection has been found to be safe and effective in the treatment of early-stage CRC (3).
Compared to laparoscopic surgery or open surgery, endoscopic treatment has a number of advantages, including minimal trauma, a rapid recovery, and a low incidence of complications (4). However, endoscopic treatment cannot be used to remove regional lymph nodes or evaluate whether regional lymph nodes are metastatic. A study showed that the regional lymph node metastasis rate in patients with T1 stage CRC is as high as 22.5% (5). If such patients are treated by endoscopy, they often need to be treated again due to regional lymph node metastasis. Thus, the accurate evaluation of the risk factors of lymph node metastasis in patients with T1 stage CRC before treatment is of great significance, and may inform decisions of whether to preform endoscopic submucosal dissection.
Research has shown that vascular tumor thrombus and histological grade 3 are risk factors of lymph node metastasis in T1 stage CRC patients (6). Another study showed that the poor differentiation of tumor cells was a risk factor of lymph node metastasis in patients with T1 stage CRC (7). However, the number of the patients included in these two studies were relatively small (6,7). In recent years, more and more studies have been conducted to explore the related risk factors of malignant tumors (8-11). A previous study also showed that a prediction model that included multiple risk factors had high value in predicting lymph node metastasis in patients with T1 stage CRC (12). However, the study had a number of obvious shortcomings, including that the cohort comprised only 97 patients, of whom only 14 had lymph node metastasis (12). Thus, the risk factors of lymph node metastasis in patients with T1 stage CRC need to be further explored. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-125/rc).
Methods
General information
The SEER*Stat version 8.3.9 software (The American National Cancer Institute) was used to extract the data of 2,085 patients who had been pathologically diagnosed with CRC from 2015 to 2017 from the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/). The diagnoses of CRC and lymph node metastasis were based on pathological diagnosis. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have a pathological diagnosis of CRC; (II) have available medical records; and (III) have a preoperative clinical evaluation T stage of T1. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had multiple tumors; and/or (II) had incomplete clinical or follow-up data. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The patient inclusion flow chart is shown in Figure 1.
Figure 1.
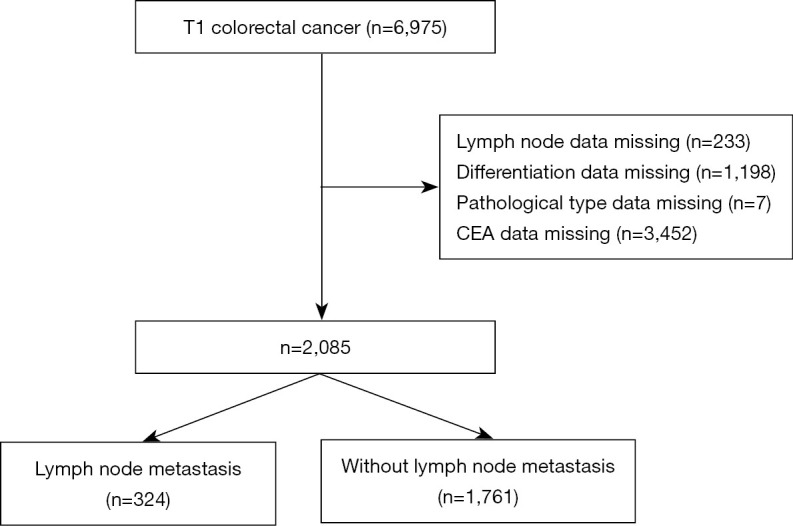
The process used to extract the data of patients who had been pathologically diagnosed with CRC from the SEER database. CEA, carcinoembryonic antigen; CRC, colorectal cancer; SEER, Surveillance, Epidemiology, and End Results.
Observation indicators
Age at diagnosis, gender, primary site, pathological type, degree of differentiation, distant metastasis, carcinoembryonic antigen (CEA) level, and overall survival were analyzed in this study.
Statistical analysis
SPSS 26.0 (IBM, Chicago, IL, USA) was used to complete the data analysis in this study, and a two-tailed P value <0.05 indicated that the difference was statistically significant. The age and other measurement data of the two groups are expressed as the mean ± standard deviation (SD), and the differences between the two groups were analyzed using an independent sample t-test. The counting data of the patients in the two groups are expressed as the number (%), and the differences between the two groups were analyzed using the chi-square test. A multivariate logistic regression analysis was conducted to explore the risk factors of lymph node metastasis in patients with T1 stage CRC [in Table 1, the P values of five variables (including age at diagnosis, the primary site, the degree of differentiation, the distant metastasis rate, the CEA increase rate) <0.1, therefore the five variables were enrolled in the multivariate logistic regression]. The R4.0.3 statistical software (R Development Core Team) was used to establish and validate a lymph node metastasis model for patients with T1 stage CRC.
Table 1. Clinical characteristics of patients with T1 stage CRC.
| Variables | Lymph node metastasis group (n=324) | Non-lymph node metastasis group (n=1,761) | t/χ2 value | P value |
|---|---|---|---|---|
| Age at diagnosis (years) | 62.31±12.54 | 65.32±12.03 | 4.104 | 0.000 |
| Gender | 0.535 | 0.465 | ||
| Male | 173 (53.40) | 979 (55.59) | ||
| Female | 151 (46.60) | 782 (44.41) | ||
| Primary site | 8.396 | 0.038 | ||
| Rectal cancer | 162 (50.00) | 987 (56.05) | ||
| Rectosigmoid cancer | 36 (11.11) | 122 (6.93) | ||
| Colon cancer | 85 (26.23) | 442 (25.10) | ||
| Ileocecum cancer | 41 (12.65) | 210 (11.93) | ||
| Pathological type | 0.923 | 0.820 | ||
| Adenocarcinoma | 320 (98.77) | 1,745 (99.09) | ||
| Squamous cell carcinoma | 1 (0.31) | 5 (0.28) | ||
| Signet-ring cell carcinoma | 2 (0.62) | 5 (0.28) | ||
| Other | 1 (0.31) | 6 (0.34) | ||
| Degree of differentiation | 37.141 | 0.000 | ||
| Well differentiated | 25 (7.72) | 295 (16.75) | ||
| Moderately differentiated | 241 (74.38) | 1,311 (74.45) | ||
| Poorly differentiated | 51 (15.74) | 132 (7.50) | ||
| Undifferentiated | 7 (2.16) | 23 (1.31) | ||
| Distant metastasis | 127.686 | 0.000 | ||
| Yes | 108 (33.33) | 175 (9.94) | ||
| No | 216 (66.67) | 1,586 (90.06) | ||
| CEA increase | 31.367 | 0.000 | ||
| Yes | 132 (40.74) | 450 (25.55) | ||
| No | 192 (59.26) | 1,311 (74.45) | ||
| Overall survival | 189 (58.33) | 1,287 (73.08) | 28.794 | 0.000 |
Data are presented as mean ± SD or n (%). CRC, colorectal cancer; CEA, carcinoembryonic antigen; SD, standard deviation.
Results
Clinical characteristics of patients with T1 stage CRC with lymph node metastasis
There were significant differences between the patients with and without lymph node metastasis in terms of age at diagnosis, the primary site, the degree of differentiation, the distant metastasis rate, the CEA increase rate, and the overall survival rate (P<0.05) (Table 1).
Risk factors of lymph node metastasis in patients with T1 stage CRC
The results of the multivariate logistic regression analysis showed that age at diagnosis, rectosigmoid cancer, poorly differentiated or undifferentiated tumor cells, and distant metastasis were independent factors of lymph node metastasis in patients with T1 stage CRC (P<0.05) (Table 2).
Table 2. Risk factors of lymph node metastasis in patients with T1 stage CRC.
| Variables | β | SE | Wald | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Age at diagnosis | 0.018 | 0.005 | 12.256 | 0.000 | 1.018 (1.008–1.028) |
| Rectosigmoid cancer | 0.496 | 0.210 | 5.567 | 0.018 | 1.642 (1.088–2.480) |
| Poorly differentiated or undifferentiated tumor cells | 0.597 | 0.179 | 11.139 | 0.001 | 1.816 (1.279–2.578) |
| CEA increase | 0.003 | 0.169 | 0.000 | 0.988 | 1.003 (0.720–1.397) |
| Distant metastasis | 1.408 | 0.186 | 57.055 | 0.000 | 4.088 |
| Constant | −4.061 | 0.662 | 37.617 | 0.000 | 0.017 |
CRC, colorectal cancer; SE, standard error; CI, confidence interval; CEA, carcinoembryonic antigen.
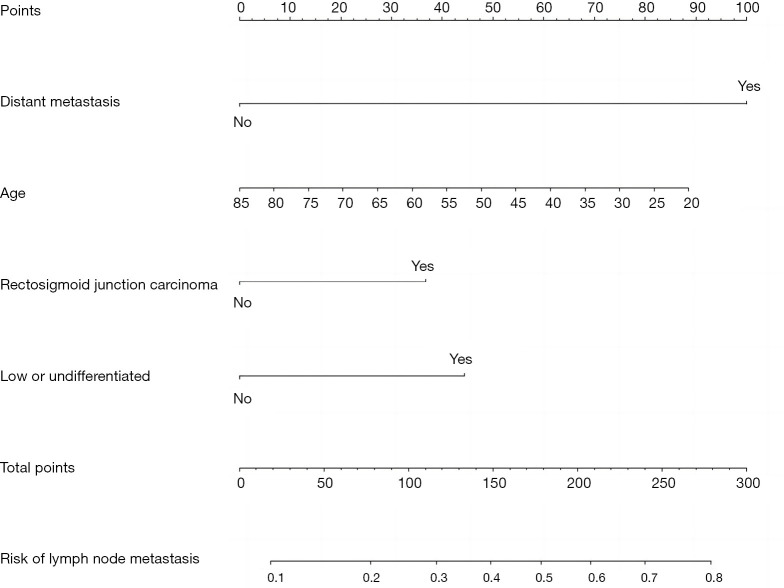
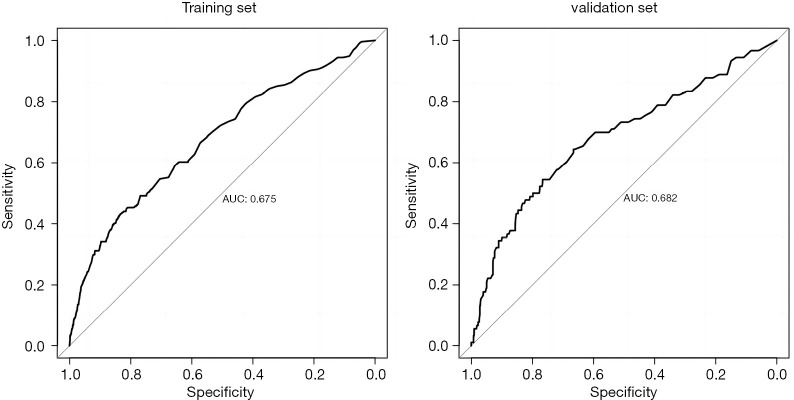
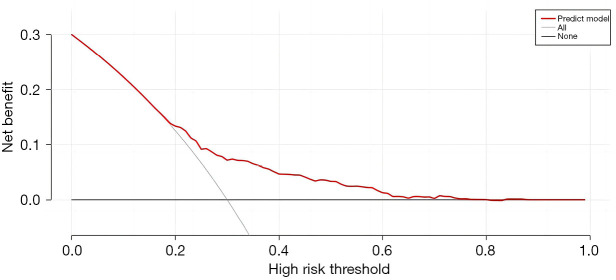
Establishment and validation of the lymph node metastasis prediction model for patients with T1 stage CRC
This study used the R4.0.3 statistical software for the statistical analysis. The dataset was randomly divided into the training set and verification set. The training set comprised 1,460 patients, and the verification set comprised 625 patients. The area under the receiver operating characteristic curve (AUC) of the training set was 0.675 [95% confidence interval (CI): 0.635–0.714], and the AUC of the verification set was 0.682 (95% CI: 0.617–0.747). In the validation set, the model was tested by the Hosmer-Lemeshow Goodness-of-Fit Test (χ2=4.018, P=0.855) (Figures 2-5).
Figure 2.
Nomogram of the lymph node metastasis prediction model for patients with T1 stage CRC. CRC, colorectal cancer.
Figure 3.
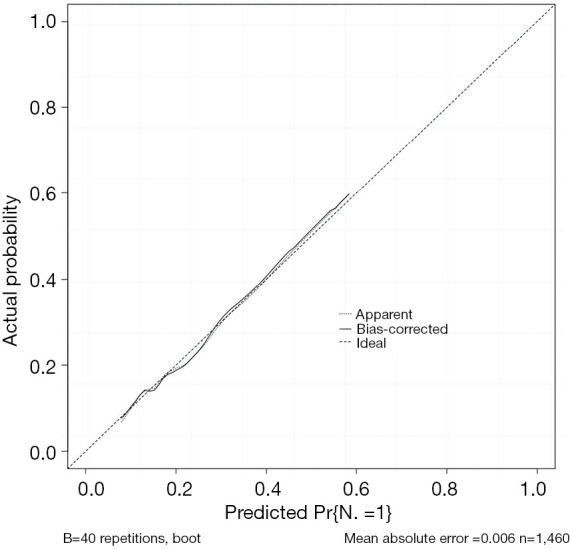
Calibration curve of the lymph node metastasis prediction model for patients with T1 stage CRC. CRC, colorectal cancer.
Figure 4.
Predictive value of the model for lymph node metastasis in patients with T1 stage CRC. AUC, area under the receiver operating characteristic curve; CRC, colorectal cancer.
Figure 5.
Decision curve analysis of the lymph node metastasis prediction model in patients with T1 stage CRC. CRC, colorectal cancer.
Discussion
The key to evaluating the applicability of endoscopic treatment is to evaluate whether patients with T1 stage CRC have lymph node metastasis (13-15). This study explored the risk factors of lymph node metastasis in patients with T1 stage CRC, and found that age at diagnosis, rectosigmoid cancer, poorly differentiated or undifferentiated tumor cells, and distant metastasis were independent factors of lymph node metastasis in patients with T1 stage CRC (P<0.05). A prediction model was established based on these risk factors. The results showed that the AUC of the training set was 0.675 (95% CI: 0.635–0.714), and the AUC of the validation set was 0.682 (95% CI: 0.617–0.747). In the validation set, the model was tested by the Hosmer-Lemeshow Goodness-of-Fit Test (χ2=4.018, P=0.855), and the results showed that the model was reliable at predicting lymph node metastasis in patients with T1 stage CRC.
Predicting the risk of lymph node metastasis in patients with T1 stage CRC can provide physicians with a reference for choosing endoscopic treatment and can also help them to formulate follow-up treatment plans for patients with T1 stage CRC after endoscopic resection. At present, the incidence rate of CRC is increasing in younger adults, and research has shown that young patients have higher grades of malignancy and a worse prognosis (16-18). The present study showed that patients with lymph node metastasis were younger, which also indicated that younger patients had a higher degree of malignant tumor cells and were more prone to lymph node metastasis. In addition, this study showed that the location of CRC also affected the lymph node metastasis rate, and the lymph node metastasis rate of rectosigmoid cancer was higher than that of other locations.
It is very easy for rectosigmoid cancer to metastasize through the lymphatic and blood channels. At present, no final conclusion has been reached about the effect of the primary site on CRC. A previous study suggested that the rate of lymph node metastasis of rectal cancer was higher than colon cancer (19). Another study showed that patients with tumors located from the descending colon to the rectum (including rectosigmoid cancer) had a higher rate of lymph node metastasis than that of other locations (20). A similar result was obtained in another study (21).
The present study also found that poorly differentiated or undifferentiated tumor cells were risk factors of lymph node metastasis in patients with T1 stage CRC. At present, studies have confirmed that poorly differentiated or undifferentiated tumor cells are risk factors for the poor prognosis of patients. Poorly differentiated or undifferentiated CRC cells grow faster and are more aggressive than other types CRC (22,23).
The present study also found that patients with distant metastasis had a higher rate of lymph node metastasis than those without distant metastasis. Distant metastasis is the manifestation of advanced disease, and some patients with T1 stage CRC have distant metastasis, which indicates that such tumor cells are highly malignant and prone to lymph node metastasis (24-26). For T1 stage CRC patients with the above-mentioned risk factors, endoscopic physicians should carefully evaluate the advantages and disadvantages of endoscopic surgery before deciding whether to perform this surgery.
The risk of lymph node metastasis is affected by multiple factors, and a single factor, histopathological feature, or biological indicator has limited value of in predicting the risk of lymph node metastasis. Thus, some scholars have proposed the use of a prediction model for lymph node metastasis in patients with T1 stage CRC. The prediction model established by the present study had high value in predicting lymph node metastasis in patients with T1 stage CRC, but the number of cases included in this study was small, and only 14 patients had lymph node metastasis (12). Another study involving 674 patients with CRC showed that the constructed prediction model, which had an AUC of 0.721, had certain value in predicting lymph node metastasis in patients with CRC. However, unlike the previous study, this study was limited to patients with T1 stage CRC.
Limitations
This study had a number of limitations. First, the AUC of the training set of the prediction model established in this study was 0.675 (95% CI: 0.635–0.714), and the AUC of the validation set was 0.682 (95% CI: 0.617–0.747). Thus, while the model had some predictive value, its AUC was not sufficiently high, which might be due to the lack of variables included in the present model. In the future, further multi-center clinical research needs to be conducted to identify more risk factors and further improve the ability of the prediction model to identify the risk of lymph node metastasis in patients with T1 stage CRC. Moreover, the data of the tumor infiltrating lymphocyte, vascular invasion and submucosa invasion depth in the SEER database was not available.
Conclusions
For CRC patients with high risk factors of lymph node metastasis, endoscopic physicians should carefully evaluate the advantages and disadvantages of the endoscopic surgery before deciding whether to perform this surgery.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the Yunnan Province High-Level Health and Family Planning Personnel Training Project (Yunwei Science and Education Development [2017] No. 14), the Basic Research Joint Special General Project of Yunnan Provincial Local Universities (part) (Nos. 2018FH001-076 and 2018FH001-080), and the 8th Research Project of Education and Teaching Reform of Dali University (Special Medical Education Reform Project, Nos. 2022JGYX08-01 and 2022JGYX08-02).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-125/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-125/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-125/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Ortega-Morán JF, Azpeitia Á, Sánchez-Peralta LF, et al. Medical needs related to the endoscopic technology and colonoscopy for colorectal cancer diagnosis. BMC Cancer 2021;21:467. 10.1186/s12885-021-08190-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okazawa Y, Sugimoto K, Ii Y, et al. Local recurrence of submucosal invasive colorectal cancer after endoscopic submucosal dissection revealed by copy number variation. DEN Open 2023;3:e208. 10.1002/deo2.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomiki Y, Kawai M, Kawano S, et al. Endoscopic Submucosal Dissection Decreases Additional Colorectal Resection for T1 Colorectal Cancer. Med Sci Monit 2018;24:6910-7. 10.12659/MSM.909380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramai D, Singh J, Facciorusso A, et al. Predictors of Lymph Node Metastasis in T1 Colorectal Cancer in Young Patients: Results from a National Cancer Registry. J Clin Med 2021;10:5511. 10.3390/jcm10235511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh JH, Han KS, Kim BC, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy 2012;44:590-5. 10.1055/s-0031-1291665 [DOI] [PubMed] [Google Scholar]
- 7.Ji X, Kang M, Zhao X, et al. Poorly differentiated cluster grade-a vital predictor for lymph node metastasis and oncological outcomes in patients with T1 colorectal cancer: a retrospective study. BMC Gastroenterol 2022;22:409. 10.1186/s12876-022-02492-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. 10.1016/j.jtemb.2021.126867 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Qi A, Teng D, et al. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol 2022;26:425-36. 10.1007/s10151-022-02585-1 [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Li Z, Zhong Q, et al. Developing and validating a multivariable machine learning model for the preoperative prediction of lateral lymph node metastasis of papillary thyroid cancer. Gland Surg 2023;12:101-9. 10.21037/gs-22-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. 10.1155/2022/6705052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macias-Garcia F, Celeiro-Muñoz C, Lesquereux-Martinez L, et al. A clinical model for predicting lymph node metastasis in submucosal invasive (T1) colorectal cancer. Int J Colorectal Dis 2015;30:761-8. 10.1007/s00384-015-2164-3 [DOI] [PubMed] [Google Scholar]
- 13.Cracco N, Todaro V, Pedrazzi G, et al. The risk of lymph node metastasis in T1 colorectal cancer: new parameters to assess the degree of submucosal invasion. Int J Colorectal Dis 2021;36:41-5. 10.1007/s00384-020-03738-0 [DOI] [PubMed] [Google Scholar]
- 14.Miyachi H, Kudo S, Mochizuki K, et al. Tumor location and patient sex are novel risk factors of lymph node metastasis in T1 colorectal cancer. J Gastroenterol Hepatol 2020;35:2292. 10.1111/jgh.15242 [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa Y, Horimatsu T, Nishizaki D, et al. Qualitative and Quantitative Analysis of Posttreatment Strategy After Endoscopic Resection for Patients with T1 Colorectal Cancer at High Risk of Lymph Node Metastasis. J Gastrointest Cancer 2020;51:242-9. 10.1007/s12029-019-00247-4 [DOI] [PubMed] [Google Scholar]
- 16.Shen L, Mo M, Jia L, et al. Poorer prognosis in young female patients with non-metastatic colorectal cancer: a hospital-based analysis of 5,047 patients in China. Cancer Manag Res 2018;10:653-61. 10.2147/CMAR.S159901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou CL, Tseng CJ, Shiue YL. The impact of young age on the prognosis for colorectal cancer: a population-based study in Taiwan. Jpn J Clin Oncol 2017;47:1010-8. 10.1093/jjco/hyx110 [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Bao F, Yan J, et al. Poor prognosis of young patients with colorectal cancer: a retrospective study. Int J Colorectal Dis 2017;32:1147-56. 10.1007/s00384-017-2809-5 [DOI] [PubMed] [Google Scholar]
- 19.Aytac E, Gorgun E, Costedio MM, et al. Impact of tumor location on lymph node metastasis in T1 colorectal cancer. Langenbecks Arch Surg 2016;401:627-32. 10.1007/s00423-016-1452-x [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki K, Kudo SE, Ichimasa K, et al. Left-sided location is a risk factor for lymph node metastasis of T1 colorectal cancer: a single-center retrospective study. Int J Colorectal Dis 2020;35:1911-9. 10.1007/s00384-020-03668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichimasa K, Kudo SE, Kouyama Y, et al. Tumor Location as a Prognostic Factor in T1 Colorectal Cancer. J Anus Rectum Colon 2022;6:9-15. 10.23922/jarc.2021-029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichimasa K, Kudo SE, Yeoh KG. Which variable better predicts the risk of lymph node metastasis in T1 colorectal cancer: Highest grade or predominant histological differentiation? Dig Endosc 2022;34:1494. 10.1111/den.14422 [DOI] [PubMed] [Google Scholar]
- 23.Qin Y, Li M, Lin Q, et al. Colorectal Cancer Cell Differentiation Trajectory Predicts Patient Immunotherapy Response and Prognosis. Cancer Control 2022;29:10732748221121382. 10.1177/10732748221121382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng J, Wu H, Huang Q, et al. Dihydropyrimidine dehydrogenase (DPYD) gene c.1627A>G A/G and G/G genotypes are risk factors for lymph node metastasis and distant metastasis of colorectal cancer. J Clin Lab Anal 2021;35:e24023. 10.1002/jcla.24023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama S, Fujita Y, Matsumura S, et al. Cribriform carcinoma in the lymph nodes is associated with distant metastasis, recurrence, and survival among patients with node-positive colorectal cancer. Br J Surg 2021;108:e111-2. 10.1093/bjs/znaa123 [DOI] [PubMed] [Google Scholar]
- 26.Newland RC, Chan C, Chapuis PH, et al. Relative effects of direct spread, lymph node metastasis and venous invasion in relation to blood borne distant metastasis present at the time of resection of colorectal cancer. Pathology 2020;52:649-56. 10.1016/j.pathol.2020.06.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as