Abstract
The prevalence of cigarette smoking in young adults is higher among those with socioeconomic disadvantage than those without. Low treatment-seeking among young adult smokers is compounded by few efficacious smoking cessation interventions for this group, particularly socioeconomically-disadvantaged young adults (SDYA) who smoke cigarettes. The goal of this study was to test a tailored smoking-cessation intervention for SDYA. 343 SDYA aged 18-30 living in the U.S. (85% female) who smoke cigarettes with access to a smartphone and interest in quitting smoking in the next six months were recruited online in Spring 2020 and randomized to referral to online quit resources (usual care control; n=171) or a 12-week tailored text message smoking-cessation program with a companion web-based intervention (n=172). Intent to treat analyses examined associations between study condition, self-reported 30-day point prevalence abstinence (PPA), and confidence to quit smoking at 12 weeks, controlling for potential confounders. Intervention group participants had greater self-reported 30-day PPA at 12-weeks than controls (adjusted relative risk 3.93, 95% CI 2.14-7.24). Among those who continued smoking, the intervention increased confidence to quit (0.81 points, 95% confidence interval 0.08-1.53). Weekly engagement in the intervention predicted greater cessation. A tailored text message intervention for SDYA increased smoking abstinence and confidence to quit at the end-of-treatment. Findings may have been influenced by recruitment at the start of the COVID pandemic but suggest that text messaging is an acceptable and efficacious cessation strategy for SDYA smokers. Future studies should examine the impact on longer-term smoking-cessation and importance of intervention tailoring for SDYA.
Keywords: Young adult, smoking cessation, text message, socioeconomic disadvantage, randomized controlled trial
INTRODUCTION
Emerging evidence suggests that smoking initiation is shifting into young adulthood (Barrington-Trimis et al., 2020; Cantrell et al., 2018). Early cessation can dramatically reduce the long-term harms of cigarette smoking, yet systematic reviews have identified a limited number of cessation interventions that are efficacious in young adults (Villanti et al., 2010; Villanti et al., 2020b), a group less likely to employ evidence-based methods to quit smoking (Curry et al., 2007). Of these, brief text message cessation interventions provide an effective and scalable means of reducing cigarette use in young adults (Buller et al., 2014; Ybarra et al., 2016), with consistent effects for hookah (Mays et al., 2021) and vaping cessation (Graham et al., 2021a). Tailored text message programs are currently available for a number of population groups, though not for socioeconomically-disadvantaged people who smoke, nor young adults who smoke (Prutzman et al., 2021).
Socioeconomically-disadvantaged young adults (SDYA) represent a population at greater risk of smoking and its long-term consequences. Consistent with data in adults (Cornelius et al., 2020), the prevalence of smoking in young adults aged 18 to 24 is higher in those with low education and subjective financial status (Villanti et al., 2017). Socioeconomic disadvantage also impacts cessation behaviors and success in young adults (Hammett et al., 2017), with young adults who have a high school education or less reporting lower cessation rates at one-year follow-up compared to those with a 2-year or 4-year college education (Solberg et al., 2007).
Technology-based interventions targeting tobacco use have generally been shown to be acceptable in young adults (An et al., 2013; Baskerville et al., 2016; Buller et al., 2014; Gulliver et al., 2015) and feasible to implement in a population that includes SDYA smokers, though the lack of subgroup analyses in these intervention studies limit understanding of their impact on SDYA smokers (An et al., 2013; Buller et al., 2014). More concerning are findings from two cessation studies in young adults that reported lower abstinence or no effect of the intervention in SDYA smokers (Baskerville et al., 2016; Haug et al., 2013).
Given the need for effective smoking-cessation interventions in SDYA who smoke cigarettes, our team undertook formative research to identify cessation-related content and delivery strategies relevant to this population (Villanti et al., 2017; Villanti et al., 2020b; West et al., 2020; West et al., 2019). Findings informed the design and implementation of a tailored text message program to address barriers and motivators to quitting in SDYA. We then conducted a randomized smoking-cessation trial to evaluate the impact of this tailored intervention for this population on short-term smoking abstinence and other cessation-related measures in SDYA who smoke cigarettes and were interested in quitting. Our hypotheses were that: 1) SDYA smokers in the tailored cessation condition would show greater increases in self-efficacy (i.e., confidence) to quit and 30-day point prevalence abstinence (PPA) compared to SDYA smokers in the control condition, and 2) SDYA smokers in the tailored intervention group who reported a greater number of weekly check-ins would report greater confidence to quit and 30-day PPA at end of treatment.
METHODS
Trial Design
A parallel, two-group individually-randomized controlled trial with 1:1 allocation compared the tailored text message program and companion web-based intervention for SDYA who smoke cigarettes with a usual-care control (referral to online quit resources) on differences in self-reported 30-day PPA and confidence to quit at week 12 (end-of-treatment). This study was approved by the host Institutional Review Board and registered on Clinical Trials.gov (NCT04379388). Sample size calculations were based on a 13% absolute difference in 30-day PPA between groups at 12-week follow-up as observed in a text messaging smoking-cessation intervention for young adults (Buller et al., 2014). For the primary outcome (self-reported 30-day PPA) at follow-up, we calculated an initial sample size of 171 participants in each group (total n=342) to achieve power=.8 with two-tailed alpha=.05, accounting for 25% attrition from the baseline survey per previous studies (An et al., 2013). Results are reported according to CONSORT eHealth recommendations (Eysenbach and Group, 2011).
Participants
Eligible participants were 437 SDYA ages 18-30 who were U.S. residents, smoked 100 lifetime cigarettes and now smoke every day or some days, reported subjective financial status of “just meets basic expenses” or “don’t meet basic expenses” (Williams et al., 2017), had access to a smartphone with internet, and reported interest in quitting in the next six months. National recruitment occurred in Spring 2020 using paid social media ads and was conducted by Hark, Inc., a digital advertising and marketing company. All study advertisements and links directed participants to the study website, where there was a brief description of the study and a link to the online screening questionnaire. Respondents who met initial screening eligibility were automatically directed to a study consent form and brief consent quiz involving multiple-choice and true/false questions to assess understanding of study procedures, followed by contact and payment forms. All administrative forms and surveys were developed and distributed using Qualtrics (Provo, UT). A series of automated and manual checks were conducted to ensure that participants were valid, as done in our other online studies (LePine et al.; Villanti et al., 2020a).
Eligible respondents were e-mailed a link to complete the baseline survey, at the end of which they were randomized by the survey platform to the control or intervention condition. Participants in the intervention condition were redirected to a unique link for the BecomeAnEX (EX) website to complete registration following the baseline survey. These individuals were required to complete two steps as part of study enrollment: 1) set up a BecomeAnEX account and provide a mobile phone number for the text messages and, 2) respond to an initial message from the text message platform to confirm enrollment. Research personnel were not involved in treatment allocation but were aware of treatment group in order to deliver assessments on a pre-defined schedule; participants may have suspected their allocation based on receipt of study text messages.
Interventions
Tailored web and text message intervention
Introduced by Truth Initiative in 2008, BecomeAnEX.org is a freely-available, evidence-based smoking-cessation program based on social cognitive theory. This multimodal intervention (Graham et al., 2021b) educates smokers about behavioral and physiological nicotine addiction using six interactive features: 1) “Set quit date” assists users in selecting a quit date; 2) “Track smoking triggers” helps users to track their cigarette use and identify personal smoking triggers; 3) “Beat smoking triggers” encourages identifying strategies to dissociate cigarettes from triggers; 4) “Choose quit smoking aid” educates users about medication and helps them create a medication plan; 5) “Build support system” presents the importance of social support and assists users in identifying supportive friends/family; and 6) “EX Community” is a large online social network for smoking-cessation where users can connect with current and former smokers. Users can sign up for an interactive and dynamically tailored text message program during website registration. The current study revised EX’s existing text message library and added new content to address SDYA barriers and motivators based on information gleaned from eight focus groups with 34 SDYA smokers (West et al., 2020) and message testing with 47 SDYA on Amazon Mechanical Turk (Supplemental Appendix). We implemented a new tool (“Quit Reasons”) that solicited reasons for quitting via SMS or web and personalized 11 text messages delivered throughout the intervention. We also added 8 new messages related to stress management based on our focus groups and prior literature (Carlson et al., 2018; Villanti et al., 2016). Twenty-eight existing messages were revised to add animated images (GIFs) to be more relevant to a young adult sample. New introductory and closing messages were added to personalize the tEXt Study (n=9) and existing BecomeAnEX messages that would be received after 84 days were removed to fit with the 12-week intervention period. Use of the website remained the same for study participants as for existing users of the site; study participants could freely engage with the intervention to their preference.
Intervention participants received daily text messages for the first 4 weeks of the 12-week intervention, with the number of messages varying based on whether or not they set a quit date. After the first 4 weeks, participants received regular (~ 5 days per week) text messages for the remaining 8 weeks of the 12-week intervention period. Consistent with the traditional BecomeAnEX text message program, participants could use keywords (e.g., CRAVE, SLIP, MOOD) to receive content on-demand in addition to the scheduled messages.
Usual care control
Those randomized to the control condition received an e-mail referral at the end of the baseline survey to a specific page of our tEXt Study website, which provided national cessation resources (e.g., Smokefree.gov). Upon completion of the 12-week assessment, control participants also received an e-mail with the opportunity to enroll in the full 12-week study intervention, although without weekly check-ins.
Participant payments
All participants received a $10 gift card for completing the baseline survey and a $20 gift card for completing the 12-week follow-up survey. Any participant who reported 30-day smoking abstinence at the 12-week follow-up was contacted by study staff to receive a saliva cotinine test by mail and reimbursed with a $50 gift card for participating in a saliva cotinine test via video call, regardless of the test results. Payments were distributed through a system (Rybbon) where participants selected online gift cards of their choice immediately upon survey completion.
Intervention participants also received a $10 payment for registration in the web and text program. They were entered into a $50 weekly lottery (1 winner per week) for completing weekly check-in surveys and received a bonus payment of $30 for completing at least 10 of the 12 weekly check-ins during the intervention period. Possible payments ranged from $30 to $80 for control participants and $40 to $170 for intervention participants.
Measures
Outcomes
The primary behavioral outcome was self-reported past 30-day PPA at 12-week follow-up. We mailed saliva cotinine tests to participants who reported 30-day PPA and responded to a request for biochemical validation via video call. A single survey item assessed self-efficacy to quit with the primary measure being change from baseline to 12-week follow-up. This item asked: “How confident are you that you will quit smoking within the next month?” with response choices on a 0–10 scale from 0 = “Not at all confident” to 10 = “100% confident” (Boudreaux et al., 2012).
Secondary outcomes included self-reported 7-day PPA and motivations to quit. Motivations to quit were assessed through motivational rulers that asked about readiness and importance to quit (Boudreaux et al., 2012) and an additional question to assess desire to quit using the same 0–10 scale, with 10 indicating higher readiness, importance, or desire.
Other Measures
Participants provided information on sociodemographic data and tobacco use at baseline. Tobacco use information included number of days smoked in the past 30 days, number of cigarettes smoked per day, time to first cigarette, past 30-day use of tobacco/nicotine products, the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991), number of quit attempts in the past year, and perceived peer smoking prevalence. Intrinsic and extrinsic motivations to quit were assessed using the Nondaily Smoking Cessation Motivation Questionnaire (NSCM; (Berg, 2013). Participants completed two-item screeners on anxiety symptoms (Kroenke et al., 2007) and depressive symptoms (Kroenke et al., 2003), reported past 30-day alcohol and marijuana use, and answered questions about the impact of the COVID-19 pandemic on their smoking behavior and motivation to quit.
Engagement in the intervention condition was assessed by the number of completed weekly check-in surveys. Other metrics of engagement were collected through automated tracking of the BecomeAnEX web and text message systems and included the number of times tools (e.g., set quit date, cigarette tracker) were used, web engagement, and use of on-demand keywords. Intervention acceptability was assessed at follow-up with four items adapted from Graham et al. 2011.
Analyses
We examined distributional properties of all variables and used t-tests and/or chi-square tests to identify any differences between study conditions on demographic or tobacco-related characteristics at baseline or in those lost-to-follow-up. This ensured baseline equivalence, and when differences were found at the p<0.10 level, outcome models included those covariates. Multivariable Poisson regression models with robust variance were used to estimate relative risks of self-reported 30-day and 7-day PPA at end-of-treatment by study condition and multivariable linear regression models were used to estimate change in continuous measures from baseline to follow-up among continuing cigarette smokers. All outcome models were run under two scenarios: an intent-to-treat analysis, in which those lost to follow-up were assumed to be smoking and assumed to have no change in their pretreatment measures of days smoked, cigarettes per days, or any motivational rulers and a complete case analysis. For each scenario, unadjusted and adjusted analyses are presented, with adjusted models controlling for variables with imbalance across study conditions (i.e., Hispanic ethnicity, current enrollment, past 30-day alcohol use, readiness to quit, the NSCM (Berg, 2013), and past 30-day electronic vapor product [EVP], snus, and nicotine replacement therapy [NRT] use at baseline). Intervention engagement was examined in the intervention condition only, with univariate assessment of continuous measures (e.g., number of weekly check-ins completed) and frequencies of program tools used (e.g., quit reasons tool). Intent-to-treat analyses examined the association between the number of weekly check-in surveys completed and the primary outcomes in intervention participants only; additional models explored the relationship between number of text messages sent, web engagement, and the primary outcomes, as well as effect of engagement on the self-reported 7-day PPA outcome.
RESULTS
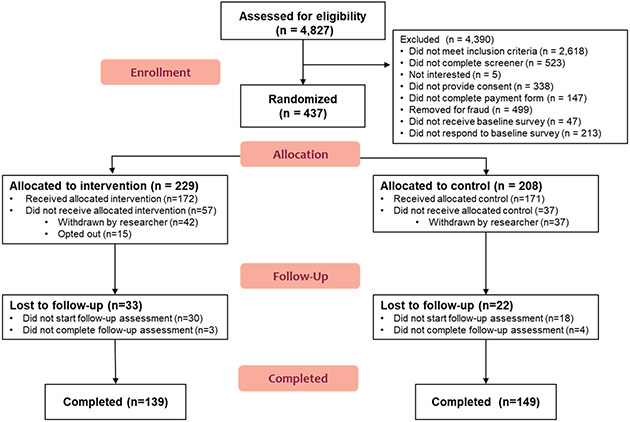
From April – June 2020, 4,827 participants were screened and 437 completed the baseline survey and were randomized to either the intervention (n=229) or control (n=208) condition (Figure 1), with 172 participants receiving the allocated intervention and 171 receiving the allocated control. Overall follow-up at 12-weeks (July – September 2020) was 84% (81% intervention vs 87% control, p = 0.11). Those retained in the study were generally similar to those lost to follow-up, though a greater proportion of those lost to follow-up reported the lowest subjective financial status (p=0.04) and endorsed past 30-day marijuana use (p=0.02) at baseline compared to those retained (Table S1).
Figure 1. tEXt Study CONSORT Diagrama.
a 79 individuals (42 intervention, 37 control) were withdrawn by researcher after completing the baseline assessment but before receipt of the allocated condition after additional manual and automated checks identified probable fraudulent responding (e.g., suspicious patterns in reporting email and mailing addresses, likely duplicate or bot responses).
The mean age of participants was 25.6 (SD 3.3), with 64% between the ages of 25 and 30. The sample largely identified as female (85%) and white (77%), with 12% identifying as Hispanic (Table 1). Consistent with our inclusion criteria for socioeconomic disadvantage, few participants had completed a Bachelor’s degree (6%) or were currently enrolled in school or a degree program (23%); approximately half of our sample (48%) was not employed and 22% reported not meeting basic expenses (Williams et al., 2017). Approximately half of the sample reported depressive (46%) or anxiety symptoms (56%) in the past two weeks, as well as past 30-day alcohol (56%) or marijuana use (53%). Approximately one-quarter (24%) reported increasing their cigarette use since the start of the COVID pandemic, with 59% reporting no change, and 16% decreasing cigarette use. A greater proportion of intervention participants reported increased motivation to quit smoking since the start of the pandemic (54% vs. 40% control; p = 0.01).
Table 1.
Characteristics of participants by study condition
| Control n (%) |
Intervention n (%) |
Total n (%) |
p- value |
|
|---|---|---|---|---|
| Age group | 0.42 | |||
| 18-20 | 14 (8.2) | 21 (12.2) | 35 (10.2) | |
| 21-24 | 44 (25.7) | 46 (26.7) | 90 (26.2) | |
| 25-30 | 113 (66.1) | 105 (61.1) | 218 (63.6) | |
| Sex | 0.39 | |||
| Male | 29 (17.0) | 23 (13.4) | 52 (15.2) | |
| Female | 141 (82.5) | 149 (86.6) | 290 (84.6) | |
| Other | 1 (0.6) | 0 (0.0) | 1 (0.3) | |
| Sexual identity | 0.20 | |||
| Straight/Heterosexual | 108 (63.5) | 104 (60.8) | 212 (62.2) | |
| Gay or Lesbian | 7 (4.1) | 10 (5.9) | 17 (5.0) | |
| Bisexual | 48 (28.2) | 41 (24.0) | 89 (26.1) | |
| Other sexuality | 7 (4.1) | 16 (9.4) | 23 (6.7) | |
| Race | 0.43 | |||
| White | 132 (77.2) | 132 (76.7) | 264 (77.0) | |
| Black or African American | 17 (9.9) | 15 (8.7) | 32 (9.3) | |
| Other | 12 (7.0) | 8 (4.7) | 20 (5.8) | |
| More than 1 race | 10 (5.9) | 17 (9.9) | 27 (7.9) | |
| Hispanic ethnicity | 0.05 | |||
| No | 144 (84.2) | 157 (91.3) | 301 (87.8) | |
| Yes | 27 (15.8) | 15 (8.7) | 42 (12.2) | |
| Education completed | 0.34 | |||
| Less than HS | 20 (11.7) | 23 (13.4) | 43 (12.5) | |
| HS/GED | 67 (39.2) | 52 (30.2) | 119 (34.7) | |
| Some college/AA | 74 (43.3) | 88 (51.2) | 162 (47.2) | |
| BA or greater | 10 (5.9) | 9 (5.2) | 19 (5.5) | |
| Currently enrolled in school/program | 0.08 | |||
| No | 138 (81.2) | 125 (73.1) | 263 (77.1) | |
| Yes | 32 (18.8) | 46 (26.9) | 78 (22.9) | |
| Employment status | 0.91 | |||
| Full time | 43 (25.3) | 43 (25.2) | 86 (25.2) | |
| 15-34 hours | 29 (17.1) | 34 (19.9) | 63 (18.5) | |
| <15 hours | 16 (9.4) | 14 (8.2) | 30 (8.8) | |
| Not employed | 82 (48.2) | 80 (46.8) | 162 (47.5) | |
| Subjective financial status | 0.20 | |||
| Just meet basic expenses | 138 (80.7) | 129 (75.0) | 267 (77.8) | |
| Don't meet basic expenses | 33 (19.3) | 43 (25.0) | 76 (22.2) | |
| Financially dependent on parents | 0.94 | |||
| Completely/almost completely | 15 (8.8) | 16 (9.4) | 31 (9.1) | |
| Partially | 26 (15.3) | 24 (14.0) | 50 (14.7) | |
| No | 129 (75.9) | 131 (76.6) | 260 (76.3) | |
| Financially dependent on others | 0.64 | |||
| Completely/almost completely | 23 (13.5) | 20 (11.7) | 43 (12.6) | |
| Partially | 50 (29.4) | 58 (33.9) | 108 (31.7) | |
| No | 97 (57.1) | 93 (54.4) | 190 (55.7) | |
| Depressive symptoms | 0.87 | |||
| No | 92 (53.8) | 94 (54.7) | 186 (54.2) | |
| Yes | 79 (46.2) | 78 (45.4) | 157 (45.8) | |
| Anxiety symptoms | 0.26 | |||
| No | 81 (47.4) | 71 (41.3) | 152 (44.3) | |
| Yes | 90 (52.6) | 101 (58.7) | 191 (55.7) | |
| Past 30 day alcohol use | 0.07 | |||
| No | 67 (39.2) | 84 (48.8) | 151 (44.0) | |
| Yes | 104 (60.8) | 88 (51.2) | 192 (56.0) | |
| Past 30 day marijuana use | 0.26 | |||
| No | 85 (49.7) | 75 (43.6) | 160 (46.7) | |
| Yes | 86 (50.3) | 97 (56.4) | 183 (53.4) |
Missing: sexual identity (2), currently enrolled (2), employment status (2), financially dependent on parents (2), financially dependent on others (2)
Tobacco-related characteristics were generally similar across study conditions, with participants smoking an average of 29 of the past 30 days and 84% being daily smokers (Table 2). Participants smoked an average of 12 cigarettes per day and while 41% smoked within five minutes of waking, overall nicotine dependence was low (mean 2.0, SD 1.2). Mean readiness to quit and scores on the NSCM were slightly higher in the intervention compared with the control condition. Past 30-day use of other nicotine products ranged from 1% for dissolvable or heated tobacco products to 52% for EVPs. Compared to the control condition, the intervention group reported higher prevalence of past 30-day EVP use (58% vs. 47%; p = 0.04), snus (4% vs. 1%; p = 0.09), and NRT use (21% vs. 12%; p = 0.03).
Table 2.
Tobacco-related characteristics of the sample at baseline by study condition
| Control Mean (SD) |
Intervention Mean (SD) |
p-value | |
|---|---|---|---|
| Days smoked cigarettes in past 30 days | 28.3 (4.9) | 28.7 (4.3) | 0.40 |
| Daily cigarette use (n, %) | 144 (84.7) | 144 (83.7) | 0.80 |
| Cigarettes smoked per day | 12.7 (10.3) | 12.2 (10.7) | 0.64 |
| Time to first cigarette (n, %) | 0.37 | ||
| Within 5 min | 67 (39.2) | 72 (41.9) | |
| 6-30 min | 78 (45.6) | 67 (39.0) | |
| 31-60 min | 11 (6.4) | 19 (11.1) | |
| After 60 min | 15 (8.8) | 14 (8.1) | |
| Fagerstrom test for nicotine dependence | 2.1 (1.2) | 1.9 (1.3) | 0.32 |
| Number of quit attempts in past year | 5.8 (26.9) | 3.7 (5.4) | 0.32 |
| Readiness rulers (Range: 0 - 10) | |||
| Want to quit | 7.9 (1.8) | 8.2 (2.0) | 0.26 |
| Confidence to quit | 5.8 (2.6) | 5.6 (2.7) | 0.49 |
| Readiness to quit | 7.4 (2.4) | 8.0 (2.3) | 0.04 |
| Importance of quitting | 8.0 (1.8) | 8.1 (1.9) | 0.52 |
| Nondaily Smoking Cessation Motivation Scale (Range: 13-91) | 65.6 (14.1) | 68.4 (14.3) | 0.08 |
| Past 30-day nicotine use | |||
| Dissolvables | 3 (1.8) | 2 (1.2) | 0.65 |
| Cigars | 20 (11.7) | 20 (11.6) | 0.98 |
| Pipe | 18 (10.5) | 15 (8.7) | 0.57 |
| Cigarillos | 55 (32.2) | 53 (30.8) | 0.79 |
| Electronic vapor products | 80 (46.8) | 100 (58.1) | 0.04 |
| Smokeless tobacco | 6 (3.5) | 13 (7.6) | 0.10 |
| Snus | 2 (1.2) | 7 (4.1) | 0.09 |
| Hookah | 20 (11.7) | 23 (13.4) | 0.64 |
| Nicotine replacement therapy | 21 (12.3) | 36 (20.9) | 0.03 |
| Heated tobacco products | 2 (1.2) | 2 (1.2) | 1.00 |
| Perceived smoking prevalence among peers | 0.25 | ||
| 20% or less | 23 (13.5) | 31 (18.0) | |
| 21% and over | 148 (86.6) | 141 (82.0) |
Missing: days smoked cigarettes (1), daily cigarette use (1), want to quit (2), confidence to quit (2), readiness to quit (2), important to quit (1)
Table 3 presents the outcomes at end-of-treatment. Results from adjusted intent-to-treat analyses showed that the intervention increased self-reported 30-day PPA four-fold (adjusted relative risk [RR] 3.93, 95% CI 2.14, 7.24) and self-reported 7-day PPA three-fold at 12 weeks (adjusted RR 3.03, 95% CI 1.96, 4.67) compared with the control condition (Figure S1). Of the 57 participants reporting 30-day PPA, only 15 completed a saliva cotinine test (1 control, 14 intervention), with 9 (60%) having biochemically-verified abstinence, all in the intervention group. Similar analyses in those who reported past 30-day cigarette use indicated that the intervention reduced the mean number of days smoked at follow-up (adjusted b = −5.23, 95% CI −7.73, −2.73) compared with the control condition. Among past 30-day cigarette smokers at follow-up, those in the intervention condition also reported a modest increase in confidence to quit (adjusted b = 0.81, 95% CI 0.08, 1.53) and desire to quit (adjusted b = 0.65, 95% CI 0.18, 1.11) compared with the control condition. Changes in cigarettes per day, importance of quitting, and readiness to quit were similar at follow-up across study conditions.
Table 3.
Outcomes at end of treatment (12 weeks) by study condition
| Outcome | Control n = 171 |
Intervention n = 172 |
Unadjusted RR (95% CI) |
Adjusted RRa (95% CI) |
|---|---|---|---|---|
| 7-day point prevalence abstinence | ||||
| Number of responses | 153 | 142 | ||
| Number abstinent | 22 | 68 | ||
| Complete case analysis, % | 14.4 | 47.9 | 3.33 (2.18, 5.09) | 3.31 (2.17, 5.06) |
| Intent-to-treat analysis, % | 12.9 | 39.5 | 3.07 (1.99, 4.73) | 3.03 (1.96, 4.67) |
| 30-day point prevalence abstinence | ||||
| Number of responses | 153 | 142 | ||
| Number abstinent | 12 | 45 | ||
| Complete case analysis, % | 7.8 | 31.7 | 4.04 (2.23, 7.33) | 4.30 (2.34, 7.89) |
| Intent-to-treat analysis, % | 7.0 | 26.2 | 3.73 (2.04, 6.80) | 3.93 (2.14, 7.24) |
| Outcome | Mean (95% CI) | Mean (95% CI) | Unadjusted b (95% CI) |
Adjusted ba (95% CI) |
| Days smoked in past 30 days b | ||||
| Complete case analysis | 19.8 (18.1, 21.5) | 15.9 (13.8, 18.0) | −4.04 (−6.73, −1.34) | −3.64 (−6.36, −0.92) |
| Intent-to-treat analysis | 20.7 (19.1, 22.3) | 19.3 (17.3, 21.2) | −5.42 (−7.89, −2.95) | −5.23 (−7.73, −2.73) |
| Cigarettes per day in past 30 days b | ||||
| Complete case analysis | 8.5 (6.6, 10.4) | 5.6 (4.5, 6.7) | −3.06 (−5.37, −0.75) | −2.37 (−4.75, 0.01) |
| Intent-to-treat analysis | 9.4 (7.4, 11.5) | 7.3 (6.0, 8.5) | −2.09 (−4.20, 0.03) | −1.78 (−3.99, 0.43) |
| Want to quit c | ||||
| Complete case analysis | 7.8 (7.4, 8.1) | 8.4 (7.9, 8.8) | 0.70 (0.16, 1.24) | 0.71 (0.15, 1.27) |
| Intent-to-treat analysis | 7.8 (7.4, 8.1) | 8.5 (8.1, 8.8) | 0.69 (0.24, 1.14) | 0.65 (0.18, 1.11) |
| Confidence to quit c | ||||
| Complete case analysis | 5.5 (5.0, 6.1) | 6.5 (5.8, 7.2) | 1.22 (0.39, 2.05) | 1.02 (0.16, 1.88) |
| Intent-to-treat analysis | 5.5 (5.0, 6.0) | 6.2 (5.6, 6.8) | 0.96 (0.27, 1.66) | 0.81 (0.08, 1.53) |
| Importance of quitting c | ||||
| Complete case analysis | 8.1 (7.7, 8.4) | 8.1 (7.6, 8.5) | 0.03 (−0.47, 0.54) | 0.03 (−0.49, 0.55) |
| Intent-to-treat analysis | 8.1 (7.8, 8.4) | 8.2 (7.8, 8.5) | 0.07 (−0.35, 0.49) | −0.01 (−0.44, 0.43) |
| Readiness to quit c | ||||
| Complete case analysis | 7.2 (6.7, 7.7) | 7.7 (7.2, 8.2) | 0.47 (−0.24, 1.17) | 0.40 (−0.33, 1.14) |
| Intent-to-treat analysis | 7.1 (6.7, 7.6) | 7.8 (7.3, 8.3) | 0.53 (−0.06, 1.12) | 0.46 (−0.16, 1.07) |
Adjusted models control for Hispanic ethnicity, current enrollment, past 30-day alcohol use, readiness to quit, Nondaily Smoking Cessation Motivation Scale, past 30-day electronic vapor product use, past 30-day snus use, and past 30-day nicotine replacement therapy use at baseline
Analyses limited to participants who reported past 30-day cigarette use at follow-up and control for baseline assessments of these outcomes
Analyses limited to participants who reported past 7-day or past 30-day cigarette use at follow-up and control for baseline assessments of these outcomes
Intervention participants completed a mean of 4.4 weekly check-in surveys (range 0–12), with 42 participants (24%) completing at least 9 check-ins (Table S2). More than three-quarters of intervention participants set a quit date (82%), used the cigarette tracker tool (77%), or used the trigger tool (81%) via web or text and more than half used the meds tool, support tool, or quit reasons tool. The top key words used by intervention participants were “SMOKED” (58%) and “COPE” (35%). For each additional check-in completed, there was a 9% increase in 30-day PPA (RR 1.09, 95% CI 1.04, 1.15) and a 0.14 mean increase in confidence to quit (b = 0.14, 95% CI 0.03, 0.24; Table S3); there was also a 7% increase in 7-day PPA (RR 1.07, 95% CI 1.03, 1.11). Due to an error in survey skip logic, only 123 of the 172 intervention participants (72%) received the four items on intervention acceptability; the majority agreed that “the text messages I received helped me cope with cravings” (80%), “I got advice and support from the text messages that I could not find anywhere else” (76%), “It was comforting to know that I wasn’t alone in the struggle to get and stay quit” (93%), and “the fact that the text messages were available whenever I needed them, night or day, was important to me” (89%).
DISCUSSION
In this randomized controlled trial of a tailored text message intervention for SDYA who smoke cigarettes, the intervention produced greater smoking abstinence across self-reported 7-day and 30-day PPA at 12-week follow-up compared with the usual care control. Among SDYA who continued to smoke cigarettes, those in the intervention group also reported reductions in number of days smoked and increases in confidence and desire to quit at follow-up compared with the control. Completing a greater number of weekly check-ins was associated with higher self-reported 30-day PPA and confidence to quit in the intervention group at end of treatment. Our findings support the feasibility and efficacy of a text message cessation intervention for SDYA smokers in contrast to prior studies showing negative or no effect of cessation interventions in this group (Baskerville et al., 2016; Haug et al., 2013). These results also extend findings from other text message cessation trials in general population samples of young adults (Buller et al., 2014; Graham et al., 2021a; Mays et al., 2021), highlighting the potential for tailored text message programs to reduce smoking disparities in this age group. The large proportion of female participants in this sample suggests that this approach may be especially relevant for SDYA women, counter to prior work showing larger effects in text messaging cessation trials with fewer women (Scott-Sheldon et al., 2016).
Our short-term results were consistent with prior studies using text messaging in young adults (Buller et al., 2014; Graham et al., 2021a; Mays et al., 2021), though the magnitude of the treatment effect on self-reported 30-day PPA exceeded earlier work for several possible reasons. First, the imbalance on baseline covariates suggested that participants randomized to the intervention group were more motivated to quit or using nicotine products that could support their quit (e.g., e-cigarettes, snus, NRT); while we used an intent-to-treat approach and multivariable adjustment, the magnitude of these results may still reflect some uncontrolled bias in favor of the intervention. Future studies may want to employ methods (e.g., stratified randomization) to reduce these imbalances. Second, our online recruitment may have produced higher smoking abstinence rates, though we would have expected this to affect the intervention and control groups equally (Scott-Sheldon et al., 2016). Finally, samples in prior studies generally reflect a range of socioeconomic disadvantage and without subgroup analyses, the magnitude of the effect in SDYA may be masked. Our findings are consistent with other types of interventions that increase access to cessation treatment, showing a greater impact of the intervention on those with greater socioeconomic disadvantage (Dahne et al., 2020).
Strengths of our study included a focus on SDYA who smoke, the integration of findings from formative research with an evidence-based cessation platform to tailor an intervention, and recruitment of a national sample. Limitations of the study include potential knowledge of study condition by participants, lack of biochemically-verified abstinence, and end-of-treatment outcome assessment instead of the recommended six-month follow-up (Hughes et al., 2003). Similar to prior work (Cha et al., 2017) and consistent with guidelines in the field (Benowitz et al., 2020), we found it infeasible to biochemically-verify abstinence in our sample. Given that our intervention was designed to be low-burden and remote, scheduling a video call may have proven too onerous for our participants. Our study may be limited in generalizability due to SDYA who could not access the program due to not having a smartphone. However, 97% of people aged 18–29 have a smartphone (Pew Research Center, 2021), thereby increasing the likelihood that SDYA were able to participate in the text message intervention. The rapidity of recruitment in Spring 2020 may have reflected greater motivation to quit due to COVID (Klemperer et al., 2020); if this had influenced study findings, we would have expected greater cessation in both study arms and an attenuation of the intervention effects. The magnitude of our short-term effects are similar to those seen in a prior young adult smoking-cessation trial that tailored digital content for this age group (An et al., 2013), supporting the generalizability of our findings to the broader population of SDYA.
CONCLUSION
Boosting smoking-cessation in SDYAs is essential to reducing long-term tobacco-related health disparities in this group. This study demonstrates strong effects of a brief, low-touch tailored text message smoking-cessation intervention on self-reported smoking abstinence, reduction in days smoked, and confidence to quit at 12 weeks. Even if abstinence is not sustained, boosting quit attempts in this group may improve quit success in the future, as well as overall population quit rates (Abrams et al., 2010). Future studies are needed to assess the effect of dual use of e-cigarettes and cigarettes on cessation in this age group (Klemperer and Villanti, 2021), the durability of intervention effects over time, and whether intervention tailoring is needed to produce smoking abstinence in SDYA.
Supplementary Material
DISCLOSURE OF FUNDING AND CONFLICTS OF INTEREST
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103644. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SC and ALG are employees of Truth Initiative, a non-profit public health foundation that sells digital tobacco cessation programs to support its mission-driven work. CP's spouse is employed by Perrigo, which markets consumer smoking cessation products. The other authors have no conflicts of interest to disclose.
REFERENCES
- Abrams DB, Graham AL, Levy DT, Mabry PL, Orleans CT, 2010. Boosting population quits through evidence-based cessation treatment and policy. Am J Prev Med 38:S351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An LC, Demers MR, Kirch MA, Considine-Dunn S, Nair V, Dasgupta K, Narisetty N, Resnicow K, Ahluwalia J, 2013. A randomized trial of an avatar-hosted multiple behavior change intervention for young adult smokers. J Natl Cancer Inst Monogr 2013:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Braymiller JL, Unger JB, McConnell R, Stokes A, Leventhal AM, Sargent JD, Samet JM, Goodwin RD, 2020. Trends in the Age of Cigarette Smoking Initiation Among Young Adults in the US From 2002 to 2018. JAMA Netw Open 3:e2019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville NB, Azagba S, Norman C, McKeown K, Brown KS, 2016. Effect of a Digital Social Media Campaign on Young Adult Smoking Cessation. Nicotine Tob Res 18:351–60. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, Joseph A, Oncken C, Piper ME, 2020. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob Res 22:1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, 2013. Nondaily Smoking Cessation Motivation for Young Adults: Scale Development and Validation. J Smok Cessat 8:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreaux ED, Sullivan A, Abar B, Bernstein SL, Ginde AA, Camargo CA Jr., 2012. Motivation rulers for smoking cessation: a prospective observational examination of construct and predictive validity. Addict Sci Clin Pract 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE, 2014. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed J E Health 20:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell J, Bennett M, Mowery P, Xiao H, Rath J, Hair E, Vallone D, 2018. Patterns in first and daily cigarette initiation among youth and young adults from 2002 to 2015. PLoS One 13:e0200827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S, Widome R, Fabian L, Luo X, Forster J, 2018. Barriers to Quitting Smoking Among Young Adults: The Role of Socioeconomic Status. Am J Health Promot 32:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S, Ganz O, Cohn AM, Ehlke SJ, Graham AL, 2017. Feasibility of biochemical verification in a web-based smoking cessation study. Addict Behav 73:204–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ, 2020. Tobacco Product Use Among Adults - United States, 2019. MMWR Morb Mortal Wkly Rep 69:1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SJ, Sporer AK, Pugach O, Campbell RT, Emery S, 2007. Use of tobacco cessation treatments among young adult smokers: 2005 National Health Interview Survey. Am J Public Health 97:1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahne J, Wahlquist AE, Smith TT, Carpenter MJ, 2020. The differential impact of nicotine replacement therapy sampling on cessation outcomes across established tobacco disparities groups. Prev Med 136:106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenbach G, Group C-E, 2011. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res 13:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Amato MS, Cha S, Jacobs MA, Bottcher MM, Papandonatos GD, 2021a. Effectiveness of a Vaping Cessation Text Message Program Among Young Adult e-Cigarette Users: A Randomized Clinical Trial. JAMA internal medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Cha S, Amato MS, Jacobs MA, Cohn AM, Abroms LC, Whittaker R, 2021b. Effectiveness of an optimized text message and Internet intervention for smoking cessation: A randomized controlled trial. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Kang H, Moreno JL, Abrams DB, 2011. Development and validation of the online social support for smokers scale. J Med Internet Res 13:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver A, Farrer L, Chan JK, Tait RJ, Bennett K, Calear AL, Griffiths KM, 2015. Technology-based interventions for tobacco and other drug use in university and college students: a systematic review and meta-analysis. Addict Sci Clin Pract 10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammett PJ, Fu SS, Burgess DJ, Nelson D, Clothier B, Saul JE, Nyman JA, Widome R, Joseph AM, 2017. Treatment barriers among younger and older socioeconomically disadvantaged smokers. Am J Manag Care 23:e295–e302. [PMC free article] [PubMed] [Google Scholar]
- Haug S, Schaub MP, Venzin V, Meyer C, John U, 2013. Efficacy of a text message-based smoking cessation intervention for young people: a cluster randomized controlled trial. J Med Internet Res 15:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction 86:1119–27. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE, 2003. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 5:13–25. [PubMed] [Google Scholar]
- Klemperer EM, Villanti AC, 2021. Why and how do dual users quit vaping? Survey findings from adults who use electronic and combustible cigarettes. Tob Induc Dis 19:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemperer EM, West JC, Peasley-Miklus C, Villanti AC, 2020. Change in Tobacco and Electronic Cigarette Use and Motivation to Quit in Response to COVID-19. Nicotine Tob Res 22:1662–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2003. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 41:1284–92. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B, 2007. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 146:317–25. [DOI] [PubMed] [Google Scholar]
- LePine SE, Peasley-Miklus C, Farrington ML, Young WJ, Bover Manderski MT, Hrywna M, Villanti AC, Ongoing Refinement and Adaptation are Required to Address Participant Deception in Online Nicotine and Tobacco Research Studies. Nicotine Tob Res. 25(1):170–172, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays D, Johnson AC, Phan L, Sanders C, Shoben A, Tercyak KP, Wagener TL, Brinkman MC, Lipkus IM, 2021. Tailored Mobile Messaging Intervention for Waterpipe Tobacco Cessation in Young Adults: A Randomized Trial. Am J Public Health 111:1686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center, 2021. Mobile Fact Sheet. [Google Scholar]
- Prutzman YM, Wiseman KP, Grady MA, Budenz A, Grenen EG, Vercammen LK, Keefe BP, Bloch MH, 2021. Using Digital Technologies to Reach Tobacco Users Who Want to Quit: Evidence From the National Cancer Institute's Smokefree.gov Initiative. Am J Prev Med 60:S172–S84. [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Lantini R, Jennings EG, Thind H, Rosen RK, Salmoirago-Blotcher E, Bock BC, 2016. Text Messaging-Based Interventions for Smoking Cessation: A Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 4:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LI, Asche SE, Boyle R, McCarty MC, Thoele MJ, 2007. Smoking and cessation behaviors among young adults of various educational backgrounds. Am J Public Health 97:1421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Bover Manderski MT, Gundersen DA, Steinberg MB, Delnevo CD, 2016. Reasons to quit and barriers to quitting smoking in US young adults. Fam Pract 33:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Rath JM, 2017. Beyond education and income: Identifying novel socioeconomic correlates of cigarette use in U.S. young adults. Prev Med 104:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, McKay HS, Abrams DB, Holtgrave DR, Bowie JV, 2010. Smoking-cessation interventions for U.S. Young adults: a systematic review. Am J Prev Med 39:564–74. [DOI] [PubMed] [Google Scholar]
- Villanti AC, Vallencourt CP, West JC, Peasley-Miklus C, LePine SE, McCluskey C, Klemperer E, Priest JS, Logan A, et al. , 2020a. Recruiting and Retaining Youth and Young Adults in the Policy and Communication Evaluation (PACE) Vermont Study: Randomized Controlled Trial of Participant Compensation. J Med Internet Res 22:e18446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, West JC, Klemperer EM, Graham AL, Mays D, Mermelstein RJ, Higgins ST, 2020b. Smoking-Cessation Interventions for U.S. Young Adults: Updated Systematic Review. Am J Prev Med 59:123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JC, Peasley-Miklus C, Graham AL, Mays D, Mermelstein R, Higgins ST, Villanti AC, 2020. Impact of alcohol and drug use on smoking and cessation in socioeconomically disadvantaged young adults. Addict Behav 110:106486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JC, Villanti AC, Graham AL, Mays D, Mermelstein RJ, Higgins ST, 2019. Tobacco Use and Cessation Behaviors in Young Adults: 2016 National Health Interview Survey. Am J Public Health 109:296–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VF, Smith AA, Villanti AC, Rath JM, Hair EC, Cantrell J, Teplitskaya L, Vallone DM, 2017. Validity of a Subjective Financial Situation Measure to Assess Socioeconomic Status in US Young Adults. J Public Health Manag Pract 23:487–95. [DOI] [PubMed] [Google Scholar]
- Ybarra ML, Jiang Y, Free C, Abroms LC, Whittaker R, 2016. Participant-level meta-analysis of mobile phone-based interventions for smoking cessation across different countries. Prev Med 89:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.