Abstract
Psychophysics measures the relationship between a stimulus’s physical magnitude and its perceived magnitude. Because decisions are based on perception of stimuli, this relationship is critical to understanding decision-making. We tested whether psychophysical laws explain how female túngara frogs (Physalaemus pustulosus) and frog-eating bats (Trachops cirrhosus) compare male frog calls, and how this imposes selection on call evolution. Although both frogs and bats prefer more elaborate calls, they are less selective as call elaboration increases, because preference is based on stimulus ratios. Thus, as call elaboration increases, both relative attractiveness and relative predation risk decrease because of how receivers perceive and compare stimuli. Our data show that female cognition can limit the evolution of sexual signal elaboration.
Comparing stimuli such as communication signals depends on how an individual perceives the physical properties of those stimuli. The field of psychophysics demonstrates that actual stimulus value does not scale with perceived stimulus value in a simple linear fashion (1, 2). This can inform an understanding of how exaggerated male traits evolve under sexual selection, because female mate choice depends on how females perceive and compare male signals, and female preference for elaborate signals often leads to the evolution of signal exaggeration (3). Yet such exaggeration does not evolve without limit. Although psychophysics may explain how cognitive constraints on female discrimination could broadly impose a selective force that limits signal elaboration (4, 5), it has rarely been applied to the evolution of reproductive decisions (6). Here, we measured how female túngara frogs (Physalaemus pustulosus) and frog-eating bats (Trachops cirrhosus) respond to frog mating signals (i.e., calls) in binary choice tests, and found that female cognition limits the evolution of signal elaboration.
Túngara frog males advertise with calls of variable complexity; females prefer greater complexity (7, 8). Mating calls can have two components: a low-frequency “whine” and a terminal high-frequency “chuck” (7). Each male produces both simple calls (a single whine) and complex calls (a single whine with one to seven chucks), and males add chucks through vocal competition (7). We investigated how preference strength—the proportion of females choosing the more attractive of two signals—relates to the different numbers of chucks in those signals, and tested hypotheses that might explain these patterns of preference.
We predicted that female ability to discriminate between two calls depends on their proportional rather than absolute difference, as predicted by Weber’s law: ΔI/I = k, where ΔI is the minimum difference required to discriminate from a stimulus of magnitude I, and k is a constant (2). Therefore, a greater difference is required to discriminate between stimuli of greater magnitude. Cohen (4) suggested that Weber’s law could influence the evolution of male traits to the extent that it constrains females’ ability to discriminate increasingly exaggerated signals. Female choice constrained by Weber’s law would pressure males to produce more elaborate signals while limiting the relative benefits of increasing elaboration.
Following previously published methods (7, 8), we tested whether Weber’s law or alternative hypotheses explain túngara frog mate choice (9). Wild-caught females were placed in a sound chamber and presented with two call types broadcast alternately from two speakers on opposite sides of the chamber. Choice was quantified as walking to within 10 cm of either speaker. We broadcast pairs of calls that had variable numbers of chucks as follows: 1 vs. 2, 1 vs. 3, 1 vs. 4, 1 vs. 5, 2 vs. 3, 2 vs. 4, and 3 vs. 4 (≥40 replicates per stimulus pair; 151 females). To these results we added previously published data (8) from the same females, testing call pairs 0 vs. 1 and 0 vs. 3 (25 replicates per stimulus pair), for an overall N = 331 choices.
We tested three hypotheses that could explain the observed strength of preference for greater complexity, which ranged from near 0.5 (no preference) to 1 (all prefer more chucks). First we tested the possibility that preference strength is based on the ratio of the total acoustic energy (sum of whine and chucks) in each stimulus. Least-squares fit of the psychometric function (10) shows that this independent variable explains only 16.5% of the data (P = 0.278; Fig. 1A and table S1), which suggests that decisions are based on the difference in chucks per se.
Fig. 1.
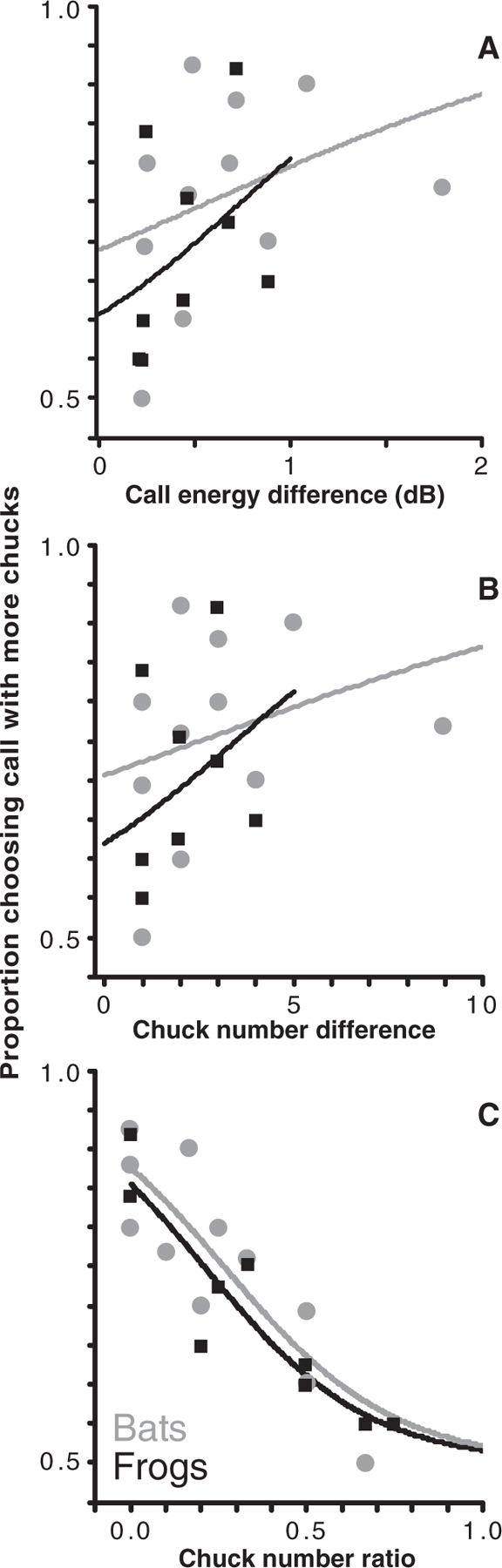
Preference response in frogs and bats. Proportions of frogs (black) and bats (gray) choosing the more complex call are shown as a function of chuck number relationships. Independent variables are (A) total call energy difference (dB), (B) chuck number difference, and (C) chuck number ratio. Curves are the least-squares fit of the psychometric function for data bound by 0.5 and 1.0 (10).
We next tested whether preference strength varies according to the difference between the number of chucks in each call (i.e., chucks in stimulus A minus chucks in stimulus B). This hypothesis is equally valid for preference based on the absolute amount of acoustic energy in the chucks. This variable explains only 11.8% of the variation in female choice (P = 0.366; Fig. 1B and table S1).
Weber’s law predicts that discrimination between two stimuli should depend on the ratio of the two stimulus quantities rather than the difference. Thus, we tested a third prediction: that preference strength varies with the ratio of the number of chucks in each stimulus (i.e., the ratio of chucks in stimulus A to chucks in stimulus B). This variable explains 84.4% of the response (P < 0.0005; Fig. 1C and table S1). Ratio-based preference explains why an increase in male attractiveness from additional chucks wanes as calls become increasingly elaborate.
These data suggest that female cognition could limit the evolution of signal elaboration, because females frequently face conditions in which Weber’s law could constrain discrimination ability in nature. Males increase their number of chucks per call one at a time, and they do so in response to neighboring males doing likewise (11). Therefore, calls of neighboring males usually differ by one chuck (if any), and females are less likely to distinguish between males producing many chucks.
Alternatively, costs due to factors such as increasing predation risk might limit the evolution of male signal elaboration. If so, then female choice itself might not be limiting. Understanding how predators choose between elaborate frog calls can help us understand females’ selective influence.
Fringe-lipped bats (T. cirrhosus) eat túngara frogs and use the frog’s mating call to localize prey (12). The bats preferentially approach complex calls (13), but how their preference varies among calls varying in chuck number has not been established. We tested bat preferences in a binary choice test between two speakers broadcasting túngara frog calls of variable complexity, following previously published methods (9, 14). Wild-caught bats were released into a flight cage and presented with two speakers concealed under screens in opposite corners of the cage. We broadcast the two stimuli and quantified choice as flight within 1 m of a speaker. We tested bats with pairs of calls that varied in chuck number as follows: 0 vs. 1, 0 vs. 2, 0 vs. 3, 1 vs. 2, 1 vs. 3, 1 vs. 4, 1 vs. 5, 1 vs. 6, 1 vs. 10, 2 vs. 3, and 2 vs. 4 (N = 219 choices, N = 26 bats).
Applying our three hypotheses to the bat data reveals that chuck number ratio is the only variable that provides a significant fit [total call energy: R2 = 0.118 (Fig. 1A); chuck number difference: R2 = 0.083 (Fig. 1B); chuck number ratio: R2 = 0.739, P = 0.0007 (Fig. 1C); table S1]. Thus, preference strength is based on chuck number ratio, not absolute difference.
Our data demonstrate that natural selection may not have as strong an influence on limiting túngara frog signal elaboration as previously thought. As males increase chucks, so do their neighbors, and the difference between neighbors remains small. With a fixed difference of one chuck between neighbors, both the risks and benefits of adding chucks decrease with increasing elaboration. Adding one chuck to many chucks adds less risk than adding one chuck to few chucks. Adding multiple chucks to outcompete neighbors will not succeed, because males maintain a fixed difference. Therefore, signal elaboration is more likely limited by reduced attractiveness than by the risk of predation.
Selective forces could possibly cause the preference patterns shown in our data for reasons other than cognitive constraints. For example, male quality differences could coincidentally scale with chuck number ratio, such that diminishing selectivity between more elaborate signals could reflect motivation rather than discrimination. Females accrue fitness benefits from mating with larger males (7), but chuck number does not indicate size (15). Similarly, prey quality might scale in this manner by chance, but chuck number does not signal male condition (15). Localization could also scale with chuck ratio, but chucks do not consistently reduce phonotactic error (12, 16). We cannot, however, test every conceivable male quality (17).
Support that Weber’s law explains our data comes from the fact that bats and frogs translate actual stimulus value to behavioral preference along the same scale (Fig. 1C). That is, these two disparate taxa have the same constant (k) in Weber’s law. Auditory mechanisms in bats and frogs differ; bats have one rather than two inner ear organs, they are more sensitive to ultrasonic frequencies, and they have a cortex (18). Thus, it is astounding that they use the same perceptual scale, suggesting generality in how animals compare stimuli. The most parsimonious explanation points to a shared perceptual mechanism, as it is unlikely that mate value and meal value scale with chuck ratio identically.
Applying Weber’s law to the evolution of signals enhances our understanding of both elaboration and innovation. Elaboration increases the magnitude of a signal, whereas innovation is the emergence of a novel signal along a separate perceptual axis (19, 20). The diminishing benefits of further elaboration imposed by Weber’s law could provide the impetus to switch from elaboration to innovation and thus incorporate new signal components or modalities in complex displays (21).
Weber’s law has been applied to several aspects of behavioral ecology (22), but few studies have tested whether it explains female preferences between sexually selected traits (23–25). This relationship has long been predicted, and our data show that female cognition can limit the evolution of signal elaboration. The diminishing returns of increased elaboration precisely fit the predictions of Weber’s law (i.e., discriminating through stimulus ratios). Although other factors can influence limits to elaboration (26–29), our data explain cases where females show diminished selectivity with increasing elaboration (30) by using a psychophysics approach to uncover broad cognitive constraints that can shape the evolution of behavioral mechanisms.
Supplementary Material
Acknowledgments:
We thank J. Saunders, S. Ozeroff, A. Shah, K. Miller, K. Loukes, S. Troxell, S. Griffin, M. Moscoso, V. Fugère, and W. Wohlwend for help conducting experiments. A. Mason and WABC 2011 participants gave helpful suggestions. STRI provided excellent logistical support. Supported by an NSF graduate research fellowship (K.L.A.), NIH grant P20RR016816 (H.E.F.), NSF grant IBN0078150 (M.J.R.), STRI, NSF grant 0608131, the American Association of University Women, and a P.E.O. Scholar award (R.A.P.).
Footnotes
Supporting Online Material
References and Notes
- 1.Fechner GT, in The Classical Psychologists, Rand B, Ed. (Houghton Mifflin, Boston, 1912), pp. 562–572. [Google Scholar]
- 2.Stevens SS, Psychophysics: Introduction to its Perceptual, Neural, and Social Prospects (Transaction, New Brunswick, NJ, 1986). [Google Scholar]
- 3.Andersson M, Sexual Selection (Princeton Univ. Press, Princeton, NJ, 1994). [Google Scholar]
- 4.Cohen JA, Z. Tierpsychol 64, 1 (1984). [Google Scholar]
- 5.Ryan MJ, Oxford Surv. Evol. Biol 7, 157 (1990). [Google Scholar]
- 6.Bateson M, Healy SD, Trends Ecol. Evol 20, 659 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Ryan MJ, The Túngara Frog, A Study in Sexual Selection and Communication (Univ. of Chicago Press, Chicago, 1985). [Google Scholar]
- 8.Akre KL, Ryan MJ, Ethology 116, 1138 (2010). [Google Scholar]
- 9.See supporting material on Science Online.
- 10.Green DM, Richards VM, Forrest TG, J. Acoust. Soc. Am 86, 629 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Bernal XE, Akre KL, Baugh AT, Rand AS, Ryan MJ, Behav. Ecol. Sociobiol 63, 1269 (2009). [Google Scholar]
- 12.Page RA, Ryan MJ, Anim. Behav 76, 761 (2008). [Google Scholar]
- 13.Ryan MJ, Tuttle MD, Rand AS, Am. Nat 119, 136 (1982). [Google Scholar]
- 14.Page RA, Ryan MJ, Proc. Biol. Sci 272, 841 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal XE, Page RA, Rand AS, Ryan MJ, Am. Nat 169, 409 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Bonachea LA, Ryan MJ, Ethology 117, 56 (2011). [Google Scholar]
- 17.Prum RO, Evolution 64, 3085 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Popper AN, Fay RR, Eds., Hearing by Bats, Springer Handbook of Auditory Research (Springer-Verlag, New York, 1995). [Google Scholar]
- 19.Endler JA, Westcott DA, Madden JR, Robson T, Evolution 59, 1795 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Ryan MJ, Bernal XE, Rand AS, Current Zool 56, 343 (2010). [Google Scholar]
- 21.Rowe C, Anim. Behav 58, 921 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Shettleworth SJ, Cognition, Evolution, and Behavior (Oxford Univ. Press, Oxford, ed. 2, 2010). [Google Scholar]
- 23.Bee MA, Anim. Behav 76, 845 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerhardt HC, Tanner SD, Corrigan CM, Walton HC, Behav. Ecol 11, 663 (2000). [Google Scholar]
- 25.Wyttenbach RA, Farris HE, Microsc. Res. Tech 63, 375 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Lande R, Proc. Natl. Acad. Sci. U.S.A 78, 3721 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland B, Rice WR, Evolution 52, 1 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Pryke SR, Andersson S, Behav. Ecol 19, 1116 (2008). [Google Scholar]
- 29.Price DK, Burley NT, Am. Nat 144, 908 (1994). [Google Scholar]
- 30.Jennions MD, Petrie M, Biol. Rev. Camb. Philos. Soc 72, 283 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


