Abstract
Laryngeal squamous cell carcinoma (LSCC) is one of the most aggressive cancers, and its early diagnosis is urgent. Exosomes are believed to have diagnostic significance in cancer. However, the role of serum exosomal microRNAs, miR-223, miR-146, and miR-21, and phosphatase and tensin homologue (PTEN) and hemoglobin subunit delta (HBD) mRNAs in LSCC is unclear. Exosomes were isolated from the blood serum of 10 LSCC patients and 10 healthy controls to perform scanning electron microscopy and liquid chromatography quadrupole time-of-flight mass spectrometry analyses to characterize them and to undergo reverse transcription polymerase chain reaction to identify miR-223, miR-146, miR-21, and PTEN and HBD mRNA expression phenotypes. Biochemical parameters, including serum C-reactive protein (CRP) and vitamin B12, were also obtained. Serum exosomes of 10–140 nm were isolated from LSCC and controls. Serum exosomal miR-223, miR-146, and PTEN were found to be significantly decreased (p < 0.05), in contrast to serum exosomal miRNA-21 (p < 0.01), and serum vitamin B12 and CRP (p < 0.05) were found to be significantly increased, in LSCC vs controls. Our novel data show that the combination of reduced serum exosomal miR-223, miR-146, and miR-21 profiles and biochemical alterations in CRP and vitamin B12 levels may be useful indicators of LSCC that could be validated by large studies. Our findings also suggest a possible negative regulatory effect of miR-21 on PTEN in LSCC, encouraging a more extensive investigation of its role.
Keywords: exosome, miRNAs, miR-223, miR146, miR-21, LSCC, CRP, vitamin B12
Laryngeal squamous cell carcinoma (LSCC) constitutes 1% of all cancers and 13% of head and neck cancers. However, it is the most common cause of mortality among head and neck malignancies.1 Even though patients are treated with surgery and/or radiotherapy, the 5 year survival rate is 60%. Laryngeal cancers are most seen in the glottic region. It can be seen less frequently in the supraglottic and subglottic regions.2,3 Due to the aggressiveness of the disease, it is more successfully treated if diagnosed early. Therefore, there is a need to identify molecular signatures or biochemical parameters associated with LSCC.
Small non-coding regulatory molecules, such as miRNA markers, are considered as potential diagnostic and prognostic biomarkers in head and neck cancer. Specifically, miRNAs are small non-coding RNA molecules (18 to 24 nt), and their expression is often deregulated in head and neck cancer.4 Deregulation of miRNAs in the early carcinogenic stages of HNSCC has been previously documented by preclinical studies.5−9 Specifically, previous in vitro and in vivo studies documented that miR-21 expression can be progressively altered from precancerous lesions into invasive head and neck squamous cell carcinoma (HNSCC),5−9 while the cancer-related miR-21 expression phenotype can be effectively reversed by inhibiting key cancer-related molecules.7,10,11 In addition, clinical data from a previous study supported the idea that the head and neck tumor microenvironment may be equally important to its tumor, therefore playing an important role in tumor development and progression.10
MicroRNAs can be secreted by tumor cells to the extracellular fluid and hence the microenvironment or can be injected into the circulation.12−15 The majority of extracellular miRNAs are in a non-vesicle-associated form; however, their secretion may also be mediated by a protein-complex or membrane-bound vesicle, such as exosomes or micro-vesicles,16,17 characterizing tissue/disease specificity of extracellular miRNAs. Exosomes are small vesicles bounded by lipid bilayer membranes, about 30–150 nm in diameter, distributed in body fluids such as peripheral blood, urine, saliva, acid, and amniotic fluid.18−21 Different cell types including LSCC have been identified to secrete various molecules such as proteins, lipids, and nucleic acids, including miRNA.22 Exosomes contain important miRNAs that can contribute to intercellular communication. To date, studies focusing on exosome miRNAs have shown that circulating exosome miRNAs have diagnostic and prognostic significance in head and neck carcinoma, including nasopharyngeal, oral squamous cell carcinoma, and LSCC.23−25
Recent findings have shown that miR-21 inhibition can negatively affect the cell viability of tobacco smoke-related head and neck cancer cells.7 Liu et al.26 found that miR-21 can also inhibit the proliferation and invasion ability of laryngeal cancer cells by negatively regulating the expression of the phosphatase and tensin homolog (PTEN) gene, which is a tumor suppressor gene. The results showed that miR-21 was negatively correlated with PTEN gene expression.27PTEN can inhibit cell migration, cancer cell growth, and invasion by blocking the PI3K/AKT bypass. MicroRNA-21, on the other hand, can inhibit PTEN expression by targeting the 3′UTR of PTEN mRNA.28
In addition to miR-21, miR-146 has been described to play a regulatory role in various diseases, including cancer. Interestingly, miR-146a inhibited invasion, metastasis, and cell growth, as well as increased cellular apoptosis in cancer cell lines.22,29 Furthermore, transfection with mimic miR-146a inhibited cell proliferation and increased apoptosis in cancer cell lines, suggesting that miR-146a like PTEN signals a potential tumor suppressor function.30
Despite the growing interest in studying the exosome miRNA difference between cancer cells and normal cells, we still lack an understanding of the role of exosomal miRNAs in cancer disease. In this study, we aimed to examine the expression levels of 3 exosomal miRNAs (miR-21, miR-223, and miR-146), as well as of hemoglobin subunit delta (HBD) and PTEN genes circulating in the serum of LSCC patients, and to explore their expression phenotypes in laryngeal cancer. In addition, biochemical parameters and hematological evaluations of routinely collected blood samples were examined. Findings from this pilot study could suggest potential prognostic or predictive biomarkers for LSCC that could be validated by large studies.
Results and Discussion
Clinicopathological Evaluation of LSCC
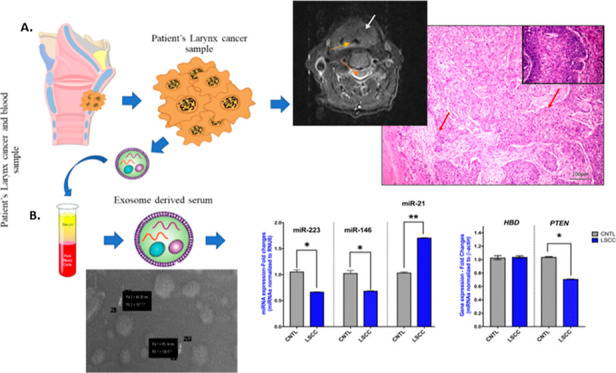
Laryngeal cancer is commonly diagnosed in the neck and throat region. Although the diagnosis process starts with the complaint of difficulty breathing, it is very important to diagnose cancer in its early stages. The first aim of this study was to evaluate the clinicopathological characteristics of the laryngeal mass found by endoscopic examination in the patients of our study. Magnetic resonance imaging (MRI) and pathological images of laryngeal cancer patients are shown in Figures 1 and 2. Specifically, image analysis by MRI revealed laryngeal tumor (Figure 1), while histopathological evaluation revealed LSCC of stage I–III, T1-3N0-1M0 (Table 1).
Figure 1.
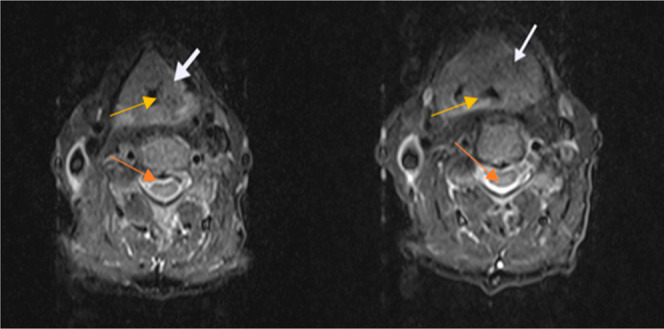
Representative MRI findings in the right section of the neck. Diagnosis; laryngeal tumor. White arrows show the larynx cancer area, orange arrows show the vertebra, and yellow arrows show the larynx.
Figure 2.
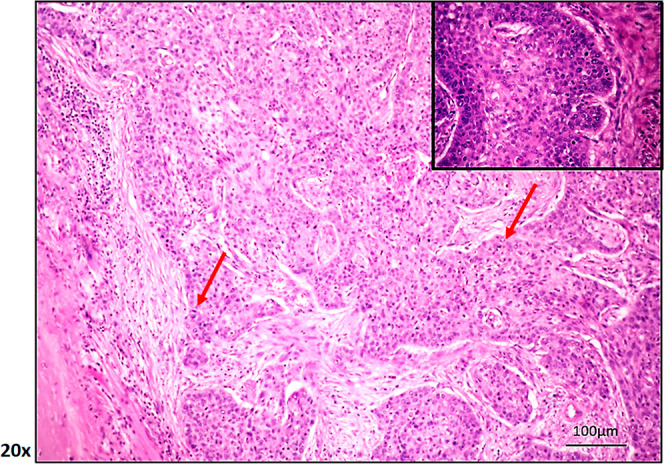
Representative pathological image of a patient with LSCC diagnosis showing invasiveness, heterogeneity, and amorphous cell formation (100 μm hematoxylin and eosin staining; by ImageScope). Red arrow: infiltrative cancer cells.
Table 1. Histopathological Characteristics of Patients with Laryngeal Cancera.
| stage of LSCC | tumor (T) | lymph nodes (N) | metastasis (M) | number of patients | smoking history | alcohol history | HPV history |
|---|---|---|---|---|---|---|---|
| I | T1 | N0 | M0 | 2 | 1 | 1 | 0 |
| II | T2 | N0 | M0 | 4 | 1 | 2 | 0 |
| III | T3 | N0 | M0 | 2 | 1 | 0 | 0 |
| III | T1 | N1 | M0 | 1 | 1 | 1 | 0 |
| III | T2 | N1 | M0 | 1 | 1 | 1 | 0 |
| Total | 10 |
LSCC: laryngeal squamous cell carcinoma. Note: staged according to AJCC (American Joint Committee on Cancer). Numbers show patient size.
Serum Exosomal Characterization in LSCC
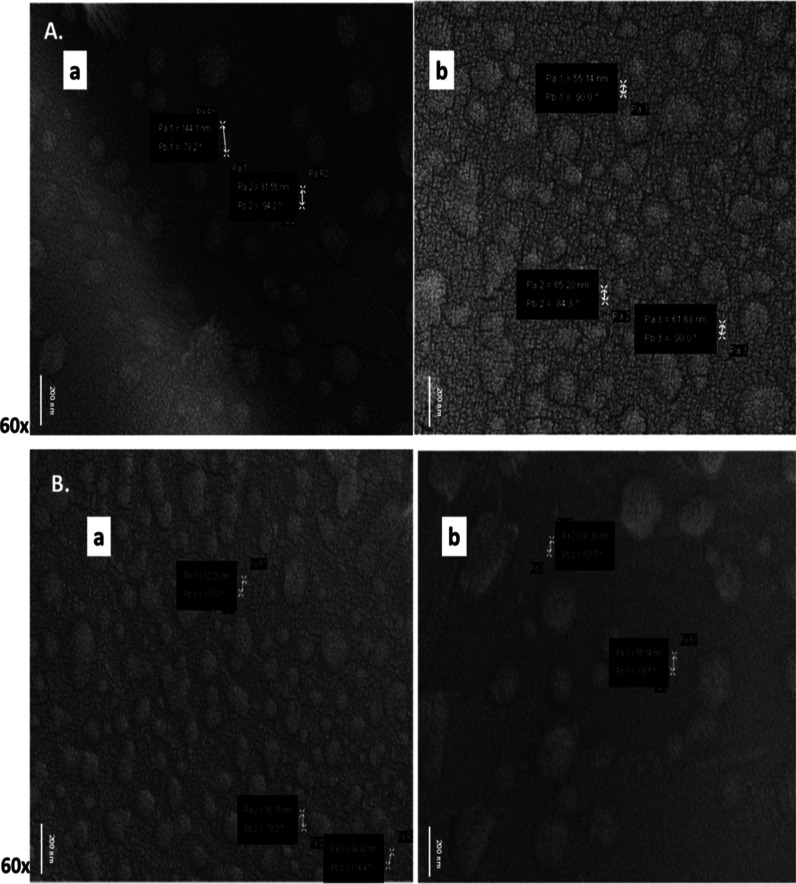
Identification of tumor-specific extracellular molecules may be used as a valuable diagnostic tool for the early detection of malignancy.31−34 To identify extracellular molecular phenotypes associated with laryngeal tumors, we isolated serum exosomes from LSCC patients and healthy volunteers. First, serum exosomes were characterized by scanning electron microscopy (SEM) analysis, and exosome shape and size were confirmed as shown in Figure 3. Specifically, under a scanning electron microscope, exosomes from LSCC patients (Figure 3A) and healthy controls (Figure 3B) were found to be between 10 and 140 nm in diameter and round-shaped cystic vesicles, in line with previous studies.35,36
Figure 3.
Controls (A) and LSCC (B) patient serum exosomes examined by SEM. The white arrow shows particle size. P: particle, Pa: particle size in nm, Pb: particle angle and, Pa R: particle radius. (by Carl Zeiss Evo 40 SEM; Jena Germany).
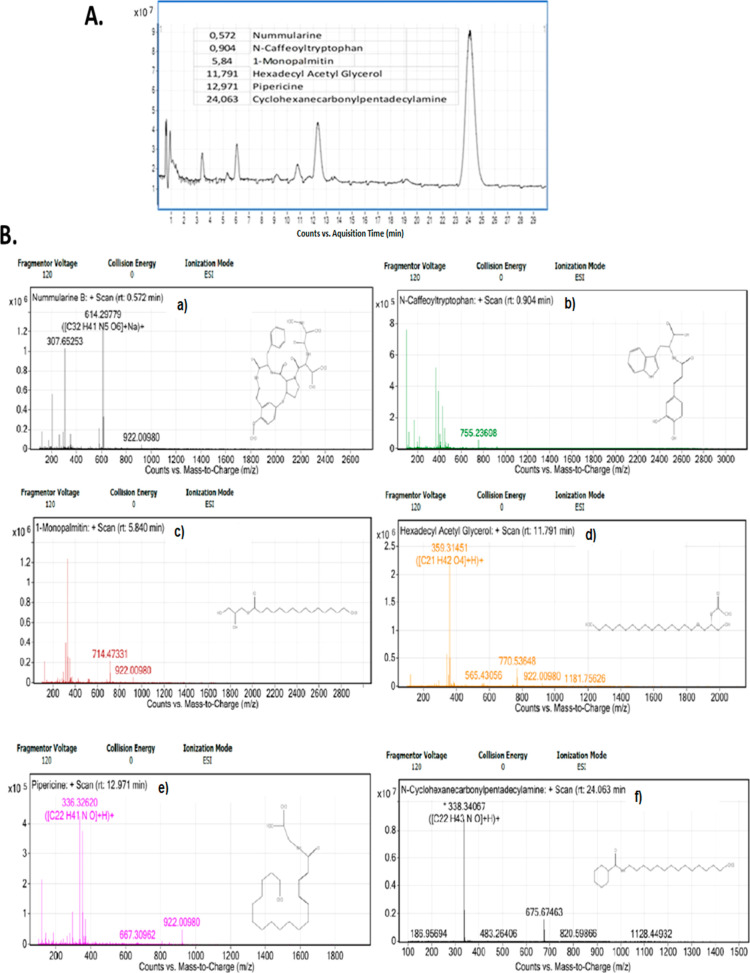
Subsequently, to determine exosome content of the obtained high-purity exosome, quadrupole time-of-flight (QTOF) mass spectrometry was performed in the serum of LSCC patients. The QTOF result is shown in Figure 4. According to the results, we present novel findings of 6 new lipid species, such as nummularine, N-caffeoyltryptophan, 1-monopalmitin, hexadecyl acetyl glycerol, pipericine, and cyclohexanecarbonyl-pentadecylamine (Figure 4A,B). While some of them like nummularine and N caffeoyltryptophan can be found in the diet of patients, we need future experiments to clarify whether they came from cancer or diet of patients.
Figure 4.
Diagram depicts the total results from QTOF analysis (A). Diagrams (B) depict the QTOF of each molecule: (a) nummularine, (b) N-caffeoyltryptophan, (c) 1-monopalmitin, (d) hexadecyl acetyl glycerol, (e) pipericine, and (f) cyclohexanecarbonylpentadecylamine (Agilent 6530 QTOF).
Biochemical Parameters in the Serum of LSCC Patients
We performed a biochemical analysis to evaluate several serum biochemical parameters for LSCC diagnosis. Specifically, biochemical analyses were performed to examine whether serum obtained from the LSCC patients differed from that of healthy individuals. All data are given in Table 2.
Table 2. Biochemical Results of Serum Parameters in LSCC and Healthy Subjects.
| biochemical parameters | control (mean ± SD) | LSCC (mean ± SD) | p value (by t-test) |
|---|---|---|---|
| ALT | 28.37 ± 1.3 | 23.55 ± 0.84 | |
| AST | 24.06 ± 1.02 | 19.44 ± 0.97 | |
| LDH | 195.62 ± 8.65 | 204.44 ± 18.24 | |
| GGT | 22.81 ± 4.36 | 36.66 ± 2.1 | 0.45 |
| ALP | 63.27 ± 6.31 | 80 ± 6.45 | |
| CRP | 3.2 ± 0.26 | 8.59 ± 0.54 | 0.001 |
| ferritin | 37.75 ± 1.78 | 110.5 ± 9.4 | 0.001 |
| vitamin B12 | 288.81 ± 21.23 | 408.66 ± 27.6 | 0.001 |
| T3 | 2.59 ± 0.15 | 2.41 ± 0.12 | |
| T4 | 1.04 ± 0.1 | 1.09 ± 0.09 | |
| TSH | 1.52 ± 0.06 | 2.79 ± 0.23 | 0.001 |
Aminotransaminases, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), are expressed by non-cancerous and cancerous cells by different mechanisms and are strongly involved in cellular metabolism and cancer cell turnover.37 For this reason, changes in ALT and AST values were examined in our study. First, when the ALT values were examined, it was observed that there was no difference between the cancer group and the control group. There was a slight decrease in the ALT value of the LSCC group (23.55 ± 0.84 IU/L), but the findings were not statistically significant. AST values show decreased 19.44 ± 0.97 IU/L in the LSCC group (p > 0.5; by t-test).
The lactate dehydrogonase (LDH) level is a marker of many diseases and is among the parameters routinely examined in disease pathogenesis. There are many studies in which an increase in the LDH level is associated with cancer.38 When the LDH levels of LSCC groups were compared with the control group, a significant increase was observed (204.44 ± 18.24 IU/L). However, the increase was not above the normal level of human LDH (90–220 IU/L). Therefore, we cannot associate our findings with LSCC.
The γ-glutamyl transferase (GGT) level showed an increase in the LSCC group compared to the control group (36.66 ± 2.1 IU/L P < 0.05). The GGT value in the control group was 22.81 ± 4.36 IU/L.
Alkaline phosphatase (ALP) contains a group of enzymes that catalyze the hydrolysis of phosphate esters in an alkaline environment.39 Changes in serum ALP levels are disease-related like other parameters. Our analysis revealed an increase in the ALP value in the LSCC group compared to the control group. This increase was determined as approximately 0.78-fold (p < 0.05; by t-test).39
C-reactive protein (CRP), like other parameters, is routinely measured before and during clinical treatments.40 CRP values in the LSCC group were increased to 8.59 ± 0.54 mg/dL and were statistically significant compared to controls (p < 0.01; by t-test).
Serum ferritin is elevated during chronic and acute inflammation.40,41 The ferritin value was found as 37.75 ± 1.78 μg/L in the control group, while the ferritin value in the LSCC group was found as 110.5 ± 9.4 μg/L. Although a significant increase was observed in the LSCC group compared to the controls, this value was within the normal ferritin values.41
High levels of vitamin B12 are associated with solid cancers. According to the findings we obtained, there was an approximately 2-fold increase in the level of vitamin B12 in the LSCC group compared to the control group. Specifically, the value of vitamin B12 in the LSCC group was determined as 408.66 ± 27.6 pg/mL (p < 0.05 by t-test).
Thyroid hormones: No change was observed in T3 and T4 levels between our control and patient groups. Both the T3 and T4 values of LSCC and control groups were found to be within the normal range. While the TSH level was 1.52 ± 0.06 ng/mL in the control group, it was 2.79 ± 0.23 ng/mL in the LSCC group. Although there appears to be a significant increase in the TSH levels of the LSCC group compared to the control, the TSH level remains within normal limits (0.35–4.5 mU/mL).
Overall, our data from the biochemical analysis showed that CRP and vitamin B12 levels may be useful parameters for LSCC diagnosis. Although AST, ALT, LDH, GGT, ALP, ferritin, T3, T4, and TSH represent easily measurable, blood-based biomarkers that are routinely analyzed before initiating therapy and have important roles in cancer diagnosis,42 our findings show that only vitamin B12 and CRP parameters were statistically significantly increased (>2-fold higher) in LSCC patients compared to the control group. Based on other investigators, it was found that CRP and vitamin B12 levels may increase dramatically in patients with cancer.43,44 In particular, several recent studies have investigated the relationship between serum CRP levels and survival of HNSCC patients and showed elevated CRP levels.45−48 In addition, it was reported that there is a higher prevalence of cancer in patients with vitamin B12 levels compared with patients with normal vitamin B12 levels.49−51 Two cohort studies have shown that patients with high plasma vitamin B12 levels have a 6 to 15 times higher risk of short-term cancer compared to the general population in Denmark.51,52 Here, we show an association between vitamin B12 increase and LSCC. Our findings may suggest that elevated vitamin B12 levels may be a prodromal sign of undiagnosed cancer.
Serum Exosomal mRNA and miRNA Phenotypes in LSCC
To identify serum exosomal phenotypes of specific miRNA and mRNA markers, we performed qPCR analyses. Our analysis revealed significant deregulations of specific miRNA and mRNA phenotypes in LSCC compared to healthy controls. Specifically, exosomal HBD and PTEN expression analysis by qPCR revealed a significant decrease of PTEN transcriptional levels in LSCC versus controls (p < 0.05; PTEN mRNAs; p = 0.79; HBD mRNAs; by t-test; GraphPad Prism 7.0) (Figure 5). Like the PTEN transcriptional levels, qPCR analysis revealed a significant decrease of serum exosomal miR-223 and miR-146 in LSCC compared to healthy controls (p < 0.05; by t-test) (Figure 5). However, unlike miR-223 and miR-146, our analysis showed a significant increase of serum exosomal miR-21 levels in LSCC versus controls (p < 0.001; by t-test; GraphPad Prism 7.0) (Figure 5).
Figure 5.
Serum exosomal miRNA-223, miRNA-146, and miRNA-21 levels (A), and PTEN and HBD mRNA levels (B) in serum LSCC and healthy controls (*p < 0.05; **p < 0.001; by t-test; GraphPad Prism 7.0). (miRNA levels were normalized to the RNU6 reference control; mRNA levels were normalized to the β-actin reference gene).
Overall, our novel data show significantly decreased levels of exosomal miR-223 and miR-146 in LSCC relative to healthy controls, similar to PTEN transcriptional levels, suggesting a possible tumor suppressor role of these two markers. MiR-223 is an evolutionarily conserved anti-inflammatory microRNA expressed primarily in myeloid cells.53 Recent studies suggest that miR-223 is expressed endogenously or transferred from exosomes to non-phagocytic cells.53 In cancerous cells, miR-223 acts as a tumor suppressor, suppressing the inflammatory tumor microenvironment and modulating the malignancy of cancer cells, thereby inhibiting tumorigenesis.53 Similarly, the tumor suppressor role of miR-146a has been previously suggested by others.22,29,30 Although extensive analysis is required to verify our data, the decreased expression profiles of serum exosomal miRNA-223 and miR-146 may be characteristic indicators of LSCC.
On the other hand, our data showed that the miR-21 expression level is significantly increased in LSCC, consistent with previous explorations.6,8,10,54,55 Upregulation of miR-21 has been previously shown to affect the proliferation of head and neck cancer cells,54 supporting the role of miR-21 as an “oncomir” in LSCC. In parallel, our data showed that PTEN mRNAs are downregulated in LSCC compared to controls. The PTEN is a known tumor suppressor that negatively regulates PI3K/AKT signaling, and thus its downregulation can increase cell proliferation in LSCC.56 Previous studies have supported that miR-21 can negatively regulate PTEN expression.12−14 Here, our data support a possible negative regulatory role of miR-21 on PTEN in LSCC, encouraging further investigation. Identification of negative regulators of PTEN is of high importance, particularly in the prevention and management of LSCC.
Conclusions
Although limitations of the study may include the small number of subjects, the analyzed miRNA markers, and genes, our data support that the combination of reduced serum exosomal miRNA-223 and miR-146 expression levels and biochemical alterations in CRP and vitamin B12 levels may be useful indicators of LSCC. The role of specific miRNAs, including miR-21, has been previously discussed in HNSCC and laryngeal carcinogenesis.5,6,10 However, here, we show that exosomal miR-21 is significantly increased in serum of LSCC patients. Our findings suggest the role of miRNA-21 in monitoring LSCC patients, which needs to be validated by more extensive studies. In parallel, our observations suggest a possible negative regulatory effect of miR-21 on PTEN in LSCC, encouraging a more extensive investigation on its role. Our findings also suggest a broader investigation of exosomal miRNA-223, miR-146, and miR-21 profiles and biochemical changes in laryngeal cancer including different classifications, such as severity, diversity, malignancy, and volume of cancer.
Methods
Chemicals and Reagents
Exosome isolation was performed with the total exosome isolation reagent (from serum) (Thermo Fisher; Massachusetts, ABD). MicroRNA synthesis was performed with a TaqMan microRNA reverse transcription kit (Thermo Fisher; Massachusetts, ABD). Primers and regents for gene expression and miRNA analyses by real-time PCR were obtained from Roche (Darmstadt, Germany) and described below (gene expression analysis and microRNA expression analysis, respectively).
Selection of Laryngeal Cancer Patients
Endoscopic examination revealed a laryngeal mass in 10 patients [6 males and 4 females; mean age 60.9 (SD ± 10.2)] who were admitted to the Bilecik Training and Research Hospital Otorhinolaryngology-Head and Neck Surgery outpatient clinic with complaints of hoarseness and a feeling of fullness in the throat. Criteria for selection of LSCC patients or healthy subjects were included, such as smoking history but no chronic or acute disease or human papilloma virus diagnosis. Stage I–III patients who received radiotherapy with or without chemotherapy, patients with a history of cancer in another organ, and patients with acute or chronic diseases were excluded from the study. Healthy controls of 10 patients without current history of head and neck malignancy were also included [5 males and 5 females; mean age 58.2 (SD ± 7.5). Health control and LSCC groups were of similar sex (p = 0.65, by χ2 test) and age (p = 0.57 by Student’s t-test; p < 0.05 represents the statistical significance level). A neck MRI was performed, while biopsies obtained during direct laryngoscopy were submitted for pathologic evaluation to reveal diagnosis of LSCC.
Ethical Permission
This study was approved by the Ethics Committee of Bilecik Seyh Edebali University Faculty of Medicine as non-invasive clinical research. All patients gave informed consent. The ethical approval number of this study is E-10333602-050.01.04-126102.
Blood Collection and Serum Isolation
Blood collection was performed after the diagnosis was received and before any treatment was initiated. Specifically, after the histopathological diagnosis, the blood obtained from patients was divided into two parts. One part was used for hemogram and biochemical analysis (Beckman coulter au5800, Brea CA, USA), and the other part was used for exosome and miRNA analysis.
Specifically, we collected 2 × 2 mL of whole blood from each patient and kept at room temperature for 30 min until clotting. Clotted blood was centrifuged at 2600 rpm for 10 min, and serum was shared in aliquots and used for subsequent exosome isolation and biochemical and molecular analysis.
Exosome Isolation and Characterization
Blood serum obtained from patients and healthy controls was first centrifuged at 1000g to remove debris. Then, after applying the exosome isolation kit procedure (Total Exosome Isolation Reagent; Thermo Fisher; Massachusetts, ABD), isolation was performed by centrifugation at 10,000g for 30 min.35
Pelleted exosomes were re-suspended in PBS. The solution was coated to an aluminum plate (SPI Supplies/Structure Probe, Inc., USA) and dried at 30°. The dimensions of exosomes were assessed by SEM, and images were taken with a Carl Zeiss Evo 40 SEM device (Jena, Germany) under high vacuum and 20 kV EHT.
Biochemical Analysis
An experienced team of clinicians from Bilecik Seyh Edebali University Faculty of Medicine prepared the patients’ medical records. Analyses of serum biochemical parameters, such as ALT, AST, LDH, GGT, ALP, CRP, ferritin, vitamin B12, T3, and T4, were performed after the diagnosis was received and before any treatment was initiated. Data were obtained from 20 subjects (10 patients and 10 healthy subjects).
Gene Expression Analysis
Total RNA was extracted from the exosomes with the High Pure RNA isolation kit (Roche Mannheim Germany). The exosome capsule was removed with absolute methanol. mRNA was purified by special filters. We performed a real-time polymerase chain reaction (PCR) using a high-capacity first-strand cDNA synthesis kit for RT-PCR (AMW) by Roche (Darmstadt, Germany) and specific primers, and their sequences are listed below (Roche; Darmstadt, Germany) (protocol: initial step of 94 °C for 5 min followed by 40 cycles of 94 °C for 30 s, 56 °C for 45 s, and 72 °C for 60 s). Results were expressed as relative mRNA expression changes (fold changes) between the study group (patients) and the control group (healthy subjects). We normalized the mRNA expression of target genes to beta-actin (reference control gene) using the ΔΔCt method (Table 3).39,41
Table 3. Human Primers for Target and Reference Genes and miRNA Expression and Their Amplicon Size, as Well as PCR Conditions.
| target and control | primer sequence 5′–3′ | amplicon size bp |
|---|---|---|
| β-actin | forward: 5′-CCAACCGCGAGAAGATGA-3′ | 163 |
| reverse: 5′-CCAGAGGCGTACAGGGATAG-3′ | ||
| HBD-1 | forward: 5′-CTTGACTGTGGCACCTCCCTTCAG-3′ | 184 |
| reverse: 5′-GCAGCTACAAGCCATGAGTCTG-3′ | ||
| PTEN | forward: 5′-TGAGTTCCCTCAGCCGTTACCT-3′ | 181 |
| reverse: 5′-GAGGTTTCCTCTGGTCCTGGTA-3′ | ||
| RNU6 | forward: 5′-GTGCTCGCTTCGGCAGCA-3′ | 93 |
| reverse: 5′-CAAAATATGGAACGCTT-3′ | ||
| miR-146 | forward: 5′-TGAGAACTGAATTCCATGGGTT-3′ | 54 |
| reverse: 5′-CTGAAGAACTGAATTTCAGAGG-3′ | ||
| miR-223 | forward: 5′-ATTCCGGTAGTAACGTTGCGGGGTATTTG-3′ | 60 |
| reverse: 5′-ATTCCGGTAG-TAACGTTGC-3′ |
MicroRNA Expression Analysis
Total miRNA was extracted from serum exosomes. Total miRNA was used to synthesize complementary DNA (cDNA) using a high-capacity miRNA reverse transcription kit (TaqMan microRNA reverse transcription kit, Thermo Fisher Massachusetts, ABD). MicroRNA analysis was performed by real-time PCR, using TaqMan microRNA assays (Thermo Fisher, Massachusetts, ABD) and specific mature miRNA primers, and their sequences are listed below (Roche; Darmstadt, Germany) (protocol: initial step of 94 °C for 5 min followed by 40 cycles of 94 °C for 30 s, 56 °C for 35 s, and 72 °C for 30 s). Results were expressed as relative miRNA expression changes (fold changes) between the study group (patients) and the control group (healthy subjects). We normalized the miRNA expression [10] of target miRNAs to the RNU6 reference control using the ΔΔCt method, as previously described (Table 3).57,58
QTOF Mass Spectrometry
Liquid chromatography QTOF mass spectrometry analysis was performed with an Agilent 6530 QTOF (Agilent Technologies, Kista, Sweden). QTOF analyses of exosome samples were performed for both the study group (LSCC patients) and the control group. Analyzes and database searches were performed as service procurement from Atatürk University Eastern Anatolia High Technology Application and Research Center (DAYTAM). Mass spectrometric data were obtained in the positive electrospray ionization mode.
Statistical Analyses
Statistical comparison between groups was calculated using the t-test and Tukey HSD method (p < 0.05). All calculations were performed using SPSS 26 software for statistical analysis. Mean and standard deviation (mean ± SD) were calculated for serum biochemical parameters.
Author Contributions
Conceptualization, A.T., S.G., R.D., D.P.V, P.G.D, S.G.D, A.T, and N.C; methodology, S.G., T.Y., D.P.V, N.C., and M.T.; formal analysis, A.T., R.D., T.Y., M.T., D.P.V, P.G.D, S.G.D, N.C., and A.T.; investigation, A.T. S.G, D.P.V, N.C, S.G.D., and P.G.D; resources, S.G., T.Y., and M.T.; data curation, A.T., R.D., M.T., and A.T.; writing—original draft preparation, S.G., T.Y., R.D., D.P.V., P.G.D., and N.C.; writing—review and editing, D.P.V., A.T., and S.G.D.; and visualization, S.G., D.P.V., and A.T.
This research received no external funding.
The authors declare no competing financial interest.
Notes
Institutional Review Board Statement: This study was performed and approved by the Ethics Committee of BilecikSeyhEdebali University, Bilecik, Turkey (date of approval: 06.10.2022/protocol code no. E-10333602-050.01.04-126102).
Notes
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Notes
Raw data are available on request.
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68, 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Rubinstein M.; Armstrong W. b. Transoral laser microsurgery for laryngeal cancer: a primer and review of laser dosimetry. Lasers Med. Sci. 2011, 26, 113–124. 10.1007/s10103-010-0834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Fisher S. G.; Mohideen N.; Emami B. Second primary cancers in patients with laryngeal cancer: a population-based study. Int. J. Radiat. Oncol., Biol., Phys. 2003, 56, 427–435. 10.1016/s0360-3016(02)04613-8. [DOI] [PubMed] [Google Scholar]
- Sethi N.; Wright A.; Wood H.; Rabbitts P. MicroRNAs and head and neck cancer: reviewing the first decade of research. Eur. J. Cancer 2014, 50, 2619–2635. 10.1016/j.ejca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Vageli D. P.; Doukas S. G.; Doukas P. G.; Judson B. L. Bile reflux and hypopharyngeal cancer (Review). Oncol. Rep. 2021, 46, 244. 10.3892/or.2021.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C. T.; Vageli D. P. miR-21, miR-155, miR-192, and miR-375 Deregulations Related to NF-kappaB Activation in Gastroduodenal Fluid-Induced Early Preneoplastic Lesions of Laryngeal Mucosa In Vivo. Neoplasia 2016, 18, 329–338. 10.1016/j.neo.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas P. G.; Vageli D. P.; Doukas S. G.; Sasaki C. T. Temporal characteristics of NF-kappaB inhibition in blocking bile-induced oncogenic molecular events in hypopharyngeal cells. Oncotarget 2019, 10, 3339–3351. 10.18632/oncotarget.26917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C. T.; Doukas S. G.; Doukas P. G.; Vageli D. P. The Progressive Mutagenic Effects of Acidic Bile Refluxate in Hypopharyngeal Squamous Cell Carcinogenesis: New Insights. Cancers 2021, 13, 852. 10.3390/cancers13040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C. T.; Doukas S. G.; Doukas P. G.; Vageli D. P. Weakly Acidic Bile Is a Risk Factor for Hypopharyngeal Carcinogenesis Evidenced by DNA Damage, Antiapoptotic Function, and Premalignant Dysplastic Lesions In Vivo. Cancers 2021, 13, 852. 10.3390/cancers13040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki C. T.; Doukas S. G.; Costa J.; Vageli D. P. Biliary reflux as a causal factor in hypopharyngeal carcinoma: New clinical evidence and implications. Cancer 2019, 125, 3554–3565. 10.1002/cncr.32369. [DOI] [PubMed] [Google Scholar]
- Vageli D. P.; Kasle D.; Doukas S. G.; Doukas P. G.; Sasaki C. T. The temporal effects of topical NF-kappaB inhibition, in the in vivo prevention of bile-related oncogenic mRNA and miRNA phenotypes in murine hypopharyngeal mucosa: a preclinical model. Oncotarget 2020, 11, 3303–3314. 10.18632/oncotarget.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N. J.; Zhou H.; Elashoff D.; Henson B. S.; Kastratovic D. A.; Abemayor E.; Wong D. T. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. 10.1158/1078-0432.ccr-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. H.; Yi H. S.; Kim Y.; Kroh E. M.; Chien J. W.; Eaton K. D.; Goodman M. T.; Tait J. F.; Tewari M.; Pritchard C. C. Plasma processing conditions substantially influence circulating microRNA biomarker levels. PLoS One 2013, 8, e64795 10.1371/journal.pone.0064795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes L. M. R. B.; De Carvalho A. C.; Melendez M. E.; Lopes-Carvalho A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. 10.1080/14737159.2017.1404906. [DOI] [PubMed] [Google Scholar]
- Nowicka Z.; Stawiski K.; Tomasik B.; Fendler W. Extracellular miRNAs as Biomarkers of Head and Neck Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 4799. 10.3390/ijms20194799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo J. D.; Chevillet J. R.; Kroh E. M.; Ruf I. K.; Pritchard C. C.; Gibson D. F.; Mitchell P. S.; Bennett C. F.; Pogosova-Agadjanyan E. L.; Stirewalt D. L.; Tait J. F.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 5003–5008. 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. C.; Xu Z.; Zhang T. F.; Wang Y. L. Circulating microRNAs as diagnostic and prognostic tools for hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 9853–9862. 10.3748/wjg.v21.i34.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear J. W.; Street J. M.; Bailey M. A. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 2013, 13, 1572–1580. 10.1002/pmic.201200285. [DOI] [PubMed] [Google Scholar]
- Gallo A.; Tandon M.; Alevizos I.; Illei G. G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 2012, 7, e30679 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.; Gu Y.; Du Y.; Liu J. Exosomes: Diagnostic Biomarkers and Therapeutic Delivery Vehicles for Cancer. Mol. Pharm. 2019, 16, 3333–3349. 10.1021/acs.molpharmaceut.9b00409. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Liu T.; Zhou M. Immune-Cell-Derived Exosomes for Cancer Therapy. Mol. Pharm. 2022, 19, 3042–3056. 10.1021/acs.molpharmaceut.2c00407. [DOI] [PubMed] [Google Scholar]
- Zhang H. G.; Grizzle W. E. Exosomes and cancer: a newly described pathway of immune suppression. Clin. Cancer Res. 2011, 17, 959–964. 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S. B.; Zhang H.; Cai T. T.; Liu Y. N.; Ni J. J.; He J.; Peng J. Y.; Chen Q. Y.; Mo H. Y.; Jun-Cui; Zhang X. S.; Zeng Y. X.; Li J. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340. 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- Takeuchi T.; Kawasaki H.; Luce A.; Cossu A. M.; Misso G.; Scrima M.; Bocchetti M.; Ricciardiello F.; Caraglia M.; Zappavigna S. Insight toward the MicroRNA Profiling of Laryngeal Cancers: Biological Role and Clinical Impact. Int. J. Mol. Sci. 2020, 21, 3693. 10.3390/ijms21103693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Liu M.; Tang Q.; Qiu M.; Lang N.; Li M.; Zheng Y.; Bi F. miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett. 2011, 585, 2998–3005. 10.1016/j.febslet.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Si M. L.; Zhu S.; Wu H.; Lu Z.; Wu F.; Mo Y. Y. miR-21-mediated tumor growth. Oncogene 2007, 26, 2799–2803. 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- Xiong B.; Cheng Y.; Ma L.; Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int. J. Oncol. 2013, 42, 219–228. 10.3892/ijo.2012.1707. [DOI] [PubMed] [Google Scholar]
- Huang S.; He R.; Rong M.; Dang Y.; Chen G. Synergistic effect of MiR-146a mimic and cetuximab on hepatocellular carcinoma cells. BioMed Res. Int. 2014, 2014, 384121–384215. 10.1155/2014/384121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z.; Yin H.; Chen C.; Dai X.; Li X.; Liu B.; Fang X. microRNA-146a targets the L1 cell adhesion molecule and suppresses the metastatic potential of gastric cancer. Mol. Med. Rep. 2012, 6, 501–506. 10.3892/mmr.2012.946. [DOI] [PubMed] [Google Scholar]
- Głuszko A.; Szczepański M. J.; Whiteside T. L.; Reichert T. E.; Siewiera J.; Ludwig N. Small Extracellular Vesicles from Head and Neck Squamous Cell Carcinoma Cells Carry a Proteomic Signature for Tumor Hypoxia. Cancers 2021, 13, 4176. 10.3390/cancers13164176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiah S. G.; Chou S. T.; Chang J. Y. MicroRNAs: Their Role in Metabolism, Tumor Microenvironment, and Therapeutic Implications in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5604. 10.3390/cancers13225604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C.; Song F.; Zheng Y. L.; Lv J.; Wang Q. F.; Xu N. Exosomes in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 894. 10.3389/fonc.2019.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. H.; Chen Y. C. Clinical significance of exosomes as potential biomarkers in cancer. World J. Clin. Cases 2019, 7, 171–190. 10.12998/wjcc.v7.i2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S.; Pennisi M.; Yeni Y.; Yildirim S.; Gattuso G.; Altinoz M. A.; Taghizadehghalehjoughi A.; Bolat I.; Tsatsakis A.; Hacımüftüoğlu A.; Falzone L. Potential Neurotoxic Effects of Glioblastoma-Derived Exosomes in Primary Cultures of Cerebellar Neurons via Oxidant Stress and Glutathione Depletion. Antioxidants 2022, 11, 1225. 10.3390/antiox11071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun Z.; Bhat A.; Sharma S.; Sharma A. Elucidating diversity of exosomes: biophysical and molecular characterization methods. Nanomedicine 2016, 11, 2359–2377. 10.2217/nnm-2016-0192. [DOI] [PubMed] [Google Scholar]
- Chen W.; Wang W.; Zhou L.; Zhou J.; He L.; Li J.; Xu X.; Wang J.; Wang L. Elevated AST/ALT ratio is associated with all-cause mortality and cancer incident. J. Clin. Lab. Anal. 2022, 36, e24356 10.1002/jcla.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic V.; Radenkovic S.; Konjevic G. The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 115–124. 10.1007/978-94-017-7215-0_8. [DOI] [PubMed] [Google Scholar]
- Celebi D.; Taghizadehghalehjoughi A.; Baser S.; Genc S.; Yilmaz A.; Yeni Y.; Yesilyurt F.; Yildirim S.; Bolat I.; Kordali S.; Yilmaz F.; Hacimuftuoglu A.; Celebi O.; Margina D.; Nitulescu G. M.; Spandidos D. A.; Tsatsakis A. Effects of boric acid and potassium metaborate on cytokine levels and redox stress parameters in a wound model infected with methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2022, 26, 294. 10.3892/mmr.2022.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria T. G.; Vasileios K. E.; Panagiotis P. S.; Kostas S. N. Changes of acute-phase protein levels in the serum of lung cancer patients following radiotherapy. Int. J. Clin. Exp. Med. 2013, 6, 50–56. [PMC free article] [PubMed] [Google Scholar]
- Yeni Y.; Cakir Z.; Hacimuftuoglu A.; Taghizadehghalehjoughi A.; Okkay U.; Genc S.; Yildirim S.; Saglam Y. S.; Calina D.; Tsatsakis A.; et al. A Selective Histamine H4 Receptor Antagonist, JNJ7777120, Role on glutamate Transporter Activity in Chronic Depression. J. Pers. Med. 2022, 12, 246. 10.3390/jpm12020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajertehran F.; Ayatollahi H.; Jafarian A. H.; Khazaeni K.; Soukhtanloo M.; Shakeri M. T.; Mohtasham N. Overexpression of Lactate Dehydrogenase in the Saliva and Tissues of Patients with Head and Neck Squamous Cell Carcinoma. Rep. Biochem. Mol. Biol. 2019, 7, 142–149. [PMC free article] [PubMed] [Google Scholar]
- Hart P. C.; Rajab I. M.; Alebraheem M.; Potempa L. A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. 10.3389/fimmu.2020.595835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe V.; Chabrun F.; Lacout C.; Ghali A.; Capitain O.; Patsouris A.; Lavigne C.; Urbanski G. Persistent elevation of plasma vitamin B12 is strongly associated with solid cancer. Sci. Rep. 2021, 11, 13361. 10.1038/s41598-021-92945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE Paz D.; Young C. K.; Chien H. T.; Tsao C. K.; Fok C. C.; Fan K. H.; Liao C. T.; Wang H. M.; Kang C. J.; Chang J. T.; Huang S. F. Prognostic Roles of SCC Antigen, CRP and CYFRA 21-1 in Oral Cavity Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 2025–2033. 10.21873/anticanres.13313. [DOI] [PubMed] [Google Scholar]
- Lino-Silva L. S.; Salcedo-Hernández R. A.; García-Pérez L.; Meneses-García A.; Zepeda-Najar C. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res. 2017, 27, 140–144. 10.1097/CMR.0000000000000333. [DOI] [PubMed] [Google Scholar]
- Diem S.; Schmid S.; Krapf M.; Flatz L.; Born D.; Jochum W.; Templeton A. J.; Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Bagley S. J.; Kothari S.; Aggarwal C.; Bauml J. M.; Alley E. W.; Evans T. L.; Kosteva J. A.; Ciunci C. A.; Gabriel P. E.; Thompson J. C.; Stonehouse-Lee S.; Sherry V. E.; Gilbert E.; Eaby-Sandy B.; Mutale F.; DiLullo G.; Cohen R. B.; Vachani A.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Arendt J. F.; Nexo E. Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS One 2012, 7, e45979 10.1371/journal.pone.0045979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brah S.; Chiche L.; Mancini J.; Meunier B.; Arlet J. B. Characteristics of patients admitted to internal medicine departments with high serum cobalamin levels: results from a prospective cohort study. Eur. J. Intern. Med. 2014, 25, e57–e58. 10.1016/j.ejim.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Chiche L.; Jean R.; Romain F.; Roux F.; Thomas G.; Canavese S.; Branger S.; Harlé J. R.; Durand J. M. Clinical implications of high cobalamin blood levels for internal medicine]. Rev. Med. Interne 2008, 29, 187–194. 10.1016/j.revmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ryg J.; Nybo M.; Hallas J. Cancer incidence in persons with elevated cobalamin levels. Eur. J. Clin. Invest. 2013, 43, 557–561. 10.1111/eci.12076. [DOI] [PubMed] [Google Scholar]
- Jeffries J.; Zhou W.; Hsu A. Y.; Deng Q. miRNA-223 at the crossroads of inflammation and cancer. Cancer Lett. 2019, 451, 136–141. 10.1016/j.canlet.2019.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas S. G.; Vageli D. P.; Lazopoulos G.; Spandidos D. A.; Sasaki C. T.; Tsatsakis A. The Effect of NNK, A Tobacco Smoke Carcinogen, on the miRNA and Mismatch DNA Repair Expression Profiles in Lung and Head and Neck Squamous Cancer Cells. Cells 2020, 9, 1031. 10.3390/cells9041031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas S. G.; Vageli D. P.; Doukas P. G.; Nikitovic D.; Tsatsakis A.; Judson B. L. The Effect of Tobacco Smoke N-Nitrosamines, NNK and NDEA, and Nicotine, on DNA Mismatch Repair Mechanism and miRNA Markers, in Hypopharyngeal Squamous Cell Carcinoma: An In Vivo Model and Clinical Evidence. Curr. Oncol. 2022, 29, 5531–5549. 10.3390/curroncol29080437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. Y.; Zhang Y. Y.; Zhu B. L.; Feng F. Z.; Yan H.; Zhang H. Y.; Zhou B. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4149–4155. 10.26355/eurrev_201905_17917. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Zhang Q.; Gu M.; Zhang K.; Xia T.; Zhang S.; Chen W.; Yin H.; Yao H.; Fan Y.; Pan S.; Xie H.; Liu H.; Cheng T.; Zhang P.; Zhang T.; You B.; et al. MIR106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma. Autophagy 2021, 17, 1667–1683. 10.1080/15548627.2020.1781368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.; Wan X.; Alvarez A. A.; James C. D.; Song X.; Yang Y.; Sastry N.; Nakano I.; Sulman E. P.; Hu B.; et al. MIR93 (microRNA -93) regulates tumorigenicity and therapy response of glioblastoma by targeting autophagy. Autophagy 2019, 15, 1100–1111. 10.1080/15548627.2019.1569947. [DOI] [PMC free article] [PubMed] [Google Scholar]