Abstract
Objective
Prior to the coronavirus disease 2019 (COVID-19) pandemic, influenza was the most frequent cause of viral respiratory pneumonia requiring intensive care unit (ICU) admission. Few studies have compared the characteristics and outcomes of critically ill patients with COVID-19 and influenza.
Methods
This was a French nationwide study comparing COVID-19 (March 1, 2020–June 30, 2021) and influenza patients (January 1, 2014–December 31, 2019) admitted to an ICU during pre-vaccination era. Primary outcome was in-hospital death. Secondary outcome was need for mechanical ventilation.
Results
105,979 COVID-19 patients were compared to 18,763 influenza patients. Critically ill patients with COVID-19 were more likely to be men with more comorbidities. Patients with influenza required more invasive mechanical ventilation (47 vs. 34%, p < 0·001), vasopressors (40% vs. 27, p < 0·001) and renal-replacement therapy (22 vs. 7%, p < 0·001). Hospital mortality was 25% and 21% (p < 0·001) in patients with COVID-19 and influenza, respectively. In the subgroup of patients receiving invasive mechanical ventilation, ICU length of stay was significantly longer in patients with COVID-19 (18 [10–32] vs. 15 [8–26] days, p < 0·001). Adjusting for age, gender, comorbidities, and modified SAPS II score, in-hospital death was higher in COVID-19 patients (adjusted sub-distribution hazard ratio [aSHR]=1.69; 95%CI=1.63–1.75) compared with influenza patients. COVID-19 was also associated with less invasive mechanical ventilation (aSHR=0.87; 95%CI=0.85–0.89) and a higher likelihood of death without invasive mechanical ventilation (aSHR=2.40; 95%CI=2.24–2.57).
Conclusion
Despite younger age and lower SAPS II score, critically ill COVID-19 patients had a longer hospital stay and higher mortality than patients with influenza.
Keywords: Intensive care unit, COVID-19, Influenzae, Nationwide study
Introduction
After the first cases were diagnosed in December, 2019, coronavirus disease 2019 (COVID-19) rapidly emerged worldwide. It was recognized as a pandemic by the World Health Organization (WHO) on March 11, 2020.1 Although most patients with COVID-19 remained asymptomatic or presented with a mild-course form, about 5% of symptomatic patients required admission to the intensive care unit (ICU) for acute respiratory failure (ARF) and received supportive care.2, 3 Among all hospital admissions for COVID-19, 18% of patients had been admitted in ICU in France during the first (between March 1 and June 30, 2020) and second (between July 1 and December 31, 2020) surges4 and 23% during the third surge (between January 1 to June 30, 2021).5 COVID-19 became one of the main reasons for admission to an ICU in France between March, 2020 and June, 2021.6, 7
Before COVID-19, influenza was the most frequent viral etiology of ARF in the ICU. As such, a previous influenza pandemic was used for modeling and preparing plans for future epidemics.8, 9 Person-to-person transmission by droplets and severe forms of ARF leading to acute respiratory disease syndrome (ARDS) are characteristics common to both influenza and COVID-19.10, 11 However, some discrepancies, such as baseline characteristics and outcomes, have been recorded in monocenter studies of patients displaying heterogeneous severity of wild-type SaRS CoV-2.12, 13, 14, 15, 16, 17, 18, 19, 20, 21
By using the French administrative health care database, we aimed to compare the clinical characteristics and outcomes of critically ill COVID-19 patients with a historical cohort admitted to an ICU for influenza pneumonia.
Material and method
Study design and participants
This claims study was performed using the French administrative health care database (Système National des Données de Santé, SNDS). The SNDS contains data on outpatient care (medical consultation, paramedical interventions, dispensing of reimbursed drugs) as well as data from the program for the medicalization of the information system (admission date, duration, ICD-10 codes for main and associated diagnosis, medical interventions) collected during hospital stay.22 All data were linked by means of a unique personal identification number.
We included all adult patients hospitalized in French ICUs from March 1, 2020 to June 30, 2021, for whom a complete hospital course was available. For the comparative group (influenza cohort), we included all adult patients hospitalized in an ICU from January 1, 2014 to December 31, 2019. To be included in one of these two groups, the patient should have had either one ICD-10 diagnosis code of COVID-19 or influenza. The complete list of ICD-10 diagnosis codes is available in Appendix 1.
Variables
Age, gender, and Simplified Acute Physiology Score (SAPS) II score23 at admission were collected for each inpatient stay. The SAPS II score is a severity score and mortality estimation tool developed from a large sample of medical and surgical patients in North America and Europe. It includes 17 variables: 12 physiology variables, age, type of admission (scheduled surgical, unscheduled surgical, or medical), and three underlying disease variables.23
We calculated the Charlson Comorbidity Index (CCI)24 based on all ICD-10 diagnoses collected. Several comorbidities were also recorded: arterial hypertension, diabetes mellitus, heart disease, chronic lung disease, cirrhosis, cancer, hematological malignancies, chronic kidney disease, and immunosuppression. Immunocompromised patients were defined as patients with agranulocytosis, medullar aplasia, immunodeficiency, cancer treated by chemotherapy, or solid organ transplants (ICD-10 diagnosis codes used to identify patients are available in Appendix 1).
We recorded the oxygenation and ventilation procedures used during hospitalization (according to the French Common Classification of Medical Procedures [CCAM]25): invasive mechanical ventilation, non-invasive mechanical ventilation (NIV), and high-flow nasal cannula therapy (HFNC). Some patients received more than one of the three oxygenation techniques, in which case the most invasive was retained for further analyses, assuming invasive mechanical ventilation to be more invasive than NIV and NIV to be more invasive than HFNC. We also recorded patients who received prone position or extracorporeal membrane oxygenation (ECMO).
We additionally documented whether the patients required renal replacement therapy (RRT) or shock-requiring vasopressors, or presented with venous thrombosis events, including pulmonary embolism, acute liver failure, and disseminated intravascular coagulation. The list of CCAM codes used to identify advanced life support therapies is available in Appendix 2. Patient outcomes included mechanical ventilation duration, ICU and hospital length of stay (LOS), and vital status at hospital discharge.
For patients admitted after January 1, 2021, we collected vaccination status. A full vaccination scheme was defined as more than 28 days after a single dose of Ad26. COV2-S vaccine (Covid-19 Vaccine Janssen®) or more than 7 days after the second dose of vaccine other than Ad26. COV2-S vaccine. A partial vaccination was defined as fewer than 28 days after a single dose of Ad26. COV2-S vaccine or fewer than 7 days after the second and/or after the first dose of vaccine other than Ad26. COV2-S. Patients were considered non-vaccinated if they did not receive any dose of any vaccine against Covid-19.
Ethics
The SNDS database was created by French law n°2016–41 on 26 January, 2016.26 The purpose of the database is to reuse claims data for research after names and social security numbers have been removed. The condition of use and forms of security that apply to the database are defined by the French government regulation dated 22 March, 2017.27 As part of its public statistics mission, the Directorate for Research, Studies, Assessment and Statistics (DREES) of the French Ministry of Health, has permanent access to the SNDS database. An internet page has information available to the public about the reuse of data from the database and their rights according to the European General Data Protection Regulation n° UE 2016/679 dated 27 April, 2016.28
Statistical analysis
Characteristics of patients were reported as frequencies and percentages for categorical variables and as medians and interquartile ranges for continuous variables. A Chi-square test and a Wilcoxon test were used, as appropriate, to compare characteristics between COVID-19 patients and influenza patients.
Risk factors of treatment with mechanical ventilation were identified through a competing risk framework (i.e., the Fine and Gray model) with ICU discharge alive or death in the ICU without intubation as competing events.29, 30 The strength of the association between a specific risk factor and the event of interest in the Fine-Gray model was measured by the sub-hazard ratio (SHR), which is the ratio of hazards associated with the cumulative incidence function in the presence and absence of the risk factor. We first computed SHR for invasive mechanical ventilation and 95% confidence intervals (CIs) associated with each of the risk factors in univariate analysis. Then we performed a multivariable analysis to adjust for the following predefined potential confounding factors: type of infection (COVID-19 vs. influenza), age, sex, arterial hypertension, diabetes mellitus, heart disease, chronic lung disease, cirrhosis, cancer, hematological malignancies, chronic kidney disease, immunosuppression, and modified SAPS II score. No pre-selection covariate procedure was performed because of the high number of events limiting the risk of overfitting. In the same way, we assessed the association between the type of infection (COVID-19 vs. influenza) and in-hospital mortality (considering that being discharged alive was a competing risk). We also performed a sensitivity analysis modeling the likelihood of death only in patients who received invasive mechanical ventilation. And finally, to specifically assess the impact of the vaccination programme, we performed an analysis including only patients admitted after January, 2021, when the vaccine was available to the entire French population.
A P value< 0.05 was considered significant. Analyses were computed using the SAS 2017 software (SAS Institute, Cary, NC, USA).
Results
Between March 1, 2020 and June 30, 2021, 105,979 COVID-19 patients were admitted to an ICU in France. These patients were compared to 18,763 patients with influenza admitted between January 1, 2014, and December 31, 2019.
Patients’ characteristics
The baseline characteristics of the influenza and COVID-19 population are described in Table 1. Most patients with COVID-19 were men (64%, n = 67,951) and 30% (n = 32,044) were younger than 60 years. Their median SAPS II score at admission was 3224, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 (Table 1). Patients with COVID-19 were more frequently men, and had a lower SAPS II score at ICU admission than patients with influenza (Table 1). The type of comorbidities differed between the two populations. Arterial hypertension, diabetes mellitus, chronic kidney disease, and solid tumors were more frequent in COVID-19 patients, while congestive heart disease, chronic respiratory disease, cirrhosis, and malignancies were more frequent in influenza patients.
Table 1.
Main characteristics at intensive care unit (ICU) admission.
| COVID-19 | INFLUENZA | p | |
|---|---|---|---|
| N = 105 979 | N = 18 763 | ||
| Age (years) | 67 (57–76) | 68 (58–78) | < 0·001 |
| Male gender | 67,951 (64·12%) | 10,590 (56·44%) | < 0·001 |
| Charlson comorbidity index | |||
| 0 | 69,886 (65·94%) | 12,006 (63·99%) | < 0·001 |
| 1–2 | 24,887 (23·48%) | 4870 (25·96%) | |
| 3–4 | 6748 (6·37%) | 1197 (6·38%) | |
| 5 and more | 4458 (4·21%) | 690 (3·68%) | |
| Comorbidities | |||
| Arterial hypertension | 38,608 (36·43%) | 4898 (26·10%) | < 0·001 |
| Diabetes mellitus | 5139 (4·85%) | 818 (4·36%) | 0·0038 |
| Congestive heart disease | 12,934 (12·20%) | 2978 (15·87%) | < 0·001 |
| Chronic respiratory disease | 10,766 (10·16%) | 3470 (18·49%) | < 0·001 |
| Chronic kidney disease | 8685 (8·20%) | 348 (1·85%) | < 0·001 |
| Cirrhosis | 957 (0·90%) | 209 (1·11%) | 0·0057 |
| Solid tumor | 2431 (2·29%) | 317 (1·69%) | < 0·001 |
| Malignancy | 1895 (1·79%) | 525 (2·80%) | < 0·001 |
| SAPS II score at ICU admission | 32 (24–41) | 39 (29–52) | < 0·001 |
SAPS II, Simplified Acute Physiology Score; ICU, intensive care unit.
Organ failure and support
The maximal level of respiratory support for patients with COVID-19 was invasive mechanical ventilation for 34% (n = 36,185), non-invasive mechanical ventilation for 6% (n = 6749), and HFNC therapy for 18% (n = 19,024) ( Table 2). Patients with influenza were more frequently treated with invasive mechanical ventilation (47% vs. 34%, p < 0·001). After adjustment for age, sex, comorbidities, and modified SAPS II score at admission, the Fine-Gray model revealed that COVID-19 was associated with a lower likelihood of treatment with invasive mechanical ventilation (aSHR = 0·87; 95%CI=0·85–0·89) as well as higher likelihood of death without invasive mechanical ventilation (aSHR = 2·40; 95%CI = 2·24–2·57) ( Table 3).
Table 2.
Management and outcomes.
| COVID-19 | INFLUENZA | p | |
|---|---|---|---|
| N = 105 979 | N = 18 763 | ||
| Maximal level of respiratory support | |||
| Invasive mechanical ventilation, n (%) | 36,185 (34·14%) | 8742 (46·59%) | < 0·001 |
| Non-invasive mechanical ventilation, n (%) | 6749 (6·37%) | 1578 (8·41%) | |
| High flow nasal cannula therapy, n (%) | 19,024 (17·95%) | 1625 (8·66%) | |
| Other oxygen therapy, n (%) | 44,021 (41·54%) | 6818 (36·34%) | |
| Organ failure and support during ICU stay | |||
| Tracheotomy, n (%) | 2297 (2·17%) | 524 (2·79%) | < 0·001 |
| Prone position, n (%) | 20,231 (19·09%) | 2239 (11·93%) | < 0·001 |
| Extra-corporeal membrane oxygenation, n (%) | 1125 (1·06%) | 282 (1·5%) | < 0·001 |
| Vasopressors use, n (%) | 28,943 (27·31%) | 7448 (39·7%) | < 0·001 |
| Renal replacement therapy, n (%) | 7358 (6·94%) | 2116 (22·33%) | < 0·001 |
| Acute liver failure, n (%) | 1806 (1·70%) | 668 (3·56%) | < 0·001 |
| Pulmonary embolism, n (%) | 7981 (7·53%) | 556 (2·96%) | < 0·001 |
| Venous thrombosis, n (%) | 3969 (3·75%) | 614 (3·27%) | 0·001 |
| In-hospital death, n (%) | 26,407 (24·9%) | 3966 (21·1%) | < 0·001 |
| Duration of invasive mechanical ventilation (days) | 13 (6–26) | 10 (4–19) | < 0·001 |
| Survivors only | 13 (6–26) | 10 (5–19) | < 0·001 |
| ICU length of stay (days) | 7 (3–16) | 7 (4–15) | < 0·001 |
| Survivors only | 7 (3–14) | 7 (4–14) | 0·007 |
| Hospital length of stay (days) | 14 (8–24) | 14 (8–25) | < 0·001 |
| Survivors only | 14 (8–24) | 14 (8–26) | < 0·001 |
| Disseminated intravascular coagulation, n (%) | 446 (0·42%) | 212 (1·13%) | < 0·001 |
ICU, intensive care unit.
Table 3.
Fine and Gray models of invasive mechanical ventilation.
| Invasive MV |
Death without invasive MV |
|||
|---|---|---|---|---|
| aSHR | 95%CI | aSHR | 95%CI | |
| Age (+10 years) | 0·90 | 0·90–0·91 | 2·68 | 2·63–2·74 |
| Male gender | 1·21 | 1·18–1·23 | 1·06 | 1·02–1·11 |
| Arterial hypertension | 1·25 | 1·23–1·28 | 0·72 | 0·69–0·76 |
| Diabetes mellitus | 1·01 | 0·96–1·05 | 1·27 | 1·17–1·38 |
| Heart disease | 0·99 | 0·96–1·02 | 1·05 | 0·99–1·11 |
| Lung disease | 1·02 | 0·99–1·04 | 1·07 | 1·01–1·14 |
| Cirrhosis | 0·92 | 0·85–1·01 | 2·12 | 1·76–2·57 |
| Cancer | 0·57 | 0·53–0·62 | 2·11 | 1·91–2·32 |
| Hematological malignancies | 0·86 | 0·81–0·91 | 1·20 | 1·07–1·34 |
| Chronic kidney disease | 0·69 | 0·66–0·72 | 1·21 | 1·14–1·29 |
| Immunodepression | 0·87 | 0·83–0·90 | 1·76 | 1·63–1·91 |
| Modified SAPS II | ||||
| ≤ 14 | Ref | Ref | ||
| 15 – 20 | 2·00 | 1·93–2·07 | 1·32 | 1·25–1·40 |
| 21–28 | 3·41 | 3·29–3·53 | 1·53 | 1·45–1·63 |
| ≥ 29 | 6·95 | 6·72–7·19 | 1·70 | 1·60–1·80 |
| Type of infection | ||||
| Influenza | Ref | Ref | ||
| COVID-19 | 0·87 | 0·85–0·89 | 2·40 | 2·24–2·57 |
aSHR, adjusted sub distribution hazard ratio; CI, confidence interval; MV, mechanical ventilation; SAPS II, Simplified Acute Physiology Score.
Among Covid-19 patients, prone position and ECMO were used in 20,231 (19%) and 1125 (1%) patients, corresponding to 56% and 3% of patients who received invasive mechanical ventilation, respectively. Among influenza patients, it concerned 2239 (12%) and 282 (1%) patients, respectively.
Compared to influenza patients, COVID-19 patients were less likely to receive vasopressors (27% vs. 40% p < 0·001) and RRT (7% vs. 22%, p < 0·001). In the subgroup of patients who received invasive mechanical ventilation, COVID-19 patients were still less likely to receive vasopressors (74% vs. 77%, p < 0·001) and RRT (18% vs. 22% p < 0·001) than the cohort with influenza (Supplementary Table 1).
Pulmonary embolism was more frequently observed in COVID-19 patients than in influenza patients (7.5% vs. 3%, p < 0·001) (Table 2). This was also the case in patients who received invasive mechanical ventilation (10% vs. 4%, p < 0·001) (Supplementary Table 1).
Outcome analysis
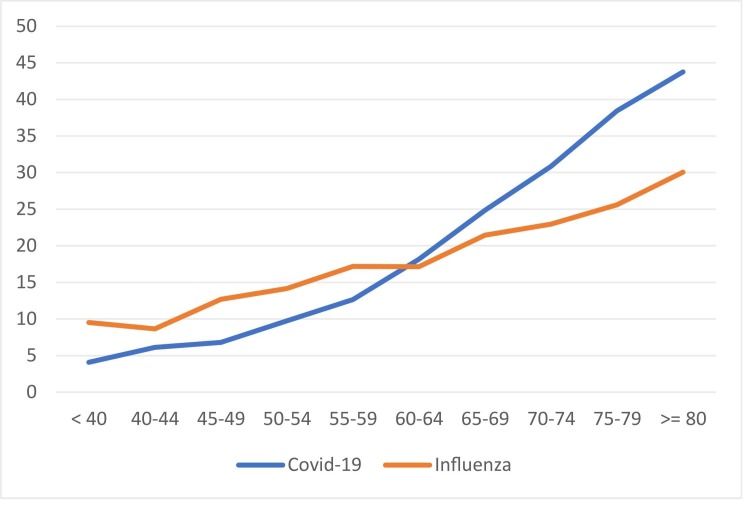
Of the entire study group, hospital mortality was higher in patients with COVID-19 than in patients with influenza (25% vs. 21%, p < 0·001) (Table 2), both in those who received invasive mechanical ventilation (40% vs. 33%, p < 0·001) (Supplementary Table 1), and in subjects who did not receive invasive mechanical ventilation (17% vs. 11%, p < 0·001). Age-specific mortality rates for COVID-19 and influenza are shown in Fig. 1. For patients younger than 60 years, mortality was higher in patients with influenza than in those with COVID-19 (13% vs. 9%, p < 0·001). Conversely, mortality rates were higher in COVID-19 patients who were 60 or older (32% vs. 24%, p < 0·001).
Fig. 1.
In-hospital mortality rate according to age and type of infection. Blue line represented COVID-19 patients. Orange line represents Influenzae patients. X-axis correspond to age. Y-axis correspond to in-hospital mortality rate.
After adjustment for age, sex, comorbidities, and modified SAPS II score at admission, the Fine-Gray model demonstrated that the likelihood of in-hospital death was higher in patients with COVID-19 (aSHR = 1.69; 95%CI = 1.63–1.75) compared to patients with influenza, especially among patients 65 years and older (aSHR = 1.91; 95%CI = 1.83–1.99) ( Table 4). In the subgroups of patients who required invasive mechanical ventilation, the likelihood of in-hospital death was also higher in patients with COVID-19 (aSHR = 1.46; 95%CI = 1.40–1.53) than in those with influenza (Supplementary Table 2).
Table 4.
Fine and Gray models of in-hospital death.
| In-hospital death |
|||||||
|---|---|---|---|---|---|---|---|
| All patients |
Patients < 65 years |
Patients ≥ 65 years |
|||||
| aSHR | 95%CI | aSHR | 95%CI | aSHR | 95%CI | ||
| Age (+ 10 years) | 1·69 | 1·67–1·71 | 1·57 | 1·51–1·62 | 1·65 | 1·62–1·68 | |
| Male gender | 1·19 | 1·16–1·22 | 1·07 | 1·01–1·13 | 1·23 | 1·20–1·27 | |
| Arterial hypertension | 0·79 | 0·77–0·81 | 0·82 | 0·77–0·87 | 0·79 | 0·77–0·81 | |
| Diabetes mellitus | 1·17 | 1·11–1·24 | 1·10 | 0·97–1·25 | 1·17 | 1·11–1·24 | |
| Heart disease | 0·99 | 0·95–1·02 | 1·15 | 1·05–1·26 | 0·97 | 0·93–1·00 | |
| Lung disease | 0·99 | 0·95–1·03 | 0·90 | 0·83–0·98 | 1·00 | 0·96–1·05 | |
| Cirrhosis | 1·83 | 1·64–2·04 | 2·04 | 1·75–2·38 | 1·59 | 1·37–1·85 | |
| Cancer | 1·37 | 1·27–1·47 | 1·98 | 1·76–2·27 | 1·22 | 1·12–1·32 | |
| Hematological malignancies | 1·16 | 1·08–1·24 | 1·30 | 1·13–1·51 | 1·11 | 1·02–1·20 | |
| Chronic kidney disease | 1·04 | 0·99–1·08 | 1·17 | 1·05–1·30 | 1·00 | 0·96–1·05 | |
| Immunodepression | 1·45 | 1·38–1·53 | 1·61 | 1·46–1·77 | 1·33 | 1·25–1·41 | |
| Modified SAPS II | |||||||
| ≤ 14 | Ref | Ref | Ref | ||||
| 15 – 20 | 1·56 | 1·50–1·63 | 2·00 | 1·79–2·23 | 1·50 | 1·44–1·58 | |
| 21–28 | 2·20 | 2·11–2·29 | 3·09 | 2·77–3·43 | 2·05 | 1·95–2·14 | |
| ≥ 29 | 4·09 | 3·93–4·25 | 7·12 | 6·45–7·87 | 3·54 | 3·39–3·69 | |
| Type of infection | |||||||
| Influenza | Ref | Ref | Ref | ||||
| COVI-19 | 1·69 | 1·63–1·75 | 1·16 | 1·09–1·25 | 1·91 | 1·83–1·99 | |
aSHR, adjusted sub distribution hazard ratio; CI, confidence interval; SAPS II, Simplified Acute Physiology Score.
Although the difference between groups was not clinically relevant, ICU length of stay was statistically longer in patients with COVID-19 than in those with influenza (7 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 days vs. 7 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 days) (p < 0·001) (Table 2). However, in the subgroups of patients receiving invasive ventilation, ICU length of stay was 18 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 days for patients with COVID-19 vs. 15 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 days for patients with influenza (p < 0·001). In the whole population, total hospital LOS was 25 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 days vs. 21 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 days (p < 0·001) in patients with COVID-19 and in patients with influenza, respectively.
Sars-CoV-2 vaccination
In a sensitivity analysis, we restricted the analysis to patients with COVID-19 admitted after January, 2021 for whom vaccine status was available (n = 48,140). Adjusting for age, sex, comorbidities, immunocompromised status, and SAPS-II score at admission, a multivariate model showed that COVID-19 patients had a higher risk of death compared to influenza patients regardless of the vaccination status (COVID-19 non-vaccinated aSHR = 1.73; 95%CI = 1.66–1.80; COVID-19 partially vaccinated: aSHR = 1.79; 95%CI = 1.67–1.93; COVID-19 fully vaccinated: aSHR = 1.48; 95%CI = 1.26–1.75) (Supplementary Table 3).
Discussion
In this French nationwide observational study, we showed major differences between critically ill patients with severe COVID-19 and influenza pneumonia admitted to an ICU. The two categories differed in baseline characteristics, ICU management, and outcomes. Patients with COVID-19 were less likely to receive invasive mechanical ventilation but had a higher likelihood of in-hospital death. Whether or not they received invasive mechanical ventilation, mortality was consistently higher in patients with COVID-19 than in patients with influenza, especially among those who were 65 years and older and independently of the invasive mechanical ventilation status.
Patients with COVID-19 were younger, more frequently male, and had a SAPS II at ICU admission. However, whilst CCI is a useful tool to synthesize comorbid conditions, it does not capture all important chronic diseases, such as arterial hypertension, and one cannot conclude that patients with COVID-19 admitted to the ICU had fewer comorbidities. For instance, patients with COVID-19 presented more often with arterial hypertension, solid tumor, and chronic kidney disease. The higher proportion of arterial hypertension has been already described31, 32, 33 and the potential role of a chronic exposure of renin-angiotensin system (RAS) acting agent on the severity of COVID19 has been questioned. Indeed, RAS blockers might upregulate the expression of angiotensin-converting enzyme 2 (ACE2) receptor, which acts as a co-receptor for human cell infection by SARS-CoV-2 through the binding with the spike protein. However, several studies, included RCTs concluded to an absence of effect of chronic exposure of RAS blocked and/or RAS blocked discontinuation.31, 32, 33
Conversely, patients with influenza had higher instances of chronic respiratory disease, congestive heart disease, and malignancy. The greater proportion of male gender and respiratory chronic disease in patients with influenza are common observations reported in observational studies,13, 14, 15, 17, 18, 19, 34 suggesting that the presence of lung damage from a preexisting condition might be necessary for ICU admission in the case of influenza but not in the case of COVID-19. The role of age in these infections remains debated, especially in studies focusing on ICU patients.35 Indeed, there has been intense research into the management of patients with ARDS over the last decade, leading to improved outcomes and, consequently, a broadening of the criteria for admission to intensive care for older patients.36, 37, 38, 39 Concerning the others comorbidities, we observed that our study is distinguished by a lower proportion of many comorbidities as diabetes, chronic lung disease, congestive heart failure, chronic kidney disease and solid tumor.14, 15, 16, 17, 18, 20, 39 There are several reason for this. First, we previously published, in a study comparing the management and outcome of COVID-19 patients admitted of ICU according time (March 2020 and June 2021), a significant decreasing of all patient’s comorbidities. Majority of studies comparing COVID-19 and influenza have been limited to the first wave of COVID-19 patients, which were exposed to a higher proportion of comorbidities. Second, comorbidities were identified in our study according to coding diagnosis registered in the French nationwide administrative health care database. This could limit the comparison with others studies, especially monocentric observational cohorts, which do not use ICD-10 classification and collected data from medical reports or medication. Third, there are already reported some discrepancies between characteristics COVID-19 populations depending on the origin of study suggested the importance of ethnic origin in incidence and severity of the disease.
While most studies comparing COVID-19 to influenza have reported greater use of invasive respiratory, hemodynamic and renal supportive care in patients with COVID-19,14, 19, 34, 40 these studies looked at all patients admitted to hospital and did not focus on patients specifically admitted to the ICU, thus exposing statistical bias due to censoring. Once focused on ICU patients, this difference is either attenuated15, 34 or reversed.18
Most epidemiological studies of COVID-19 were performed within the first surge, which may have differed from the subsequent waves regarding treatment, ventilation management, and ICU admission capacity. In our study, although invasive mechanical ventilation during the first surge remained lower in patients with COVID-19 than in those with influenza, this difference was much smaller and might not have been highlighted in a smaller cohort. Because initial recommendations warned of the potential risk of aerosolisation that, in the case of HFNC or NIV, could have increased the risk of contamination for healthcare workers,41 studies performed during the first surge of COVID-19 reported that only 19% of patients received HFNC in the ICU.6 Thereafter, it has been shown that the risk of aerosolisation with HFNC was similar to that with standard oxygen therapy.42 Moreover, it has been suggested that HFNC use might be associated with a lower intubation rate during the first pandemic wave.43, 44 All combined, international guidelines recommended the use of HFNC and NIV as first-line therapy in COVID-19 patients with ARF for the subsequent pandemic surges.45 Later, the results of the RECOVERY-RS study found no significant association between initial strategy of HFNC and the risk of tracheal intubation.46 The main consequence of the first data concerning HFNC was the decreasing rate of intubation in COVID-19 patients over time as reported in our previous study, with a decrease of 10% associated with a lower use of vasopressor therapy and lower rate of renal failure.47
The use of prone positioning was doubled during COVID-19 compared to influenza. This might not only be because of more severe hypoxemia in patients with COVID but also because, pre-pandemic, ventilation management trends in the ICU prone position were already being more frequently used following the PROSEVA study published in 2013.37 In a previous international study, only 13.7% of patients with ARDS had at least one session in the prone position, and the most prevalent reason for not using prone positioning was that hypoxemia was not considered sufficiently severe.48 Though the severity of the ARDS (PaO2/FiO2 ratio) was not available in our database, many reports on COVID-19-related ARDS showed that these patients were highly hypoxemic.49 Patients with COVID-19 had a median duration of invasive mechanical ventilation longer than patients with influenza. As ARDS is known to be associated with a higher risk of prolonged duration of invasive mechanical ventilation, it might explain a higher use of the prone position in COVID-19 patients.50 Finally, prone positioning in non-ventilated patients has been demonstrated to reduce the need for intubation, supporting the use of this procedure, including in less severe patients.51, 52, 53
Despite the lower rate of organ support, we found that COVID-19 was strongly associated with a higher likelihood of in-hospital death after adjustment for age, gender, comorbidities, and severity at ICU admission. Previous reports also found that patients admitted for COVID-19 had higher mortality than those admitted for influenza, whatever the severity.50, 54, 55 In our study, the excess of in-hospital deaths of COVID-19 patients compared with influenza was more significant in patients aged 60 years and older. The association between age and the likelihood of in-hospital death is consistent with previous studies.50, 56 Among patients who did not receive invasive mechanical ventilation, mortality was higher in patients with COVID-19 than in patients with influenza. This result suggests that there were probably more patients with COVID-19 admitted to the ICU but for whom life support was more limited (in particular for invasive mechanical ventilation) than in patients with influenza. Indeed, the type of patients who received invasive mechanical ventilation is different between the two groups, as Charlson comorbidity index was lower in patients with COVID-19 than in patients with influenza. It might reflect that selection and therapeutic limitation based on frailty and/or comorbidities was stricter in patients with COVID-19 than in patients with influenza, probably due to epidemic pressure and limited resources.
Finally, anti-virus treatment used in the ICU, and vaccination against influenza before ICU admission, could explain the lower mortality in the influenza cohort compared to those with COVID-19. However, in our study, most of patients have been admitted in ICU during the pre-vaccination era and the current cohort of patients with COVID-19 might have different characteristics to the group studied here. We assessed patients with COVID-19 admitted at the beginning of the French vaccine campaign in those for whom vaccine status was available. We observed that, even though full vaccination decreased the risk of death, patients admitted with COVID-19 still had worse outcomes than those with influenza. However, these data only concerned a few number of patients, at the beginning of the vaccine campaign, and further studies are needed in order to compare vaccinated COVID-19 patients to patients with influenza.
Our study has some limitations. First, clinical data (such as arterial blood pressure), as well as laboratory results (particularly arterial blood gas), were not collected in the SNDS and thus could not be used to describe respiratory and cardiovascular failures at admission. But details regarding SAPS II, as well as respiratory and hemodynamic support, were available from our data and were used as a surrogate of severity. In the same way, data concerning COVID-19 variants as well as common drugs used (steroids for example) are not available. Indeed, it would have been interested to compare the risk for each outcomes according to COVID-19 but also influenza variants. It should be noted that in a previous publication, we have analyzed the trends during different surges which could be used here as a proxy for the different variants.47 Second, we did not collect data on pressures on hospital admission or availability of beds in the ICU. This is critical because it could have led to patient selection and early discharge from the ICU and thus contribute to suboptimal care. Unfortunately, our data did not capture ICU bed occupancy during this period. Thirdly, only patients admitted to ICU were included in our study. Because of epidemic pressure, some patients that would usually been admitted to ICU for NIV or HFNC might have been admitted to another ward (for example infectious disease). This could lead to a selection bias as we do not have data for all severe Covid-19 patients but we assume that it concerns a small number of patients, compared with the large volume of patients in our study.
Our study has some strengths. To the best of our knowledge, our work is the largest study focused on ICU patients comparing COVID-19 with influenza. Indeed, we included all patients with COVID-19 admitted to an ICU between March, 2020 and June, 2021, corresponding to more than 100,000 patients. These patients were compared to all patients with influenza admitted to an ICU over a 5-year period. In addition, because SNDS is a national database, we were able to include all inpatients in the country without center selection. Finally, we were not limited to the first surge but could use patients from successive surges, including the one after the start of the vaccination program.
Conclusion
In a nationwide observational study, critically ill COVID-19 patients had a higher likelihood of death than patients with influenza despite a lower use of respiratory and hemodynamic invasive supportive care. The excess of in-hospital mortality persisted in COVID vaccinated patients.
Funding
This study received no support from any funding source.
CRediT authorship contribution statement
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Naouri Diane. The first draft of the manuscript was written by Naouri Diane and Jamme Matthieu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jinf.2023.05.011.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Aziz S., Arabi Y.M., Alhazzani W., Evans L., Citerio G., Fischkoff K., et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46(7):1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallet F. (DREES/OSAM/LABSANTE/EXTERNES). Parcours hospitaliers des patients atteints de la Covid-19 de mars 2020 à janvier 2021; 2020.
- 5.Deltour Q. (DREES/OSAM/LABSANTE). Parcours hospitaliers des patients atteints du Covid-19 lors des troisième et quatrième vagues épidémiques; 2021.
- 6.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med, 47(1); 2021, p. 60–73. [DOI] [PMC free article] [PubMed]
- 7.Reyes L.F., Murthy S., Garcia-Gallo E., Irvine M., Merson L., Martin-Loeches I., et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res. 2022;8(1):00552–02021. doi: 10.1183/23120541.00552-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gacouin A., Lesouhaitier M., Reizine F., Pronier C., Grégoire M., Painvin B., et al. Short-term survival of acute respiratory distress syndrome patients due to influenza virus infection alone: a cohort study. ERJ Open Res. 2020;6(4):00587–02020. doi: 10.1183/23120541.00587-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicoll A., Brown C., Karcher F., Penttinen P., Hegermann-Lindencrone M., Villanueva S., et al. Developing pandemic preparedness in Europe in the 21st century: experience, evolution and next steps. Bull World Health Organ. 2012;90(4):311–317. doi: 10.2471/BLT.11.097972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X., Wang H., Su Z., Li W., Yang D., Deng F., et al. Co-infection of SARS-CoV-2 and Influenza virus in Early Stage of the COVID-19 Epidemic in Wuhan, China. J Infect. 2020;81(2):e128–e129. doi: 10.1016/j.jinf.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coronavirus disease (COVID-19): similarities and differences between COVID-19 and influenza [Internet]. [cité 26 févr 2022]. Disponible sur: 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/coronavirus-disease-covid-19-similarities-and-differences-with-influenza〉.
- 12.Faury H., Courboulès C., Payen M., Jary A., Hausfater P., Luyt C., et al. Medical features of COVID-19 and influenza infection: a comparative study in Paris, France. J Infect. 2021;82(2):e36–e39. doi: 10.1016/j.jinf.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piroth L., Cottenet J., Mariet A.S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y., Bowe B., Maddukuri G., Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. 2020;371:m4677. doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roedl K., Kahn A., Jarczak D., Fischer M., Boenisch O., de Heer G., et al. Clinical characteristics, complications and outcomes of patients with severe acute respiratory distress syndrome related to COVID-19 or influenza requiring extracorporeal membrane oxygenation-a retrospective cohort study. J Clin Med. 2021;10(22):5440. doi: 10.3390/jcm10225440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X., Du R.H., Wang R., Cao T.Z., Guan L.L., Yang C.Q., et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot H.K., Martin E.T., Gaglani M., Middleton D.B., Ghamande S., Silveira F.P., et al. Coronavirus disease 2019 (COVID-19) versus influenza in hospitalized adult patients in the United States: differences in demographic and severity indicators. Clin Infect Dis. 2021;73(12):2240–2247. doi: 10.1093/cid/ciab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serpa Neto A., Burrell A.J.C., Bailey M., Broadley T., Cooper D.J., French C.J., et al. Comparison of critical care occupancy and outcomes of critically Ill patients during the 2020 COVID-19 winter surge and 2009 H1N1 influenza pandemic in Australia. Ann ATS. 2021;18(8):1380–1389. doi: 10.1513/AnnalsATS.202010-1311OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi Y, Kuno T, Komiyama J, Adomi M, Suzuki T, Abe T, et al. Comparison of patient characteristics and in-hospital mortality between patients with COVID-19 in 2020 and those with influenza in 2017–2020: a multicenter, retrospective cohort study in Japan. Lancet Reg Health West Pac; 2022 [Internet] [cité 26 févr 2022]; 20. Disponible sur: 〈https://www.thelancet.com/journals/lanwpc/article/PIIS2666-6065(21)00274-1/fulltext〉. [DOI] [PMC free article] [PubMed]
- 20.Oliva A., Ceccarelli G., Borrazzo C., Ridolfi M., D.’Ettorre G., Alessandri F., et al. Comparison of clinical features and outcomes in COVID-19 and influenza pneumonia patients requiring intensive care unit admission. Infection. 2021;49(5):965–975. doi: 10.1007/s15010-021-01624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marois C., Nedelec T., Pelle J., Rozes A., Durrleman S., Dufouil C., et al. Comparison of clinical profiles and mortality outcomes between influenza and COVID-19 patients invasively ventilated in the ICU: a retrospective study from all Paris public hospitals from 2016 to 2021. Crit Care Explor. 2022;4(7) doi: 10.1097/CCE.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scailteux L.M., Droitcourt C., Balusson F., Nowak E., Kerbrat S., Dupuy A., et al. French administrative health care database (SNDS): the value of its enrichment. Therapie. 2019;74(2):215–223. doi: 10.1016/j.therap.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 23.Le Gall J.R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 24.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 25.Bousquet C., Trombert B., Souvignet J., Sadou E., Rodrigues J.M. Evaluation of the CCAM hierarchy and semi structured code for retrieving relevant procedures in a hospital case mix database. AMIA Annu Symp Proc. 2010;2010:61–65. [PMC free article] [PubMed] [Google Scholar]
- 26.LOI no. 2016-41 du 26 janvier 2016 de modernisation de notre système de santé (1). 2016-41; 2016.
- 27.Arrêté du 22 mars 2017 relatif au référentiel de sécurité applicable au Système national des données de santé.
- 28.Le règlement général sur la protection des données – RGPD. CNIL [Internet]. [cité 14 mars 2022]. Disponible sur: 〈https://www.cnil.fr/fr/reglement-europeen-protection-donnees〉.
- 29.Andersen P.K., Abildstrom S.Z., Rosthøj S. Competing risks as a multi-state model. Stat Methods Med Res. 2002;11(2):203–215. doi: 10.1191/0962280202sm281ra. [DOI] [PubMed] [Google Scholar]
- 30.Pepe M.S., Mori M. Kaplan-Meier. marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12(8):737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes R.D., Macedo A.V.S., de Barros E., Silva P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brehm T.T., van der Meirschen M., Hennigs A., Roedl K., Jarczak D., Wichmann D., et al. Comparison of clinical characteristics and disease outcome of COVID-19 and seasonal influenza. Sci Rep. 2021;11(1):5803. doi: 10.1038/s41598-021-85081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dres M., Hajage D., Lebbah S., Kimmoun A., Pham T., Béduneau G., et al. Characteristics, management, and prognosis of elderly patients with COVID-19 admitted in the ICU during the first wave: insights from the COVID-ICU study: prognosis of COVID-19 elderly critically ill patients in the ICU. Ann Intensive Care. 2021;11(1):77. doi: 10.1186/s13613-021-00861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 37.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 38.Frat J.P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 39.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani D de M, Damiani LP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low peep on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA, 318(14); 2017, p. 1335–45. [DOI] [PMC free article] [PubMed]
- 40.Piroth L., Cottenet J., Mariet A.S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons C., Callaghan M. The use of high-flow nasal oxygen in COVID-19. Anaesthesia. 2020;75(7):843–847. doi: 10.1111/anae.15073. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J; 2020 [Internet] [cité 28 févr 2022]; Disponible sur: 〈https://erj.ersjournals.com/content/early/2020/04/08/13993003.00892-2020〉. [DOI] [PMC free article] [PubMed]
- 43.Demoule A., Vieillard Baron A., Darmon M., Beurton A., Géri G., Voiriot G., et al. High-flow nasal cannula in critically III patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet N., Martin O., Boubaya M., Levy V., Ebstein N., Karoubi P., et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. 2021;11(1):37. doi: 10.1186/s13613-021-00825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SCCM. COVID-19 guidelines [Internet]. Society of Critical Care Medicine (SCCM); 2022. Disponible sur: 〈https://sccm.org/SurvivingSepsisCampaign/Guidelines/COVID-19〉.
- 46.Perkins G.D., Ji C., Connolly B.A., Couper K., Lall R., Baillie J.K., et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proceedings of reanimation 2022, the French Intensive Care Society International Congress. Ann Intensive Care, 12(1); 2022, p. 54. [DOI] [PMC free article] [PubMed]
- 48.Guérin C., Beuret P., Constantin J.M., Bellani G., Garcia-Olivares P., Roca O., et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44(1):22–37. doi: 10.1007/s00134-017-4996-5. [DOI] [PubMed] [Google Scholar]
- 49.Estenssoro E., Loudet C.I., Ríos F.G., Edul V.S.K., Plotnikow G., Andrian M., et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9(9):989–998. doi: 10.1016/S2213-2600(21)00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ludwig M., Jacob J., Basedow F., Andersohn F., Walker J. Clinical outcomes and characteristics of patients hospitalized for influenza or COVID-19 in Germany. Int J Infect Dis. 2021;103:316–322. doi: 10.1016/j.ijid.2020.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrmann S., Li J., Ibarra-Estrada M., Perez Y., Pavlov I., McNicholas B., et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fazzini B., Page A., Pearse R., Puthucheary Z. Prone positioning for non-intubated spontaneously breathing patients with acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Br J Anaesth. 2022;128(2):352–362. doi: 10.1016/j.bja.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Luo J., Pavlov I., Perez Y., Tan W., Roca O., et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: a systematic review and meta-analysis. Lancet Respir Med. 2022;10(6):573–583. doi: 10.1016/S2213-2600(22)00043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma A.A., Hora T., Jung H.Y., Fralick M., Malecki S.L., Lapointe-Shaw L., et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ. 2021;193(12):E410–E418. doi: 10.1503/cmaj.202795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donnino MW, Moskowitz A, Thompson GS, Heydrick SJ, Pawar RD, Berg KM, et al. Comparison between influenza and COVID-19 at a tertiary care center. medRxiv; 2020 [Internet] [cité 18 févr 2022]. p. 2020.08.19.20163857. Disponible sur: 〈https://www.medrxiv.org/content/10.1101/2020.08.19.20163857v1〉.
- 56.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material